Abstract
The survival of enteric bacteria was measured in bovine feces on pasture. In each season, 11 cow pats were prepared from a mixture of fresh dairy cattle feces and sampled for up to 150 days. Four pats were analyzed for Escherichia coli, fecal streptococci, and enterococci, and four inoculated pats were analyzed for Campylobacter jejuni and Salmonella enterica. Two pats were placed on drainage collectors, and another pat was fitted with a temperature probe. In the first 1 to 3 weeks, there were increases (up to 1.5 orders of magnitude) in the counts of enterococci (in four seasons), E. coli (three seasons), fecal streptococci (three seasons), and S. enterica (two seasons), but there was no increase in the counts of C. jejuni. Thereafter, the counts decreased, giving an average ranking of the times necessary for 90% inactivation of C. jejuni (6.2 days from deposition) < fecal streptococci (35 days) < S. enterica (38 days) < E. coli (48 days) < enterococci (56 days). The pat temperature probably influenced bacterial growth, but the pattern of increases and decreases was primarily determined by desiccation; growth occurred when the water content was greater than 80%, but at a water content of 70 to 75% counts decreased. E. coli and enterococcus regrowth appeared to result from pat rehydration. Of 20 monthly leaching losses of E. coli, 16 were <10% of the total counts in the pat, and 12 were <1%. Drainage losses of C. jejuni (generally <1%) were detected for only 1 to 2 months. Although enterococci exhibited the best survival rate, higher final counts suggested that E. coli is the more practical indicator of bovine fecal pollution.
New Zealand has a population of around 4 million people, but there are far higher numbers of farm animals. For example, in July 2006 the number of sheep was estimated to be 39.8 million, the number of beef cattle was estimated to be 4.5 million, and the number of dairy cattle was estimated to be 5.1 million (28). Because these animals graze on open land throughout the year, they constitute a far greater potential source of fecal pollution than human effluents. Cattle farming, in particular, has been linked with degradation of the microbial quality of surface waters in New Zealand (7, 9, 43).
Management and mitigation strategies for animal pollution of water, as well as zoonotic pathogen risk assessments, would benefit from the ability to gauge the size of the enteric microbe “reservoir” in feces deposited on a given area of pasture. Thus, reliable data are needed for microbial outputs from each livestock category, survival rates in deposited feces, and leaching rates under different irrigation and rainfall regimens.
Reviews of the survival of fecal indicators and pathogens from animal (mostly bovine) feces have focused primarily on studies of manure and slurries applied to soil (14, 19, 37, 40). However, composting and spreading procedures are not widely practiced in New Zealand. Furthermore, these procedures may not represent conditions inside naturally deposited cow pats, because they are designed to enhance feces breakdown and increase the inactivation of enteric bacteria by subjecting them to elevated composting temperatures or the antagonistic microbiota, pH changes, and desiccation associated with mixing with soil.
Comparatively few studies of the survival of enteric bacteria in cow pats on pasture have been reported. Coliforms and fecal coliforms survived for up to 18 weeks in cattle feces in hot, dry summers (5), and release of fecal coliforms and Escherichia coli from cattle feces has been recorded for up to 30 days (29, 38) and 100 days (24). Campylobacter spp. have been detected in cattle feces after incubation for 1 to 3 weeks at 5°C and after incubation for 1 week at 30°C, and Salmonella spp. have been detected for 12 to 28 weeks after incubation at 5°C and for 4 weeks after incubation at 30°C (30).
More recent survival studies have focused primarily on E. coli O157 and related strains in recognition of the importance of bovine feces as a major source of these pathogens (12). Most of these studies either have involved manure and slurries (16, 25) or have been laboratory based and have produced extinction times ranging from 24 h to 100 days, with faster inactivation generally associated with higher temperatures (13, 17, 21, 25, 27, 41). There appear to have been few field-based studies of survival in cow pats, although a 4- to 5-log10 decrease in E. coli O157:H7 within 50 days was recorded in inoculated cow pats placed on grassland (3).
This paper describes a field study of the comparative survival of bacterial indicators and pathogens in bovine feces on pasture over four seasons. The selected indicators were E. coli, fecal streptococci, and enterococci. Fecal streptococci were included in addition to enterococci because a previous survey showed that Streptococcus bovis (a non-Enterococcus species) comprised over one-half of the fecal streptococci in local cattle feces (32). The selected pathogens were Salmonella enterica and Campylobacter jejuni. New Zealand has reported annual incidences of salmonellosis and campylobacteriosis of 39 and 432 per 100,000, respectively (1), and the latter rate is very high compared to the rates in other developed countries. Both internationally and in New Zealand, cattle are widely regarded as important reservoirs for Salmonella and Campylobacter genotypes pathogenic to humans (10, 23, 26, 39).
MATERIALS AND METHODS
Field site.
This study was conducted at a unshaded experimental site (10 by 7 m) at Lincoln, 10 km south of Christchurch, New Zealand. The site pasture, which was a typical New Zealand sward of ryegrass and white clover, was hand mown throughout the study.
General approach and preparatory work.
The study involved the use of simulated, composite cow pats, each 30 cm in diameter and weighing 2.1 kg (the mean parameters for 20 fresh cow pats collected from local dairy farms). Preliminary sampling indicated that the counts of Salmonella spp. and Campylobacter spp. were too low and/or variable to provide reliable survival curves. Accordingly, one-half of the pats in each experiment were inoculated with both pathogens (see below).
A composite of the collected cow pats was gently mixed using a modified, hand-operated clothes tumbler/washer. The mixing efficiency was tested using five pats inoculated with S. enterica by comparing the counts for five samples from the center and perimeter of each pat. The results showed that 10 min of tumbler operation completely mixed the S. enterica with the fecal material (P = 0.001, chi-square test).
Experimental setup.
Four experiments were conducted, starting in the middle month of each (Southern Hemisphere) season, in January, April, July, and October. Although designated summer, autumn, winter, and spring experiments, respectively, each experiment extended over two or three seasons.
About 24 kg of feces was collected from up to 15 different cows within 30 s of deposition onto the concrete pad outside the milking shed on a dairy farm at Te Pirita, 30 km south of Christchurch. The material was then either transported directly to the field site for setup or stored overnight in the dark at 5 to 6°C (see below).
For each experiment, 11 replicate pats were placed on the pasture in separate, pegged plots. Ten pats were set out 1.5 m apart in two rows of five. Two fecal mixtures were prepared (one for each row), as follows. (i) Approximately 11 kg of the composite sample of fresh feces (direct from the farm) was mixed for 10 min, and 2.1-kg portions were weighed and poured into a 30-cm-diameter circular mold, which was then removed, giving a pat depth of approximately 2 cm. These pats were used for measuring indicator survival. (ii) The following day, using feces stored overnight, the procedure was repeated but with addition of a mixture of S. enterica serovar Typhimurium and C. jejuni, each cultured from a mixture of six strains isolated from New Zealand rivers, as described by Sinton et al. (31). This set of pats was used to measure the survival of these pathogens (the mixtures of six strains were used to obtain a better average inactivation rate for each pathogen).
One pat in each row of five pats was placed on a removable drainage collector, a pad of natural turf (diameter, 350 mm; depth, 20 mm) from the site sitting on a buried funnel and discharging into a collection vessel. This pat (not sampled) was used to gauge bacterial leaching losses. The eleventh pat (also not sampled) was placed nearby and fitted with a temperature probe connected to an Onset HOBO data logger.
Sample collection.
Immediately after the mold was removed, a 12- to 15-g (wet weight) sample was scooped from each pat using a sterile spatula. After several days, a hard crust formed on the pats, so they were sampled using a set of four sharp, bent knives. These knives were inserted to delineate a fecal plug (20 by 20 mm), which was removed with a spatula down to soil level. A numbered wooden peg was placed in the delineated square and carefully hammered down to the pat surface. This procedure identified all sample locations and reduced any artificial increase in pat disintegration from rainfall entry. Samples were collected in sterile plastic pots and transported to the laboratory in a cooled, dark container.
Sampling of the pats continued until they had disintegrated and/or were indistinguishable from the underlying soil, a period ranging from 5 to 6 months. The sampling frequency varied from daily for C. jejuni in fresh pats to monthly for the fecal indicators at the end of each season. Immediately after rainfall, the volume of water collected in the drainage collectors was measured, and samples were analyzed for only E. coli and C. jejuni. At the end of each experiment, the remains of the undisturbed pat on the top of each collection funnel were removed, weighed, and analyzed for indicators or pathogens.
Laboratory methods.
The moisture content of each fecal sample was determined by drying 2 g at 103 to 105°C for 18 h (6). All microbial counts were then expressed per gram of dry weight.
For each microbiological analysis, 2 g (wet weight) of fecal sample was added to 18 ml of sterile water, blended, and processed as follows.
(i) E. coli.
Samples were analyzed using the Colilert Quanti Tray system (IDEXX Laboratories) according to the manufacturer's instructions.
(ii) Fecal streptococci and enterococci.
Following tests with selective Pfizer enterococcus agar (6) and the Enterolert system (IDEXX Laboratories), the procedure of Bordner et al. (4) was adopted because it was found to give better separation of Enterococcus and non-Enterococcus species in bovine feces. Blended samples were dispensed into 5 × 5 most-probable-number (MPN) dilution series of azide dextrose broth (Difco) and incubated at 35°C for 48 h. The contents of all tubes were (i) streaked onto plates of KF streptococcus (KFS) agar (Difco) and incubated at 35°C for 24 to 48 h and (ii) inoculated into vials of brain heart infusion broth (BBL) containing 6.5% NaCl and incubated at 45°C for 24 h. All red colonies growing on KFS agar were counted as fecal streptococci. Red colonies surrounded by yellow zones of KFS agar that corresponded to growth in brain heart infusion broth containing 6.5% NaCl were counted as enterococci. This procedure was calibrated using cultures of Enterococcus faecium ATCC 19434, Enterococcus faecalis ATCC 19433, Enterococcus durans ATCC 19432, and S. bovis ATCC 33317.
(iii) C. jejuni.
For each experiment, the six C. jejuni strains were cultured in 250 ml of modified Exeter broth at 42°C for 48 h. Tests showed that there were large losses of C. jejuni with washing steps, so the broth culture was centrifuged once and the pellet was resuspended in 40 ml of modified Exeter broth. The suspension was stored overnight at 6°C and mixed into the composite cow feces as described above.
The fecal material was analyzed for C. jejuni using the method described by Hudson et al. (22). Samples were dispensed into 5 × 5 MPN dilution series of modified Exeter broth prepared from Oxoid media and supplements and incubated at 42°C for 48 h. The contents of all tubes were streaked onto plates of modified Exeter agar and incubated at 42°C for 48 h. All pink or brown colonies were counted as C. jejuni, and randomly selected colonies were confirmed to be this species by the PCR method of Eyers et al. (15).
(iv) S. enterica.
For each experiment, the S. enterica inoculum was cultured in 40 ml of selenite cysteine broth at 35°C for 24 h. The culture was washed and resuspended twice in a sterile saline solution, stored overnight at 6°C, and then mixed into the composite cow feces as described above.
The fecal material was analyzed for salmonellae using the method described by Cook (8). Samples were dispensed into 5 × 5 MPN dilution series of selenite cysteine broth (Merck) and incubated at 35°C for 18 to 24 h. The contents of all tubes were streaked onto XLD agar (Oxoid) containing novobiocin (Appli Chem) and incubated at 35°C for 18 to 24 h. Deep red colonies with a black center were counted as S. enterica. In preliminary tests, randomly selected colonies were confirmed to be salmonellae by streaking on triple sugar iron agar (Merck) and lysine decarboxylase agar (Oxoid) and were confirmed to be urease negative by using the procedures described in the Compendium of Methods for the Microbiological Examination of Foods (11). After this, since no naturally occurring salmonellae were found in the pats prior to inoculation, no further confirmatory steps were used.
Meteorological data.
Data for the following meteorological parameters were obtained from a weather station that was 200 m from the site in order to determine their influence on microbial survival in each experiment: rainfall (in millimeters), air temperature (in degrees centigrade), hourly solar radiation (in megajoules per square meter), soil temperature at 10 cm, daily wind run (a measure of the average wind speed over the previous 24 h [in kilometers]), and Penman evapotranspiration (in millimeters per day).
RESULTS
The initial E. coli and fecal streptococcal counts in the cow pats (Table 1) were all around 105 or 106 CFU g−1. In contrast, the initial enterococcus counts were highly variable, ranging from over 105 CFU g−1 in the spring to 99 CFU g−1 in the autumn experiment. As previously noted, preliminary sampling showed that the natural counts of C. jejuni in individual pats were highly variable, ranging from <1 to 1.8 × 107 CFU g−1. This necessitated inoculation of the pats to consistently provide the initial counts in the composite samples shown in Table 1. The natural S. enterica counts were low, ranging from 0 to 8 CFU g−1, which again necessitated inoculation.
TABLE 1.
Initial, peak, and final counts of indicators and pathogens in cow pats placed on pasture in each season
| Measurement | Concn (CFU/g [dry wt] of feces)a
|
||||
|---|---|---|---|---|---|
| E. coli | Fecal streptococci | Enterococci | C. jejuni | S. enterica | |
| Winter | |||||
| Initial | 2.2 × 106 | 6.9 × 105 | 3.2 × 102 | 6.7 × 106 | 1.1 × 107 |
| Peak | 1.0 × 106 (21) | 1.3 × 103 (21) | 2.4 × 107 (7) | ||
| Final | 91 (149) | 1.3 × 103 (149) | 4.7 × 102 (149) | 3.1 × 102 (76)b | 5.0 × 102 (140) |
| Spring | |||||
| Initial | 3.3 × 105 | 7.2 × 106 | 1.3 × 105 | 6.3 × 106 | 1.7 × 107 |
| Peak | 5.0 × 105 (7) | 3.4 × 107 (7) | 3.5 × 106 (7) | ||
| Final | 1.4 × 103 (148) | 1.4 × 103 (148) | 71 (148) | 46 (16)b | 40 (153) |
| Summer | |||||
| Initial | 3.0 × 106 | 1.4 × 106 | 2.8 × 104 | 2.7 × 107 | 1.7 × 107 |
| Peak | 4.8 × 107 (7) | 5.0 × 107 (7) | 1.3 × 105 (7) | ||
| Final | 2.9 × 104 (141) | 1.3 × 104 (141) | 1.5 × 103 (141) | 4 (10)b | 1.5 × 104 (146) |
| Autumn | |||||
| Initial | 2.2 × 105 | 4.0 × 106 | 99 | 6.1 × 106 | 3.2 × 107 |
| Peak | 1.9 × 106 (14) | 9.6 × 102 (7) | 6.5 × 107 (5) | ||
| Final | 1.7 × 103 (155) | 1.6 × 102 (155) | 1 (155) | 54 (27)b | 1.6 × 103 (153) |
Each count is the mean for four samples (one sample from each cow pat). Peak counts are given only where they were greater than the initial count. The number in parentheses is the day on which the count was recorded.
Last day on which C. jejuni was detected.
C. jejuni became undetectable before the end of each seasonal experiment, and the final enterococcal counts were as low as 1 CFU g−1 (autumn experiment). In contrast, E. coli and fecal streptococci persisted to the end of all of the experiments, with counts ranging from 102 to 104 CFU g−1, and the final S. enterica counts ranged from 40 to 104 CFU g−1.
The percent survival curves for the winter, spring, summer, and autumn experiments are shown in Fig. 1 to 4, respectively, together with water content curves and rainfall data. In all seasons, there was an initial increase in most of the indicator counts, followed by a decrease. To characterize and compare these phases, regression analyses were performed on the loge-transformed data (Table 2). One regression line (for the increase phase, where present) was fitted to the data up to and including the peak count, and a second line (for the decrease phase) was fitted to the data after and including the peak count. These regressions were used to derive increase and decrease coefficients (in day−1) (Table 2). In addition, the times necessary for 90% inactivation (T90) were calculated both for the decrease phase only and for all data points from the time of deposition (Table 2).
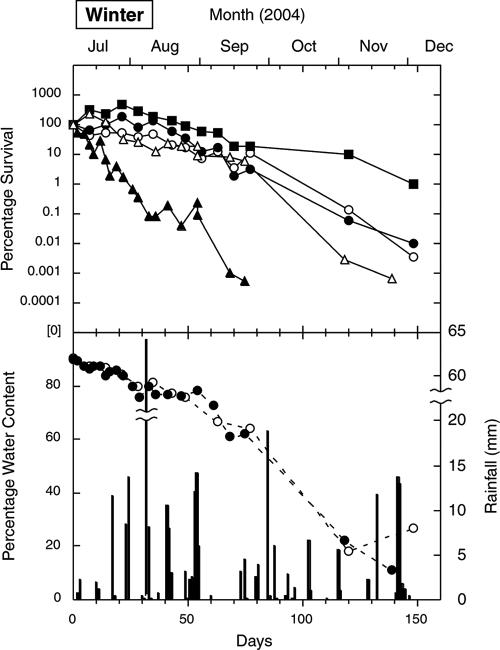
FIG. 1.
Survival of E. coli (○), fecal streptococci (•), enterococci (▪), S. enterica (▵), and C. jejuni (▴) in cow pats deposited on pasture in the winter, as well as the water contents of the indicator (○) and pathogen (•) pats and rainfall (columns).
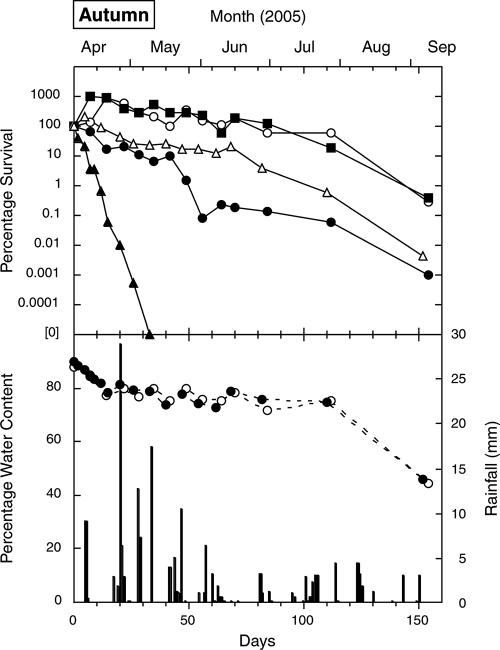
FIG. 4.
Survival of E. coli (○), fecal streptococci (•), enterococci (▪), S. enterica (▵), and C. jejuni (▴) in cow pats deposited on pasture in the autumn, as well as the water contents of the indicator (○) and pathogen (•) pats and rainfall (columns).
TABLE 2.
Growth and decrease phase coefficients and T90 values (calculated for both the total survival curve and for the decrease phase only) for indicator and pathogenic bacteria in cow patsa
| Season | Indicator or pathogen | Increase coefficient
|
Decrease coefficient
|
T90 for decrease phase (days)b | T90 for deposition (days) | ||
|---|---|---|---|---|---|---|---|
| Value (day−1) | R2 | Value (day−1) | R2 | ||||
| Winter | E. coli | 0.06 | 0.89 | 38 | 38 | ||
| Fecal streptococci | 0.03 | 0.80 | 0.08 | 0.91 | 29 | 34 | |
| Enterococci | 0.06 | 0.85 | 0.04 | 0.98 | 58 | 62 | |
| C. jejuni | 0.14 | 0.97 | 16 | 16 | |||
| S. enterica | 0.11 | (1)c | 0.09 | 0.99 | 26 | 26 | |
| Spring | E. coli | 0.06 | (1)c | 0.06 | 0.89 | 38 | 39 |
| Fecal streptococci | 0.23 | (1)c | 0.07 | 0.92 | 33 | 30 | |
| Enterococci | 0.48 | (1)c | 0.06 | 0.83 | 38 | 37 | |
| C. jejuni | 0.87 | 0.97 | 2.7 | 2.7 | |||
| S. enterica | 0.08 | 0.96 | 29 | 29 | |||
| Summer | E. coli | 0.40 | (1)c | 0.05 | 0.74 | 46 | 48 |
| Fecal streptococci | 0.53 | (1)c | 0.06 | 0.77 | 38 | 42 | |
| Enterococci | 0.25 | (1)c | 0.03 | 0.82 | 77 | 69 | |
| C. jejuni | 1.91 | 0.99 | 1.2 | 1.2 | |||
| S. enterica | 0.04 | 0.87 | 58 | 58 | |||
| Autumn | E. coli | 0.15 | 0.98 | 0.05 | 0.93 | 46 | 66 |
| Fecal streptococci | 0.07 | 0.98 | 33 | 33 | |||
| Enterococci | 0.33 | (1)c | 0.05 | 0.94 | 46 | 57 | |
| C. jejuni | 0.49 | 0.99 | 4.7 | 4.7 | |||
| S. enterica | 0.15 | (1)c | 0.06 | 0.95 | 38 | 38 | |
The coefficients (k) were derived from a regression of the natural logarithms: N = ekt, where N is the count and t is time. T90 values were calculated from T90 = 2.303/k.
Same as the T90 for deposition when there was no increase phase.
There were only two data points.
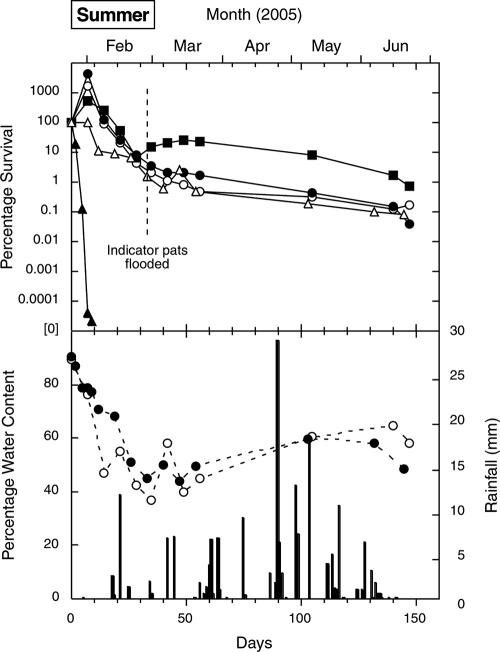
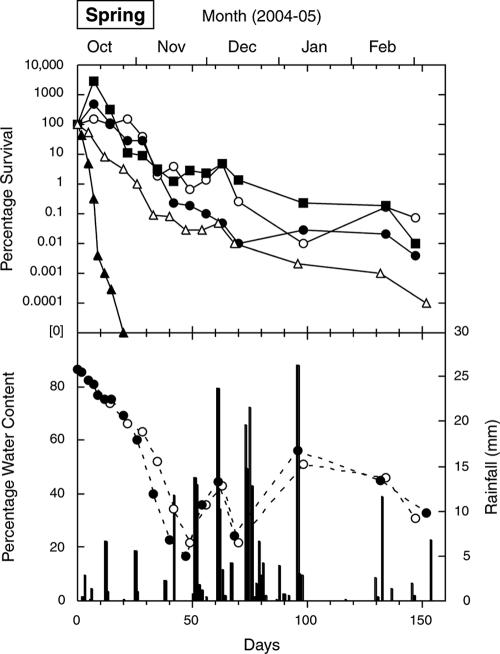
Enterococci exhibited an increase phase in all four seasons, and increases were also recorded for E. coli and fecal streptococci (three seasons) and for S. enterica (two seasons). Although increases of around 1.5 orders of magnitude were recorded (in the spring and summer), most increases were around 1 order of magnitude. The increases usually occurred between the first and second sampling times (7 days apart), although peak counts in the winter were not recorded for fecal streptococci and enterococci until the fourth sampling time (21 days). The concentrations of S. enterica increased slightly within the first 7 days in two seasons but fell below the initial counts thereafter. No increases in C. jejuni counts were recorded. Peak counts (where present) are shown in Table 1.
The overall decrease phase ranking (from the greatest inactivation to the least inactivation), based on averages for the decrease coefficient values in Table 2, was C. jejuni (0.853 day−1) > S. enterica (0.068 day−1) ≥ fecal streptococci (0.070 day−1) > E. coli (0.055 day−1) > enterococci (0.045 day−1). The equivalent T90 ranking was C. jejuni (6.2 days from deposition) < fecal streptococci (35 days) < S enterica (38 days) < E. coli (48 days) < enterococci (56 days).
In all seasons, the initial water content of the cow pats was around 90%. Figures 1 to 4 show that periods of rainfall appeared to decrease desiccation and sometimes rehydrated the pats. The final water contents varied from 15 to 60%, depending on the season and the elapsed time since the last rainfall.
The initial pat desiccation rate (i.e., the rate in the first 30 days) was highest in the summer, followed by the spring, autumn, and winter (Fig. 3, 2, 4, and 1, respectively). This ranking broadly corresponded to the average daily wind run for the month in which the experiment started: 365 km in January 2005 (summer), 380 km in October 2004 (spring), 343 km in April 2005 (autumn), and 291 km in July 2004 (winter). There was better correspondence between the initial desiccation rate and the average daily Penman evapotranspiration for the month: 4.9 mm in January 2005 (summer), 2.9 mm in October 2004 (spring), 1.8 mm in April 2005 (autumn), and 0.73 mm in July 2004 (winter).
FIG. 3.
Survival of E. coli (○), fecal streptococci (•), enterococci (▪), S. enterica (▵), and C. jejuni (▴) in cow pats deposited on pasture in the summer, as well as the water contents of the indicator (○) and pathogen (•) pats and rainfall (columns).
FIG. 2.
Survival of E. coli (○), fecal streptococci (•), enterococci (▪), S. enterica (▵), and C. jejuni (▴) in cow pats deposited on pasture in the spring, as well as the water contents of the indicator (○) and pathogen (•) pats and rainfall (columns).
Rainfall, drainage, and percent leaching losses for E. coli are shown in Table 3. The monthly drainage was for the most part less than 45 mm. The average drainage was 22% of the rainfall in the winter experiment, 45% of the rainfall in the spring experiment, 50% of the rainfall in the summer experiment, and 49% of the rainfall in the autumn experiment (excluding February 2005, when the site was flooded to a depth of around 10 mm for several hours by an overflow from a nearby water tank, causing drainage to exceed rainfall). The total monthly leaching losses of E. coli from each pat ranged from 105 to 106 CFU, decreasing to 102 to 103 CFU toward the end of the experiment. However, the percent losses remained relatively constant; of the 20 monthly E. coli leaching losses measured, 16 were <10% of the total counts in the pat and 12 were <1% of the total counts. C. jejuni (data not shown) was detected (generally accounting for <1% of the estimated counts in the pat) for only 2 months in the winter and 1 month in the summer and autumn and was not detected at all in the spring.
TABLE 3.
Monthly rainfall, drainage, and the percent loss of E. coli from undisturbed cow pat on the leachate collectora
| Month | Rainfall (mm) | Winter
|
Spring
|
Summer
|
Autumn
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Drainage (mm) | % E. coli loss from pat | Drainage (mm) | % E. coli loss from pat | Drainage (mm) | % E. coli loss from pat | Drainage (mm) | % E. coli loss from pat | ||
| July 2004 | 40.4 | 13.1 | 0.39 | ||||||
| August 2004 | 136.8 | 31.3 | 0.43 | ||||||
| September 2004 | 34.2 | 7.6 | 0.03 | ||||||
| October 2004 | 25.4 | 3.1 | 0.13 | 0.1 | 0.002 | ||||
| November 2004 | 45 | 9.6 | 4.71 | 28.2 | 16.6 | ||||
| December 2004 | 131.6 | 84.3 | 57.8 | ||||||
| January 2005 | 33.6 | 17.3 | 11.4 | ||||||
| February 2005 | 18.6 | 32.5b | 6.13 | 35.7b | 26.5 | ||||
| March 2005 | 36.6 | 10.3 | 0.13 | ||||||
| April 2005 | 52.4 | 30.1 | 5.95 | 32.4 | 0.25 | ||||
| May 2005 | 61 | 47.3 | 7.79 | 41.2 | 0.03 | ||||
| June 2005 | 19 | 7.0 | 1.74 | 5.7 | 0.005 | ||||
| July 2005 | 18.2 | 7.0 | 0.001 | ||||||
| August 2005 | 13.8 | 6.0 | 0.92 | ||||||
The total number of E. coli CFU in the pat was estimated from the geometric mean of the counts in the other four pats.
Site flooded (drainage exceeded rainfall).
The average final weight of the undisturbed pats on the leachate collectors was 214 g (wet weight) (around 1/10 of the initial wet weight); the average water content was 57%, and the diameters were around 20 cm (around two-thirds of the initial diameter). The final bacterial counts in these pats were broadly the same as those in the fragments of the sampled pats.
The continuous internal cow pat temperature record (11,104 measurements) (data not shown) was compared with the equivalent weather station records for air temperature, soil temperature (at 10 cm), and hourly global solar radiation (GSR). The temperature of the pats, soil, and air was governed by solar radiation and exhibited diurnal oscillations. The pats appeared to be directly warmed by sunlight, and the pat temperatures were generally higher than those of the air or soil. This effect was more marked during the spring and summer.
A multiple regression analysis on the complete data set (all seasons) suggested that: pat temperature = 3.4 × GSR + 0.9 × soil temperature + 0.13 × air temperature (R2 = 0.94; P < 0.0001), the where temperature is expressed in degrees centigrade and the GSR is expressed in megajoules per square meter.
The average, minimum, and maximum pat temperatures for each experiment were as follows: in the summer, 19.5, 6.2, and 46.9°C, respectively; in the autumn, 6.2, 0, and 27.5°C, respectively; in the winter, 10.1, 0.3, and 41.5°C, respectively; and in the spring, 18.4, 4.2, and 46.9°C, respectively. The spring and summer experiments shared the same maximum temperature, and because of a data logger failure in the autumn, the true minimum temperature (probably around −2.0°C) was not recorded.
DISCUSSION
Increase phase.
A major finding of this study was the initial increases (up to 1.5 orders of magnitude) in the indicator and S. enterica counts in many of the cow pats. Water content appeared to be the critical factor determining the growth period and magnitude. As long as the water content remained above 80%, a period of 7 to 30 days depending on the season and weather, there was growth of enterococci, usually growth of E. coli and fecal streptococci, and sometimes growth of S. enterica.
Temperature appeared to have a secondary role in determining the growth rate and duration. Growth was slower in the cooler winter experiment, but there were no marked growth rate differences between the other seasons, when pat temperatures regularly exceeded 20°C. In controlled laboratory experiments, Wang et al. (42) also recorded initial increases of up to 2.5 orders of magnitude for E. coli and fecal streptococci in dairy cow manure but demonstrated a survival-enhancing moisture level effect only for fecal streptococci.
Freshly deposited feces contain the nutrients required by enteric bacteria, so replication presumably depends on the cow pat retaining water and attaining suitable temperatures for growth (the optimum temperature is around 35°C for most enteric bacteria, although growth occurs at higher and lower temperatures). Thus, counts for fresh cow pats are likely to underestimate the loads on pastures during this period.
When exposed to sunlight, the pats quickly formed a skin, which thickened to a well-defined crust within about 48 h. As noted by Haynes and Williams (20), this crust helps to keep the interior of the pat moist and thus assists bacterial growth. Rainfall appeared to rehydrate the pats, particularly in the spring experiment (November to December 2004), when it was associated with a small increase in E. coli and enterococcus counts, although the water content remained below 45%. The February 2005 flooding event appeared to increase the water content of the pats by at least 20% to around 60% (Fig. 3) and was followed by a small but sustained increase in enterococcus counts. The possibility that pat rehydration causes regrowth of enteric bacteria may warrant further investigation.
Decrease phase.
The most marked feature of the decrease phase was the rapid inactivation of C. jejuni (Table 2 and Fig. 1 to 4). In contrast to the data for the indicators, there was no evidence of a C. jejuni increase phase. Instead, in all seasons, C. jejuni counts were reduced by 3 orders of magnitude while the pat water content was greater than 80%, and there was a further reduction by 1 to 3 orders of magnitude while water content remained between 70 and 80%. There were also marked seasonal differences in C. jejuni inactivation coefficients (Table 2), with a decrease coefficient ranking of summer (1.91 day−1) > spring (0.87 day−1) > autumn (0.49 day−1) > winter (0.14 day−1). These values corresponded to the ranking of average pat temperatures, summer (23.1°C) > spring (13.2°C) > autumn (11.3°C) > winter (5.9°C), during the relatively short periods (Table 1) in which C. jejuni was detectable. This suggests that temperature, rather than desiccation, most strongly influenced C. jejuni survival. Postexcretion exposure to oxygen diffusing into the pat may also increase the inactivation of this oxygen-sensitive bacterium. Factors determining the rapid inactivation of C. jejuni in cow pats, such as oxygen diffusion and temperature, may warrant further study in controlled laboratory microcosms.
In each season, the decreases in counts of the indicators and S. enterica to values below the initial concentrations appeared to be associated with a decrease in pat water content to 70 to 75%. In contrast to the data for C. jejuni, there was relatively little difference in the slopes for the decrease phase between the three indicators, regardless of the season. Comparisons with average seasonal temperatures were not considered meaningful for these organisms, because their inactivation curves extended over two or three seasons.
The counts for fecal streptococci did not initially increase as often as or to the same extent as the counts for enterococci except in the summer, and the fecal streptococci were inactivated more rapidly in all seasons. Unlike enterococci, they also showed no regrowth following the site flooding event. These results probably reflect well-recognized differences in species composition. The fecal streptococci include S. bovis, which comprised over one-half the fecal streptococci in fresh cow pats on nearby farms (32). Studies reviewed by Sinton et al. (33, 34) have shown that the S. bovis-S. equinus group is more rapidly inactivated in the environment than the enterococcal species E. faecium and E. faecalis.
Although enterococci have largely superseded fecal streptococci as an alternative to E. coli as an indicator, particularly in marine waters, low enterococcus counts at the end of each season (as low as 1 CFU g−1 [Table 1]) suggest that these organisms may have limited value as indicators of the presence of bovine feces. Notwithstanding the more rapid inactivation of streptococci, the final fecal streptococcus counts exceeded those of enterococci by 1 to 2 orders of magnitude. However, these values were matched or exceeded by the final E. coli counts in three seasons, suggesting that E. coli may be the more useful indicator of bacterial pollution from bovine feces.
Except for the winter experiment, E. coli counts remained high in the dried cow pat remnants after 150 days on pasture (103 to 104 CFU g−1 [Table 1]). Thelin and Gifford (38) also found high fecal coliform counts in a cow pat long after it had been thoroughly dried. The E. coli results in our study are assumed to be applicable to verotoxigenic E. coli, including E. coli O157:H7, because the survival rates of naturally occurring indicator and O157 strains in cattle manure are very similar (2). Of the various laboratory and field methods used to study extinction times in bovine feces, our procedures are most comparable to those of Bolton et al. (3), who inoculated E. coli O157 into cattle fecal material placed outdoors on grassland. These authors recorded T90s ranging from 10 to 12.5 days, which are significantly lower (i.e., faster inactivation) than the E. coli T90s in Table 2 (average, 47 days). However, they used a modified (nontoxigenic) NTCC O157 strain, and small (100-ml) fecal samples, which may have desiccated more rapidly than the 2,100-ml pats used in our study.
Overall, S. enterica was inactivated only slightly more rapidly than E. coli and the other indicators. Himathongkham et al. (21) similarly found that E. coli and Salmonella spp. survival rates were similar in cow manure. Although this implies that cow pats are a potentially greater reservoir for S. enterica than for the rapidly inactivated organism C. jejuni, no Salmonella spp. were detected in a recent survey of 155 fresh cow pats on four New Zealand dairy farms (ESR, Christchurch, New Zealand, unpublished data).
Desiccation was more rapid in the spring and summer, when pat temperatures were higher and when direct warming of the pats by sunlight was more marked. Thus, the effects of sunlight on bacterial survival in cow pats are complex. Our study suggests that sunlight is likely to initially enhance survival rates in the pat interior by helping to form a protective crust and by warming the moist pats to near-optimum growth temperatures. However, sunlight is likely to inactivate bacteria on the pat surface, as demonstrated for natural waters (35), and eventually contributes to pat desiccation through heating.
Although there was only a broad relationship between initial pat desiccation and wind run, there was a better correlation with evapotranspiration. Wind run is an input to the Penman-Monteith equation, on which the weather station evapotranspiration data are based. Other inputs include solar radiation, soil temperature, and vapor pressure. This equation is designed around living plants, but the correlation with the initial rate of water loss in the pats shown in Fig. 1 to 4 suggests that it may also predict pat desiccation rates. The evapotranspiration ranking (summer > spring > autumn > winter) reflects the predominant Canterbury weather pattern of warm summer winds and calmer, cool winters.
Drainage and leaching.
No consistent bacterial leaching patterns were found when individual rainfall events were compared. However, in each experiment, the monthly data showed that reductions in the numbers of E. coli recorded in the drainage matched the decreases in the counts in the overlying pats. The percent losses (Table 3) remained reasonably consistent; of the 20 monthly E. coli leaching losses measured, 16 were <10% of the total counts in the pat and 12 were <1% of the total counts. The only significant percent loss of E. coli (57.8% in December; spring experiment) occurred during the time when there was the highest recorded monthly drainage (84.3 mm). The short period of recovery of C. jejuni in the drainage reflects rapid inactivation in the pat.
The results suggest that once a crust has formed, leaching losses tend be low if subsequent rainfall is <50 mm per month, although intense rainfall events within this regimen may erode the pat surface. In our study, no rainfall was recorded prior to crust formation, whereas other studies have shown that heavy rain or irrigation on a fresh cow pat is likely to result in far greater microbial mobilization and leaching (24, 36).
Although the drainage collectors provided a useful estimate of microbial leaching losses, the results should be treated with caution. Only one collector was used for each row of replicate pats, and considerable variation between some collectors was likely. For example, although the drainage volumes from the two indicator organism collectors for the summer-autumn overlap period in April to June 2005 were very similar (Table 3), the drainage volumes were substantially different in the winter-spring overlap period (October to November 2004). This variability may also have contributed to the far lower drainage (excluding the flooding event) per millimeter of rainfall in winter-spring than in summer-autumn, an average from all the collectors of 30% versus 60%, which is the opposite of what would be expected. However, three other factors may have contributed to this difference: (i) increased drainage through cracking of the dry turf pads in the summer, (ii) the summer-autumn rainfall occurring in more intense events, and (iii) an atypically dry winter in 2005.
The drainage losses (22 to 50%) were likely to be higher than those for normal pasture, because the 20-mm-deep turf pads on the collection funnels allowed only limited evapotranspiration. Some bacterial retention in the turf pads was likely, although it would be unlikely to represent losses from filtration in deeper soil profiles. Drainage losses were not included in the decrease phase or T90 parameters in Table 2, but are currently under more detailed investigation in our laboratory using cow pats placed on 700-mm-deep soil cores in a lysimeter facility.
Artifacts.
The use of standardized, composite cow pats was assumed to have introduced artifacts that would both increase and decrease bacterial survival. The principal survival-enhancing factor was likely to be the protection of the pats from trampling by cattle. However, this was probably offset by pat disintegration due to the destructive sampling approach. The mixing procedure did not appear to alter the physical composition of the fecal material. Kress and Gifford (24) similarly concluded that manipulation did not change the structure of bovine feces and found no difference in fecal coliform release rates between naturally deposited cattle feces and constructed “cowpies”.
Low S. enterica counts and highly variable C. jejuni counts in fresh cow pats necessitated inoculation of the pats with higher concentrations than would naturally be encountered. This allowed reliable establishment of inactivation rates but may have extended the period over which these pathogens would normally be detected. The possibility that laboratory-cultured inocula exhibited decreased environmental resistance (18) was reduced by selection of strains isolated from natural waters and by minimizing subculturing.
The herd was grazed on turnips shortly before the summer experiment, resulting in pats that were less viscous and lighter in color. However, the initial water content was similar to the initial water contents in the other seasons, and there was no obvious effect of this feed change on initial bacterial counts or survival rates.
Conclusions.
Counts of E. coli, fecal streptococci, enterococci, and possibly S. enterica in fresh cow pats are likely to underestimate loads on pasture for 1 to 3 weeks after deposition. Increases of up to 1.5 orders of magnitude may occur depending on the season and as long as the pat water content remains above 80%.
When the pat water content falls below 70 to 75%, the counts of enteric bacteria are likely to fall, and the order of the overall rate of decrease from the date of deposition (i.e., including the increase phase) is C. jejuni > S. enterica ≥ fecal streptococci > E. coli > enterococci. However, the E. coli counts in the dried pat remnants are likely to be higher than those of enterococci, suggesting that E. coli may be the more useful indicator of bovine fecal pollution.
C. jejuni appears to be very rapidly inactivated in cow pats on pasture, and the rate apparently is determined by the average seasonal temperature, even when the water content remains above 80%. Thus, although C. jejuni has been detected at high concentrations in cow pats (ESR, Christchurch, New Zealand, unpublished data), only freshly deposited pats are likely to be a significant reservoir for this pathogen.
The effect of sunlight on cow pats is complex. It initially assists bacterial replication by warming the pats to optimum growth temperatures and by assisting in the formation of a moisture-retaining crust on the pat. Thereafter, sunlight contributes to bacterial inactivation through pat dehydration.
The effect of rainfall is also complex. It leaches bacteria from cow pats, but once a crust has formed, under the rainfall regimen encountered in this study, leaching rates are likely to be low (mostly <1%). Conversely, rainfall rehydrates pats, slowing inactivation rates and possibly causing some bacterial regrowth.
The results of this study, in combination with those of an associated survey of dairy farms, are currently being incorporated into a “fecal microbe reservoir” model to help ascertain microbial loads from cow pats on New Zealand pastures.
Acknowledgments
We acknowledge the invaluable assistance of Brendan Barnes, manager of the Te Pirita dairy farm, from which the cow pat material was collected. We also thank Crop and Food Ltd. for allowing use of their land for the experimental site. Rod Dann and David Woods, ESR, Christchurch, New Zealand, assisted with data analysis. Richard Muirhead and Andrea Donnison (Ag Research), Robert Davies-Colley and Graham McBride (NIWA), Murray Close, Brent Gilpin, and Hilary Michie (ESR), and the journal's referees provided valuable review comments.
This research was funded by the New Zealand Public Good Science Fund, administered by the Foundation for Research, Science and Technology.
Footnotes
Published ahead of print on 19 October 2007.
REFERENCES
- 1.Anonymous. 2006. New Zealand Public Health surveillance report, September 2006. Institute of Environmental Science & Research Ltd., Wellington, New Zealand. www.surv.esr.cri.nz.
- 2.Berry, E. D., and D. N. Miller. 2005. Cattle feedlot soil moisture and manure content. II. Impact on Escherichia coli O157. J. Environ. Qual. 34:656-663. [DOI] [PubMed] [Google Scholar]
- 3.Bolton, D. J., C. M. Byrne, J. J. Sheridan, D. A. McDowell, and I. S. Blair. 1999. The survival characteristics of a non-toxigenic strain of Escherichia coli O157:H7. J. Appl. Microbiol. 86:407-411. [DOI] [PubMed] [Google Scholar]
- 4.Bordner, R. H., J. A. Winter, and P. Scarpino. 1978. Microbiological methods for monitoring the environment: water and wastes. EPA publication 600/8-78-017. U.S. EPA Environmental Monitoring and Support Laboratory, Cincinnati, OH.
- 5.Buckhouse, J. C., and G. F. Gifford. 1976. Water quality implications of grazing on a semiarid watershed in southeastern Utah. J. Range Manag. 29:109-113. [Google Scholar]
- 6.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 7.Collins, R. 2004. Fecal contamination of pastoral wetlands. J. Environ. Qual. 33:1912-1918. [DOI] [PubMed] [Google Scholar]
- 8.Cook, R. L. 1993. Microbiological methods for the meat industry, 2nd ed. Technical report 873. MIRINZ Food Technology and Research, AgResearch, Hamilton, New Zealand.
- 9.Davies-Colley, R. J., J. W. Nagels, R. Smith, R. Young, and C. Phillips. 2004. Water quality impact of a dairy cow herd crossing a stream. N. Z. J. Mar. Freshw. Res. 38:569-576. [Google Scholar]
- 10.Devane, M., C. Nicol, A. Ball, J. D. Klena, P. Scholes, J. A. Hudson, M. G. Baker, B. J. Gilpin, N. Garrett, and M. G. Savill. 2005. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J. Appl. Microbiol. 98:980-990. [DOI] [PubMed] [Google Scholar]
- 11.Downes, F. P., and K. Ito. 2001. Compendium of methods for the microbiological examination of foods, 4th ed. American Public Health Association, Washington, DC.
- 12.Duffy, G. 2003. Verocytoxigenic Escherichia coli in animal faeces, manures and slurries. J. Appl. Microbiol. 94:94S-103S. [DOI] [PubMed] [Google Scholar]
- 13.Echeverry, A., G. H. Loneragan, and M. M. Brashears. 2006. Survival of Escherichia coli O157:H7 in bovine faeces over time under various temperature conditions. J. Food Prot. 69:2851-2855. [DOI] [PubMed] [Google Scholar]
- 14.Ellis, J. R., and T. M. McCalla. 1978. Fate of pathogens in soils receiving animal wastes—a review. Trans. ASAE 21:309-319. [Google Scholar]
- 15.Eyers, M., S. Chapelle, G. Van Camp, H. Goossens, and R. De Wachter. 1993. Discrimination among thermophilic Campylobacter species by polymerase chain reaction amplification of 23S rRNA gene fragments. J. Clin. Microbiol. 31:3340-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremaux, B., C. Prigent-Combaret, M. L. Delignette-Muller, M. Dothal, and C. Vernozy-Rozand. 2007. Persistence of Shiga toxin-producing Escherichia coli O26 in cow slurry. Lett. Appl. Microbiol. 45:55-61. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima, H., K. Hoshina, and M. Gomyoda. 1999. Long-term survival of Shiga toxin-producing Escherichia coli O26, O111, and O157 in bovine feces. Appl. Environ. Microbiol. 65:5177-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fux, C. A., M. Shirtliff, P. Stoodley, and J. W. Costerton. 2005. Can reference laboratory strains mirror ‘real-world’ pathogenesis? Trends Microbiol. 13:58-63. [DOI] [PubMed] [Google Scholar]
- 19.Guan, T. Y., and R. A. Holley. 2003. Pathogen survival in swine manure environments and transmission of human enteric illness: a review. J. Environ. Qual. 32:383-392. [DOI] [PubMed] [Google Scholar]
- 20.Haynes, R. J., and P. H. Williams. 1993. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv. Agron. 49:119-199. [Google Scholar]
- 21.Himathongkham, S., S. Bahari, H. Riemann, and D. Cliver. 1999. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 22.Hudson, J. A., C. Nicol, J. Wright, R. Whyte, and S. K. Hasell. 1999. Seasonal variation of Campylobacter types from human cases, veterinary cases, raw chicken, milk and water. J. Appl. Microbiol. 87:115-124. [DOI] [PubMed] [Google Scholar]
- 23.Jones, K. 2001. Campylobacters in water, sewage, and the environment. J. Appl. Microbiol. 90:68S-79S. [DOI] [PubMed] [Google Scholar]
- 24.Kress, M., and G. F. Gifford. 1984. Fecal coliform release from cattle fecal deposits. AWRA Water Resour. Bull. 20:61-66. [Google Scholar]
- 25.Kudva, I. T., K. Blanch, and C. J. Houde. 1998. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 64:3166-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lightfoot, D. 2004. Salmonella and other enteric organisms, p. 228-241. In J. A. Cotruvo, A. Dulfour, G. Rees, J. Bartram, R. Carr, D. O. Cliver, G. F. Craun, R. Fayer, and V. P. J. Gannon (ed.), Waterborne zoonoses: identification, causes and control. World Health Organization. IWA Publishing, London, United Kingdom.
- 27.Maule, A. 2000. Survival of verotoxigenic Escherichia coli O157 in soil, water and on surfaces. J. Appl. Microbiol. Symp. Suppl. 88:71S-78S. [DOI] [PubMed] [Google Scholar]
- 28.Meat and Wool New Zealand. 2006. Sheep and beef new season outlook 2006-07. Paper P06008. Meat and Wool New Zealand, Wellington, New Zealand.
- 29.Muirhead, R. W., R. P. Collins, and P. J. Bremer. 2005. Erosion and subsequent transport state of Escherichia coli from cowpats. Appl. Environ. Microbiol. 71:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson, M. E. 2001. Human and animal pathogens in manure. In Livestock Options for the Future National Conference, Winnipeg, Manitoba, Canada, June 25-27, 2001. Agriculture and Agri-Food Canada. http://www.gov.mb.ca/agriculture/livestock/livestockopt/papers/olson.pdf.
- 31.Sinton, L., C. Hall, and R. Braithwaite. 2007. Sunlight inactivation of Campylobacter jejuni and Salmonella enterica, compared with Escherichia coli, in seawater and river water. J. Water Health 5:357-365. [DOI] [PubMed] [Google Scholar]
- 32.Sinton, L. W., and A. M. Donnison. 1994. Characterisation of faecal streptococci from some New Zealand effluents and receiving waters. N. Z. J. Mar. Freshw. Res. 28:145-158. [Google Scholar]
- 33.Sinton, L. W., A. M. Donnison, and C. M. Hastie. 1993. Faecal streptococci as faecal pollution indicators: a review. Part I. Taxonomy and enumeration. N. Z. J. Mar. Freshw. Res. 27:101-115. [Google Scholar]
- 34.Sinton, L. W., A. M. Donnison, and C. M. Hastie. 1993. Faecal streptococci as faecal pollution indicators: a review. Part II. Sanitary significance, survival and use. N. Z. J. Mar. Freshw. Res. 27:117-137. [Google Scholar]
- 35.Sinton, L. W., C. H. Hall, P. A. Lynch, and R. J. Davies-Colley. 2002. Sunlight inactivation of fecal bacteria and bacteriophages from waste stabilization pond effluent in fresh and saline waters. Appl. Environ. Microbiol. 68:1122-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoddard, C. S., M. S. Coyne, and J. H. Grove. 1998. Fecal bacteria survival and infiltration through a shallow agricultural soil: timing and tillage effects. J. Environ. Qual. 27:1516-1523. [Google Scholar]
- 37.Strauch, D. 1996. Occurrence of microorganisms pathogenic for man and animals in source separated biowaste and compost—importance, controls, limits, epidemiology, p. 224-232. In M. de Bertoldi, P. Sequi, B. Lemmes, and T. Papi (ed.), The science of composting. CEC, Blackie Academic and Professional, London, United Kingdom.
- 38.Thelin, R., and G. F. Gifford. 1983. Fecal coliform release patterns from fecal material of cattle. J. Environ. Qual. 12:57-63. [Google Scholar]
- 39.Till, D. G., and G. B. McBride. 2004. Potential public health risk of Campylobacter and other zoonotic waterborne infections in New Zealand, p. 191-208. In J. A. Cotruvo, A. Dulfour, G. Rees, J. Bartram, R. Carr, D. O. Cliver, G. F. Craun, R. Fayer, and V. P. J. Gannon (ed.), Waterborne zoonoses: identification, causes and control. World Health Organization. IWA Publishing, London, United Kingdom.
- 40.Unc, A., and M. J. Goss. 2004. Transport of bacteria from manure and protection of water resources. Appl. Soil Ecol. 25:1-18. [Google Scholar]
- 41.Wang, G., T. Zhao, and M. P. Doyle. 1996. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol. 62:2567-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, L., K. R. Mankin, and G. L. Marchin. 2004. Survival of fecal bacteria in dairy cow manure. Trans. ASAE 47:1239-1246. [Google Scholar]
- 43.Wilcock, R. J., J. W. Nagels, H. J. E. Rodda, M. B. O'Connor, and B. S. Thorrold. 1999. Water quality of a lowland stream in a New Zealand dairy farming catchment. N. Z. J. Mar. Freshw. Res. 33:683-696. [Google Scholar]