Abstract
A molecular method based on PCR-restriction fragment length polymorphism (RFLP) analysis of internal transcribed spacer (ITS) ribosomal DNA sequences was designed to rapidly identify fungal species, with members of the genus Pleurotus as an example. Based on the results of phylogenetic analysis of ITS sequences from Pleurotus, a PCR-RFLP endonuclease autoscreening (PRE Auto) program was developed to screen restriction endonucleases for discriminating multiple sequences from different species. The PRE Auto program analyzes the endonuclease recognition sites and calculates the sizes of the fragments in the sequences that are imported into the program in groups according to species recognition. Every restriction endonuclease is scored through the calculation of the average coefficient for the sequence groups and the average coefficient for the sequences within a group, and then virtual electrophoresis maps for the selected restriction enzymes, based on the results of the scoring system, are displayed for the rapid determination of the candidate endonucleases. A total of 85 haplotypes representing 151 ITS sequences were used for the analysis, and 2,992 restriction endonucleases were screened to find the candidates for the identification of species. This method was verified by an experiment with 28 samples representing 12 species of Pleurotus. The results of the digestion by the restriction enzymes showed the same patterns of DNA fragments anticipated by the PRE Auto program, apart from those for four misidentified samples. ITS sequences from 14 samples (of which nine sequences were obtained in this study), including four originally misidentified samples, confirmed the species identities revealed by the PCR-RFLP analysis. The method developed here can be used for the identification of species of other living microorganisms.
The PCR-restriction fragment length polymorphism (RFLP) technique, consisting of PCR amplification of target DNA fragments and subsequent digestion of the PCR products with restriction endonucleases to obtain band patterns, has been used extensively for molecular identification of living organisms, especially microorganisms (25, 37). The selection of suitable endonucleases is the key step of the technique. Endonucleases have been selected through extensive experimentation with restriction enzyme cleavage to distinguish different species of bacteria (31, 34), yeasts (9, 30), filamentous fungi (21-23), and other organisms (4, 5) in the recent years. As more DNA sequences are becoming readily available, the selection of endonucleases can be based on sequence analyses through the examination of recognition sites of restriction enzymes from the alignment of sequences, e.g., from bacteria (10), fungi (7, 41), and other living organisms (1, 48).
In recent years, various computer programs have been used for the selection of these restriction endonucleases. Programs have been designed to search for the recognition sites of endonucleases, to locate the positions of these sites in sequences, and to calculate the sizes of fragments after digestion. Some of the software packages, e.g., Gene Runner version 3.05 (24), Genetyx version 6.1 (26), MapDraw of DNASTAR version 3.14 (16, 32), NEBcutter version 2.0 (35), Webcutter version 2.0 (40), BioEdit (45), DNA Club (47), the Infobiogen restriction program (29), and REBsites (14), have been adopted to assist in the selection of endonucleases in the PCR-RFLP experimental design. However, these programs perform restriction analysis only for individual sequences. Although the SNP Cutter software (51) can analyze the data from multiple sequences, the subsequent comparison of banding profiles of different sequences after digestion is done manually. Therefore, employing these programs is time-consuming, tedious, and often error prone, especially when a large number of taxa with different sequences are involved. There is a need to develop a strategy to select the endonucleases rapidly through the restriction analysis of a large number of sequences.
The purpose of this work was to develop a method of rapid selection of restriction endonucleases for PCR-RFLP identification of fungal species with the aid of a computer program based on the analysis of a considerably large number of DNA sequences. Species of Pleurotus (Fr.) P. Kumm., a fungal genus, are used as examples owing to the reasonable number of species in the genus and the considerable molecular data on internal transcribed spacer (ITS) sequences.
Species of Pleurotus, commercially called oyster mushrooms, are a group of the most important edible fungi, accounting for 25% of the total world production of commercially cultivated mushrooms (17). In addition, Pleurotus species are also used as a tool for environmental control in the biotransformation-biodegradation of industrial effluents owing to their unique ligninolytic system (6). Further, some species of Pleurotus are known as predaceous fungi (39) and have the potential for controlling nematode diseases of agricultural crops. Traditionally, species-level identification of Pleurotus has been based mainly on morphology and compatibility relationships (3, 18, 49). However, many difficulties have been associated with the identification of species of this genus, especially those of commercially cultivated strains, due to their similar morphological characteristics and morphological plasticity in cultivation (19). The long period from the inoculation of spawn to the production of fruiting bodies of these fungi also delays any positive determination (33). Various molecular methods, such as DNA sequencing and RFLP and PCR-RFLP techniques, have been used to identify Pleurotus species. The application of analyses of sequences of ITS regions and large subunits (LSU) of nuclear ribosomal DNA (rDNA) to the determination of Pleurotus species (42, 43, 50) is very powerful at the species level, but these analyses are often used for particular research and have not been used for routine or rapid identification. RFLP and PCR-RFLP methods have also been applied to the study of molecular systematics and the genotyping of Pleurotus (2, 12). In the two latter studies (2, 12), however, the numbers of restriction enzymes screened (four and seven, respectively) and species identified (5 and 10, respectively) were limited because the selection of restriction endonucleases was based on results from extensive experiments. It is necessary to establish a rapid, accurate, and simple molecular method for the identification of Pleurotus species, based on the analysis of a large number of sequences and the selection of restriction enzymes from thousands of candidate endonucleases.
There are about 20 Pleurotus species recognized worldwide (15). Recent molecular phylogenetic analysis of Pleurotus provides a useful framework for understanding species concepts and taxonomy. Relationships between species and species determination have also been studied, and the results of systematic analyses of LSU and ITS sequences in nuclear rDNA (42, 43, 50) and of small-subunit sequences in mitochondrial rDNA (8) show that ITS sequences are an ideal marker for species identification. A large number of ITS sequences from this genus have been accumulated, and a molecular identification method using the PCR-RFLP technique to distinguish Pleurotus species can be developed through the DNA sequence analyses.
MATERIALS AND METHODS
Fungal material.
A total of 17 living strains and 11 dried specimens were used for this study and are listed in Table 1. The strains were stored at 4°C on potato dextrose agar medium and subcultivated at 25°C in liquid potato dextrose medium for 10 days to collect the mycelia for extracting DNA. Strains and specimens of fungal material were designated according to the acquisition sources, as indicated in Table 1.
TABLE 1.
Fungal material used in this study
| Species name | Samplea | Origin | GenBank accession no.b for ITS sequence | Reidentification of speciesc |
|---|---|---|---|---|
| P. abalonus Han, Chen, et Cheng | MG 005 | China | ||
| CGMCC 5.409 | China | |||
| P. calyptratus (Lindblad) Saccardo | HMAS 63355 | China | AY562495 | |
| HMAS 77117 | China | |||
| P. cornucopiae (Paulet) Rolland | ATCC 38547 | Germany | ||
| HMAS 76520 | China | EF514242 | P. ostreatus | |
| MG 504 | China | |||
| P. cystidiosus Mill | ATCC 28597 | United States | EF514244 | |
| CGMCC 5.467 | China | AY540320 | P. abalonus | |
| CGMCC 5.494 | China | AY540321 | P. abalonus | |
| P. djamor (Rumphius ex Fries) Boedijn | CGMCC 5.600 | China | ||
| CGMCC 5.407 | China | |||
| P. dryinus (Persoon) Kummer | F 14011 | Finland | ||
| CBS 44977 | Former Czechoslovakia | EF514249 | ||
| HKAS 17450 | China | EF514243 | P. pulmonarius | |
| P. eryngii (Lanzi) Saccardo | MG 497 | China | ||
| HMAS 25978 | China | EF514246 | ||
| P. nebrodensis (Inzenga) Quélet | MG 500 | China | ||
| HMAS 86357 | China | EF514245 | ||
| P. pulmonarius (Fries) Quélet | MG 502 | China | ||
| HMAS 76672 | China | |||
| HMAS 72869 | China | |||
| P. ostreatus (Jacquin: Fries) Kummer | HMAS 66080 | China | EF514248 | |
| CGMCC 5.37 | China | EF514247 | ||
| CGMCC 5.344 | China | AY540325 | ||
| P. tuber-regium (Rumphius ex Fries) Singer | MG 506 | China | ||
| HMAS 84647 | China | EF514250 | ||
| P. smithii Guzmán | IE 74 | Mexico | AY315779 |
The prefixes in the sample designations indicate the sources, as follows: ATCC, American Type Culture Collection, Rockville, MD; CBS, Centraalbureau voor Schimmelcultures, Baarn, The Netherlands; CGMCC, China General Microbiological Culture Collection Center, Beijing, China; F, National Museum of Natural Science, Taiwan, China; HKAS, Herbarium, Kunming Institute of Botany, Academia Sinica, Kunming, China; HMAS, Mycological Herbarium, Institute of Microbiology, Academia Sinica, Beijing, China; IE, Instituto de Ecologia, Xalapa, Mexico; and MG, Macrofungi Group, Chinese Academy of Science, China.
GenBank accession numbers in bold represent sequences obtained in this study.
For samples for which no reidentification is listed, the original determination was confirmed.
DNA isolation, PCR amplification, and sequencing.
Genomic DNA was isolated from well-preserved herbarium specimens and fresh fungal cultures by using the modified cetyltrimethylammonium bromide method as described by Li and Yao (20). The ITS region of rDNA was amplified using the primers ITS4 and ITS5 (46). PCR amplification was carried out in a 25-μl reaction volume containing 12.5 μl of 2× reaction mix (200 M deoxynucleoside triphosphates, 4.0 mM MgCl2, 2.5 U of Taq DNA polymerase), 50 pmol of each primer, and 2 μl of template DNA. The thermal cycling conditions consisted of an initial denaturation at 95°C for 2 min; 30 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 45 s; and a final extension at 72°C for 7 min. The PCR products were analyzed by electrophoresis in a 1.0% (wt/vol) agarose gel in 10× Tris-borate-EDTA buffer and were subsequently visualized by UV illumination after ethidium bromide staining.
PCR products were purified using cleanup plates (Millipore Corporation). Sequencing was performed by the cyclic reaction termination method on an ABI Prism 3100 genetic analyzer (Applera Corporation), and data were collected on a Dell computer with the ABI Prism DNA sequencing analysis software, version 3.7. Each fragment was sequenced in both directions for confirmation, and the sequences of the two strands were assembled with ABI Prism SeqScape software, version 1.1.
Sequence analysis.
The retrieval tool from the National Centre of Biotechnology Information was used to search ITS sequences by using the phrase “Pleurotus internal transcribed spacer 1 internal transcribed spacer 2” as keywords. All available ITS sequences from GenBank were aligned using Clustal X 1.81 (38) and then further manually adjusted using BioEdit 5.0.6 (11) to reduce some obvious mismatching of sequences created by computer alignment. Sequences with regions of consecutive ambiguous bases or with potentially confounding ambiguous bases, i.e., unresolved bases or ambiguous bases in a position that may affect a restriction site, were excluded. Only sequences from material identified to the species level were used, and pairs of identical sequences were represented by one haplotype. A total of 259 ITS sequences were retrieved, and 85 haplotypes representing 151 sequences met all of the above criteria of selection for study. These sequences were used for phylogenetic analysis and for the selection of restriction enzymes (Table 2). Phylogenetic analyses were performed by PAUP 4.0b10 for Macintosh (36) by using ITS sequences from Hohenbuehelia grisea as the outgroup. A parsimony analysis was performed using a heuristic search, with the random addition of sequences with 1,000 replicates, tree bisection-reconnection as the branch-swapping algorithm, one tree held at each step during stepwise addition, and the MULTREES option off. Gaps were treated as missing data. Bootstrap values were calculated from 1,000 replicates.
TABLE 2.
Representative sequences of ITS haplotypes used for the phylogenetic analyses and the autoscreening of restriction endonucleases for PCR-RFLP
| Species | GenBank voucher | Origin | GenBank accession no. | Taxon name used in GenBank |
|---|---|---|---|---|
| P. abalonus Han, Chen, et Cheng | CBS 61580 | India | AY315792 | P. cystidiosus subsp. abalonus |
| ASIK 3 | Taiwan | AY315794 | P. cystidiosus subsp. abalonus | |
| FCUP 661 | Philippines | AY315801 | P. cystidiosus subsp. abalonus | |
| VT 2476 | Hawaii | AY315802 | P. cystidiosus subsp. abalonus | |
| IFO 31074 | Japan | AY315804 | P. cystidiosus subsp. abalonus | |
| CBS 80391 | China | AY315806 | P. cystidiosus subsp. abalonus | |
| DSM 5340 | Thailand | AY315808 | P. cystidiosus subsp. abalonus | |
| ZA 472 | Thailand | AY315809 | P. cystidiosus subsp. abalonus | |
| CBS 61580 | India | AY315810 | P. cystidiosus subsp. abalonus | |
| Blao | Vietnam | DQ882571 | P. cystidiosus subsp. abalonus | |
| S 396 | Japan | DQ882573 | P. cystidiosus subsp. abalonus | |
| P. calyptratus (Lindblad) Saccardo | HMAS 63355 | China | AY562495 | P. djamor f. calyptratus |
| CBS 325.85 | Unknown | AY265814 | P. calyptratus | |
| TENN 57451 | Austria | AY450338 | P. calyptratus | |
| P. cornucopiae (Paulet) Rolland | ATCC 42045 | Unknown | AB115037 | P. cornucopiae |
| wc 608 | Unknown | AF079582 | P. cornucopiae | |
| IFO 30528 | Unknown | AY265816 | P. cornucopiae | |
| CBS 383.80 | Unknown | AY265817 | P. cornucopiae | |
| ASI 2011 | Unknown | AY265852 | P. citrinopileatus | |
| S 033 | China | AY540318 | P. citrinopileatus | |
| HMAS 63344 | China | AY696301 | P. citrinopileatus | |
| PHZAU 1 | Unknown | DQ077889 | P. citrinopileatus | |
| P. cystidiosus Mill | ATCC 28598 | Unknown | AY265818 | P. cystidiosus |
| D 420 | United States | AY315767 | P. cystidiosus | |
| VT 1780 | United States | AY315769 | P. cystidiosus | |
| D 412 | United States | AY315770 | P. cystidiosus | |
| D 417 | United States | AY315773 | P. cystidiosus | |
| CBS 297.35 | United States | AY315776 | P. cystidiosus | |
| ATCC 28598 | South Africa | AY315777 | P. cystidiosus | |
| P. djamor (Rumphius ex Fries) Boedijn | IFO 9573 | Unknown | AB115053 | P. djamor |
| IFO 31859 | Unknown | AY265843 | P. salmoneostramineus | |
| ASI 2104 | Unknown | AY265844 | P. salmoneostramineus | |
| ASI 2172 | Unknown | AY265845 | P. salmoneostramineus | |
| P. dryinus (Persoon) Kummer | 7947 | Denmark | AY450343 | P. dryinus |
| P. eryngii (Lanzi) Saccardo | TFM-M-E 856 | Unknown | AB115042 | P. eryngii |
| IFO 32798 | Unknown | AY265825 | P. eryngii | |
| WU 13414 | Austria | AY450347 | P. eryngii | |
| S 607 | China | AY540333 | P. eryngii | |
| J 2 | Unknown | AY589046 | P. eryngii | |
| tw 1 | Unknown | AY589047 | P. eryngii | |
| P. nebrodensis (Inzenga) Quélet | S 498 | China | AY540331 | P. nebrodensis |
| W | Unknown | AY581426 | P. nebrodensis | |
| W 1 | Unknown | AY581427 | P. nebrodensis | |
| W 3 | Unknown | AY581429 | P. nebrodensis | |
| W 4 | Unknown | AY581430 | P. nebrodensis | |
| No. 4 Bailing | Unknown | AY720935 | P. nebrodensis | |
| P. ostreatus (Jacquin: Fries) Kummer | wc 534 | Unknown | AF079583 | P. ostreatus |
| ATCC 38538 | Unknown | AY265828 | P. ostreatus f. florida | |
| ASI 2029 | Unknown | AY368665 | P. ostreatus | |
| TENN 53662 | Austria | AY450345 | P. ostreatus | |
| S 039 | China | AY540322 | P. floridanus | |
| S 474 | China | AY540332 | P. ostreatus | |
| OE-43 | Unknown | AY636055 | P. ostreatus | |
| TENN 53662 | Unknown | AY854077 | P. ostreatus | |
| P. pulmonarius (Fries) Quélet | TFM-M-C960 | Unknown | AB115046 | P. pulmonarius |
| IFO 31345 | Unknown | AB115052 | P. pulmonarius | |
| HMAS 76474 | China | AY696298 | P. pulmonarius | |
| HMAS 76672 | China | AY696299 | P. pulmonarius | |
| HMAS 86396 | China | AY696300 | P. pulmonarius | |
| NZFRI 3528 | New Zealand | U60648 | P. pulmonarius | |
| P. smithii Guzmán | CBS 689.82 | Unknown | AY265851 | P. smithii |
| IE 74 | Mexico | AY315779 | P. smithii | |
| ATCC 46391 | Mexico | AY315781 | P. smithii | |
| ATCC 46391 | Mexico | AY315782 | P. smithii | |
| ATCC 46391 | Mexico | AY315783 | P. smithii | |
| ATCC 46391 | Mexico | AY315784 | P. smithii | |
| ATCC 46391 | Mexico | AY315786 | P. smithii | |
| P. tuber-regium (Rumphius ex Fries) Singer | TFM-M-D 779 | New Caledonia | AB115045 | P. tuber-regium |
| RV 95/174.15 | Australia | AF109964 | P. tuber-regium | |
| RV 95/175.1 | Australia | AF109965 | P. tuber-regium | |
| RV 95/947.1 | Papua New Guinea | AF109966 | P. tuber-regium | |
| RV 95/949.2 | Papua New Guinea | AF109970 | P. tuber-regium | |
| RV 95/950.2 | Papua New Guinea | AF109971 | P. tuber-regium | |
| Pt 5 | New Caledonia | AF109972 | P. tuber-regium | |
| Pt 5.1 | New Caledonia | AF109973 | P. tuber-regium | |
| NedaS 467 | Indonesia | AF109975 | P. tuber-regium | |
| PTR 5 | Ghana | AF109976 | P. tuber-regium | |
| PTV 2 | Ghana | AF109978 | P. tuber-regium | |
| Pt 3 | Nigeria | AF109982 | P. tuber-regium | |
| Pt 1 | Nigeria | AF109983 | P. tuber-regium | |
| Pt 8 | Nigeria | AF109986 | P. tuber-regium | |
| Pt 9 | Nigeria | AF109987 | P. tuber-regium | |
| PtWat | Gameroon | AF109988 | P. tuber-regium | |
| PTR 1 | Ghana | AF109989 | P. tuber-regium | |
| DSH-92-155 | Papua New Guinea | AY450344 | P. tuber-regium |
Architectural structure of PRE Auto program.
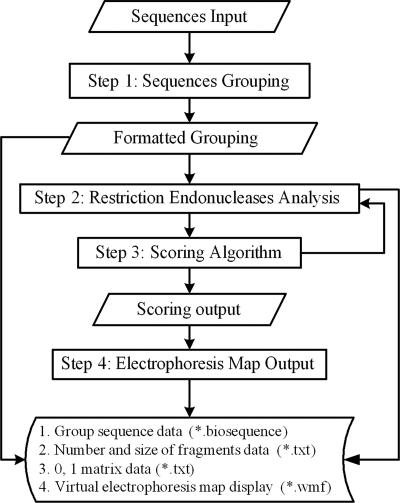
The PCR-RFLP endonuclease autoscreening (PRE Auto) program was written using Borland Delphi 6.0. A schematic diagram of the architectural framework of the program is shown in Fig. 1. The program consists of four main modular components, i.e., sequence grouping, restriction enzyme analysis, scoring algorithm, and electrophoresis map output. A restriction endonuclease database including listings for 2,992 enzymes was downloaded from REBASE, the restriction enzyme database of New England Biolabs, Inc. (http://rebase.neb.com). Endonucleases listed in the database can also be added to the PRE Auto program manually. In the sequence grouping module, the user can divide the sequences into different groups according to the results of phylogenetic analysis. The sequence data can be imported into the program in FASTA format. In the restriction enzyme analysis module, the PRE Auto program searches for recognition sites, calculates the restriction fragment sizes for different sequences, and transforms the data into a (0,1) matrix. In the scoring algorithm module, the program computes the Dice coefficient [2a/(2a + b + c)] (27) through the (0,1) matrix for the sequences, and every restriction enzyme is scored through the calculation of the average coefficient for the groups and the average coefficient for the sequences within a group. In the electrophoresis map output module, a virtual electrophoresis map of fragments after digestion with different enzymes can be displayed.
FIG. 1.
The PRE Auto work flow includes steps 1 through 4, as shown in the figure. Rectangles show the tasks that the program performs. Parallelograms show the data that the user supplies or that every step produces. The program may produce four files, as shown: *.biosequence in step 1, *.txt in steps 2 and 3, and *.wmf in step 4, where * represents an optional string for writing the file name.
Selection of restriction endonucleases and PCR-RFLP procedure.
Restriction enzymes were selected using the PRE Auto program developed in this study. The conservative partial 18S sequences from the annealing position of the primer ITS5 and partial 28S sequences from the position of ITS4 were added to both ends of the ITS sequences, if they were incomplete at the ends when downloaded from GenBank, to ensure the accuracy of the prediction of fragment sizes after digestion with restriction endonucleases. Data for all the 85 ITS sequence haplotypes were input into the PRE Auto program as FASTA files.
The screening for a single enzyme was followed by a further search for a combination of two enzymes, because all 12 species could not be distinguished by any one enzyme. Candidate restriction enzymes were determined based on two parameters, the scoring and the degree of cleavage site coverage, after the autoscreening of restriction enzymes was implemented. The predicted band patterns after digestion by candidate enzymes having a coefficient above 0.1 and a cleavage site coverage of 100% for a single enzyme and 50% for a combination of two enzymes were examined through the electrophoresis map output from the PRE Auto program. The major selection criteria for enzymes were based on the minimum number of enzymes and the steps required to produce discriminating band patterns. In addition, enzymes producing too many small fragments (<50 bp) or showing intraspecific polymorphism were avoided. The availability and the cost of enzymes were also taken into account. Rare enzymes difficult to procure were also avoided, and common and low-cost enzymes were selected when multiple candidates with the same recognition site were available.
Digestion reactions were performed for each of the selected restriction enzymes, i.e., HaeIII, AluI, and HpyCH4IV (all from New England Biolabs, Beverly, MA), in a total volume of 20 μl containing 2 μl of 10× reaction buffer, 0.5 μl of each restriction enzyme (10 U/μl), and 17.5 μl (for a single enzyme) or 17 μl (for a combination of two enzymes) of the PCR products of each sample. Digestions were incubated overnight at the optimal temperature (37°C). Digested products were separated by 3% agarose gel electrophoresis in 10× Tris-borate-EDTA buffer for 3 h at 80 V. The gels were stained with 0.1 g of ethidium bromide/liter and visualized with UV light. Sizes of restriction fragments were determined by comparison with a standard DNA molecular mass marker, a 100-bp DNA ladder (Beijing Yuanchen Bio Company, Beijing, China).
Experimental validation of PCR-RFLP species identification.
A total of 28 samples representing 12 Pleurotus species were selected for PCR-RFLP identification and validation of the method (Table 1). ITS sequences from five of these samples had been sequenced previously and submitted to GenBank (accession no. AY562495, AY540320, AY540321, AY540325, and AY315779). Of the five sequenced samples, two (CGMCC 5.467 and CGMCC 5.494, identified as P. cystidiosus) were proven to be misidentified, as revealed by their ITS sequences (AY540320 and AY540321, deposited in GenBank under the name P. cystidiosus), and were used as negative controls, while the other three (HMAS 63355 [P. calyptratus], CGMCC 5.344 [P. ostreatus], and IE 74 [P. smithii]) were used as positive controls. Additional samples were randomly selected from materials available in our laboratory to make two to three samples for each species. After the PCR products from the selected samples were confirmed by electrophoresis, the PCR-RFLP procedure described above was performed for the molecular identification of the samples through the comparison of the actual band patterns after digestion by the tested restriction endonucleases with the patterns predicted by the PRE Auto program. The species identification of the samples was further confirmed by sequencing the PCR products from ITS sequences from nine samples representing six species of Pleurotus, in addition to the five sequenced samples used as controls (Table 1).
Nucleotide sequence accession numbers.
The sequences determined in this study have been deposited in GenBank under accession numbers EF514242 to EF514250.
RESULTS
Phylogenetic analysis of ITS sequences from Pleurotus species.
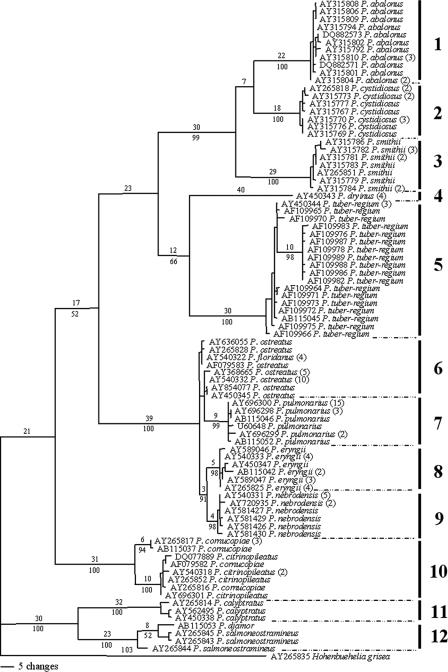
The phylogenetic analysis of a total of 85 haplotypes representing 151 ITS sequences from Pleurotus taxa indicated 12 groups, as shown in Fig. 2. The determination of Pleurotus species for these groups and the application of species names were based on results of molecular phylogeny and mating compatibility analyses (13, 43, 50), although various names have been used for the sequences submitted to GenBank. In particular, there was considerable sequence variation in groups 5, 6, and 10 (Fig. 2; see also the supplemental material). Although the degree of molecular variation among the group 5 sequences was higher than that among sequences of other groups, P. tuber-regium is morphologically distinctive owing to its tuberous sclerotium, from which the basidiomata are produced. It is treated here as one species according to Isikhuemhen et al. (13), who were able to demonstrate that the molecular variations are associated with geographic isolation. A similar situation was applicable to group 10, which is treated here as P. cornucopiae (see also references 43 and 49). The paraphyletic status of group 6, P. ostreatus, in the phylogenetic analysis was also found by Vilgalys and Sun (43) with the support of mating test results. P. ostreatus was considered as a species for the selection of restriction endonucleases to test the tolerability and sensitivity of the PRE Auto program. In addition, P. floridanus in group 6 was considered to be synonymy for P. ostreatus (18), P. salmoneostramineus was treated as synonymous with P. djamor in group 12 (18), and P. citrinopileatus was considered to be a morphological variant of P. cornucopiae in group 10 (28). More data on the taxonomic and nomenclatural treatment of these species will be reported in a separate paper on the molecular phylogeny of Pleurotus.
FIG. 2.
One of the 1,288 most parsimonious trees obtained from the analysis of nucleotide sequences of ITS regions (nuclear rDNA). The upper and lower numbers on each branch denote the number of estimated substitutions and the percentage of bootstrap replicates, respectively. Only bootstrap values higher than 50% are shown. Numbers in the brackets after species names are the numbers of sequences that the haplotypes represented. The length of the tree is 701 steps, with a consistency index of 0.6904 and a retention index of 0.9469. Grouping is as follows: group 1, P. abalonus; group 2, P. cystidiosus; group 3, P. smithii; group 4, P. dryinus; group 5, P. tuber-regium; group 6, P. ostreatus; group 7, P. pulmonarius; group 8, P. eryngii; group 9, P. nebrodensis; group 10, P. cornucopiae; group 11, P. calyptratus; and group 12, P. djamor.
Autoscreening and selection of restriction endonucleases for PCR-RFLP by the PRE Auto program.
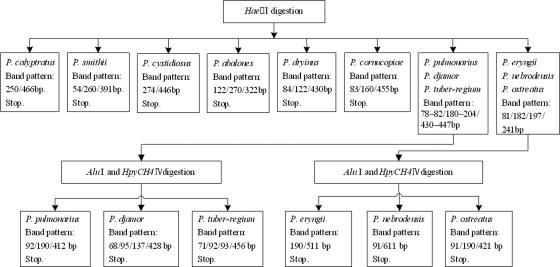
The ITS sequence haplotypes were input into the PRE Auto program and divided into 12 groups based on the phylogenetic analysis. The restriction endonucleases were autoscreened in the PRE Auto program. The ranges of coefficients and degrees of cleavage site coverage for all 2,992 endonucleases were 0.08 to 0.24 and 1.2 to 100%, respectively, in the autoscreening of single enzymes. The predicted band patterns of 569 restriction enzymes with a coefficient above 0.1 and a degree of cleavage site coverage up to 100%, sorted into 23 different groups based on recognition sites, were examined by eye. The enzyme group of HaeIII, with 179 enzymes in total, was found to have the highest resolution among all the 23 groups, distinguishing 6 of the 12 Pleurotus species and dividing the remaining 6 species into two groups. As a single enzyme could not discriminate among all the Pleurotus species, further screening of combinations of two enzymes was performed. There were 221 enzyme combinations with coefficients above 0.1 and a degree of cleavage site coverage of 50%. The combination of AluI and HpyCH4IV was found to have the highest score, 0.33, distinguishing the remaining six species, which could not be identified by digestion with the single enzyme HaeIII. With the criteria for the selection of restriction endonucleases described above, a two-stage digestion using the single enzyme HaeIII followed by the combination of AluI and HpyCH4IV was finally designed to obtain the best species-specific band patterns with minimal testing.
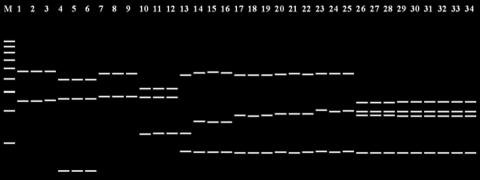
The expected enzyme digestion band patterns for HaeIII are shown in Fig. 3, with representative sequences from the 12 species. Among the patterns, six were species-specific profiles, representing P. calyptratus (lanes 1 to 3), P. smithii (lanes 4 to 6), P. cystidiosus (lanes 7 to 9), P. abalonus (lanes 10 to 12), P. dryinus (lane 13), and P. cornucopiae (lanes 14 to 16). There were some differences in band patterns of P. pulmonarius (lanes 17 to 19), P. tuber-regium (lanes 20 to 22), and P. djamor (lanes 23 to 25), but the sizes of the fragments were very similar. The band patterns of the remaining three species, P. eryngii (lanes 26 to 28), P. nebrodensis (lanes 29 to 1), and P. ostreatus (lanes 32 to 34), were identical.
FIG. 3.
Predicted band patterns produced by the PRE Auto program after digestion with HaeIII. Lane 1, P. calyptratus AY562495; lane 2, P. calyptratus AY265814; lane 3, P. calyptratus AY450338; lane 4, P. smithii AY315779; lane 5, P. smithii AY315781; lane 6, P. smithii AY315786; lane 7, P. cystidiosus AY315767; lane 8, P. cystidiosus AY315770; lane 9, P. cystidiosus AY315773; lane 10, P. abalonus AY315794; lane 11, P. abalonus AY315806; lane 12, P. abalonus AY315808; lane 13, P. dryinus AY450343 (one haplotype with four sequences); lane 14, P. cornucopiae AB115037; lane 15, P. cornucopiae AY265817; lane 16, P. citrinopileatus DQ077889; lane 17, P. pulmonarius AB115046; lane 18, P. pulmonarius AY696300; lane 19, P. pulmonarius U60648; lane 20, P. tuber-regium AB115045; lane 21, P. tuber-regium AF109983; lane 22, P. tuber-regium AY450344; lane 23, P. djamor AB115053; lane 24, P. salmoneostramineus AY265843; lane 25, P. salmoneostramineus AY265845; lane 26, P. eryngii AB115042; lane 27, P. eryngii AY450347; lane 28, P. eryngii AY540333; lane 29, P. nebrodensis AY540331; lane 30, P. nebrodensis AY581427; lane 31, P. nebrodensis AY720935; lane 32, P. ostreatus AY368665; lane 33, P. ostreatus AY540332; and lane 34, P. ostreatus AY636055. Lane M, 100 bp DNA ladder.
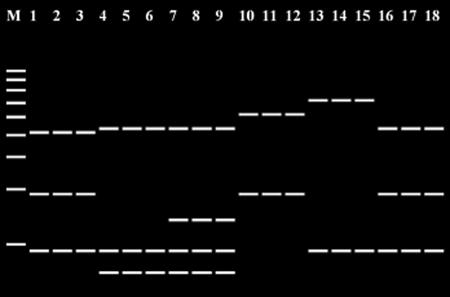
The further digestion with the combination of two endonucleases, AluI and HpyCH4IV, differentiated the three species with the similar HaeIII bands, i.e., P. pulmonarius (Fig. 4, lanes 1 to 3), P. tuber-regium (lanes 4 to 6), and P. djamor (lanes 7 to 9), and the other three species with the identical HaeIII band profiles, i.e., P. eryngii (lanes 10 to 12), P. nebrodensis (lanes 13 to 15), and P. ostreatus (lanes 16 to 18). Through the virtual sequence analysis generated by the PRE Auto program, the 12 Pleurotus species could be distinguished by the two-stage digestion of ITS fragments. The procedures for the detection and identification of Pleurotus species are summarized in Fig. 5.
FIG. 4.
Predicted band pattern after digestion with AluI and HpyCH4IV produced by the PRE Auto program. Lane 1, P. pulmonarius AB115046; lane 2, P. pulmonarius AY696300; lane 3, P. pulmonarius U60648; lane 4, P. tuber-regium AB115045; lane 5, P. tuber-regium AF109983; lane 6, P. tuber-regium AY450344; lane 7, P. djamor AB115053; lane 8, P. salmoneostramineus AY265843; lane 9, P. salmoneostramineus AY265845; lane 10, P. eryngii AB115042; lane 11, P. eryngii AY450347; lane 12, P. eryngii AY540333; lane 13, P. nebrodensis AY540331; lane 14, P. nebrodensis AY581427; lane 15, P. nebrodensis AY720935; lane 16, P. ostreatus AY368665; lane 17, P. ostreatus AY540332; and lane 18, P. ostreatus AY636055. Lane M, 100-bp DNA ladder.
FIG. 5.
Optimized flow chart for identification of Pleurotus species using three restriction endonucleases.
PCR-RFLP identification of Pleurotus species and validation by ITS sequencing.
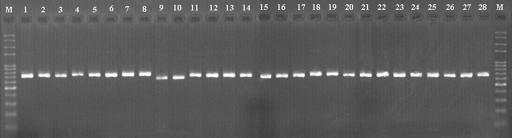
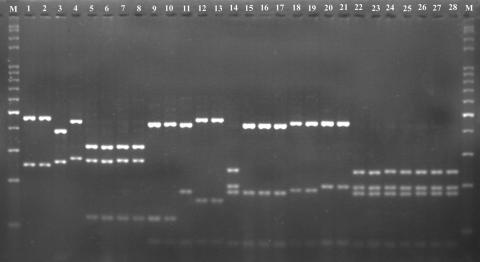
PCR amplification using the primers ITS4 and ITS5 resulted in products of approximately 720 bp (Fig. 6) from 28 tested Pleurotus samples. According to the flow chart for the identification of Pleurotus species (Fig. 5), PCR products of the tested samples were first digested with HaeIII. The RFLP band patterns on an agarose gel are shown in Fig. 7. The observed band patterns of 24 samples were in accordance with those predicted from ITS sequences by the PRE Auto program (Fig. 3.). These samples included P. calyptratus (Fig. 7, lanes 1 and 2), P. smithii (lane 3), P. cystidiosus (lane 4), P. abalonus (lanes 7 and 8), P. dryinus (lanes 9 and 10), P. cornucopiae (lanes 12 and 13), P. pulmonarius (lanes 15 to 17), P. tuber-regium (lanes 18 and 19), P. djamor (lanes 20 and 21), P. eryngii (Fig. 7, lanes 22 and 23), P. nebrodensis (lanes 24 and 25), and P. ostreatus (lanes 26 and 28). However, two of the three P. cystidiosus samples (lanes 5 and 6), used as negative controls, produced the species-specific band pattern of P. abalonus (lanes 7 and 8), in agreement with the pattern predicted for the sequences (AY540320 and AY540321) by the PRE Auto program. The species in these two samples were renamed, as noted in Table 1. Additionally, one of the three samples of P. dryinus (lane 11) produced a band pattern similar to that of P. pulmonarius (lanes 15 to 17), and one of the three P. cornucopiae samples (lane 15) produced the same pattern as P. eryngii, P. nebrodensis, and P. ostreatus (lanes 22 to 28). Using HaeIII, six species, P. calyptratus, P. smithi, P. cystidiosus, P. abalonus, P. dryinus, and P. cornucopiae, were successfully identified based on the species-specific PCR-RFLP band profiles predicted by the PRE Auto program (Fig. 3), and four samples misidentified as P. cystidiosus, P. dryinus, and P. cornucopiae were also detected (Fig. 7, lanes 5 and 6, 11, and 14, respectively).
FIG. 6.
PCR amplification products from Pleurotus species. Lane 1, Pleurotus calyptratus HMAS 63355; lane 2, P. calyptratus HMAS 77117; lane 3, P. smithii IE 74; lane 4, P. cystidiosus ATCC 28597; lane 5, P. cystidiosus CGMCC 5.467; lane 6, P. cystidiosus CBS 80391; lane 7, P. abalonus MG 005; lane 8, P. abalonus CGMCC 5.409; lane 9, P. dryinus F 14011; lane 10, P. dryinus CBS 44977; lane 11, P. dryinus HKAS 17450; lane 12, P. cornucopiae ATCC 38547; lane 13, P. cornucopiae MG 504; lane 14, P. cornucopiae HMAS 76520; lane 15, P. pulmonarius MG 502; lane 16, P. pulmonarius HMAS 76672; lane 17, P. pulmonarius HMAS 72869; lane 18, P. tuber-regium MG 506; lane 19, P. tuber-regium HMAS 84647; lane 20, P. djamor CGMCC 5.600; lane 21, P. djamor CGMCC 5.407; lane 22, P. eryngii MG 497; lane 23, P. eryngii HMAS 25978; lane 24, P. nebrodensis MG 500; lane 25, P. nebrodensis HMAS 86357; lane 26, P. ostreatus CGMCC 5.344; lane 27, P. ostreatus CGMCC 5.37; and lane 28, P. ostreatus HMAS 66080. Lane M, 100-bp DNA ladder.
FIG. 7.
PCR-RFLP band patterns observed with HaeIII. Lane 1, Pleurotus calyptratus HMAS 63355; lane 2, P. calyptratus HMAS 77117; lane 3, P. smithii IE 74; lane 4, P. cystidiosus ATCC 28597; lane 5, P. cystidiosus CGMCC 5.467; lane 6, P. cystidiosus CBS 80391; lane 7, P. abalonus MG 005; lane 8, P. abalonus CGMCC 5.409; lane 9, P. dryinus F 14011; lane 10, P. dryinus CBS 44977; lane 11, P. dryinus HKAS 17450; lane 12, P. cornucopiae ATCC 38547; lane 13, P. cornucopiae MG 504; lane 14, P. cornucopiae HMAS 76520; lane 15, P. pulmonarius MG 502; lane 16, P. pulmonarius HMAS 76672; lane 17, P. pulmonarius HMAS 72869; lane 18, P. tuber-regium MG 506; lane 19, P. tuber-regium HMAS 84647; lane 20, P. djamor CGMCC 5.600; lane 21, P. djamor CGMCC 5.407; lane 22, P. eryngii MG 497; lane 23, P. eryngii HMAS 25978; lane 24, P. nebrodensis MG 500; lane 25, P. nebrodensis HMAS 86357; lane 26, P. ostreatus CGMCC 5.344; lane 27, P. ostreatus CGMCC 5.37; and lane 28, P. ostreatus HMAS 66080. Lane M, 100-bp DNA ladder.
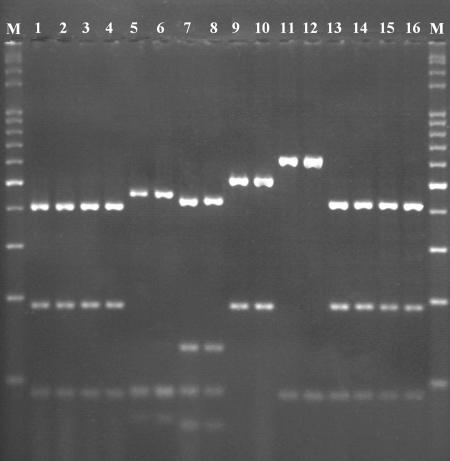
Because samples in lanes 15 to 21 of Fig. 7 (P. pulmonarius, P. tuber-regium, and P. djamor) produced similar patterns, as predicted by the PRE Auto program (Fig. 3), these samples were further treated with the combination of AluI and HpyCH4IV, as were the samples in lanes 22 to 28 of Fig. 7 (P. eryngii, P. nebrodensis, and P. ostreatus), which displayed the same band profiles, also as predicted in Fig. 3, to obtain better resolution for species identification. In addition, two of the four misidentified samples (Fig. 7, lanes 11 and 14), which could not be identified at the species level after the digestion by HaeIII, were also further digested with the two enzymes. Figure 8 shows the resulting band patterns after digestion by the combination of the two selected enzymes. Samples with similar HaeIII band patterns in lanes 11 and 15 to 21 of Fig. 7 were clearly distinguished from one another in three groups in Fig. 8, representing P. pulmonarius (lanes 1 to 4), P. tuber-regium (lanes 5 and 6), and P. djamor (lanes 7 and 8). Samples with the same HaeIII band patterns in lanes 14 and 22 to 28 of Fig. 7 were also separated without any ambiguity into P. eryngii (Fig. 8, lanes 9 and 10), P. nebrodensis (Fig. 8, lanes 11 and 12), and P. ostreatus (Fig. 8, lanes 13 to 16), as predicted from the sequence information (Fig. 4).
FIG. 8.
PCR-RFLP band patterns observed after digestion with the combination of AluI and HpyCH4IV. Lane 1, P. dryinus HKAS 17450; lane 2, P. pulmonarius MG 502; lane 3, P. pulmonarius HMAS 76672; lane 4, P. pulmonarius HMAS 72869; lane 5, P. tuber-regium MG 506; lane 6, P. tuber-regium HMAS 84647; lane 7, P. djamor CGMCC 5.600; lane 8, P. djamor CGMCC 5.407; lane 9, P. eryngii MG 497; lane 10, P. eryngii HMAS 25978; lane 11, P. nebrodensis MG 500; lane 12, P. nebrodensis HMAS 86357; lane 13, P. ostreatus CGMCC 5.344; lane 14, P. ostreatus CGMCC 5.37; lane 15, P. ostreatus HMAS 66080; lane 16, P. cornucopiae HMAS 76520; and lane M, 100-bp DNA ladder.
To confirm PCR-RFLP species identification, the PCR products of ITS regions from nine samples, including two misidentified samples of P. pulmonarius (previously misidentified as P. dryinus, in lane 11 of Fig. 7 and lane 1 of Fig. 8) and P. ostreatus (previously misidentified as P. cornucopiae, in lane 14 of Fig. 7 and lane 16 of Fig. 8), were subjected to DNA sequencing. The GenBank accession numbers for ITS regions from the sequenced samples are listed in Table 1. The results of sequencing showed the same conclusion obtained by the PCR-RFLP analysis described here. The redetermination of fungal names for the four previously misidentified samples, based on the results of both PCR-RFLP analysis and ITS sequencing, is indicated in Table 1.
DISCUSSION
The method developed in this study for the autoscreening of restriction endonucleases for the PCR-RFLP technique using sequence analyses and especially the PRE Auto software is considerably efficient and accurate within a short period of time. It took less than 1 min for the PRE Auto program to perform the calculations for and select candidate endonucleases from a total of 2,992 endonucleases for 85 haplotype sequences. The display of the predicted band patterns from digestion with an enzyme made the selection of a particular enzyme and the estimate of results more intuitive. In this study, every band of DNA fragments predicted by the PRE Auto program after digestion by the selected enzymes was confirmed by actual sample experiments (compare Fig. 3 with Fig. 7 and Fig. 4 with Fig. 8). The unequal densities of DNA in the bands in Fig. 7 and 8 reflect the different sizes of the fragments after digestion. The smaller the fragment, the fainter the band appears under UV illumination after ethidium bromide staining.
Although the existing design tools for the PCR-RFLP assay, e.g., DNA CLUB (47), NEBcutter version 2.0 (44), Genetyx version 6.1 (26), and SNP Cutter (51), have been used to search for cleavage sites and to calculate the sizes of restriction fragments, the selection of restriction endonucleases by manual comparison of restriction fragment profiles of different sequences with various restriction enzymes is still very time-consuming. For example, using the DNA CLUB and Origin 5.0 computer packages, Wright and Pimm (47) had to manually compare the restriction fragment patterns of 29 sequences with 55 different restriction enzymes and finally selected 10 endonucleases to digest the 16S gene for the molecular identification of methanogens. The PRE Auto software reported here is a much more efficient and informative program than the other computer packages for PCR-RFLP experiment design, especially for the functions of multiple-sequence input, autoscreening of endonucleases, and production of an electrophoresis gel map. After the autocomparison and scoring of restriction fragment patterns of each restriction enzyme for all the input ITS sequences, the selection of restriction endonucleases was very efficient with the guidance of the electrophoresis map. Further, the treatment of grouped sequences is a novel function that makes it possible to consider the sequence variation within a species for the autoscreening of restriction enzymes. Although the haplotype sequences from the Pleurotus species used were considerably variable, e.g., groups 5 and 10 (Fig. 2), or very conservative, e.g., groups 8 and 9 (Fig. 2), within a group, the PRE Auto program could still find the restriction endonucleases to differentiate the groups, which represent different species. This result will avoid the possible restriction sites within an individual sequence but without the capability to separate species. The new method developed in this study for the rapid selection of restriction enzymes can be applied widely in the molecular identification of living organisms using the PCR-RFLP technique when a large number of target sequences are considered. However, it is worth notice that group 6, representing P. ostreatus, is a paraphyletic group in the ITS sequence analysis (Fig. 2), closely related to group 7, P. pulmonarius. Because groups 6 and 7 were input into PRE Auto as separate groups, they were successfully distinguished from each other by the restriction digestion by HaeIII. As a computer program, the PRE Auto program is able to search for the most effective enzymes to separate the sequence groups input by the researcher, but it cannot make the taxonomic decision on the grouping of the sequences.
In addition, all other available computer packages, except the SNP Cutter, take only an individual sequence representative of a species to select restriction endonucleases. It is an advantage of both the PRE Auto program and the SNP Cutter to be able to analyze multiple sequences from a species for searching the cleavage sites of restriction enzymes. However, SNP Cutter is unable to do the autoscreening of restriction endonucleases and to output the predicted electrophoresis gel map after digestion.
The newly developed technique for the rapid selection of restriction enzymes has been validated by experiments using 17 living strains and 11 dried specimens, representing 12 Pleurotus species. This method could unequivocally identify all the 28 Pleurotus samples, including 24 in conformity with the previous species determination based on the morphological method and four in discordance with the original identification. Among the 28 tested samples, the five samples used as controls (three as positive and two as negative controls) based on ITS sequences previously submitted to GenBank were verified by the PCR-RFLP method. ITS sequences from two misidentified samples detected here (HMAS 76520 and HKAS 17450) (Table 1) and from seven correctly named samples (ATCC 28597, CBS 44977, HMAS 25978, HMAS 86357, HMAS 66080, CGMCC 5.37, and HMAS 84647) also confirmed the determination of species using the PCR-RFLP method. The results indicate that the method developed in this study is accurate and reliable.
Many efforts have been devoted to the identification of Pleurotus species, including the application of molecular methods. In the work on RFLP and PCR-RFLP by Bao et al. (2) and Iracabal et al. (12), extensive experiments were conducted to screen the restriction enzymes from a very limited set of enzyme candidates based on a few DNA sequences. The autoscreening method reported here, supported by the PRE Auto program and based on the analysis of a large number of sequences, can select restriction enzymes from thousands of candidate endonucleases, and the 12 Pleurotus species were unambiguously identified with only two steps and three restriction endonucleases. In comparison with previous studies identifying Pleurotus species by the PCR-RFLP technique, this study used a minimal number of restriction endonucleases and required minor costs and a minor amount of time for the selection of enzymes. Further, there is much scope for increase in the number of species for identification and the number of endonucleases for screening in the PRE Auto program.
In conclusion, a new method for the rapid selection of restriction endonucleases with the aid of the PRE Auto computer program was established for the molecular identification of living organisms using the PCR-RFLP technique. The method is based on the sequence analysis of target DNA fragments of species for screening of restriction enzymes from a large number of candidate endonucleases, and the autoscreening is performed by the PRE Auto program. This is a genotypic identification approach that can be applied for the discrimination of a large number of species, confirmed with at least 12 Pleurotus species in this study, and is flexible to take more species and more endonucleases into its database. The computer autoscreening of restriction enzymes for PCR-RFLP identification of species is a very efficient and time- and cost-saving method for the characterization of living organisms at the species level.
Supplementary Material
Acknowledgments
This work was supported by the National Science Funds for Distinguished Young Scholars of China (30025002), the Key Project of the Knowledge Innovation of the Chinese Academy of Sciences (KSCX2-SW-101C), and the program Introduction of Overseas Outstanding Talents, operated by the Chinese Academy of Sciences.
Footnotes
Published ahead of print on 26 October 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aranishi, F. 2005. Rapid PCR-RFLP method for discrimination of imported and domestic mackerel. Mar. Biotechnol. 7:571-575. [DOI] [PubMed] [Google Scholar]
- 2.Bao, D., H. Ishihara, N. Mori, and Y. Kitamoto. 2004. Phylogenetic analysis of oyster mushrooms (Pleurotus spp.) based on restriction fragment length polymorphisms of the 5′ portion of 26S rDNA. J. Wood Sci. 50:169-176. [Google Scholar]
- 3.Corner, E. J. H. 1981. The agaric genera Lentinus, Panus, and Pleurotus: with particular reference to Malaysian species. Nova Hedwigia 69:1-169. [Google Scholar]
- 4.de Andrade, H. M., A. B. Reis, S. L. dos Santos, A. C. Volpini, M. J. Marques, and A. J. Romanha. 2006. Use of PCR-RFLP to identify Leishmania species in naturally-infected dogs. Vet. Parasitol. 140:231-238. [DOI] [PubMed] [Google Scholar]
- 5.Demkin, V. V., and A. L. Zimin. 2005. A new amplification target for PCR-RFLP detection and identification of Chlamydiaceae species. Arch. Microbiol. 183:169-175. [DOI] [PubMed] [Google Scholar]
- 6.Eichlerová, I., L. Homolka, and F. Nerud. 2006. Ability of industrial dyes decolorization and ligninolytic enzymes production by different Pleurotus species with special attention on Pleurotus calyptratus, strain CCBAS 461. Process Biochem. 41:941-946. [Google Scholar]
- 7.Gaitanis, G., A. Velegraki, E. Frangoulis, A. Mitroussia, A. Tsigonia, A. Tzimogianni, A. Katsambas, and N. J. Legakis. 2002. Identification of Malassezia species from patient skin scales by PCR-RFLP. Clin. Microbiol. Infect. 8:162-173. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez, P., and J. Labarère. 2000. Phylogenetic relationships of Pleurotus species according to the sequence and secondary structure of the mitochondrial small-subunit rRNA V4, V6 and V9 domains. Microbiology 146:209-221. [DOI] [PubMed] [Google Scholar]
- 9.Granchi, L., M. Bosco, A. Messini, and M. Vincenzini. 1999. Rapid detection and quantification of yeast species during spontaneous wine fermentation by PCR-RFLP analysis of the rDNA ITS region. J. Appl. Microbiol. 87:949-956. [DOI] [PubMed] [Google Scholar]
- 10.Gurtler, V., C. Harford, J. Bywater, and B. C. Mayall. 2006. Direct identification of slowly growing Mycobacterium species by analysis of the intergenic 16S-23S rDNA spacer region (ISR) using a GelCompar II database containing sequence based optimization for restriction fragment site polymorphisms (RFLPs) for 12 enzymes. J. Microbiol. Methods 64:185-199. [DOI] [PubMed] [Google Scholar]
- 11.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 12.Iracabal, B., G. Zervakis, and J. Labarère. 1995. Molecular systematics of the genus Pleurotus: analysis of restriction polymorphisms in ribosomal DNA. Microbiology 141:1479-1490. [DOI] [PubMed] [Google Scholar]
- 13.Isikhuemhen, O. S., J. M. Moncalvo, F. Nerud, and R. Vilgalys. 2000. Mating compatibility and phylogeography in Pleurotus tuberregium. Mycol. Res. 104:732-737. [Google Scholar]
- 14.Jang, J., B. Kim, J. Lee, and H. Han. 2003. A rapid method for identification of typical Leuconostoc species by 16S rDNA PCR-RFLP analysis. J. Microbiol. Methods 55:295-302. [DOI] [PubMed] [Google Scholar]
- 15.Kirk, P. M., P. F. Cannon, J. C. David, and J. A. Stalpers. 2001. Ainsworth and Bisby's dictionary of the fungi, 9th ed. CAB International, Surrey, United Kingdom.
- 16.Ko, K. S., S. K. Hong, K. H. Lee, H. K. Lee, M. Y. Park, H. Miyamoto, and Y. H. Kook. 2003. Detection and identification of Legionella pneumophila by PCR-restriction fragment length polymorphism analysis of the RNA polymerase gene (rpoB). J. Microbiol. Methods 54:325-337. [DOI] [PubMed] [Google Scholar]
- 17.Kues, U., and Y. Liu. 2000. Fruiting body production in Basidiomycetes. Appl. Microbiol. Biotechnol. 54:141-152. [DOI] [PubMed] [Google Scholar]
- 18.Lechner, B. E., J. E. Wright, and E. Albertó. 2004. The genus Pleurotus in Argentina. Mycologia 96:845-858. [DOI] [PubMed] [Google Scholar]
- 19.Lewinsohn, D., S. P. Wasser, Y. Hadar, S. P. Wasser, and A. Beharav. 2000. Ecogeographical variation in the Pleurotus eryngii complex in Israel. Mycol. Res. 104:1184-1196. [Google Scholar]
- 20.Li, X. L., and Y. J. Yao. 2005. Revision of the taxonomic position of the Phoenix Mushroom. Mycotaxon 91:61-73. [Google Scholar]
- 21.Llorens, A., M. J. Hinojo, R. Mateo, M. T. González-Jaén, F. M. Valle-Algarra, A. Logrieco, and M. Jimenéz. 2006. Characterization of Fusarium spp. isolates by PCR-RFLP analysis of the intergenic spacer region of the rRNA gene (rDNA). Int. J. Food Microbiol. 106:297-306. [DOI] [PubMed] [Google Scholar]
- 22.Loppnau, P. A., and C. Breuil. 2003. Species level identification of conifer associated Ceratocystis sapstain fungi by PCR-RFLP on a beta-tubulin gene fragment. FEMS Microbiol. Lett. 222:143-147. [DOI] [PubMed] [Google Scholar]
- 23.Mabru, D., J. P. Douet, A. Mouton, C. Dupré, J. M. Ricard, B. Médina, M. Castroviejo, and G. Chevalier. 2004. PCR-RFLP using a SNP on the mitochondrial Lsu-rDNA as an easy method to differentiate Tuber melanosporum (Perigord truffle) and other truffle species in cans. Int. J. Food Microbiol. 94:33-42. [DOI] [PubMed] [Google Scholar]
- 24.Marcilla, A., M. D. Bargues, and S. Mas-Coma. 2002. A PCR-RFLP assay for the distinction between Fasciola hepatica and Fasciola gigantica. Mol. Cell. Probes 16:327-333. [DOI] [PubMed] [Google Scholar]
- 25.McEwen, J. G., J. W. Taylor, D. Carter, J. Xu, M. S. Felipe, R. Vilgalys, T. G. Mitchell, T. Kasuga, T. White, T. Bui, and C. M. Soares. 2000. Molecular typing of pathogenic fungi. Med. Mycol. 38(Suppl. 1):189-197. [PubMed] [Google Scholar]
- 26.Mochizuki, T., H. Tanabe, M. Kawasaki, H. Ishizaki, and C. J. Jackson. 2003. Rapid identification of Trichophyton tonsurans by PCR-RFLP analysis of ribosomal DNA regions. J. Dermatol. Sci. 32:25-32. [DOI] [PubMed] [Google Scholar]
- 27.Nei, M., and W. H. Li. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76:5269-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohira, I. 1990. A revision of the taxonomic status of Pleurotus citrinopileatus. Rep. Tottori Mycol. Inst. 28:143-150. [Google Scholar]
- 29.Paillard, D., V. Dubois, R. Duran, F. Nathier, C. Guittet, P. Caumette, and C. Quentin. 2003. Rapid identification of Listeria species by using restriction fragment length polymorphism of PCR-amplified 23S rRNA gene fragments. Appl. Environ. Microbiol. 69:6386-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quirós, M., P. Martorell, M. J. Valderrama, A. Querol, J. M. Peinado, and M. I. de Silóniz. 2006. PCR-RFLP analysis of the IGS region of rDNA: a useful tool for the practical discrimination between species of the genus Debaryomyces. Antonie Leeuwenhoek 90:211-219. [DOI] [PubMed] [Google Scholar]
- 31.Rachman, C., P. Kabadjova, R. Valcheva, H. Prévost, and X. Dousset. 2004. Identification of Carnobacterium species by restriction fragment length polymorphism of the 16S-23S rRNA gene intergenic spacer region and species-specific PCR. Appl. Environ. Microbiol. 70:4468-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridgway, K. P., J. M. Duck, and J. P. Young. 2003. Identification of roots from grass swards using PCR-RFLP and FFLP of the plastid trnL (UAA) intron. BMC Ecol. 3:8. http://www.biomedcentral.com/1472-6785/3/8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmones, D., G. Mata, and K. N. Waliszewski. 2005. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: biomass production and substrate biodegradation. Bioresour. Technol. 96:537-544. [DOI] [PubMed] [Google Scholar]
- 34.Singh, R. K., R. P. Mishra, H. K. Jaiswal, V. Kumar, S. P. Pandey, S. B. Rao, and K. Annapurna. 2006. Isolation and identification of natural endophytic rhizobia from rice (Oryza sativa L.) through rDNA PCR-RFLP and sequence analysis. Curr. Microbiol. 52:345-349. [DOI] [PubMed] [Google Scholar]
- 35.Steuber, S., A. Abdel-Rady, and P. H. Clausen. 2005. PCR-RFLP analysis: a promising technique for host species identification of blood meals from tsetse flies (Diptera: Glossinidae). Parasitol. Res. 97:247-254. [DOI] [PubMed] [Google Scholar]
- 36.Swofford, D. L. 2001. PAUP: Phylogenetic Analysis Using Parsimony, version 4.0b10. Sinauer Associates, Sunderland, MA.
- 37.Thompson, F. L., T. Iida, and J. Swings. 2004. Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 68:403-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorn, R. G., and G. L. Barron. 1984. Carnivorous mushrooms. Science 224:76-78. [DOI] [PubMed] [Google Scholar]
- 40.Traversa, D., D. Otranto, R. Iorio, and A. Giangaspero. 2005. Molecular characterization of Thelazia lacrymalis (Nematoda, Spirurida) affecting equids: a tool for vector identification. Mol. Cell. Probes 19:245-249. [DOI] [PubMed] [Google Scholar]
- 41.Trost, A., B. Graf, J. Eucker, O. Sezer, K. Possinger, U. B. Göbel, and T. Adam. 2004. Identification of clinically relevant yeasts by PCR/RFLP. J. Microbiol. Methods 56:201-211. [DOI] [PubMed] [Google Scholar]
- 42.Vilgalys, R., J. M. Moncalvo, S. R. Liou, and M. Volovsek. 1996. Recent advances in molecular systematics of the genus Pleurotus, p. 91-101. In D. J. Royse (ed.), Mushroom biology and mushroom products. Proceedings of the 2nd International Conference. Pennsylvania State University, University Park.
- 43.Vilgalys, R., and B. L. Sun. 1994. Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proc. Natl. Acad. Sci. USA 91:4599-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vincze, T., J. Posfai, and R. J. Roberts. 2003. NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Res. 31:3688-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpini, Â. C., V. M. Passos, G. C. Oliveira, and A. J. Romanha. 2004. PCR-RFLP to identify Leishmania (Viannia) braziliensis and L. (Leishmania) amazonensis causing American cutaneous leishmaniasis. Acta Trop. 90:31-37. [DOI] [PubMed] [Google Scholar]
- 46.White, T. J., T. Bruns, S. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, London, United Kingdom.
- 47.Wright, A. D. G., and C. Pimm. 2003. Improved strategy for presumptive identification of methanogens using 16S riboprinting. J. Microbiol. Methods 55:337-349. [DOI] [PubMed] [Google Scholar]
- 48.Yang, D. Y., H. Fushimi, S. Q. Cai, and K. Komatsu. 2004. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and amplification refractory mutation system (ARMS) analyses of medicinally used Rheum species and their application for identification of Rhei Rhizoma. Biol. Pharm. Bull. 27:661-669. [DOI] [PubMed] [Google Scholar]
- 49.Zervakis, G., and C. Balis. 1996. A pluralistic approach in the study of Pleurotus species with emphasis on compatibility and physiology of the European morphotaxa. Mycol. Res. 100:717-731. [Google Scholar]
- 50.Zervakis, G. I., J. M. Moncalvo, and R. Vilgalys. 2004. Molecular phylogeny, biogeography and speciation of the mushroom species Pleurotus cystidiosus and allied taxa. Microbiology 150:715-726. [DOI] [PubMed] [Google Scholar]
- 51.Zhang, R., Z. Zhu, H. Zhu, T. Nguyen, F. Yao, K. Xia, D. Liang, and C. Liu. 2005. SNP Cutter: a comprehensive tool for SNP PCR-RFLP assay design. Nucleic Acids Res. 33:489-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.