Abstract
One of the pathways involved in the acquisition of the essential metal iron by bacteria involves the reduction of insoluble Fe3+ to soluble Fe2+, followed by transport of Fe2+ to the cytoplasm. Flavins have been implicated as electron donors in this poorly understood process. Ferrous iron uptake is essential for intestinal colonization by the important pathogen Campylobacter jejuni and may be of particular importance under low-oxygen conditions. In this study, the links among riboflavin biosynthesis, ferric reduction, and iron acquisition in C. jejuni NCTC11168 have been investigated. A riboflavin auxotroph was generated by inactivation of the ribB riboflavin biosynthesis gene (Cj0572), and the resulting isogenic ribB mutant only grew in the presence of exogenous riboflavin or the riboflavin precursor diacetyl but not in the presence of the downstream products flavin adenine dinucleotide and flavin mononucleotide. Riboflavin uptake was unaffected in the ribB mutant under iron-limited conditions but was lower in both the wild-type strain and the ribB mutant under iron-replete conditions. Mutation of the fur gene, which encodes an iron uptake regulator of C. jejuni, resulted in an increase in riboflavin uptake which was independent of the iron content of the medium, suggesting a role for Fur in the regulation of the as-yet-unknown riboflavin transport system. Finally, ferric reduction activity was independent of iron availability in the growth medium but was lowered in the ribB mutant compared to the wild-type strain and, conversely, increased in the fur mutant. Taken together, the findings confirm close relationships among iron acquisition, riboflavin production, and riboflavin uptake in C. jejuni.
The bioavailability of iron is recognized to be a significant factor which potentially limits bacterial growth and depends on environmental conditions, particularly pH and oxygen tension, which affect iron solubility and oxidation state (1). Soluble ferrous iron (Fe2+) is readily available to bacteria and, in gram-negative bacteria, requires only transport across the inner membrane (7, 19). However, in the presence of oxygen at a pH of ≥7, ferrous iron is rapidly converted into ferric iron (Fe3+), which is almost completely insoluble at a pH of ≥7. In mammalian tissues, iron is also complexed into hemoglobin, stored intracellularly in ferritin, or chelated by transferrin in serum and by lactoferrin at mucosal surfaces (40). The resulting free-iron concentration is far too low to support bacterial growth, and consequently, bacteria have evolved different mechanisms to acquire host-complexed iron compounds, including siderophore production and outer-membrane transport mechanisms and the production of ferric reductases (1, 9, 40).
One process linked with the formation of ferrous iron is ferric reduction by the action of flavin-cofactored ferric reductases (9, 29), which reduce flavins using reduced nicotinamide nucleotides as hydride donors, with the reduced flavins subsequently being used to reduce Fe3+ (15, 29). In a number of pathogenic bacteria, ferric (flavin) reductases have been found to be secreted extracellularly (4, 10, 11, 35), but in general, these proteins are poorly characterized. A close interrelationship of flavin status and iron uptake has been suggested in a wide range of organisms, including yeasts (14), higher plants (30), and mammals (25). A role for ferric reduction and riboflavin in iron uptake was previously suggested for several pathogenic bacteria, including the gastric pathogen Helicobacter pylori, which produces substantially more riboflavin under iron-limited conditions than under iron-replete conditions (6, 13, 41). In addition, ferrous iron uptake was shown to be required for virulence of H. pylori (36).
Campylobacter jejuni is a major cause of food poisoning in the developed parts of the world (39), and its sequelae include Guillain-Barré syndrome, an acute demyelinating disease of the peripheral nervous system (31). C. jejuni is normally found in the avian gut and cecum, and this environment is thought to be low in oxygen, favoring the formation of ferrous iron. While mechanisms for the acquisition of ferric iron complexes (heme, siderophores) by C. jejuni have been described previously (23, 27), it was recently demonstrated that ferrous iron uptake also plays an important role in avian gut colonization by C. jejuni (22).
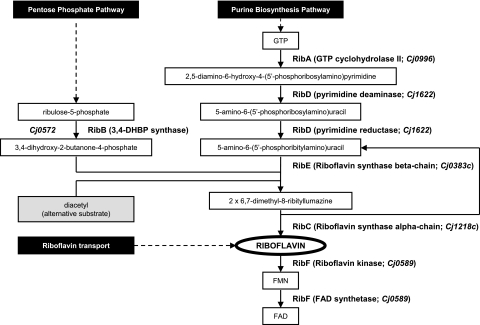
In view of the importance of ferrous iron uptake in gut colonization by C. jejuni, we have initiated a study investigating the possible role of flavins and ferric reduction in iron uptake in this organism. Analysis of the genome sequence of C. jejuni strain NCTC11168 (17, 24) indicated the presence of two genes (Cj0572 and Cj0996), both designated ribA and both putatively encoding orthologs of GTP cyclohydrolase II, the enzyme that mediates the initial step of riboflavin biosynthesis from GTP (Fig. 1). The Cj0572 protein has also been annotated as a bifunctional RibBA protein, which suggests that it also encodes RibB activity, which is the enzyme 3,4-dihydroxy-2-butanone-4-phosphate synthase that furnishes a four-carbon unit for incorporation into the riboflavin molecule (Fig. 1). In this study, it is demonstrated that the Cj0572 gene encodes the RibB enzyme of C. jejuni, that there are close links between riboflavin synthesis and ferrous and ferric iron acquisition by C. jejuni, and that these pathways are associated with the major iron-responsive regulator Fur.
FIG. 1.
Overview of the different enzymatic steps in the riboflavin biosynthetic pathway resulting in the biosynthesis of riboflavin and the coenzymes FAD and FMN. Two distinct branches of the pathway interact at the formation of 3,4-dihydroxy-2-butanone-4-phosphate (DHBP), which undergoes condensation with 5-amino-6-(5′-phosphoribitylamino)uracil to yield the riboflavin precursor 6,7-dimethyl-8-ribityllumazine. One molecule of riboflavin is formed from one molecule of GTP and two molecules of ribulose-5-phosphate in a series of enzyme-catalyzed reactions. The enzymes and corresponding genes listed are those generally found in E. coli (2, 37). The Cj gene numbers given with the RibA, RibD, RibE, RibC, and RibF steps are based on the annotation of the C. jejuni genome sequence (17, 24) and require experimental validation. The mechanism whereby the DHBP analogue diacetyl can complement an ribB deficiency is indicated by the gray box.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
C. jejuni strain NCTC11168 was obtained from the National Collection of Type Cultures, Central Public Health Laboratory, Colindale, London, United Kingdom. The fur mutant AV17 (NCTC11168 fur::Kanr) was described previously (18, 33). Campylobacter strains were maintained and grown at 37°C in a microaerophilic cabinet (Don Whitley, Shipley, United Kingdom) with a constant gas supply (10% CO2, 5% O2, 85% N2). C. jejuni strains were routinely cultured on Skirrow agar (Oxoid) and subcultured every 48 h onto fresh plates. The chemically defined tissue culture medium MEMα (Invitrogen) contains riboflavin (0.27 μM) but does not contain any added iron source (3) and supports the growth of C. jejuni (3, 18, 34). Fifty milliliters of standard MEMα or of MEMα without riboflavin (Invitrogen) was routinely supplemented with 20 μM FeCl3 and 20 mM HEPES and shaken at 200 rpm under microaerophilic conditions. Escherichia coli TOP10 (Invitrogen) was used as the host for cloning experiments and was routinely grown at 37°C in Luria-Bertani medium (28). When indicated, growth media were supplemented with kanamycin or ampicillin to final concentrations of 50 μg/ml and 25 μg/ml, respectively.
Construction of riboflavin auxotrophs by disruption of Cj0572.
The Cj0572 gene of C. jejuni strain NCTC11168 was amplified with primers 0571forward and 0573reverse (Table 1) and cloned into pGEM-Teasy (Promega). A BamHI restriction site was introduced by inverse PCR (42) with primers 0572Bamforward and 0572Bamreverse (Table 1). This inverse PCR also deleted a 502-bp internal fragment of the Cj0572 gene, which was subsequently interrupted by insertion of the kanamycin resistance gene from pJMK30 (34) into this unique BamHI site. The resulting plasmids, pCj0572CK and pCj0572KC, were subsequently introduced into C. jejuni NCTC11168 by electroporation (33). Two colonies derived from independent transformations were tested, and both colonies gave identical results in all experiments. Correct allelic replacement of the wild-type Cj0572 gene with the interrupted version was confirmed by PCR with combinations of the primers 0570forward, 0574reverse, CKRout, and CKFout (Table 1).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′ → 3′) |
|---|---|
| 0571forward | GGAAAGTCTAAGCGCCCAAG |
| 0573reverse | AAATTAAGTCTTTTGCCATCAACACT |
| 0572Bamforwarda | TGTGGATCCTTATAGAATATCGCCTAAAACATGA |
| 0572Bamreversea | TGTGGATCCATCTTCTGCATCTACCATAACAAGCAT |
| 0570forward | GGTGAGGGAAGATTTTAGTCG |
| 0574reverse | CTATAAGCCGTAGCTAGACC |
| CKFout | GGTTCGCTGGGTTTATCGGAT |
| CKRout | AGATGTCTAAAAAGCTTGGA |
Primer contains a 5′ extension with a BamHI restriction site (underlined).
Chemicals.
Unless stated otherwise, laboratory chemicals were purchased from Sigma Aldrich (Poole, Dorset, United Kingdom). Restriction enzymes were obtained from Promega (Southampton, United Kingdom). Oligonucleotide primers were synthesized by Sigma-Genosys (Pampisford, Cambridge, United Kingdom).
Riboflavin uptake.
The measurement of riboflavin uptake was adapted from the assay of Fe3+ uptake described previously (26). Late-exponential-phase cultures of strains grown in MEMα lacking riboflavin or in MEMα with additional riboflavin supplementation were harvested and washed twice in 50 mM phosphate buffer, pH 7.4. The cells were resuspended in 50 mM phosphate buffer (pH 7.4) to an optical density at 600 nm of 0.8, and 200 μl of this suspension was then added to 1 ml of standard MEMα. Samples were mixed and left to incubate microaerobically at 37°C for 1 to 2 h. The assay was initiated by adding [G-3H]riboflavin (9,874 Bq; Tocris Cookson Ltd., Bristol, United Kingdom). Samples (200 μl) were withdrawn at 1, 5, and 10 min and washed twice with 0.5 ml of 50 mM phosphate buffer (pH 7.4). The pellet was resuspended in 100 μl of Triton X-100 solution (0.5%, vol/vol, in water), mixed with 5 ml of a Quicksafe A scintillant-water cocktail (containing 1 part of scintillant to 10 parts of water, by volume [Zinsser Analytic, Maidenhead, Berks, United Kingdom]), and shaken vigorously. Radioactivity was determined by scintillation counting with radioactively labeled stock solutions as standards.
Fe3+ and Fe2+ uptake.
Iron uptake was measured by an adaptation of previously described methods (26, 36). A stock solution of Fe2+ was prepared by diluting 10 mM FeCl3 10-fold in 1 M ascorbate, and a stock solution of Fe3+ was prepared by diluting 10 mM FeCl3 10-fold into 1 M sodium citrate. 55FeCl3 (Perkin-Elmer, Waltham, MA) was added such that each assay mixture subsequently contained 7,750 Bq (the specific radioactivity therefore varied with the Fe2+ or Fe3+ concentration). Labeled Fe3+ and Fe2+ solutions were prepared and kept overnight at 4°C before use. Nitrilotriacetate (1.2 mM), which prevents nonspecific binding of iron to the bacterial membrane (16), was mixed with MEMα (1 part of nitrilotriacetate solution to 10 parts of medium, by volume) and kept overnight at 37°C before use. Late-exponential-phase cultures were harvested and washed twice in 50 mM phosphate buffer, pH 7.4, and the cells were resuspended in 50 mM phosphate buffer, pH 7.4, to an optical density at 600 nm of 0.8. Two hundred microliters was added to 1.1 ml of the nitrilotriacetate-MEMα solution and incubated microaerobically at 37°C for 1 to 2 h. The assay was initiated by adding the cell suspension in nitrilotriacetate-MEMα to the labeled Fe2+ or Fe3+ solution and mixing it thoroughly. Samples (200 μl) were withdrawn at 1, 5, and 10 min and treated as described above for measurement of riboflavin uptake.
Measurement of Fe3+ reduction.
Fe3+ reduction activity was determined as described previously (36).
Measurement of protein concentrations.
Protein concentrations of cell extracts were determined by a modified Lowry method (21) with bovine serum albumin as the standard.
RESULTS
Inactivation of the C. jejuni Cj0572 gene results in auxotrophy for riboflavin.
The requirement of C. jejuni NCTC11168 for riboflavin was investigated by two independent means, first by inactivation of genes putatively involved in riboflavin biosynthesis and second by investigation of growth kinetics of C. jejuni supplemented with different levels of exogenous riboflavin. Two C. jejuni genes annotated as ribA orthologs (Cj0572 and Cj0996, respectively) (17, 24) were targeted by insertional inactivation with a kanamycin resistance cassette. In view of the predicted auxotrophy for riboflavin, growth media used for isolation of these mutants were supplemented with riboflavin to final concentrations of 5, 50, and 500 μM.
Mutants containing a kanamycin resistance cassette in the Cj0572 gene were readily isolated on media containing high concentrations of riboflavin. Colonies were only recovered on Skirrow agar or blood agar containing >5 μM riboflavin, with the highest numbers of colonies appearing on plates containing 50 μM and 500 μM riboflavin. While mutants in Cj0572 were readily isolated with the antibiotic resistance cassette in either transcriptional orientation, we were unable to isolate Cj0996 mutants, despite repeated attempts (data not shown). This suggests that either the Cj0996 gene product or proteins encoded by genes in the proximity of Cj0996 may be essential for C. jejuni.
The C. jejuni Cj0572 gene encodes a RibB orthologue.
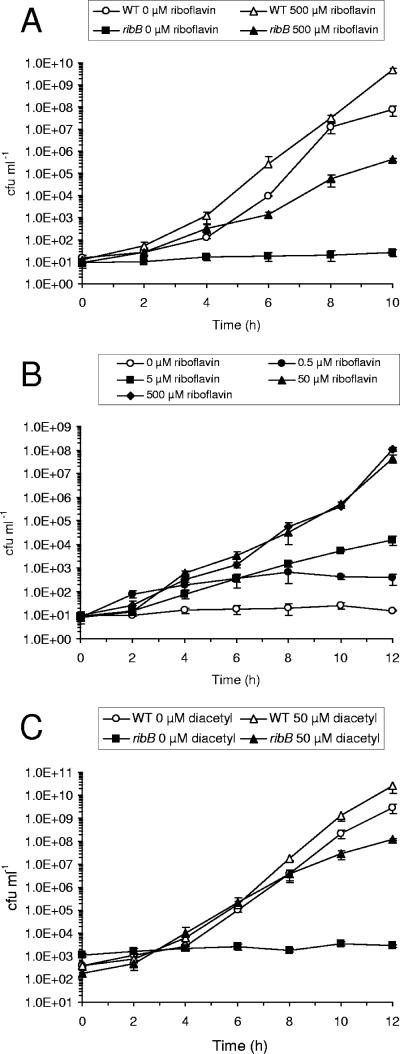
The growth kinetics of the C. jejuni Cj0572 mutant at different levels of riboflavin supplementation was determined with the defined tissue culture medium MEMα (3). Neither of two independent Cj0572 mutants was able to grow satisfactorily in the absence of added Fe3+ in MEMα and required supplementation with FeCl3 to a final concentration of 20 μM (data not shown). Even in the presence of 50 μM or 500 μM riboflavin, the Cj0572 mutant grew slowly compared to the wild-type strain (Fig. 2A). Growth of the Cj0572 mutant was optimal with riboflavin supplementation to 50 and 500 μM, and growth was significantly reduced with 5 and 0.5 μM riboflavin (Fig. 2B). Finally, the presence of the riboflavin precursor molecule diacetyl, an analogue of 3,4-dihydroxy-2-butanone-4-phosphate (2), allowed growth of the Cj0572 mutants and thus could substitute for riboflavin (Fig. 2C). In contrast, neither flavin mononucleotide (FMN) nor flavin adenine dinucleotide (FAD) could support growth (data not shown). The effect of diacetyl supplementation strongly suggests that the Cj0572 gene encodes a RibB orthologue (Fig. 1), and hence we have annotated the Cj0572 gene as ribB and its mutant as an ribB mutant in the remainder of this report.
FIG. 2.
Mutation of the Cj0572 gene in C. jejuni NCTC11168 results in riboflavin auxotrophy, which can be overcome by supplementation with exogenous riboflavin or the riboflavin precursor diacetyl. (A) Growth curves of the wild-type (WT) strain and the ribB (Cj0572) mutant in riboflavin-free MEMα with 20 μM FeCl3, with and without supplementation with 500 μM riboflavin. (B) Growth curves of the ribB (Cj0572) mutant strain in riboflavin-free MEMα supplemented with 20 μM FeCl3 and a range of riboflavin concentrations. (C) Growth curves of the C. jejuni NCTC11168 wild-type strain (WT) and ribB mutant in response to exogenous diacetyl. Cultures were grown in MEMα without riboflavin, supplemented with 20 μM FeCl3 at 37°C, with or without 50 μM diacetyl. Data shown are from two independent growth experiments, each performed in triplicate. Error bars indicate standard deviations.
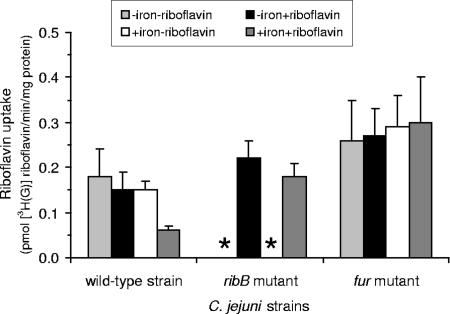
C. jejuni possesses a riboflavin uptake activity that is influenced by mutation of the fur gene.
The auxotrophy of the ribB mutant indicated that the cells were able to assimilate riboflavin. Furthermore, even the wild-type strain showed a small but consistent stimulation of growth in response to riboflavin (Fig. 2A). Riboflavin assimilation was studied directly by measuring the uptake of tritiated riboflavin in the wild-type strain and the ribB mutant (Fig. 3). To investigate the relationship between iron acquisition and riboflavin, we also included a fur mutant lacking the ferric uptake regulator (Fur), the main iron-responsive regulator of C. jejuni (18, 23, 34). Riboflavin uptake activities were comparable in all of the strains, although values were found to be higher in the fur mutant than in the other strains (Fig. 3). In the wild-type strain, and to a lesser degree in the ribB mutant, riboflavin uptake was lower under iron-replete conditions.
FIG. 3.
Riboflavin uptake by C. jejuni NCTC11168 is iron and riboflavin responsive and is increased in the fur mutant. Shown are rates of tritiated [G-3H]riboflavin uptake in the C. jejuni wild-type strain, ribB mutant, and fur mutant. Cultures were grown in riboflavin-free MEMα under iron-limited (1 μM FeCl3; indicated as −iron) and iron-replete (40 μM FeCl3, indicated as +iron) conditions with and without supplementation with 50 μM riboflavin (indicated as −riboflavin and +riboflavin). Data shown are from two independent experiments performed in triplicate. The ribB mutant could not be tested in medium without riboflavin because of the riboflavin auxotrophy; the absence of data for these samples is indicated by asterisks.
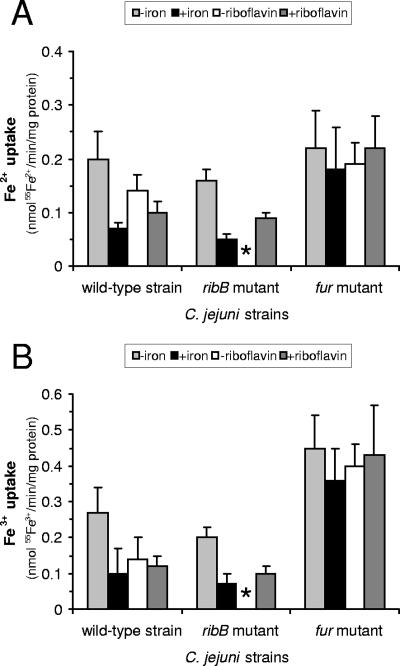
Riboflavin auxotrophy does not alter ferric or ferrous iron uptake.
The effects of riboflavin status upon cellular iron uptake were studied by measuring the uptake of radiolabeled ferrous and ferric iron in cells grown under iron-limited and iron-replete conditions (1 μM and 40 μM FeCl3 in MEMα, respectively). The wild-type strain and the fur mutant were also grown with and without 50 μM riboflavin (Fig. 4), but this was not possible with the ribB mutant because of its requirement for exogenous riboflavin. It was found that the values for Fe3+ uptake were at least as large as those for Fe2+, particularly in the case of the fur mutant, where the Fe3+ uptake values were about twice those of Fe2+ uptake and around two- to fourfold greater than those of Fe3+ uptake in the wild-type strain (Fig. 4). However, there was no appreciable difference between the ribB mutant and the wild-type strain with respect to either Fe2+ uptake or Fe3+ uptake, regardless of the level of Fe3+ under which the cells were grown (Fig. 4). There was therefore no evidence from these studies of any influence of riboflavin status on the uptake of Fe3+ or Fe2+ per se.
FIG. 4.
Ferric and ferrous iron uptake by C. jejuni NCTC11168 is iron and riboflavin responsive and is increased in the fur mutant. Rates of 55Fe2+ (A) and 55Fe3+ (B) uptake in the wild-type, ribB mutant, and fur mutant strains. Cultures were grown in iron-limited (1 μM FeCl3; indicated as −iron) and iron-replete (40 μM FeCl3, indicated as +iron) MEMα or in standard (20 μM FeCl3) MEMα without and with additional supplementation with 50 μM riboflavin (indicated as −riboflavin and +riboflavin). Data shown are from two independent experiments performed in triplicate. The ribB mutant could not be tested in MEMα without added riboflavin because of the riboflavin auxotrophy; the absence of data for this sample is indicated by an asterisk.
Ferric reduction activity requires riboflavin synthesis and is increased in a fur mutant.
The lack of an observable effect of riboflavin status upon the uptake of Fe3+ or Fe2+ was unsurprising. It was more likely that there would be an effect upon Fe3+ reduction (15, 29). Accordingly, Fe3+ reduction activity was compared among the wild-type strain, the fur mutant, and the ribB mutant (Table 2). Rates of ferric iron reduction were generally twice as high in the fur mutant than in the wild-type strain, whereas rates of ferric iron reduction were significantly reduced in the ribB mutant, which is surprising since the ribB mutant was grown in medium supplemented with riboflavin to a final concentration of 50 μM. Fe3+ reduction activity was not substantially affected by the level of Fe3+ supplied in the growth medium. Lowering iron availability from medium levels (20 μM FeCl3) to low levels (1 μM FeCl3) increased ferric reduction activity by about 15% in both the wild-type strain and the fur mutant (data not shown).
TABLE 2.
Fe3+ reduction activity in C. jejuni NCTC11168 and its ribB and fur mutants
| Strain | Mean Fe3+ reduction (nmol min−1 mg protein−1) ± SEMa
|
||
|---|---|---|---|
| Whole culture | Lysed cell pellet | Culture supernatant | |
| Wild type | 0.58 ± 0.01 | 0.38 ± 0.03 | 0.10 ± 0.01 |
| ribB mutant | 0.14 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
| fur mutant | 1.10 ± 0.01 | 0.80 ± 0.01 | 0.58 ± 0.01 |
Standard errors are for triplicate determinations on three sets of data.
DISCUSSION
The riboflavin biosynthetic pathway remains, in principle, a potential target for new antibacterial strategies (5), but aside from this, the possibility that riboflavin availability influences bacterial growth and competitiveness, particularly through effects on iron assimilation, attracts investigation. This is perhaps especially so in the case of food-borne pathogens such as C. jejuni, which, in principle, might produce riboflavin endogenously or, alternatively, might assimilate it from the host environment, including the gut luminal contents. In this study, we have investigated the processes involved in the initiation of the riboflavin biosynthesis pathway in C. jejuni by identification of the ribA (Cj0996) and ribB (Cj0572) genes. The ability of the ribB mutant to grow in the presence of diacetyl instead of riboflavin demonstrates that Cj0572 encodes a 3,4-dihydroxy 2-butanone-4-phosphate synthase, analogous to the ribBA gene of H. pylori (6, 13, 41). There is thus far no physiological evidence that the Cj0572 gene encodes a bifunctional RibBA enzyme. Similar findings have been reported for the riboflavin biosynthesis pathway in the related bacterium H. pylori (13).
The results presented here show that C. jejuni strain NCTC11168 can grow independently of exogenous riboflavin (Fig. 2A). It is also able to take up riboflavin from the medium, and exogenous riboflavin stimulated the growth of the wild-type and ribB mutant strains (Fig. 2). Riboflavin transport was confirmed directly by demonstrating the uptake of tritiated riboflavin, and rates of riboflavin uptake were somewhat higher in the ribB mutant than in the wild-type strain (Fig. 3). This suggests the possibility that the uptake mechanism—whatever this may be—may be responsive to the cellular demand for riboflavin and to the capability of the cell to fulfill it. In addition, riboflavin uptake in the fur mutant was found to be generally greater than in the wild type or the ribB mutant and unaffected by growth under riboflavin supplementation or under iron-replete conditions, suggesting an involvement of fur in the mechanism. Genes encoding specific bacterial riboflavin transporters (ypaA, ribU, pnuX, impX, and rfnT) have been identified in a number of bacteria, and expression of ypaA was found to be regulated by an RFN element, in concert with regulation of the expression of the rib operon (37); the RibU, YpaA, and PnuX riboflavin transport proteins of Lactococcus lactis, Bacillus subtilis, and Corynebacterium glutamicum, respectively, have recently been studied in detail (8, 12, 38). The genome sequences of C. jejuni, however, do not encode orthologs of these riboflavin transporter systems (17, 20, 24). It will therefore be of considerable interest to investigate further the biochemical and genetic properties of riboflavin uptake in C. jejuni. The results presented here also clearly show that neither FMN nor FAD supports the growth of the ribB mutant, suggesting that neither compound was taken up appreciably by the cells or that neither compound is hydrolyzed to riboflavin itself extracellularly.
Uptake systems potentially responsible for the assimilation of Fe3+ and Fe2+ in C. jejuni have been characterized, and it seems likely that their relative contributions will depend upon the particular iron sources available, the redox potential, and other environmental and physiological variables (18, 22, 23, 26, 32). Irrespective of the form in which iron is assimilated, there is a probable role for ferric (flavin) reductases, acting intracellularly in the case of the uptake of iron as Fe3+ or extracellularly (or periplasmically) in the case of uptake as Fe2+, for example, via the action of the FeoB transporter (22, 36). Riboflavin auxotrophy or (in the wild-type strain) supplementation with exogenous riboflavin had little effect on the uptake of Fe2+ or of Fe3+, but riboflavin auxotrophy was associated with diminished Fe3+ reduction activity. As the amount of riboflavin available to the ribB mutant was much greater than that available to the wild-type cultures (probably because of the supplementation with exogenous riboflavin), a simple insufficiency of riboflavin could not have been responsible for this diminished activity in the auxotrophs. Although riboflavin provided exogenously was sufficient to support the growth of the auxotrophic cultures, it did not support ferric (flavin) reductase activity to the levels of the wild-type strain (Table 2). This suggests that exogenous supplementation with riboflavin can only partially complement the riboflavin auxotrophy of the ribB mutant. This could be for a variety of potential reasons, including repression of the synthesis of ferric (flavin) reductase in the absence of a functioning riboflavin biosynthetic pathway, or conversely, high levels of exogenous riboflavin might act to repress the synthesis of ferric (flavin) reductase. The identity of the putative ferric reductase is still unknown, and there are unfortunately no obvious candidates present in the C. jejuni genome sequence (17, 24).
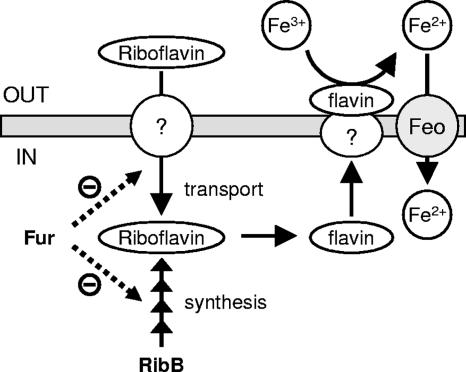
Our work unequivocally supports the links among riboflavin status, ferric reductase activity, and iron assimilation in C. jejuni, and these relationships are altered in the absence of the Fur iron-responsive regulator (Fig. 5). We have demonstrated riboflavin uptake by this organism and have shown that this process too is connected to regulation by Fur. Exactly how riboflavin uptake might be regulated in response to the cellular demand for riboflavin and to its role in iron assimilation is an intriguing question now demanding further investigation.
FIG. 5.
Graphic representation of our hypothetical model illustrating the proposed links among riboflavin biosynthesis, assimilatory ferric reduction, and iron acquisition in C. jejuni. Riboflavin biosynthesis via RibB (Fig. 1 and 2) or riboflavin uptake (Fig. 3) allows the activation of an as-yet-unknown flavin/ferric reductase, which mediates extracellular or membrane-bound reduction of ferric iron (Fe3+) to ferrous iron (Fe2+) (Table 2) (9, 15, 29). Ferrous iron may subsequently be transported by the Feo ferrous iron transporter system (Fig. 4) (22, 36). The ferric uptake regulator Fur is depicted as a negative regulator both of riboflavin synthesis and of an uncharacterized riboflavin uptake mechanism (Fig. 3 and 4; Table 2). The dashed lines indicate that it is not known whether the observed phenotypes are due to direct or indirect regulation by Fur.
Acknowledgments
This work was supported by a research studentship from the Biotechnology and Biological Sciences Research Council to R.A.C.
We thank J. M. Gee for helpful discussions.
Footnotes
Published ahead of print on 26 October 2007.
REFERENCES
- 1.Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. [DOI] [PubMed] [Google Scholar]
- 2.Bacher, A., S. Eberhardt, and G. Richter. 1996. Biosynthesis of riboflavin, p. 657-664. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC.
- 3.Baillon, M.-L. A., A. H. M. van Vliet, J. M. Ketley, C. Constantinidou, and C. W. Penn. 1999. An iron-regulated alkyl hydroperoxide reductase (AhpC) confers aerotolerance and oxidative stress resistance to the microaerophilic pathogen Campylobacter jejuni. J. Bacteriol. 181:4798-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barchini, E., and R. E. Cowart. 1996. Extracellular iron reductase activity produced by Listeria monocytogenes. Arch. Microbiol. 166:51-57. [DOI] [PubMed] [Google Scholar]
- 5.Becker, D., M. Selbach, C. Rollenhagen, M. Ballmaier, T. F. Meyer, M. Mann, and D. Bumann. 2006. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature 440:303-307. [DOI] [PubMed] [Google Scholar]
- 6.Bereswill, S., F. Fassbinder, C. Volzing, A. Covacci, R. Haas, and M. Kist. 1998. Hemolytic properties and riboflavin synthesis of Helicobacter pylori: cloning and functional characterization of the ribA gene encoding GTP-cyclohydrolase II that confers hemolytic activity to Escherichia coli. Med. Microbiol. Immunol. 186:177-187. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., K. Hantke, and W. Koster. 1998. Bacterial iron transport: mechanisms, genetics, and regulation. Met. Ions Biol. Syst. 35:67-145. [PubMed] [Google Scholar]
- 8.Burgess, C. M., D. J. Slotboom, E. R. Geertsma, R. H. Duurkens, B. Poolman, and D. van Sinderen. 2006. The riboflavin transporter RibU in Lactococcus lactis: molecular characterization of gene expression and the transport mechanism. J. Bacteriol. 188:2752-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowart, R. E. 2002. Reduction of iron by extracellular iron reductases: implications for microbial iron acquisition. Arch. Biochem. Biophys. 400:273-281. [DOI] [PubMed] [Google Scholar]
- 10.Cox, C. D. 1980. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J. Bacteriol. 142:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deneer, H. G., V. Healey, and I. Boychuk. 1995. Reduction of exogenous ferric iron by a surface-associated ferric reductase of Listeria spp. Microbiology 141:1985-1992. [DOI] [PubMed] [Google Scholar]
- 12.Duurkens, R. H., M. B. Tol, E. R. Geertsma, H. P. Permentier, and D. J. Slotboom. 2007. Flavin binding to the high affinity riboflavin transporter RibU. J. Biol. Chem. 282:10380-10386. [DOI] [PubMed] [Google Scholar]
- 13.Fassbinder, F., M. Kist, and S. Bereswill. 2000. Structural and functional analysis of the riboflavin synthesis genes encoding GTP cyclohydrolase II (ribA), DHBP synthase (ribBA), riboflavin synthase (ribC), and riboflavin deaminase/reductase (ribD) from Helicobacter pylori strain P1. FEMS Microbiol. Lett. 191:191-197. [DOI] [PubMed] [Google Scholar]
- 14.Fedorovich, D., O. Protchenko, and E. Lesuisse. 1999. Iron uptake by the yeast Pichia guilliermondii. Flavinogenesis and reductive iron assimilation are co-regulated processes. Biometals 12:295-300. [DOI] [PubMed] [Google Scholar]
- 15.Fontecave, M., J. Coves, and J. L. Pierre. 1994. Ferric reductases or flavin reductases? Biometals 7:3-8. [DOI] [PubMed] [Google Scholar]
- 16.Guignet, E. G., R. Hovius, and H. Vogel. 2004. Reversible site-selective labeling of membrane proteins in live cells. Nat. Biotechnol. 22:440-444. [DOI] [PubMed] [Google Scholar]
- 17.Gundogdu, O., S. D. Bentley, M. T. Holden, J. Parkhill, N. Dorrell, and B. W. Wren. 2007. Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence. BMC Genomics 8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes, K., F. Mulholland, B. M. Pearson, C. Pin, J. McNicholl-Kennedy, J. M. Ketley, and J. M. Wells. 2005. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology 151:243-257. [DOI] [PubMed] [Google Scholar]
- 19.Kammler, M., C. Schon, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour, N. M., M. Sawhney, D. G. Tamang, C. Vogl, and M. H. Saier, Jr. 2007. The bile/arsenite/riboflavin transporter (BART) superfamily. FEBS J. 274:612-629. [DOI] [PubMed] [Google Scholar]
- 21.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 22.Naikare, H., K. Palyada, R. Panciera, D. Marlow, and A. Stintzi. 2006. Major role for FeoB in Campylobacter jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect. Immun. 74:5433-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palyada, K., D. Threadgill, and A. Stintzi. 2004. Iron acquisition and regulation in Campylobacter jejuni. J. Bacteriol. 186:4714-4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. M. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 25.Powers, H. J. 1995. Riboflavin-iron interactions with particular emphasis on the gastrointestinal tract. Proc. Nutr. Soc. 54:509-517. [DOI] [PubMed] [Google Scholar]
- 26.Raphael, B. H., and L. A. Joens. 2003. FeoB is not required for ferrous iron uptake in Campylobacter jejuni. Can. J. Microbiol. 49:727-731. [DOI] [PubMed] [Google Scholar]
- 27.Ridley, K. A., J. D. Rock, Y. Li, and J. M. Ketley. 2006. Heme utilization in Campylobacter jejuni. J. Bacteriol. 188:7862-7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Schröder, I., E. Johnson, and S. de Vries. 2003. Microbial ferric iron reductases. FEMS Microbiol. Rev. 27:427-447. [DOI] [PubMed] [Google Scholar]
- 30.Susín, S., J. Abian, F. Sanchez-Baeza, M. L. Peleato, A. Abadia, E. Gelpi, and J. Abadia. 1993. Riboflavin 3′- and 5′-sulfate, two novel flavins accumulating in the roots of iron-deficient sugar beet (Beta vulgaris). J. Biol. Chem. 268:20958-20965. [PubMed] [Google Scholar]
- 31.van Vliet, A. H. M., and J. M. Ketley. 2001. Pathogenesis of enteric Campylobacter infection. Symp. Ser. Soc. Appl. Microbiol. 30:45S-56S. [DOI] [PubMed] [Google Scholar]
- 32.van Vliet, A. H. M., J. M. Ketley, S. F. Park, and C. W. Penn. 2002. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol. Rev. 26:173-186. [DOI] [PubMed] [Google Scholar]
- 33.van Vliet, A. H. M., A. C. Wood, J. Henderson, K. G. Wooldridge, and J. M. Ketley. 1998. Genetic manipulation of enteric Campylobacter species. Methods Microbiol. 27:407-419. [Google Scholar]
- 34.van Vliet, A. H. M., K. G. Wooldridge, and J. M. Ketley. 1998. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J. Bacteriol. 180:5291-5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vartivarian, S. E., and R. E. Cowart. 1999. Extracellular iron reductases: identification of a new class of enzymes by siderophore-producing microorganisms. Arch. Biochem. Biophys. 364:75-82. [DOI] [PubMed] [Google Scholar]
- 36.Velayudhan, J., N. J. Hughes, A. A. McColm, J. Bagshaw, C. L. Clayton, S. C. Andrews, and D. J. Kelly. 2000. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol. Microbiol. 37:274-286. [DOI] [PubMed] [Google Scholar]
- 37.Vitreschak, A. G., D. A. Rodionov, A. A. Mironov, and M. S. Gelfand. 2002. Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res. 30:3141-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogl, C., S. Grill, O. Schilling, J. Stülke, M. Mack, and J. Stolz. 2007. Characterization of riboflavin (vitamin B2) transport proteins from Bacillus subtilis and Corynebacterium glutamicum. J. Bacteriol. 189:7367-7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagenaar, J. A., D. J. Mevius, and A. H. Havelaar. 2006. Campylobacter in primary animal production and control strategies to reduce the burden of human campylobacteriosis. Rev. Sci. Tech. 25:581-594. [PubMed] [Google Scholar]
- 40.Wooldridge, K. G., and P. H. Williams. 1993. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 12:325-348. [DOI] [PubMed] [Google Scholar]
- 41.Worst, D. J., M. M. Gerrits, C. M. J. E. Vandenbroucke-Grauls, and J. G. Kusters. 1998. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. J. Bacteriol. 180:1473-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wren, B. W., J. Henderson, and J. M. Ketley. 1994. A PCR-based strategy for the rapid construction of defined bacterial deletion mutants. BioTechniques 16:994-996. [PubMed] [Google Scholar]