Abstract
The alkane- and alkene-degrading, marine sulfate-reducing bacterium Desulfatibacillum aliphaticivorans strain CV2803T, known to oxidize n-alkanes anaerobically by fumarate addition at C-2, was investigated for its 1-alkene metabolism. The total cellular fatty acids of this strain were predominantly C-(even number) (C-even) when it was grown on C-even 1-alkenes and predominantly C-(odd number) (C-odd) when it was grown on C-odd 1-alkenes. Detailed analyses of those fatty acids by gas chromatography-mass spectrometry after 6- to 10-week incubations allowed the identification of saturated 2- and 4-ethyl-, 2- and 4-methyl-, and monounsaturated 4-methyl-branched fatty acids with chain lengths that correlated with those of the 1-alkene. The growth of D. aliphaticivorans on (per)deuterated 1-alkenes provided direct evidence of the anaerobic transformation of these alkenes into the corresponding 1-alcohols and into linear as well as 10- and 4-methyl-branched fatty acids. Experiments performed with [13C]bicarbonate indicated that the initial activation of 1-alkene by the addition of inorganic carbon does not occur. These results demonstrate that D. aliphaticivorans metabolizes 1-alkene by the oxidation of the double bond at C-1 and by the subterminal addition of organic carbon at both ends of the molecule [C-2 and C-(ω-1)]. The detection of ethyl-branched fatty acids from unlabeled 1-alkenes further suggests that carbon addition also occurs at C-3. Alkylsuccinates were not observed as potential initial intermediates in alkene metabolism. Based on our observations, the first pathways for anaerobic 1-alkene metabolism in an anaerobic bacterium are proposed. Those pathways indicate that diverse initial reactions of 1-alkene activation can occur simultaneously in the same strain of sulfate-reducing bacterium.
Different studies during the past decades have demonstrated the utilization of hydrocarbons under anoxic conditions (32, 36). The initial reactions involved in the degradation of some aliphatic, alicyclic, and aromatic hydrocarbons by anaerobic bacteria have been subsequently elucidated elsewhere (4, 8, 24, 25, 29, 37, 38), but still, many gaps remain in the understanding of the anaerobic oxidation of some of these nonpolar molecules.
Unsaturated linear hydrocarbons (n-alkenes) are ubiquitous naturally occurring compounds that can be biosynthesized by different (micro)organisms and plants (3, 14). Phytoplankton appears, however, to be one of the major sources of these compounds in aquatic environments. Linear alkenes with 15 to 39 carbon atoms and with one to seven double bonds are known to be produced more or less specifically by microalgae (14). Short-chain n-C15, n-C17, and n-C19 monoenes as well as the highly unsaturated n-C21:6 are among the most commonly encountered. Specific bacterial n-alkenes have also been identified in microbial mats (33, 34).
Alkenes are more reactive than aromatic hydrocarbons and alkanes and, thus, are rarely identified as constituents of diagenetic organic matter. Different laboratory incubation studies as well as field studies have indeed revealed the low potential for preservation of n-alkenes during early diagenesis, although differences in the chemical structure and/or diagenetic conditions (e.g., anoxia) may be responsible for differences in reactivity between isomers (14, 18). Still, alkenes are found in some crude oils and condensates, but their origin seems to be related to an abiogenic alteration of n-alkanes in the trap rather than to a direct preservation of original molecules (12).
Although the anaerobic degradation of n-alkenes by enrichment cultures (28) and pure denitrifying (17) and sulfate-reducing bacteria (1, 2, 9, 10, 11, 30, 31) has been reported, the initial reactions of these unsaturated hydrocarbons in anaerobic bacteria are still unknown (32). The degradation of 1-hexadecene by methanogenic enrichment cultures was supposed to occur by initial oxidation to 1-hexadecanol and subsequent β-oxidation of hexadecanoic acid (28), but no intermediate metabolite other than acetate could be detected. On the other hand, in some early studies of alkane-degrading microorganisms, an oxygen-independent initial metabolism of alkanes via dehydrogenation to 1-alkenes and oxidation to primary alcohols was suggested (6, 23, 35). In recent investigations, however, alkane dehydrogenation to 1-alkenes was viewed critically (1, 2, 30), and two alternative pathways for the initial oxidation of alkanes in denitrifying or sulfate-reducing bacteria were subsequently described: the addition of fumarate at C-2 (5, 8, 24, 38) and carboxylation at C-3 (5, 29).
In the present report, we investigated the anaerobic degradation of 1-alkenes by the sulfate-reducing bacterium Desulfatibacillum aliphaticivorans strain CV2803T, recently isolated from a polluted marine sediment. This strain, which was proposed as the type strain of a novel species in a new genus of the family Desulfobacteraceae (class Deltaproteobacteria), is able to oxidize C13-C18 n-alkanes and C7-C23 n-alkenes into carbon dioxide during sulfate reduction (10). It was also recently demonstrated to transform anaerobically n-alkanes into fatty acids by the addition of fumarate at C-2 (8). By carefully examining the total cellular fatty acids of D. aliphaticivorans grown on different unlabeled 1-alkenes (C14:1 to C17:1) and on (per)deuterated 1-pentadecene and 1-hexadecene, we could demonstrate that strain CV2803T oxidizes alkenes into fatty acids by the oxidation of the double bond at C-1 (via a primary alcohol) and by the addition of an undefined carbon unit(s) at C-2 and C-3 and at the subterminal carbon of the saturated end of the molecule [C-(ω-1)]. Besides describing the first metabolic pathway of n-alkenes in an anaerobic bacterial isolate, our work demonstrates that diverse initial reactions of n-alkene activation can occur simultaneously in the same strain of sulfate-reducing bacteria.
MATERIALS AND METHODS
Chemicals.
1-Tetradecene, 1-pentadecene, 1-hexadecene, and 1-heptadecene (>99% pure) were purchased from Fluka Chemical Co. 1d2-1-pentadecene and perdeuterated 1-hexadecene (C16D32) were synthesized in three steps from unlabeled pentadecanoic acid (Aldrich Chemical Co.) and perdeuterated palmitic acid (C15D31COOH; Euriso-top), respectively: (i) reduction of the acid with LiAlD4 (Aldrich) in diethyl ether, (ii) reaction of the resulting alcohol with SOCl2 in pyridine (reflux for 1.5 h), and (iii) conversion of the produced 1-alkyl chloride into 1-alkene with potassium tert-butoxide in dry dimethyl sulfoxide (1 N at room temperature for 1 h) (39). The final synthetic (per)deuterated 1-alkenes were purified by column chromatography over silica gel using hexane-dichloromethane (9:1, vol/vol) as the eluent.
Source of bacterium and cultivation.
The sulfate-reducing bacterium D. aliphaticivorans strain CV2803T was isolated from sediment of the Gulf of Fos (Mediterranean Sea, France) (10). It belongs to the family Desulfobacteraceae in the Deltaproteobacteria and is phylogenetically closely related to a group of unnamed alkane-oxidizing strains. The most closely related species is Desulfosarcina variabilis (10). Strain CV2803 was grown in 500 ml of defined anoxic sulfate-reducing medium with pure unlabeled 1-alkene (1.1 to 1.4 mM) or 1d2-1-pentadecene (1.1 mM) as the growth substrate, as described previously (9). Precultures were all grown on unlabeled n-alkenes. Cultures with perdeuterated 1-hexadecene were prepared by mixing equal volumes of unlabeled and labeled 1-hexadecene (1 mM final concentrations) in order to facilitate growth (8, 24, 38). Cultures in the presence of [13C]bicarbonate (Aldrich) were prepared under argon with the medium buffered with 4-morpholinepropanesulfonic acid (MOPS; 3 g liter−1; pH 7.5) instead of bicarbonate buffer. [13C]bicarbonate and 1-hexadecene were added at concentrations of 5 mM and 1 mM, respectively. For each substrate, two experimental cultures and one sterile control (with autoclaved inoculum) were carried out. Cultures were analyzed after 6 to 10 weeks (depending on growth) of incubation at 30°C.
Extraction and analysis of cellular fatty acids.
Cells were collected by filtration through glass microfiber filters (GF/B; Whatman) and treated with 1 N KOH in methanol-water (1:1, vol/vol). Unsaponifiable (neutral) and saponifiable (acid) lipids were extracted from the basic and the acidified solutions, respectively, as described previously (8, 9). Neutrals were silylated by reaction with N,O-bis(trimethylsilyl)trifluoroacetanamide in pyridine (8), whereas free fatty acids were methylated with 1% sulfuric acid in methanol (7). A portion of the methylated fatty acids was hydrogenated (with PtO2 and H2 at room temperature overnight) in ethyl acetate containing one drop of acetic acid. The assignment of the methyl (Me) branch and double-bond positions in branched and/or unsaturated fatty acids was based on the formation of pyrrolidide derivatives (7), and the positions of the double bonds were further confirmed by oxidation with OsO4 and subsequent trimethylsilylation (26). Fatty acids were identified with an HP 5890 series II Plus gas chromatograph coupled to an HP 5972 mass spectrometer as described previously (8).
Nomenclature of fatty acids.
The fatty acid nomenclature recommended by the International Union of Pure and Applied Chemistry-International Union of Biochemistry (21) was adopted in this study. An n-saturated hexadecanoic acid is designated 16:0, with the first number representing the number of carbon atoms on the acyl group and the second number representing the number of double bonds present. A branched fatty acid, such as 2-ethylhexadecanoic acid, is designated 2-Et-16:0, and a monounsaturated branched fatty acid, such as 4-methylheptadec-11-enoic acid, is designated 4-Me-17:1Δ11. i- and a- refer to iso- and anteiso-fatty acids, respectively.
RESULTS
Transformation of 1-alkenes into fatty acids by D. aliphaticivorans.
Although D. aliphaticivorans strain CV2803T utilizes 1-alkenes from C7 to C23 (10), efficient growth is observed in the range from C14 to C17. This range was thus chosen for the present study (Table 1). In a preliminary report focused on the growth of D. aliphaticivorans on 1-alkenes (9), the major cellular fatty acids of strain CV2803T grown on 1-pentadecene and 1-hexadecene were described. This report already indicated that the chain length of the alkene influences the fatty acid composition of the strain. In the present study, new cultures with a broader spectrum of substrates further demonstrated that the chain lengths of the cellular fatty acids of D. aliphaticivorans are strongly dependent on those of the alkene substrates (Table 1). Cells grown on C-even alkenes contain mainly fatty acids with C-even carbon chains, and similarly, fatty acids with C-odd chains predominate when cells are grown on C-odd alkenes.
TABLE 1.
Relative abundances of cellular fatty acidsa of D. aliphaticivorans strain CV2803T
| Fatty acid | Relative abundance of fatty acid (% of total) of D. aliphaticivorans strain CV2803T grown on:
|
|||
|---|---|---|---|---|
| 1-Tetradecene | 1-Pentadecene | 1-Hexadecene | 1-Heptadecene | |
| 13:0 | 4.7 | 6.5 | ||
| 14:0 | 37.4 | 9.0 | 25.3 | 8.7 |
| 10-Me-14:0 | 3.6 | 1.0 | ||
| i-15:0 | 0.7 | 1.1 | 1.0 | 1.0 |
| a-15:0 | 0.9 | 1.0 | 1.0 | 1.0 |
| 15:0 | 2.5 | 35.8 | 1.4 | 29.3 |
| 10-Me-15:0 | 16.2 | 16.1 | ||
| 16:1Δ9 | 5.0 | 2.0 | 1.5 | |
| 16:0 | 26.7 | 14.0 | 28.6 | 11.1 |
| 10-Me-16:0 | 4.9 | 1.5 | 17.9 | 1.5 |
| 4-Me-16:0 | 0.9 | |||
| 17:0 | 1.1 | 1.9 | 0.5 | 13.2 |
| 4-Me-17:1b | 1.9 | |||
| 4-Me-17:0 | 1.0 | |||
| 18:2Δ9Δ11 | 1.6 | 1.6 | 4.5 | 1.7 |
| 18:1Δ11 | 5.4 | 1.7 | 3.5 | 1.8 |
| 18:1Δ9 | 0.7 | 1.1 | 2.3 | 1.0 |
| 18:0 | 8.8 | 5.6 | 5.5 | 5.8 |
| 4-Me-18:1b | 5.0 | |||
| 4-Me-18:0 | 1.0 | |||
| 4-Me-19:1b | 1.1 | |||
Data for acids constituting less than 0.5% of the total are not presented.
The double bond was located principally at C-11, but different positional isomers were detected.
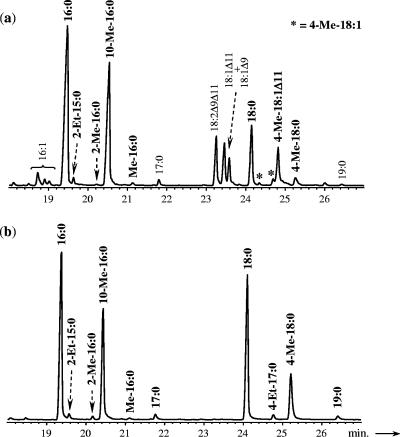
The detailed fatty acid composition of the strain grown on 1-alkenes was further analyzed by gas chromatography-mass spectrometry (GC-MS). Linear saturated fatty acids are accompanied by branched (iso-, anteiso-, and 10-Me-) and unsaturated (e.g., 18:1Δ9 and 18:1Δ11) fatty acids commonly encountered in bacteria and by less common saturated and monounsaturated 4-Me-branched fatty acids whose chain lengths are specifically correlated with those of the alkene substrates (Table 1). 4-Me-16:0, 4-Me-17:0, 4-Me-18:0, and 4-Me-19:0 and their monounsaturated homologues were detected only after growth on 1-tetradecene, 1-pentadecene, 1-hexadecene, and 1-heptadecene, respectively (Table 1 and Fig. 1a). Those saturated and monounsaturated 4-Me-branched fatty acids were detected neither in sterile controls nor in cultures grown on fatty acids (data not shown). The identification of all the saturated Me-branched fatty acids was based on the interpretation of their mass spectra, which show specific ions due to cleavage α to the branch carbon atoms, and on previously reported mass spectral data (9, 30, 38). The position of the methyl branch was further confirmed by the analysis of the pyrrolidide derivatives (data not shown) (9). Pyrrolidide derivatives were also used to determine the positions of both the methyl branch and the double bond in the monounsaturated homologues (Fig. 2a). Branching and unsaturation positions can be identified by fragments differing by 28 and 12 atomic mass units (amu), respectively, instead of the regular 14 amu (8, 26). For any alkene substrate, the monounsaturated 4-Me-branched fatty acid formed has its double bond located preferentially at C-11 (Fig. 2a), but other positional isomers (with the double bond at C-12, for instance) were sometimes observed (Fig. 1). The position of the double bond was further confirmed by oxidation with OsO4 (data not shown) (8, 9, 26), whereas that of the methyl branch was confirmed after catalytic hydrogenation. Indeed, monounsaturated methyl-branched fatty acids were all converted to the corresponding saturated 4-Me-branched fatty acids upon hydrogenation (Fig. 1).
FIG. 1.
Partial total-ion chromatograms of intact (a) and hydrogenated (b) fatty acid methyl esters of D. aliphaticivorans strain CV2803T grown on 1-hexadecene.
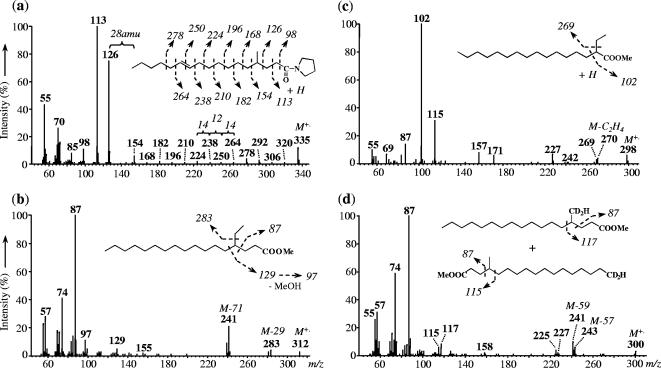
FIG. 2.
Mass spectra of 4-methylheptadec-11-enoylpyrrolidide formed after the derivatization of 4-methylheptadec-11-enoic acid from a culture of D. aliphaticivorans strain CV2803T grown on 1-pentadecene (a), 4-ethylheptadecanoic fatty acid methyl ester from a culture grown on 1-hexadecene (b), 2-ethylhexadecanoic fatty acid methyl ester from a culture grown on 1-heptadecene (c), and d2-4-methylheptadecanoic fatty acid methyl esters from a culture grown on 1d2-1-pentadecene (d).
GC-MS analysis of the minor cellular fatty acids (constituting <0.5% of the total) of D. aliphaticivorans strain CV2803T grown on 1-alkenes also allowed the identification of saturated 2-Me-branched fatty acids, as well as saturated monoethyl-branched fatty acids with an ethyl (Et) group located at C-2 or C-4 (Fig. 1, 2b, and 2c). Like the aforementioned 4-Me-branched fatty acids, these 2-Me-, 2-Et-, and 4-Et-branched fatty acids were detected neither in sterile controls nor in cultures grown on fatty acids (data not shown) and had a carbon chain length directly dependent on that of the alkene substrate (Fig. 1, 2b, and 2c). For instance, 2-Me-16:0, 2-Et-15:0, and 4-Et-17:0 were detected only after growth on 1-hexadecene (Fig. 1 and 2b), whereas 2-Me-17:0, 2-Et-16:0 (Fig. 2c), and 4-Et-18:0 were detected only after growth on 1-heptadecene. It should be noted that, due to their low abundance and their possible coelution with other major fatty acids, some of the Et-branched fatty acids may not have been detected without the catalytic hydrogenation of the fatty acid extract (Fig. 1). Mass spectra of 2-Me- and 2-Et-branched fatty acid methyl esters showed characteristic McLafferty base peaks at m/z of 88 and 102, respectively (see Fig. 2c for Et branching) (22, 30, 38). Although the mass spectra of 4-Et-branched fatty acids show similarities with those of 4-Me-branched fatty acids (Fig. 2b and d), the identification of the former compounds is based on their relative retention times (Fig. 1) and on the presence of an intense (M-71)+ ion in their mass spectra (Fig. 2b). This fragment, being analogous to the (M-57)+ ion observed for 4-Me-branched fatty acid methyl esters (Fig. 2b and d), corresponds to the elimination of the segment from C-2 to C-4 of the molecule, including the 4-ethyl group (and an additional hydrogen) (22).
The direct relationship observed between the chain lengths of the Me- and Et-branched fatty acids and those of the alkene substrates strongly suggests that 1-alkenes are (at least partly) transformed into fatty acids by the addition of exogenous carbon onto the ethylenic C-2 and onto the allylic C-3, yielding Me- and Et-branched fatty acids, respectively.
Identification of 1d2-1-pentadecene-derived fatty acids.
The growth of D. aliphaticivorans on pure 1d2-1-pentadecene yielded the same unlabeled linear, iso-, anteiso-, and 10-Me-branched fatty acids as those observed during growth on unlabeled 1-pentadecene (Table 1). However, deuterated 4-Me-17:0 (Fig. 2d) and 4-Me-17:1 (data not shown) fatty acids were also identified. The molecular ions in the mass spectra of these compounds (i.e., m/z of 300 and 298, respectively) were shifted up by 2 amu compared with those in the spectra of their unlabeled homologues (i.e., m/z of 298 and 296, respectively), indicating the presence of two deuterium atoms in the labeled compounds. On the other hand, traces of deuterated 2-Et-14:0 and 4-Et-16:0 were also detected, but clear mass spectra could not be satisfactorily recorded due to the low abundances and to coelution with major fatty acids. We failed to detect any trace of unlabeled or labeled 2-Me-15:0.
On the mass spectrum of the labeled 4-Me-17:0 obtained after catalytic hydrogenation of the acid extract, the two main peaks corresponding to those at m/z of 87 (cleavage of the C-3-C-4 bond) and m/z of 74 (McLafferty ion after the migration of a hydrogen from C-4) for the unlabeled fatty acid (30) remained the same (Fig. 2d), demonstrating that no deuterium was present between C-1 and C-4. However, doublets of peaks at m/z of 115 and 117, m/z of 225 and 227, and m/z of 241 and 243 indicate the presence of two isotopomers (Fig. 2d) (30). One contains two deuterium atoms on the methyl branch, while the other has no deuterium atom on this carbon (Fig. 2d), the ratio of the two isotopomers being 100/78. The two deuterium atoms in the latter isotopomer are most likely located at the terminal carbon (i.e., C-17), according to their original location in 1d2-1-pentadecene.
The presence of two deuterium atoms either at the Me branch or at the other end (C-ω) of the 4-Me fatty acid thus confirms that exogenous carbon is added to the alkene at C-2 and further indicates that carbon addition also occurs (albeit to a lesser extent) subterminally at the saturated end of the alkene [C-(ω-1)].
Identification of perdeuterated hexadecene-derived fatty acids.
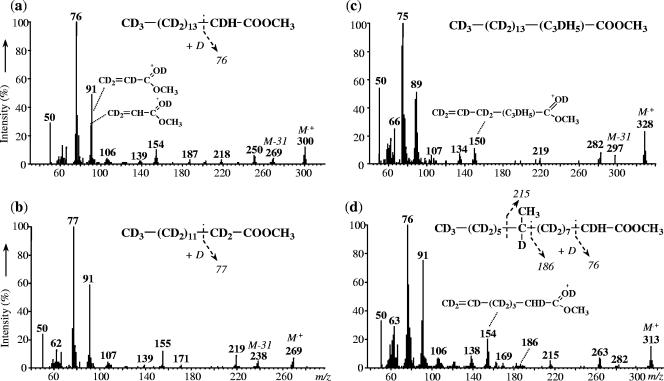
The growth of D. aliphaticivorans on d32-1-hexadecene yielded essentially deuterated 14:0, 16:0, 18:0, and 10-Me-16:0 fatty acids (Fig. 3); deuterated 4-Me-18:0, 4-Me-18:1, 2-Me-16:0, 2-Et-15:0, and 4-Et-17:0 could not be detected. Although labeling experiments were performed using a mixture of unlabeled and perdeuterated 1-hexadecene, the labeled fatty acids have shorter chromatographic retention times than the unlabeled fatty acids and, thus, generally appeared completely separated during GC-MS analyses. However, labeled Me-branched fatty acids have retention times similar to those of the unlabeled linear fatty acids, which likely induced coelution problems and, consequently, the nondetection of some minor deuterated branched fatty acids, even after catalytic hydrogenation. It should be noticed that during these labeling experiments, unlabeled 4-Me-18:1, 4-Me-18:0, 2-Me-16:0, and 4-Et-17:0 formed from unlabeled 1-hexadecene were observed.
FIG. 3.
Mass spectra and structural characteristics of d30-16:0 (a), d27-14:0 (b), d30-18:0 (c), and d29-10-Me-16:0 (d) fatty acid methyl esters from cultures of D. aliphaticivorans strain CV2803T grown on a mixture of unlabeled 1-hexadecene and d32-1-hexadecene.
Figure 3a shows the mass spectrum of the deuterated 16:0 fatty acid methyl ester. The molecular ion (m/z of 300) indicates the presence of 30 deuterium atoms in the molecule. The McLafferty ion (m/z of 76) is upshifted by 2 amu compared to the corresponding ion in the unlabeled analogue (m/z of 74), indicating the presence of two deuterium atoms in the labeled ion. Methanol used for methylation was not deuterated, and one deuterium atom would come from the McLafferty rearrangement, which indicates the presence of one deuterium and one hydrogen atom at C-2.
Similar analysis of the mass spectrum of the deuterated 14:0 fatty acid methyl ester indicates that it is fully deuterated (thus containing 27 deuterium atoms) (Fig. 3b), which suggests that it was formed by β-oxidation (loss of a C-2 unit) of the d30-16:0 fatty acid. Similar analysis of the mass spectrum of the deuterated 18:0 fatty acid shows that it contains a total of 30 deuterium atoms, together with five hydrogen atoms located between C-1 and C-5 (Fig. 3c). Although the exact positions of the hydrogen atoms cannot be deduced from the mass spectrum, the d30-18:0 fatty acid is likely formed by chain elongation of the d30-16:0 fatty acid with two nondeuterated carbon atoms. Traces of a d30-20:0 fatty acid were also detected (data not shown).
Examination of the deuterated 10-Me-16:0 fatty acid indicates that it contains a total of 29 deuterium atoms together with one hydrogen atom at C-2 and three other ones at the methyl branch (Fig. 3d). This suggests that the 10-Me-16:0 fatty acid is formed by the exogenous addition of a methyl group at C-10 of the corresponding n-saturated fatty acid (d30-16:0). Traces of d26-10-Me-14:0 fatty acid were also detected (data not shown).
These observations demonstrate that, along with the aforementioned addition of exogenous carbon at C-2, C-3, and C-(ω-1), the initial reactions of anaerobic 1-alkene degradation by D. aliphaticivorans also involve the oxidation of the double bond at C-1 and the further metabolism of the resulting fatty acid (formed via the corresponding 1-alcohol).
Fatty acids formed in the presence of [13C]bicarbonate.
The fatty acids formed in cultures of D. aliphaticivorans grown on unlabeled 1-alkene in the presence of [13C]bicarbonate were similar to those formed in the presence of unlabeled bicarbonate. Mass spectral analysis of the Me- and Et-branched fatty acids formed in the presence of [13C]bicarbonate showed that they were not 13C-labeled (data not shown), indicating that the incorporation of carbon at C-2, C-3 or C-(ω-1) in the original alkene does not proceed by incorporation of an inorganic carboxyl group.
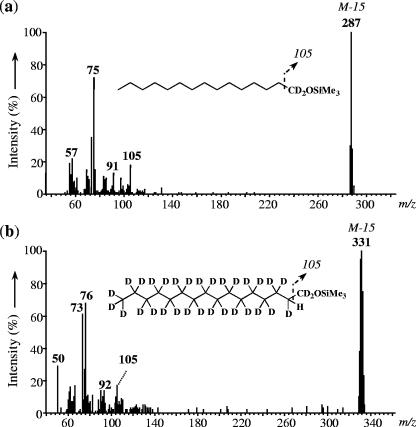
Identification of (per)deuterated fatty alcohols.
Owing to the major formation of d30-16:0 fatty acid from d32-1-hexadecene (Fig. 3a) and to the dominance of linear fatty acids with chain lengths similar to those of the substrates during growth on unlabeled 1-alkenes (Table 1), we searched for the presence of labeled fatty alcohols among the unsaponifiable lipids of D. aliphaticivorans grown on (per)deuterated 1-alkenes. 1d2-1-pentadecanol and d32-1-hexadecanol were detected after growth on 1d2-1-pentadecene and d32-1-hexadecene, respectively (Fig. 4). For both alcohols, the ion with the largest mass in the spectrum corresponds to the (M-15)+ ion resulting from the loss of one methyl from the trimethylsilyl group. This ion indicates the number of deuterium atoms present in each labeled alcohol. Moreover, for both alcohols, the ion fragment at m/z of 105 (corresponding to the ion at m/z of 103 for the unlabeled analogues) indicates the presence of two deuterium atoms at C-1 (Fig. 4).
FIG. 4.
Mass spectra of silylated 1d2-1-pentadecanol from a culture of D. aliphaticivorans strain CV2803T grown on 1d2-1-pentadecene (a) and d32-1-hexadecanol from a culture grown on a mixture of unlabeled 1-hexadecene and d32-1-hexadecene (b).
The presence of two deuterium atoms on the carbon bearing the hydroxyl group (C-1) and that of one deuterium and one hydrogen atom at C-2 in one or the other 1-alcohol further support the initial oxidation of the double bond of the alkene substrates by D. aliphaticivorans and the subsequent transformation of the resulting 1-alcohols to the corresponding linear fatty acids.
DISCUSSION
Previous studies have reported the biodegradation of 1-alkenes by pure sulfate-reducing bacteria (namely, strains Hxd3 [1, 2], Pnd3 [2], and AK-01 [30]), and partial information on the cellular fatty acid compositions of these strains grown on 1-alkenes in the range from C15 to C17 was given. Like D. aliphaticivorans, strains Hxd3, Pnd3, and AK-01 form essentially C-even fatty acids from C-even 1-alkenes and C-odd fatty acids from C-odd 1-alkenes. Aeckersberg et al. (2) further showed that the major fatty acid of strain Hxd3 grown on 1-hexadecene is 16:0, whereas 15:0 and 17:0 fatty acids dominate after growth on 1-heptadecene. However, these studies were essentially designed to demonstrate that 1-alkenes are not intermediates of the anaerobic n-alkane metabolism in strains Pnd3, Hxd3, and AK-01 (which were all originally isolated on n-alkanes), and the initial reactions of 1-alkene degradation were not studied in detail.
In the present study, we demonstrated that D. aliphaticivorans strain CV2803T transforms a 1-alkene into the corresponding 1-alcohol and into linear and branched fatty acids on the basis of the recovery of (per)deuterated fatty alcohols and fatty acids from cultures grown on labeled 1-alkenes. D. aliphaticivorans metabolizes 1-alkenes by both the oxidation of the double bond at C-1 and the addition of organic carbon at C-2, C-3, and C-(ω-1). The relationship between the carbon numbers of 1-alkene substrates and the predominant cellular n-saturated fatty acids (Table 1) may indicate that the oxidation of the double bond is the major pathway. However, our experimental approach of taking a single snapshot of metabolites rather than conducting time series or in vitro (e.g., fumarate addition) studies of metabolites may have resulted in our overlooking key transient but low-concentration metabolites involved in alternative pathways. The exact mechanism involved in the transformation of the double bond into a primary alcohol remains unknown. The hydration of double bonds seems common in the metabolisms of many organisms (32). This reaction has been hypothesized to occur during the anaerobic biotransformation of some isoprenoid alkenes (19, 27) and during the mineralization of 1-hexadecene by a methanogenic enrichment culture that also continued to grow on primary long-chain alcohols (28). However, as far as we know, hydration of a double bond to yield a primary alcohol via an anti-Markovnikov-oriented reaction has never been proven to occur in an alkene-degrading anaerobic bacterium.
Additionally, the results from our [13C]bicarbonate experiments indicate that the initial activation of 1-alkene by carboxylation does not occur in D. aliphaticivorans. The positions of the two deuterium atoms (either at the Me branch or at the other side of the molecule [C-ω]) in the two isotopomers of d2-4-Me-17:0 formed from 1d2-1-pentadecene further demonstrate that an organic carbon unit is added to the initial 1-alkene subterminally either at the ethylenic C-2 or at the saturated terminus. D. aliphaticivorans has been previously demonstrated to activate saturated n-alkanes by the addition of a molecule of fumarate at C-2 to yield 4-Me-branched fatty acids via alkylsuccinic acids (8). In such catabolic reactions, the alkylsuccinic acid undergoes a rearrangement of the carbon skeleton to form a (2-methyl-alkyl)malonate which is subsequently decarboxylated into a 4-Me-branched fatty acid possessing three carbon atoms more than the original n-alkane (38). The lack of detection of alkylsuccinates in the present study may be indicative of an alternative pathway for organic carbon addition to the alkene. However, alkylsuccinates are transient metabolites which may have been overlooked due to the analysis of cultures at a single time point and to the absence of in vitro assays (13); it is noteworthy that alkylsuccinates were not systematically observed among the fatty acids of D. aliphaticivorans during growth on n-alkanes (V. Grossi, unpublished results). We can thus still speculate that an addition of fumarate is also involved in the activation of 1-alkene by D. aliphaticivorans. Addition at C-2 and at C-(ω-1) of the 1-alkene followed by the rearrangement of the initial intermediate would yield a 4-Me fatty acid. A similar addition reaction at the carbon adjacent to the double bond (C-3) would explain the formation of 4-Et-branched fatty acids (Fig. 2b and c). Unfortunately, experiments with labeled fumarate which may have allowed confirmation of the above-stated hypothesis (24) have not given reliable results so far, probably because D. aliphaticivorans readily uses this molecule as a carbon source (10). Thus, the possibility that D. aliphaticivorans transforms 1-alkenes into branched fatty acids by the subterminal addition of a carbon unit other than fumarate cannot be ruled out. Owing to the likely difference in reactivity between the saturated and the unsaturated ends of the alkene, the addition of different organic carbon units at each side may also be envisaged.
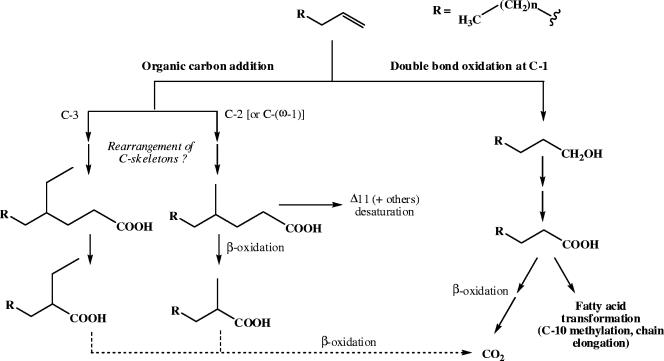
A pathway for anaerobic 1-alkene metabolism by D. aliphaticivorans strain CV2803T is proposed in Fig. 5. Although the acid metabolites were detected as free carboxylic acids, many of them presumably exist in vivo as coenzyme A thioesters (which are likely hydrolyzed during the analytical procedure) (38). The alcohol formed by the oxidation of the double bond is transformed into the corresponding linear fatty acid, which is mineralized into CO2 or, alternatively, transformed by chain elongation and/or C-10 methylation. On the other hand, the 4-Et- and 4-Me-branched fatty acids formed by the addition of organic carbon at C-3 and C-2 [or C-(ω-1)] can be degraded by β-oxidation into the corresponding 2-Et- and 2-Me-branched homologues, respectively (Fig. 5). These can be further transformed into linear fatty acids, which are subsequently mineralized into CO2 by other sequences of β-oxidation. Alternatively, the 4-Me fatty acid formed following the addition of carbon at C-2 [or C-(ω-1)] can be desaturated at C-11 (or, to a lesser extent, at other carbons), constituting anabolic reactions (Fig. 5).
FIG. 5.
Proposed pathways for anaerobic 1-alkene metabolism by the sulfate-reducing bacterium D. aliphaticivorans strain CV2803T.
Phylogenetic analyses showed that D. aliphaticivorans strain CV2803T is closely related to strains AK-01 and Pnd3, which may represent other species in the same genus (10). Moreover, as mentioned above, the carbon number of 1-alkene substrates has the same effect on the fatty acid compositions of the three strains. These similarities suggest that reactions of 1-alkene metabolism in strains AK-01 and Pnd3 are similar to those in D. aliphaticivorans. Strain Hxd3 is less phylogenetically related to D. aliphaticivorans than strains AK-01 and Pnd3 (10), but its fatty acid composition shows the same dependence on the carbon chain length of 1-alkenes when these compounds are used as growth substrates (2). It is thus possible that strain Hxd3 also possesses the same alkene-activating enzyme(s) as D. aliphaticivorans, catalyzing the oxidation of the double bond. This would not be contradictory to the fact that the initial reactions of anaerobic saturated alkane degradation in Hxd3 (involving carboxylation with inorganic carbon at C-3) (29) are different from those in D. aliphaticivorans and strain AK-01 (which oxidize n-alkanes into fatty acids by the addition of fumarate at C-2) (5, 8). Indeed, studies using dense cell suspensions have suggested that both strain Hxd3 (2) and D. aliphaticivorans (9) synthesize a specific enzyme(s) for the degradation of 1-alkenes. On the other hand, although these alkene-activating enzymes (as well as the genes encoding them) have not yet been defined, their synthesis by other alkene-degrading anaerobes which cannot utilize saturated hydrocarbons remains possible (11, 16, 17, 28).
It is noteworthy that the growth of D. aliphaticivorans on d32-1-hexadecene led to the formation of deuterated 10-methylated fatty acids (d29-10-Me-16:0 and d26-10-Me-14:0) containing only one deuterium atom less (at C-10) than their n-saturated precursors (d30-16:0 and d27-14:0, respectively) (Fig. 3). Interestingly, a similar feature (the loss of only one deuterium atom at C-10 during C-10 methylation of n-saturated fatty acids) was observed during the anaerobic metabolism of perdeuterated pentadecane by strain Hxd3 (29). Saturated midchain methyl-branched fatty acids are relatively common in bacteria (15) and are often assumed to be biosynthesized by the methylation of a monounsaturated linear fatty acid with S-adenosylmethionine as the methyl donor (2, 20). In the present case, this mechanism of methylation does not seem to be involved since desaturation and subsequent methylation at C-10 of the deuterated n-saturated fatty acids would have caused the loss of at least two deuterium atoms. Moreover, monounsaturated deuterated fatty acids that could have constituted potential intermediates in 10-Me-branched fatty acid biosynthesis were not detected. The present mechanism of methylation remains unknown but undoubtedly deserves further attention.
In summary, we provide direct evidence that 1-alkenes are anaerobically transformed into fatty acids by D. aliphaticivorans strain CV2803T and that different initial reactions of anaerobic 1-alkene degradation can occur simultaneously in this strain. The detailed characterization of labeled fatty alcohol and fatty acid metabolites demonstrates that D. aliphaticivorans metabolizes 1-alkene by the oxidation of the double bond at C-1 and by the subterminal addition of organic carbon(s) at both ends of the carbon chain. Although 1-alkenes are clearly not intermediates of the anaerobic n-alkane metabolism in D. aliphaticivorans, the present results suggest that the two classes of compounds may have some common pathways for their anaerobic oxidation.
Acknowledgments
This work was supported by an ECCO-Ecodyn grant (IndHyc project) from the Centre National de la Recherche Scientifique (CNRS) and the Institut des Sciences de l'Univers (INSU).
This is UMR5125 PEPS contribution 07.052.
Footnotes
Published ahead of print on 26 October 2007.
REFERENCES
- 1.Aeckersberg, F., F. Bak, and F. Widdel. 1991. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156:5-14. [Google Scholar]
- 2.Aeckersberg, F., F. A. Rainey, and F. Widdel. 1998. Growth, natural relationships, cellular fatty acids and metabolic adaptation of sulfate-reducing bacteria that utilize long-chain alkanes under anoxic conditions. Arch. Microbiol. 170:361-369. [DOI] [PubMed] [Google Scholar]
- 3.Bianchi, G. 1995. Plant waxes, p. 175-222. In R. J. Hamilton (ed.), Waxes: chemistry, molecular biology and functions. The Oily Press, Dundee, Scotland.
- 4.Boll, M., G. Fuchs, and J. Heider. 2002. Anaerobic oxidation of aromatic compounds and hydrocarbons. Curr. Opin. Chem. Biol. 6:604-611. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan, A. V., L. M. Gieg, K. G. Kropp, J. Suflita, and L. Y. Young. 2006. Comparison of mechanisms of alkane metabolism under sulfate-reducing conditions among two bacterial isolates and a bacterial consortium. Appl. Environ. Microbiol. 72:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chouteau, J., E. Azoulay, and J. Senez. 1962. Anaerobic formation of n-hept-1-ene from n-heptane by resting cells of Pseudomonas aeruginosa. Nature 194:576-578. [Google Scholar]
- 7.Christie, W. W. 1989. Gas chromatography and lipids: a practical guide, p. 64-184. The Oily Press, Dundee, Scotland.
- 8.Cravo-Laureau, C., V. Grossi, D. Raphel, R. Matheron, and A. Hirschler-Réa. 2005. Anaerobic n-alkane metabolism by a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T. Appl. Environ. Microbiol. 71:3458-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravo-Laureau, C., A. Hirschler-Réa, R. Matheron, and V. Grossi. 2004. Growth and cellular fatty-acid composition of a sulfate-reducing bacterium, Desulfatibacillum aliphaticivorans strain CV2803T, grown on n-alkenes. C. R. Biol. 327:687-694. [DOI] [PubMed] [Google Scholar]
- 10.Cravo-Laureau, C., R. Matheron, J.-L. Cayol, C. Joulian, and A. Hirschler-Réa. 2004. Desulfatibacillum aliphaticivorans gen. nov., sp. nov., an n-alkane- and n-alkene-degrading, sulfate-reducing bacterium. Int. J. Syst. Evol. Microbiol. 54:77-83. [DOI] [PubMed] [Google Scholar]
- 11.Cravo-Laureau, C., R. Matheron, C. Joulian, J.-L. Cayol, and A. Hirschler-Réa. 2004. Desulfatibacillum alkenivorans sp. nov., a novel n-alkene-degrading, sulfate-reducing bacterium, and emended description of the genus Desulfatibacillum. Int. J. Syst. Evol. Microbiol. 54:1639-1642. [DOI] [PubMed] [Google Scholar]
- 12.Curiale, J. A., and E. B. Frolov. 1998. Occurrence and origin of olefins in crude oils. A critical review. Org. Geochem. 29:397-408. [Google Scholar]
- 13.Davidova, I. A., L. M. Gieg, M. Nanny, K. G. Kropp, and J. M. Suflita. 2005. Stable isotopic studies of n-alkane metabolism by a sulfate-reducing bacterial enrichment culture. Appl. Environ. Microbiol. 71:8174-8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Mesmay, R., V. Grossi, D. Williamson, S. Kajula, and S. Derenne. 2007. Novel mono-, di- and tri-unsaturated very long-chain (C37-C43) n-alkenes in alkenone-free lacustrine sediments (Lake Masoko, Tanzania). Org. Geochem. 38:323-333. [Google Scholar]
- 15.Doumenq, P., M. Acquaviva, L. Asia, J.-P. Durbec, Y. Le Dréau, G. Mille, and J.-C. Bertrand. 1999. Changes in fatty acids of Pseudomonas nautica, a marine denitrifying bacterium, in response to n-eicosane as carbon source and various culture conditions. FEMS Microbiol. Ecol. 28:151-161. [Google Scholar]
- 16.Foss, S., U. Heyen, and J. Harder. 1998. Alcaligenes defragrans sp. nov., description of four strains isolated on alkenoic monoterpenes ((+)-menthene, α-pinene, 2-carene, and α-phellandrene) and nitrate. Syst. Appl. Microbiol. 21:237-244. [DOI] [PubMed] [Google Scholar]
- 17.Gilewicz, M., G. Monpert, M. Acquaviva, G. Mille, and J.-C. Bertrand. 1991. Anaerobic oxidation of 1-n-heptadecene by a marine denitrifying bacterium. Appl. Microbiol. Biotechnol. 36:252-256. [Google Scholar]
- 18.Grossi, V., S. Caradec, and F. Gilbert. 2003. Burial and reactivity of sedimentary microalgal lipids in bioturbated Mediterranean sediments. Mar. Chem. 81:57-69. [Google Scholar]
- 19.Grossi, V., A. Hirschler, D. Raphel, J.-F. Rontani, J. W. De Leeuw, and J.-C. Bertrand. 1998. Biotransformation pathways of phytol in recent anoxic sediments. Org. Geochem. 29:845-861. [Google Scholar]
- 20.Gurr, M. I., J. L. Harwood, and K. N. Frayn. 2002. Lipid biochemistry: an introduction, 5th ed. Blackwell Publishing, London, United Kingdom.
- 21.IUPAC-IUB Commission on Biochemical Nomenclature. 1978. The nomenclature of lipids. Biochem. J. 171:21-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob, J. 1978. Chemical composition of the preen gland secretions from some ciconiiform birds. Lipids 13:274-282. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa, M., M. Kanemoto, and T. Imanaka. 1996. Biological oxidation of alkane to alkene under anaerobic conditions. J. Ferment. Bioeng. 82:309-311. [Google Scholar]
- 24.Rabus, R., H. Wilkes, A. Behrends, A. Armstroff, T. Fischer, A. J. Pierik, and F. Widdel. 2001. Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl) succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J. Bacteriol. 183:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rios-Hernandez, L. A., L. M. Gieg, and J. M. Suflita. 2003. Biodegradation of an alicyclic hydrocarbon by a sulfate-reducing enrichment from a gas condensate-contaminated aquifer. Appl. Environ. Microbiol. 69:434-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rontani, J.-F. 1998. Electron ionization mass spectrometric determination of double bond position in monounsaturated α,β- and β,γ-isomeric isoprenoid acids. Rapid Commun. Mass Spectrom. 12:961-967. [Google Scholar]
- 27.Rontani, J.-F., A. Mouzdahir, V. Michotey, and P. Bonin. 2002. Aerobic and anaerobic metabolism of squalene by a denitrifying bacterium isolated from marine sediment. Arch. Microbiol. 178:279-287. [DOI] [PubMed] [Google Scholar]
- 28.Schink, B. 1985. Degradation of unsaturated hydrocarbons by methanogenic enrichment cultures. FEMS Microbiol. Ecol. 31:69-77. [Google Scholar]
- 29.So, C. M., C. D. Phelps, and L. Y. Young. 2003. Anaerobic transformation of alkanes to fatty acids by a sulfate-reducing bacterium, strain Hxd3. Appl. Environ. Microbiol. 69:3892-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.So, C. M., and L. Y. Young. 1999. Initial reactions in anaerobic alkane degradation by a sulfate reducer, strain AK-01. Appl. Environ. Microbiol. 65:5532-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.So, C. M., and L. Y. Young. 1999. Isolation and characterization of a sulfate-reducing bacterium that anaerobically degrades alkanes. Appl. Environ. Microbiol. 65:2969-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spormann, A. M., and F. Widdel. 2000. Metabolism of alkylbenzenes, alkanes, and other hydrocarbons in anaerobic bacteria. Biodegradation 11:85-105. [DOI] [PubMed] [Google Scholar]
- 33.Thiel, V., J. Peckmann, O. Schmale, J. Reitner, and W. Michaelis. 2001. A new straight-chain hydrocarbon biomarker associated with anaerobic methane cycling. Org. Geochem. 32:1019-1023. [Google Scholar]
- 34.van der Meer, M. T. J., S. Schouten, D. M. Ward, J. A. J. Geenevasen, and J. S. Sinninghe Damsté. 1999. All-cis hentriaconta-9,15,22-triene in microbial mats formed by the phototrophic prokaryote Chloroflexus. Org. Geochem. 30:1585-1587. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, F., W. Zahn, and U. Bühring. 1967. 1-Hexadecene, an intermediate in the microbial oxidation of n-hexadecane in vivo and in vitro. Angew. Chem. Int. Ed. Engl. 6:359-360. [Google Scholar]
- 36.Widdel, F., and R. Rabus. 2001. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr. Opin. Biotechnol. 12:259-276. [DOI] [PubMed] [Google Scholar]
- 37.Wilkes, H., S. Kühner, C. Bolm, T. Fischer, A. Classen, F. Widdel, and A. Rabus. 2003. Formation of n-alkane and cycloalkane-derived organic acids during anaerobic growth of a denitrifying bacterium with crude oil. Org. Geochem. 34:1313-1323. [Google Scholar]
- 38.Wilkes, H., R. Rabus, T. Fischer, A. Armstroff, A. Behrends, and F. Widdel. 2002. Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation of the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch. Microbiol. 177:235-243. [DOI] [PubMed] [Google Scholar]
- 39.Wood, N. F., and F. C. Chang. 1965. Reactions of potassium t-butoxide in dimethyl sulfoxide. IV. With primary tosylates and halides. J. Org. Chem. 30:2054-2056. [Google Scholar]