Abstract
Escherichia coli NZN111 is a pflB ldhA double mutant which loses its ability to ferment glucose anaerobically due to redox imbalance. In this study, two-stage culture of NZN111 was carried out for succinic acid production. It was found that when NZN111 was aerobically cultured on acetate, it regained the ability to ferment glucose with succinic acid as the major product in subsequent anaerobic culture. In two-stage culture carried out in flasks, succinic acid was produced at a level of 11.26 g/liter from 13.4 g/liter of glucose with a succinic acid yield of 1.28 mol/mol glucose and a productivity of 1.13 g/liter·h in the anaerobic stage. Analyses of key enzyme activities revealed that the activities of isocitrate lyase, malate dehydrogenase, malic enzyme, and phosphoenolpyruvate (PEP) carboxykinase were greatly enhanced while those of pyruvate kinase and PEP carboxylase were reduced in the acetate-grown cells. The two-stage culture was also performed in a 5-liter fermentor without separating the acetate-grown NZN111 cells from spent medium. The overall yield and concentration of succinic acid reached 1.13 mol/mol glucose and 28.2 g/liter, respectively, but the productivity of succinic acid in the anaerobic stage dropped to 0.7 g/liter·h due to cell autolysis and reduced anaplerotic activities. The results indicate the great potential to take advantage of cellular regulation mechanisms for improvement of succinic acid production by a metabolically engineered E. coli strain.
Succinic acid, a member of the C4-dicarboxylic acid family, is widely used in production of foods, pharmaceuticals, and biodegradable plastics (35). Traditionally, it is produced via chemical synthesis from petrochemical feedstocks that are nonrenewable, and the chemical processes suffer from problems of environment pollution. Therefore, great attention has been paid to use of renewable feedstocks through anaerobic fermentation for production of succinic acid (28), which is among the 12 top value-added chemicals produced from biomass (33).
Several bacteria, such as Mannheimia succiniciproducens (14), Actinobacillus succinogenes (31), and Anaerobiospirillum succiniciproducens (23), are effective natural succinate producers with high productivity and tolerance to osmotic pressure caused by glucose and succinic acid. Recently studies on metabolic engineering of Escherichia coli for succinic acid production have been carried out. E. coli is a facultative anaerobe and has the advantages of fast growth, simple requirements for nutrients, detailed knowledge about its genetic background, and abundant strains and vectors available for metabolic engineering studies. It proceeds with mixed-acid fermentation under anaerobic conditions, but succinic acid is a minor product (6). To improve the efficiency of succinic acid production in E. coli, one strategy is to block the competition pathways to succinic acid, such as by inactivation of pyruvate:formate lyase (PFL) and lactate dehydrogenase (LDH) (3, 30, 32), the protein EIICBglc in the phosphotransferase system (PTS) (5, 9), alcohol dehydrogenase (ADH) (25), phosphate acetyltransferase-acetate kinase (26), etc. The other is to overexpress endogenous or heterologous CO2-fixing enzymes, such as pyruvate carboxylase (10, 25, 32), phosphoenolpyruvate (PEP) carboxylase (PPC) (10, 17), PEP carboxykinase (PCK) (8, 12), and malic enzyme (ME) (30), to direct the carbon flow to oxaloacetate (OAA) or malate, from which succinic acid is produced.
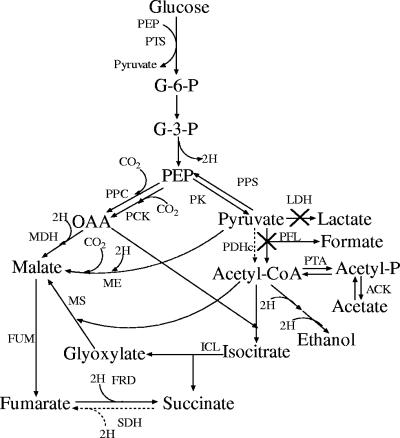
The genetic manipulation of central metabolic pathways in E. coli results in enhanced succinic acid production and reduced by-product formation. However, such manipulation often leads to the phenomena of slow growth and low metabolic ability (3, 6, 15). E. coli NZN111, a mutant of E. coli W1485, was constructed by insertional disruption of ldhA and pflB, encoding the fermentative LDH and PFL, respectively (3). It fails to grow anaerobically on glucose even though acetate is added for biosynthesis (3, 32), and this phenomenon is attributed to failure to regenerate NAD+ from NADH formed in glycolysis due to the disruption of LDH. However, in NZN111, other enzymes capable of catalyzing the reactions to regenerate NAD+ from NADH exist, including fumarate reductase (FRD), malate dehydrogenase (MDH), and ME (Fig. 1). ADH in the ethanol formation pathway may function little because of limited availability of its substrate, acetyl-coenzyme A (CoA), produced mainly by PFL under anaerobic condition (27). Therefore, NZN111 should be able to regenerate NAD+ from NADH through the reductive tricarboxylic acid (TCA) pathway. That NZN111 fails to metabolize glucose anaerobically suggests that the enzymes responsible for anaplerosis and NAD+ regeneration might not be fully induced. If these enzymes are highly activated, the cells should ferment glucose even if no further genetic manipulation is made.
FIG. 1.
Metabolic pathways of E. coli strain NZN111 under anaerobic conditions. Not all the enzyme-catalyzed steps and intermediates are shown. PTS, phosphotransferase system; PPC, PEP carboxylase; PCK, PEP carboxykinase; PK, pyruvate kinase; PPS, PEP synthetase; PDHc, pyruvate dehydrogenase complex; PTA, phosphoacetyltransferase; ACK, acetate kinase; ICL, isocitrate lyase; MS, malate synthase; MDH, malate dehydrogenase; FUM, fumarase; SDH, succinate dehydrogenase; FRD, fumarate reductase. ×, inactivated reactions. The dashed line indicates the enzyme activity formed in aerobic culture.
In E. coli, gene expression is regulated to adapt to different carbon sources under aerobic conditions (11). When E. coli is grown aerobically on glucose, acetate is the main by-product. When acetate is used as the carbon source, transcription of genes encoding anaplerotic and NAD+ regeneration enzymes is up-regulated, such as those in glyoxylate shunt (aceBAK), the reductive TCA cycle (mdh), and gluconeogenesis (pckA, ppsA, sfcA, and maeB), while transcription of those encoding the PEP utilization enzymes, such as ptsG, ptsHIccr, and pyk (11, 18, 19), is down-regulated. Such a response seems to benefit NAD+ regeneration and PEP utilization and thus is favorable to succinic acid production. This article deals with succinic acid production in two-stage (aerobic-anaerobic) cultivation of NZN111. We found that the NZN111 cells that had grown aerobically on acetate could indeed metabolize glucose quickly and produce succinic acid at a high yield in subsequent anaerobic culture. Activities of six related key enzymes, including MDH, isocitrate lyase (ICL), pyruvate kinase (PK), PCK, PPC, and ME, were measured. The data revealed that the high efficiency of succinic acid production resulted from enhanced anaplerotic activities which made regeneration of NAD+ from NADH possible.
MATERIALS AND METHODS
Strain.
E. coli strain NZN111 [F+ λ− rpoS396(Am) rph-1 ΔpflB::Cam ΔldhA::Kan] (3), which was kindly provided by D. P. Clark, Southern Illinois University, was used exclusively in this work. This strain was stored in 25% (wt/wt) glycerol at −20°C.
Media.
The medium for inoculum development was LB containing tryptone (Oxoid, United Kingdom) (10 g/liter), yeast extract (Oxoid, United Kingdom) (5 g/liter), and NaCl (10 g/liter). The salt medium (SM) that was based on M9 (22) contained (per liter) Na2HPO4·12H2O (15.12 g), KH2PO4 (3.0 g), NaCl (0.5 g), MgSO4·7H2O (0.5 g), CaCl2 (0.011 g), NH4Cl (1.0 g), 1% (wt/vol) vitamin B1 (0.2 ml), and trace elements solution (0.1 ml). The stock solution of trace elements contained the following (per liter) in 3 M HCl: FeSO4·7H2O (80 g), AlCl3·6H2O (10 g), ZnSO4·7H2O (2.0 g), CuCl2·2H2O (1.0 g), NaMoO4·2H2O (2.0 g), MnSO4·H2O (10 g), CoCl2 (4.0 g), and H3BO4 (0.5 g). The media GM and AM were prepared by supplementing the SM with 4.6 g/liter of glucose or 5.2 g/liter of sodium acetate, respectively. The medium for anaerobic fermentation was SM supplemented with 14 g/liter of glucose and 10 g/liter of NaHCO3 but without addition of NH4Cl. For culture in a 5-liter fermentor, SM was supplemented with 15 g/liter of glucose and 5 g/liter of yeast extract and the concentrations of NH4Cl, Na2HPO4·12H2O, and KH2PO4 were changed to 7.0, 3.78, and 0.75 g/liter, respectively. Antibiotics were included at the following concentrations: kanamycin, 30 mg/liter; chloramphenicol, 34 mg/liter.
Culture conditions.
The two-stage culture technique (32) was adopted with cultures carried out in flasks or a 5-liter fermentor. The primary preculture was prepared by transfer of 1 ml of the stock culture to 30 ml of LB medium in a 250-ml flask, in which the cells were aerobically incubated at 37°C and 220 rpm overnight. For experiments carried out in flasks, 2-ml aliquots of the primary preculture were transferred to 500-ml flasks containing 100 ml AM or GM, in which the cells were incubated under the same conditions for 12 or 7 h, respectively, to obtain the secondary preculture. The secondary precultures in AM or GM were combined, respectively, and the cells were harvested aseptically by centrifugation at 4°C and 3,300 × g for 10 min. Next, the cells were resuspended in the fresh anaerobic fermentation medium at a cell density around an optical density at 600 nm (OD600) of 8.5 (3.6 g [dry cell weight {DCW}]/liter), respectively. Fifty-milliliter aliquots of the cell suspension were dispensed to 100-ml bottles (Schott), and the headspace was filled with CO2 to start the anaerobic culture at 150 rpm and 37°C for 10 h.
For the experiments carried out in the fermentor, the secondary preculture was prepared by inoculating 500-ml flasks containing 75 ml GM medium with 1 ml primary preculture and by culturing at 37°C and 220 rpm for 8 h. The contents of two 500-ml flasks were combined to inoculate 3 liters of medium in a 5-liter fermentor (BIOTECH-BG-5; Baoxing Co., Shanghai, China). The cultivation process was divided into three phases. Phase I was an aerobic batch on glucose. Phase II was a fed-batch period on acetate, which was started when glucose was exhausted. After consumption of the acetate accumulated in phase I, a concentrated sodium acetate feed (450 g/liter) was added continuously to maintain a low concentration of acetate in the culture medium until the cell density reached an OD600 of around 30. Then, phase III, an anaerobic period, was started after addition of glucose (20 g/liter) and NaHCO3 (5 g/liter) to the fermentor. The anaerobic condition was achieved by sparging CO2 at 0.6 liters/min to replace air, and the flow rate was reduced to 0.2 liters/min when the anaerobic condition had been established. During the whole process, the temperature was maintained at 37°C and pH was controlled at 7.0 by automatic addition of 3 M H2SO4 or 8 M NaOH.
Analytical methods.
Cell density was estimated by measurement of the OD600 of an appropriately diluted culture sample. Optical densities were converted to DCW (g/liter) according to the relationship between the OD600 and the DCW. The concentration of glucose was assayed with a commercial analysis kit (Institute of Biological Products, Shanghai, China) containing glucose oxidase. Ethanol was analyzed by gas chromatography (GC112A; ShangFen, Shanghai, China) with a column packed with styrene-divinyl benzene (Chromosorb 101; Dikma, Lapoc, CA) and detected with a flame ionization detector. The concentrations of acetic acid, pyruvic acid, and succinic acid were determined by an ion chromatography system (ICS-1500; Dionex, Sunnyvale, CA), equipped with a 4-by-250-mm anion-exchange column (AS11-HC), a heated conductivity detector (DS6), and an anion self-regenerating suppressor (ASRS ULTRAII; 4 mm). The temperature of the column and heated conductivity detector was 30°C. The mobile phase consisted of 8.0 mM NaOH at a flow rate of 1.0 ml/min.
For measurement of intracellular enzyme activities, cells were harvested by centrifugation (9,400 × g for 10 min at 4°C) and washed twice with cold 100 mM Tris-HCl (pH 7.5). The washed cells were resuspended in the same buffer containing 0.1 mM EDTA and sonicated on ice for 90 cycles (a working period of 3 s in a 10-s interval for each cycle) at a power output of 200 W by an ultrasonic disruptor (JY92-II; Scientz Biotechnology Co., Ningbo, China). The cell debris was removed by centrifugation (13,400 × g for 20 min at 4°C), and the supernatant was used for activity determination. Enzyme activities were measured using a spectrophotometer (UV-2100; Unico, NJ) with the temperature controlled at 30°C. Reactions were initiated by adding the cell extract or substrate to give a final volume of 1 ml.
The activities of MDH, ICL, PCK, PK, and PPC were measured by monitoring the disappearance of NADH, while that of ME was measured by monitoring the formation of NADH. The assay conditions were based on the literature with some modifications of the reaction mixtures, which consisted of the following: MDH (34), 100 mM Tris-HCl (pH 8.0), 2 mM OAA, 0.2 mM NADH; ICL (31), 100 mM potassium phosphate (pH 7.0), 5.0 mM MgCl2, 1.0 mM dithiothreitol (DTT), 0.2 mM NADH, 5.0 mM isocitrate, 12.8 U LDH; PCK (31), 100 mM Tris-HCl (pH 6.6), 10 mM MgCl2, 5 mM MnCl2, 1 mM DTT, 10 mM ADP, 75 mM NaHCO3, 0.2 mM NADH, 33 U MDH, 10 mM PEP; PK (21), 100 mM Tris-HCl (pH 7.5), 5 mM ADP, 1 mM DTT, 10 mM KCl, 15 mM MgCl2, 10 mM PEP, 0.25 mM NADH, 12.8 U LDH; PPC (4), 100 mM Tris-HCl (pH 8.0), 5 mM PEP, 10 mM MgCl2, 24 mM NaHCO3, 0.15 mM NADH, 0.5 mM acetyl-CoA, 33 U MDH; ME (29), 100 mM Tris-HCl (pH 7.5), 10 mM malate, 1 mM MnCl2, 1 mM NH4Cl, 100 mM KCl, 15 mM MgCl2, 1 mM NAD+. One unit of activity was defined as the amount of enzyme needed to oxidize 1 μmol of NADH or to reduce 1 μmol of NAD+ per min. The total protein concentration in crude cell extract was measured by Bradford's method (2) with bovine serum albumin as a standard.
RESULTS
Effect of carbon source in aerobic culture.
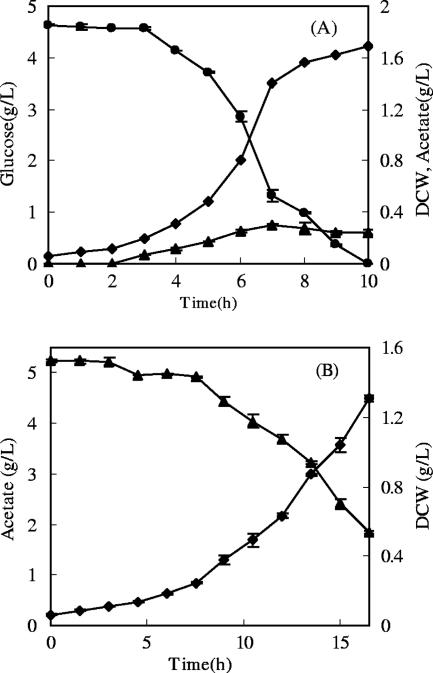
To investigate the effect of the carbon source in aerobic culture on subsequent anaerobic metabolism of glucose, NZN111 was aerobically grown in AM and GM separately. The cells grew on glucose at a maximum specific growth rate of 0.49 h−1 and produced acetate before exhaustion of glucose. When acetate was used as the carbon source, the cells grew at a maximum specific growth rate of 0.21 h−1 and achieved a lower density as shown in Fig. 2.
FIG. 2.
Growth of NZN111 in GM (A) or AM (B) in aerobic batch cultures. ⧫, DCW; •, glucose; ▴, acetate.
To prevent diauxic growth by utilization of formed acetate, the NZN111 cells grown in GM were harvested at 7 h at a DCW of 1.46 g/liter by centrifugation when glucose was not exhausted. The cells were collected at 12 h when AM was used. The glucose- and acetate-grown cells were resuspended in the medium for anaerobic culture at DCW of 3.55 and 3.74 g/liter, respectively, and were incubated anaerobically for 10 h at 37°C. The results are shown in Table 1. For comparison with other studies using the two-stage technique, the succinate productivity and glucose consumption rate were calculated for the anaerobic stage, although such rates should be greater than those based on the total culture time including both the aerobic and anaerobic stages. In addition, calculations of the product yields were also based on the data for the anaerobic stage. The acetate-grown NZN111 cells consumed glucose at an overall rate of 1.34 g/liter·h, about 4 times that of the glucose-grown cells (0.33 g/liter·h). The amount of succinic acid produced by the acetate-grown cells was 11.26 g/liter, 42 times larger than that produced by the glucose-grown cells (0.27 g/liter). The succinic acid yield of acetate-grown cells was 1.28 mol/mol, about 10-fold that of the glucose-grown cells (0.12 mol/mol). In both cases ethanol was not detected, but the yields of other by-products were discrepant. For the acetate-grown cells, the yields of pyruvic acid and acetic acid were 0.052 and 0.54 mol/mol, respectively, while those for the glucose-grown cells were 0.26 and 0.072 mol/mol. At the end of the anaerobic culture, the density of glucose-grown cells dropped from 3.55 to 2.93 g (DCW)/liter due to autolysis, but no obvious change occurred for the acetate-grown cells.
TABLE 1.
Effects of carbon source in aerobic culture of E. coli NZN111 on glucose consumption and product formation in subsequent anaerobic fermentation in flasks
| Carbon source in aerobic culture | Anaerobic fermentationa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cell concn (g [DCW]/liter)b
|
Consumed glucose (g/liter) | Amt of product (g/liter)
|
Yield of succinic acid (mol/mol) | |||||
| Initial | Final | Succinic acid | Acetic acid | Pyruvic acid | Ethanol | |||
| Glucose | 3.55 | 2.93 ± 0.06 | 3.30 ± 0.05 | 0.27 ± 0.00 | 0.079 ± 0.00 | 0.42 ± 0.04 | NDc | 0.12 ± 0.00 |
| Acetate | 3.74 | 3.69 ± 0.05 | 13.39 ± 0.04 | 11.26 ± 0.24 | 0.59 ± 0.02 | 0.34 ± 0.01 | ND | 1.28 ± 0.03 |
Data are means ± standard deviations.
In the anaerobic culture.
ND, not detected.
Key enzyme activities in two-stage experiments.
To understand the different behaviors of the acetate- and glucose-grown cells, the activities of six key enzymes related to succinic acid production were measured at the end of the aerobic phase (namely, the onset of anaerobic phase). These enzymes included the first enzyme in the glyoxylate shunt, ICL; the first enzyme in the reductive TCA pathway, MDH; the anaplerotic enzymes ME, PCK, and PPC; and the enzyme converting PEP to pyruvate, PK.
The differences in specific activities of these enzymes between the acetate- and glucose-grown cells were significant (Table 2). The specific activities of ICL, PCK, MDH, and ME were 18.1, 21.6, 3.6, and 2.1 times higher, respectively, in the cells grown on acetate, while those of PK and PPC were 3.6- and 2.8-fold higher in the glucose-grown cells. We supposed that the high PPC activity in the glucose-grown cells might exert little influence on succinic acid production because of the limited PEP pool, which was consumed by the more active PTS and PK to generate pyruvate (10).
TABLE 2.
Effects of carbon source in aerobic culture of E. coli NZN111 on activities of key enzymes in subsequent anaerobic fermentation on glucose in flasks
| Carbon source in aerobic culture | Timea | Sp act (U/mg protein) of enzymeb
|
|||||
|---|---|---|---|---|---|---|---|
| ICL | MDH | ME | PCK | PPC | PK | ||
| Glucose | Initial | 0.062 ± 0.00 | 1.46 ± 0.09 | 0.070 ± 0.01 | 0.043 ± 0.01 | 0.77 ± 0.00 | 0.80 ± 0.07 |
| Final | 0.060 ± 0.01 | 1.68 ± 0.25 | 0.077 ± 0.02 | 0.035 ± 0.01 | 0.70 ± 0.05 | 0.65 ± 0.07 | |
| Acetate | Initial | 1.12 ± 0.07 | 5.24 ± 0.40 | 0.15 ± 0.02 | 0.93 ± 0.03 | 0.28 ± 0.00 | 0.22 ± 0.04 |
| Final | 0.93 ± 0.07 | 4.85 ± 0.05 | 0.16 ± 0.01 | 0.87 ± 0.03 | 0.26 ± 0.01 | 0.18 ± 0.02 | |
“Initial” represents the data obtained at the onset of anaerobic fermentation; “final” represents those obtained at the end of anaerobic fermentation.
Data are means ± standard deviations.
Activities of these enzymes were also measured at the end of anaerobic culture as shown in Table 2. After the anaerobic phase lasted 10 h, the activities of ICL, PCK, and PK decreased while those of ME increased slightly in both acetate- and glucose-grown cells. The trends of MDH activity were different: in the acetate-grown cells, it decreased a little (by about 7.3%) at the end of anaerobic culture, while it increased in the glucose-grown cells. However, the changes in these enzyme activities were not significant at the end of anaerobic culture.
Two-stage fermentation in a 5-liter reactor.
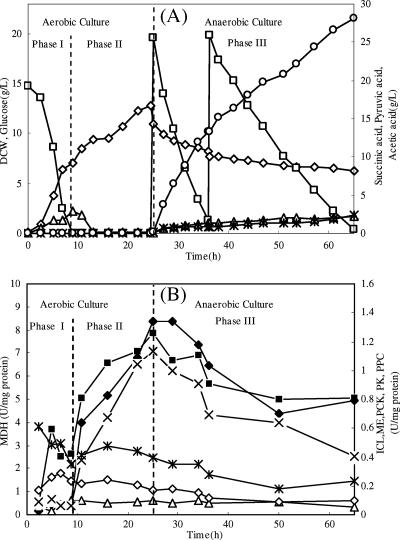
In order to increase cell density, the NZN111 cells were initially grown in the medium containing 15 g/liter of glucose and 5 g/liter of yeast extract. After the exhaustion of glucose and accumulated acetate, acetate was added continuously to induce the enzyme activities of gluconeogenesis and the cell concentration was increased from 7.11 to 12.6 g (DCW)/liter in the fed-batch phase. Next, glucose (19.6 g/liter) and NaHCO3 (5 g/liter) were added at 25 h, and the anaerobic fermentation was initiated by sparging CO2. When the glucose concentration had dropped below 2 g/liter, a concentrated glucose solution was added at 36.3 h to reach a concentration of 18.6 g/liter. The anaerobic phase lasted 40 h, and the final concentrations of succinic acid, pyruvic acid, and acetic acid were 28.2, 2.31, and 2.17 g/liter, respectively (Fig. 3A). The ethanol concentration was less than 0.2 g/liter. The glucose added at the beginning of anaerobic culture was consumed rapidly at a rate of 1.68 g/liter·h, and the succinic acid productivity was 1.21 g/liter·h with a yield of 1.10 mol/mol glucose. However, after the second addition of glucose, both the glucose consumption rate and succinic acid productivity dropped significantly, to 0.68 and 0.52 g/liter·h, respectively, even though a similar succinic acid yield (1.16 mol/mol glucose) was maintained. The overall productivity and yield of succinic acid in the whole anaerobic phase were 0.7 g/liter·h and 1.13 mol/mol, respectively. Cell growth was not observed while autolysis occurred, resulting in a rapid drop of cell density.
FIG. 3.
(A) Profiles of cell density (⋄), glucose (□), acetic acid (▵), succinic acid (○), and pyruvic acid (*) in two-stage cultivation of NZN111 in a 5-liter bioreactor. (B) Profiles of specific activities of ICL (⧫), MDH (▪), ME (▵), PCK (×), PPC (⋄) and PK (*).
Specific activities of the six enzymes were followed during the two-stage cultivation (Fig. 3B). In phase I, the specific activities of MDH and PPC increased in log phase and then dropped at the end of this phase. PK activity decreased from 0.72 to 0.35 U/mg protein, while those of PCK, ME, and ICL changed slightly. In phase II, the activities of MDH, ICL, and PCK increased significantly. At the end of this period, the specific activities of these three enzymes were 3.0, 16.6, and 18.0 times greater than those at the end of phase I, respectively. The specific activity of PK increased slightly, from 0.35 to 0.47 U/mg protein, in the first 7 h but then decreased to the original level, whereas the changes in ME and PPC activities were not observed. In the period of anaerobic fermentation (phase III), the activities of MDH, ICL, and PCK decreased quickly. The activity of PCK changed most significantly, finally decreasing to only 35.5% of that at the end of phase II. The activities of other two CO2-fixing enzymes, PPC and ME, declined by 41% and 46.6%, respectively.
DISCUSSION
Most published studies on improvement of succinic acid production in E. coli have focused on manipulating enzyme activities through deletion of enzymes in the competing pathways and overexpression of indigenous or foreign enzymes in the beneficial pathways by means of genetic manipulation (3, 5, 8, 9, 12, 17, 25, 26, 30). In addition, to redistribute the metabolic fluxes, cofactor engineering is applied by enhancing the intracellular levels of acetyl-CoA and changing the NADH/NAD+ ratio (10, 24). In fact, the metabolic fluxes are precisely controlled by cellular regulation mechanisms, and the response to inactivation or overexpression of countable enzymes may not certainly benefit production of the desired metabolite (3, 6, 12, 15). In this study, the NZN111 cells that had grown aerobically on acetate resumed the ability to ferment glucose, and the succinic acid yield and productivity were greatly improved in subsequent anaerobic culture without further genetic modification. The productivity of succinic acid in the anaerobic stage reached 1.13 g/liter·h in a nitrogen-limited, chemically defined medium. The specific productivity of succinic acid in flask cultures achieved 304 mg/g (DCW)·h, much higher than that of AFP111 overexpressing pyruvate carboxylase (about 193 mg/g [DCW]·h) (32). When the two-stage cultivation was carried out in the fermentor, the aerobically grown cells were directly brought into an anaerobic environment but the productivity in the anaerobic stage still achieved 0.7 g/liter·h even though the cells had not been separated from the spent medium. This rate was higher than 0.24 g/liter·h for NZN111 overexpressing pyruvate carboxylase in a complex medium (32).
It has been reported that the ldhA pflB double mutant NZN111 is incapable of fermenting glucose under anaerobic conditions. The reason is considered to be the imbalance between NADH formation and its reoxidation (9, 30). Disruption of LDH and PFL in E. coli NZN111 blocks the major pyruvate consumption pathways, and NADH formed in glycolysis cannot be oxidized via lactate formation; thus, NZN111 fails to ferment glucose efficiently under anaerobic condition. However, other NADH-oxidizing enzymes exist in NZN111, such as FRD, MDH, ME, etc. (Fig. 1). Therefore, the fact that NZN111 does not ferment glucose may be caused by anaplerotic enzyme activities being too low to supply substrates such as OAA and fumarate for oxidation of NADH.
Table 2 shows that the activities of the six enzymes in the cells aerobically grown on acetate were quite different from those for cells grown on glucose. It has been shown that when E. coli is cultured on acetate, the gene expression relating to glycolysis and PTS is down-regulated while that relating to glyoxylate shunt, the reductive branch of TCA, acetyl-CoA formation, and gluconeogenesis is up-regulated (11, 13, 18, 19). Van der Werf et al. (31) compared the enzyme activities in the succinic acid producer Actinobacillus sp. strain 130Z with those in E. coli K-12 grown anaerobically on glucose. In Actinobacillus sp. strain 130Z, the activities of ME, PCK, MDH, and fumarase were much higher while those of LDH, PFL, PK, and PPC were lower. In the present study, the higher PCK, ME, and MDH and lower PK and PPC activities in the acetate-grown cells were consistent with those found for Actinobacillus sp. strain 130Z. PCK catalyzes the reaction to form OAA and ATP from PEP, ADP, and CO2. Therefore, this reaction is more favorable than that catalyzed by PPC, where pyrophosphate is formed instead and energy is wasted. Thus, the enhanced PCK together with the reduction of PPC in the acetate-grown cells was energetically favorable. Considering the higher Km value for HCO3− of PCK (13.0 mM) than that of PPC (0.15 mM), Deok et al. (8) suggested that glyconeogenetic PCK is more suitable for succinic acid production at a high concentration of HCO3−. In addition, based on the comparative analysis of the genomes of E. coli W3110 and another native succinic acid producer, Mannheimia succiniciproducens, and the in silico gene knockout simulation, Lee et al. (15) predicted that disrupting the genes for three pyruvate-forming enzymes, ptsG, pykF, and pykA, allows enhanced succinic acid production. In this study, PK activity was significantly decreased in the acetate-grown cell. This resulted in a limited flux to drain PEP to pyruvate but an enhanced flux through the anaplerotic pathway. MDH is another enzyme whose transcription is remarkably up-regulated on acetate (18). Although it was reported that NZN111 could grow anaerobically on glucose when a mutant form of MDH is overexpressed (1), this was caused by the LDH activity of the MDH mutant. In Actinobacillus sp. strain 130Z, the MDH activity is 13-fold greater than that of E. coli K-12 (31). Expression of MDH is catabolite controlled under aerobic conditions (20). In the acetate-grown NZN111 cells, the MDH activity was significantly enhanced (Table 2; Fig. 3), which was favorable to oxidation of NADH to provide malate for the subsequent formation of succinic acid. ME is the only anaplerotic activity found in E. coli to provide OAA from pyruvate, but its Km for pyruvate is 16 mM when Mn2+ is added (30). At a high concentration of pyruvate, ME catalyzes the reverse reaction to form malate from pyruvate, CO2, and NADH. That the ME activity in acetate-grown cells was two times as high as that in glucose-grown cells (Table 2) favored production of succinic acid from pyruvate to some extent.
The glyoxylate shunt is an important pathway for anaerobic production of succinic acid (7, 32). If succinate is produced exclusively via the reductive arm of the TCA cycle, only 1 mol of succinate is formed from 1 mol of glucose because only 2 mol of NADH is formed in glycolysis. However, through the glyoxylate shunt, 2 mol of succinate can be formed from 2 mol of acetyl-CoA and 1 mol of OAA by consumption of only 1 mol of NADH. In addition, in the reaction catalyzed by the pyruvate dehydrogenase complex (PDHc) formed in the aerobic stage, another mole of NADH can be produced as 1 mol of acetyl-CoA is formed from pyruvate. Therefore, the maximum yield is 1.21 mol succinic acid/mol glucose when the glyoxylate shunt is active and no OAA is formed from pyruvate (32). In the present study, the ICL activity in the acetate-grown cells was 20-fold greater than that in the glucose-grown cells (Table 2; Fig. 3), and the high ICL activity favored succinic acid production. The PDH activity in the acetate-grown cells was only about half of that in the glucose-grown cells (data not shown). This was consistent with the reports that the transcription of PDHc genes in acetate-grown cells is down-regulated (18, 19). However, the PDHc activity was enough to achieve a succinate yield of 1.2 mol/mol.
The data in Table 2 suggest that the aerobically enhanced key enzyme activities still functioned in subsequent anaerobic culture. However, the activities dropped remarkably in the experiment carried out in the fermentor. In the anaerobic cultures in flasks, the aerobically grown cells had been separated from the spent medium, while in the experiment carried out in the fermentor, the aerobically grown cells were directly brought under anaerobic conditions. Therefore, factors like accumulated metabolites, increased ionic strength, and osmotic pressure in the aerobic culture might be responsible for this phenomenon. Since separation of the aerobically grown cells is not practical for large-scale fermentation, the mechanisms for reduced an aplerotic activities remain to be investigated.
Based on the redox balance in E. coli strain NZN111, Vemuri et al. (32) proposed the maximum yield of succinic acid to be 1.21 mol/mol (0.79 g/g) when both PTS and the glyoxylate shunt were active. In this study, the succinate yield obtained in the flask experiments reached 1.28 mol/mol, which was a little higher than the theoretical value. This was attributed to the increased ME activity, which converts pyruvate to OAA. In addition, the pyruvic acid yield was low in the acetate-grown cells because of the lower PK and higher ME activities (Table 2).
The acetate-grown NZN111 cells exhibited not only a high succinic acid yield but also a high glucose consumption rate and succinic acid productivity. However, in the anaerobic fermentation carried out in the 5-liter fermentor, the glucose consumption rate and succinate productivity decreased remarkably. These were attributed to decreasing cell density and key enzyme activities, even though the overall yield of succinic acid remained at a similar level of 1.13 mol/mol. Since the two-stage fermentation carried out in the fermentor has not been optimized, improvement of this process is being undergone in this laboratory.
This study demonstrated that use of a suitable gluconeogenic carbon source was very effective in enhancing anaplerotic activities and the carbon flux was effectively directed to succinic acid in a subsequent anaerobic culture, even though further genetic manipulation was not carried out. The advantages also include the high glucose consumption rate and succinate productivity. In addition to acetate, when NZN111 was aerobically grown on other gluconeogenic carbon sources, such as glycerol, pyruvate, lactate, malate, fumarate, and succinate, etc., succinic acid production was also improved significantly in subsequent anaerobic culture (unpublished data). Therefore, it is expected that the performance of metabolically engineered organisms will be improved by making use of the cellular regulation mechanisms.
Acknowledgments
We thank David P. Clark for kindly providing us with the E. coli strain NZN111. We also thank Jie Bao for his comments on the manuscript.
This work was supported by the National Natural Science Foundation of China (grant no. 20576032).
Footnotes
Published ahead of print on 19 October 2007.
REFERENCES
- 1.Boernke, W. E., C. S. Millard., P. W. Stevens, S. N. Kakar, F. J. Stevens, and M. I. Donnelly. 1995. Stringency of substrate specificity of Escherichia coli malate dehydrogenase. Arch. Biochem. Biophys. 322:43-52. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Bunch, P. K., F. Mat-Jan, N. Lee, and D. P. Clark. 1997. The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Cánovas, J. L., and H. L. Kornberg. 1969. Phosphoenolpyruvate carboxylase from Escherichia coli. Methods Enzymol. 13:288-292. [Google Scholar]
- 5.Chatterjee, R., C. S. Millard, K. Champion, D. P. Clark, and M. I. Donnelly. 2001. Mutation of the ptsG gene results in increased production of succinic acid in fermentation of glucose by Escherichia coli. Appl. Environ. Microbiol. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, D. P. 1989. The fermentation pathway of Escherichia coli. FEMS Microbiol. Rev. 63:223-234. [DOI] [PubMed] [Google Scholar]
- 7.Cox, S. J., S. S. Levanon, A. Sanchez, H. Lin, B. Peercy, G. N. Bennett, and K. Y. San. 2006. Development of a metabolic network design and optimization framework incorporating implementation constraints: a succinate production case study. Metab. Eng. 8:46-57. [DOI] [PubMed] [Google Scholar]
- 8.Deok, K. Y., S. Y. Lee, and P. Kim. 2006. Influence of gluconeogenic phosphoenolpyruvate carboxykinase (PCK) expression on succinic acid fermentation in Escherichia coli under high bicarbonate condition. J. Microbiol. Biotechnol. 16:1448-1452. [Google Scholar]
- 9.Donnelly, M., C. S. Millard, D. P. Clark, M. Chen, and J. Rathke. 1998. A novel fermentation pathway in an Escherichia coli mutant producing succinic acid, acetic acid, and ethanol. Appl. Biochem. Biotechnol. 70-72:187-198. [DOI] [PubMed] [Google Scholar]
- 10.Henry, L., V. V. Ravishankar, G. N. Bennett, and K. Y. San. 2004. Increasing the acetyl-CoA pool in presence of overexpressed phosphoenolpyruvate carboxylase or pyruvate carboxylase enhances succinate production in Escherichia coli. Biotechol. Prog. 20:1599-1604. [DOI] [PubMed] [Google Scholar]
- 11.Kao, K. C., L. M. Tran, and J. C. Liao. 2005. A global regulatory role of glyconeogenic genes in Escherichia coli revealed by transcriptome network analysis. J. Biol. Chem. 280:36079-36087. [DOI] [PubMed] [Google Scholar]
- 12.Kim, P., M. Laivenieks, V. Claire, and J. G. Zeikus. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinic acid production in Escherichia coli. Appl. Environ. Microbiol. 70:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornberg, H. 1966. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 99:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, P. C., S. Y. Lee, S. H. Hong, and H. N. Chang. 2003. Batch and continuous cultures of Mannheimia succiniciproducens MBEL55E for the production of succinic acid from whey and corn steep liquor. Bioprocess Biosyst. Eng. 26:63-67. [DOI] [PubMed] [Google Scholar]
- 15.Lee, S. J., D. Y. Lee, T. Y. Kim, B. H. Kim, J. Lee, and S. Y. Lee. 2005. Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl. Environ. Microbiol. 71:7880-7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mat-Jan, F., K. Y. Alam, and D. P. Clark. 1989. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J. Bacteriol. 171:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millard, C. S., Y. P. Chao, J. C. Liao, and M. I. Donnelly. 1996. Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl. Environ. Microbiol. 62:1808-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh, M. K., L. Rohlin, K. C. Kao, and J. C. Liao. 2002. Global expression profiling of acetate-grown Escherichia coli. J. Biol. Chem. 277:13175-13183. [DOI] [PubMed] [Google Scholar]
- 19.Oh, M. K., M. J. Cha, S. G. Lee, L. Rohlin, and J. C. Liao. 2006. Dynamic gene expression profiling of Escherichia coli in carbon source transition from glucose to acetate. J. Microbiol. Biotechnol. 16:543-549. [Google Scholar]
- 20.Park, S. J., P. A. Cotter, and R. P. Gunsalus. 1995. Regulation of malate dehydrogenase (mdh) gene expression in Escherichia coli in response to oxygen, carbon, and heme availability. J. Bacteriol. 177:6652-6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng, L., and K. Shimizu. 2003. Global metabolic regulation analysis for Escherichia coli K12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl. Microbiol. Biotechnol. 61:163-178. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Samuelov, N. S., R. Lamed, S. Lowe, and J. G. Zeikus. 1991. Influence of CO2-HCO3− levels and pH on growth, succinate production, and enzyme activities of Anaerobiospirillum succiniciproducens. Appl. Environ. Microbiol. 57:3013-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.San, K. Y., G. N. Bennett, J. B. Susana, R. V. Vadli, Y. T. Yang, E. Horton, B. R. Fred, B. Sariyar, and K. Blackwood. 2002. Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab. Eng. 4:182-192. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez, A. M., G. N. Bennett, and K. Y. San. 2005. Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant. Biotechnol. Prog. 21:358-365. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez, A. M., G. N. Bennett, and K. Y. San. 2005. Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield. Metab. Eng. 7:229-239. [DOI] [PubMed] [Google Scholar]
- 27.Sawers, G., and A. Bock. 1988. Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J. Bacteriol. 170:5330-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Songa, H., and S. Y. Lee. 2006. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 39:352-361. [Google Scholar]
- 29.Spina, J., H. J. Bright, and J. Rosenbloom. 1970. Purification and properties of L-malic enzyme from Escherichia coli. Biochemistry 9:3794-3801. [Google Scholar]
- 30.Stols, L., and M. I. Donnelly. 1997. Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl. Environ. Microbiol. 63:2695-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Werf, M. J., M. V. Guettle, M. K. Jain, and J. G. Zeikus. 1997. Environmental and physiological factors affecting the succinic acid product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch. Microbiol. 167:332-342. [DOI] [PubMed] [Google Scholar]
- 32.Vemuri, G. N., M. A. Eiteman, and E. Altman. 2002. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl. Environ. Microbiol. 68:1715-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werpy, T., and G. Petersen (ed.). 2004. Top value added chemicals from biomass, vol. 1. Results of screening for potential candidates from sugars and synthesis gas. National Renewable Energy Laboratory, Golden, CO. DOE/GO-102004-1992.
- 34.Zeikus, J. G., G. Fuchs, W. Kenealy, and R. K. Thauer. 1977. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J. Bacteriol. 132:604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeikus, J. G., M. K. Jain, and P. Elankovan. 1999. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 51:545-552. [Google Scholar]