Abstract
The association of Vibrio cholerae with zooplankton has been suggested as an important factor in transmission of human epidemic cholera, and the ability to colonize zooplankton surfaces may play a role in the temporal variation and predominance of the two different serogroups (V. cholerae O1 El Tor and O139) in the aquatic environment. To date, interactions between specific serogroups and species of plankton remain poorly understood. Laboratory microcosm experiments were carried out to compare quantitatively the colonization of two copepod species, Acartia tonsa and Eurytemora affinis, by each of the epidemic serogroups. V. cholerae O1 consistently achieved higher abundances than V. cholerae O139 in colonizing adults of each copepod species as well as the multiple life stages of E. affinis. This difference in colonization may be significant in the general predominance of V. cholerae O1 in cholera epidemics in rural Bangladesh where water supplies are taken directly from the environment.
Vibrio cholerae O1 El Tor and O139 Bengal are recognized as causative agents of cholera and are responsible for cholera epidemics in India and Bangladesh. More than 10 years ago, V. cholerae O139 Bengal was recognized as a newly emerged epidemic variant when it replaced V. cholerae O1 El Tor for two successive cholera seasons (9). It is not clear what factors contributed to the emergence of serogroup O139 as an epidemic variant or to its present coexistence with serogroup O1, but recent research provides strong evidence that horizontal transfer of genes among environmental strains of V. cholerae was a mechanism of its origin (4, 12, 15).
Since the emergence of V. cholerae O139 in the Gangetic Delta region, both V. cholerae O1 and O139 have continued to cause cholera outbreaks with regular seasonality but with temporal variation in the prevalence of the two serogroups (3, 41). Yearly differences in reported cases of cholera caused by V. cholerae O1 and O139 suggest that differences may exist in resource utilization and responses to changing environmental conditions in the aquatic habitat as well as in the immunity of susceptible human populations. Publications that compare V. cholerae O1 and O139 emphasize molecular distinctions (15, 16, 34, 36) and environmental distribution (1, 30, 43). No studies have explored the mechanisms by which the genotypes of V. cholerae may differ in their resource use. However, Simidu et al. (44) showed that different phenotypes of vibrios aggregated in different microhabitats, suggesting that temporal and spatial variation in the environment play important roles in the relative abundance of phenotypes.
Aquatic organisms such as phytoplankton and zooplankton are particularly important as microhabitats of V. cholerae (26, 27, 28, 32, 33, 47). Associations with plankton are thought to have a protective effect for attached cholera vibrios as well as providing an important source of nutrients, especially during interepidemic periods (2, 7, 14, 27, 29, 42). Furthermore, Colwell and Huq (11) proposed that transmission of the cholera vibrios via zooplankton is significant in cholera epidemics in developing countries. Recently, Huq et al. (31) demonstrated a significant correlation between zooplankton blooms and cholera cases in Bangladesh. Copepods appear to be especially important because they are the most numerous and widely dispersed zooplankton with which the incidence and distribution of V. cholerae have been associated (27, 28, 47) and because they possess a chitinous exoskeleton that is attractive to vibrios, which are known to enzymatically breakdown chitin. However, it is unclear whether these metazoans are equally as important for V. cholerae O139 as they are suggested to be for serogroup O1. Factors that contribute to or hinder such an association could greatly influence the transmission and dynamics of the two epidemic serogroups.
All species or life stages of copepods may not provide equally suitable resources for V. cholerae since space, molt frequency, nutrients, and patch distribution can differ greatly between species and life stages. Furthermore, results of previous investigations studying the colonization, interactions, and organization of zooplankton epibiont communities suggest a premium on efficient location of suitable substrates, especially when such substrates are periodically renewed and/or occur in widely dispersed patches (48). It is unknown whether V. cholerae colonizes all copepod species or copepod life stages to similar concentrations or if some serogroups are superior to others in colonizing copepods. Differences in dispersal and colonization may affect relative interstrain abundance, persistence during interepidemic periods, and, ultimately, transmission of cholera.
The objective of this study was to measure quantitatively and compare the colonization dynamics of V. cholerae epidemic serogroups O1 and O139 on two copepod species, Acartia tonsa and Eurytemora affinis. These two copepod species possess different physical characteristics and life histories and are members of genera that are predominant in the aquatic environments of the Chesapeake Bay and the Bay of Bengal. Although A. tonsa and E. affinis are both considered pelagic, calanoid copepods, they exhibit differences in habitat utilization and egg production. E. affinis is known to be demersal (attaches to substrates such as rocks and pilings) as well as planktonic, whereas A. tonsa is largely planktonic. A. tonsa is a broadcast spawner, releasing eggs one at a time, essentially “scattering” them, but E. affinis organisms brood their eggs in an exterior assemblage (egg sac) near the anal pore. Since V. cholerae can be found in bays and estuaries around the world, including tributaries of the Chesapeake Bay, and V. cholerae research has had a long history associated with the Chesapeake Bay, including field surveys of the organism's presence and laboratory studies documenting its coexistence with zooplankton, laboratory microcosm experiments were used to test for colonization by V. cholerae O1 and O139 of the different species and different life stages of copepods.
MATERIALS AND METHODS
Microcosm preparation.
Microcosms were prepared with adult copepods of either A. tonsa collected from the Baltimore Harbor or E. affinis collected from the Patuxent River, a tributary of the Chesapeake Bay, MD. Copepods were collected by towing an 80-μm-pore-size plankton net vertically through the respective bodies of water. A. tonsa and E. affinis were cultured separately as single species cultures in filtered-sterilized river water at a salinity of 15‰ in 2-liter beakers (ThermoFisher Scientific Inc., Waltham, MA). Cultures were sustained on a mixed diet of the diatom Thalassiosira sp. and the brown alga Isochyrisis galbana. All cultures were kept in a growth room, maintained at 20°C and with a 12:12 h day/night cycle prior to experiments. To ensure that only live copepods were used in experiments, the bottom of each culture was routinely cleaned by pipetting to remove any dead copepod and exoskeletons (molts).
The stock cultures used water that was collected from the Rhode River, MD, and filter sterilized by a series of filtration steps, employing filters with pore sizes of 10 μm, 5 μm, and 1 μm (Filterite Inc., Timonium, MD). The salinity was adjusted to 15‰ using Instant Ocean. All river water used for experiments was further filtered, using 0.22-μm-pore-size poretic polycarbonate filters (Osmonic Inc., Livermore, CA), and was adjusted to pH 8.5 (29). Copepods were harvested from the stock cultures by decanting the stock onto a sieve, washing the sample with phosphate-buffered saline (PBS) (0.8% [wt/vol] NaCl, 0.02% [wt/vol] KH2PO4, 0.02% [wt/vol] KCl, 0.12% [wt/vol] Na2HPO4, pH 7.0) (Sigma-Aldrich, St. Louis, MO), resuspending the copepods in filter-sterilized experiment water, and decanting 100 ml of experiment water with copepods into 200 ml of tall-form Berzelius beakers (Kimble-Kontes, Inc., Vineland, NJ), which served as the microecosystems. The copepods were exposed to as little handling as possible to maintain viability. Excessive handling resulted in mortality.
Inoculum preparation.
Prior to inoculation, V. cholerae O1 El Tor strain C6709 (ATCC 55331; Manassas, VA) and V. cholerae O139 Bengal strain M010 (ATCC 55438; Manassas, VA) were incubated individually at 37°C with shaking (200 rpm) for 6 h in 20 ml of alkaline peptone water (1% [wt/vol] peptone [Difco, Detroit, MI], 1% [wt/vol] NaCl, pH 8.5) in 50-ml Fisherbrand centrifuge tubes (ThermoFisher Scientific Inc., Waltham, MA). Both V. cholerae strains were collected during log phase at a concentration of 107 cells per ml, determined by direct fluorescence counts (21). An aliquot (1 ml) was transferred to a 1.5-ml Fisherbrand microcentrifuge tube, harvested by centrifugation (6,000 rpm for 4.5 min), washed twice in 1 ml of PBS, and resuspended in filter-sterilized river water (described above), and an inoculum of 33 μl was added to the microcosms containing copepods.
Microcosms containing either A. tonsa or E. affinis were inoculated with V. cholerae O1 El Tor strain C6709, and an equal number of microcosms were inoculated with V. cholerae O139 Bengal strain MO10, each to a final concentration of ca. 5 × 104 cells per ml. Each copepod species was also introduced into separate microcosms without the addition of V. cholerae O1 or O139 to serve as controls for environmental V. cholerae O1 or O139 possibly present on copepods at the onset of the experiments. Using this general approach, four separate experiments were conducted to evaluate colonization of the different species and life stages of copepods, as described below.
Effect of copepod species and serogroup on colonization.
Colonization of adult copepods by the two serogroups was compared in the first experiment. Microcosm experiments were carried out in a randomized complete block design, with a 2 by 3 factorial treatment structure [two copepod species × (two V. cholerae strains + no V. cholerae strains)]. Microcosms contained from 15 to 40 adult copepods of either species. Multiple replicates for each copepod-serogroup treatment were run on different days to obtain an overall total of 13 replicate microcosms per treatment. Treatments were blocked by day.
All microcosms were incubated at 25°C for 24 h, after which copepods from each microcosm were collected by filtration, using a 153-μm-pore-size sieve (made by adhering nitex mesh [Wildlife Supply Company, Buffalo, NY] to PVC couplings). The copepods were rinsed and resuspended in 1 ml of filter-sterilized water. All samples were prepared for direct viable counts (DVC) (10, 21) and fixed with 5% formalin.
The number of copepods in each sample was counted, and the external surface area of copepods was also estimated for each microcosm so that counts could be normalized by surface area present. Surface area estimates do not include internal surfaces and contents of the digestive systems, where V. cholerae can occur, representing an uncontrolled variable in this analysis. External surface area was calculated by measuring the length and width of a subsample of each copepod species (n = 50 per species) using the Scion Imaging program (NIH, Bethesda, MD) and modeling the cephalothorax as a right-angle cylinder [using the equation 2πr(r + l), where r is the radius and l is length].
After the copepods in each microcosm had been counted and measured, they were resuspended in 1 ml of PBS in 1.5-ml microcentirfuge tubes and sonicated at 40 kHz using a Branson 5510 ultrasonic cleaner (Bransonic, Danbury, CT) filled with water for 120 s in an effort to dislodge and disperse possible clumps of V. cholerae. The copepods were further homogenized in a 2-ml tissue grinder (Kontes Glass Company, Vineland, NJ) to obtain an even distribution of total Vibrio counts. Direct fluorescent antibody reagent (New Horizons, Columbia, MD) specific for serogroups O1 and O139 was added to each sample, permitting enumeration of V. cholerae O1 and O139 cells (both nonculturable and culturable cells) using an epifluorescent microscope (Leitz Dialux 20; Wetzlar, Germany). Non-O1 and non-O139 V. cholerae cells were not enumerated.
A two-way mixed-model analysis of variance ([ANOVA]SAS, version 8.0; SAS Institute Inc., Cary, NC) was used to determine differences, if any, in the number of V. cholerae cells per mm2 that were associated with A. tonsa and E. affinis. Experiments were blocked by day where each treatment (V. cholerae serogroup and copepod species) was randomly applied to the experimental units (microcosms). Replicates were pooled because there was no significant covariate effect of day in the mixed-model ANOVA. Day and day x treatments were considered random effects in the mixed model. Further analysis (two-way ANOVA) was performed to determine whether there were differences in total surface area between the two copepods that could contribute to estimated differences of attached cells per surface area of copepods. Cell counts were log-transformed to satisfy the assumption of homoscedasticity for this analysis. Data were back-transformed to present in graphic form. Significant differences were determined at an α value of 0.05.
Colonization of V. cholerae O1 and O139 to adults and eggs of E. affinis copepods over time.
To measure attachment of V. cholerae to copepods over time, V. cholerae O1 El Tor strain C6709 and O139 Bengal strain MO10 were introduced into separate microcosms containing E. affinis adults. Microcosms were prepared and inoculated as above and were sampled at 1, 25, and 49 h, such that each microcosm was sampled only once, representing a single independent replicate. Each serogroup treatment (O1, O139, and control without V. cholerae) was replicated five times at 1, 25, and 49 h.
Microcosms consisted of 50 to 100 adult copepods of E. affinis. Copepods distributed among microcosms were a mixture of males and both ovigerous and nonovigerous females. At the specified sampling times (see above), adults and eggs were collected from each microcosm by filtering the copepod fractions with a 63-μm-pore-size sieve. Copepods were rinsed with filter-sterilized water to disassociate loosely associated bacteria. Each life stage was manually separated, and the number of adult copepods and eggs were counted. Egg sacs were removed from adult females and were analyzed separately. Counts of eggs ranged from 30 to 150 per microcosm. All samples were prepared for DVC of V. cholerae according to the methods described above, namely, fixation with 5% formalin and enumeration by epifluorescent microscopy using a Leitz Dialux microscope (10, 21).
Two-way ANOVA (general linear model in SAS, version 8.0) was used to estimate the effect of time on attachment of V. cholerae O1 and O139 to adults and eggs. Statistical analyses were performed separately for adults and eggs to avoid violating the assumptions of independence of the ANOVA model. The quantity “total cells attached,” measured for V. cholerae O1 and O139 within replicate microcosms, was used as the dependent variable to estimate differences in colonization of copepod substrates. Cell counts were log transformed to satisfy homoscedasticity. Data were back-transformed to present in graphic form. Significant differences were determined at an α value of 0.05.
Effect of nauplii on colonization of adults and eggs of E. affinis.
Another microcosm experiment tested whether the presence of recently hatched copepod larvae (nauplii) affected colonization of adults and eggs by V. cholerae O1 and O139. Twenty-four hours after inoculation, effects were tested by comparing microcosms with nauplii to those without nauplii. Microcosms were prepared and analyzed as above, where each microcosm contained between 15 to 40 adults, including males as well as ovigerous and nonovigerous females. Nauplii were produced in some microcosms from hatching of eggs but not in others. Replicate microcosms were run for each serogroup treatment, allowing a comparison of the effects of nauplii presence and absence on vibrio colonization. It should be noted that the time course of E. affinis adults and eggs was a separate study from the effect of nauplii on E. affinis adults and eggs, with different numbers of copepods employed, based also on availability of copepods.
Microcosms were arranged in a random incomplete block design, with a two by three factorial treatment structure [two nauplii phases (present/absent) × three V. cholerae treatments (two V. cholerae strains + no V. cholerae strains)] to examine effects of nauplii on colonization of adults and eggs by V. cholerae O1 and O139. Experiments were blocked by day. However, nauplii did not hatch in equal numbers of microcosms. After a 24-h incubation, adults, nauplii, and eggs were collected from each microcosm by filtering the copepod fractions with a 63-μm-pore-size sieve. Adults and eggs were separated manually and were analyzed as independent components. The number of adult copepods and eggs were determined, and nauplii were treated as a qualitative measure (present or absent). The adult and egg fractions of each microcosm were prepared separately for DVC according to the methods described above.
Experimental runs were performed on different days and combined using the mixed-model ANOVA (using SAS, version 8.0). The number of replicates employed were different between days, with three for the run on the first day and four on the second. Day and day x serogroup treatments were considered random effects. There was no significant effect of day, so results were combined from two experimental runs, forming an overall total of seven replicate microcosms for adults and eggs. Adults and eggs were analyzed separately to avoid violating assumptions of independence for ANOVA. Statistical comparisons between numbers of O1 and O139 cells colonizing either adults or eggs were made using counts of total cells attached as the dependent variable. Cell counts were log transformed to satisfy homoscedasticity. Data were back-transformed to present in graphic form. Significant differences were determined at an α of 0.05.
Colonization of adults and nauplii by V. cholerae O1 El Tor.
To determine if there were quantitative differences in colonization by V. cholerae of adults and nauplii, V. cholerae O1 El Tor was added to microcosms containing either 10 to 20 E. affinis adults or 20 to 45 nauplii (all naupliar stages, N1 to N6). Microcosms were prepared, inoculated, and run under the same conditions as above and were sampled at 24 h. All samples were prepared for direct microscope counting, following methods previously described (10, 21).
Microcosms were blocked by day with a two by two factorial treatment structure [two copepod life stages (adults + nauplii) × two levels of strain (V. cholerae O1 + control, no V. cholerae strains)] to examine the difference in colonization of V. cholerae on adults and nauplii. Each microcosm represented a single independent replicate. Treatments were blocked by day. Replicates of each life stage were run on three different days, with two replicates on the first two days of the experiment and four on the third.
Total cells attached per microcosm were analyzed using a one-way ANOVA (mixed-model ANOVA using SAS, version 8.0). Since experimental runs were performed on different days, treatments were combined where day and day x copepod life stages were considered random effects in the ANOVA. There was no significant covariate effect of day. Thus, replicates were combined from the three experiments, forming an overall total of eight replicate microcosms for adults and nauplii. Cell counts were log transformed to satisfy homoscedasticity. Data were back-transformed to present in graphic form. Significant differences were determined at an α of 0.05.
RESULTS
Effect of copepod species and serogroup on colonization.
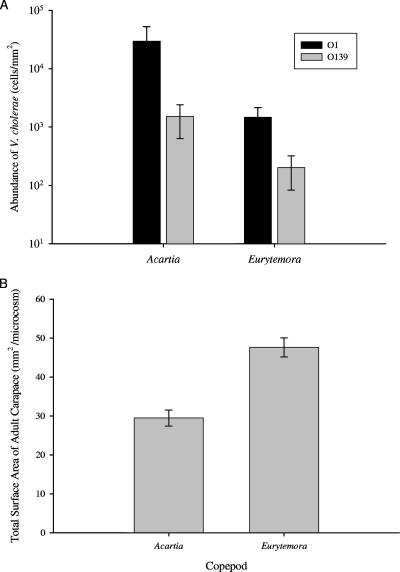
After incubation for 24 h, significant differences existed in the density of attached V. cholerae cells between the two copepod species and between the two serotypes (Fig. 1A). Overall, V. cholerae occurred in a greater density on A. tonsa than E. affinis (means ± standard error [SE] of cell counts, 1.55 × 104 ± 1.09 × 104 and 8.63 × 102 ± 3.48 × 102 per mm2 of surface area, respectively; F1,6.09 = 37.72 and P = <0.0001); F is the F statistic of the mixed-model ANOVA (MS/error MS), and the corresponding subscript is the numerator degrees of freedom and denominator degrees of freedom, respectively. Furthermore, V. cholerae O1 attached to both copepod species in significantly higher density than V. cholerae O139 (F1,41.2 = 6.11 and P = 0.0478). There was 20 times more V. cholerae O1 cells (2.95 × 104 ± 2.28 × 104) associated with A. tonsa than O139 cells (1.52 × 103 ± 8.82 × 102). For E. affinis, there was 7 times more associated V. cholerae O1 cells (1.48 × 103 ± 6.66 × 102) than O139 cells (2.02 × 102 ± 1.17 × 102). There were no significant copepod species interactions in any of the statistical models, suggesting that the differences between the colonization affinities of V. cholerae O1 and O139 were produced by factors other than copepod species (F1,41.2 = 0.06 and P = 0.8154). In this and all subsequent experiments, no measurable background V. cholerae O1 or O139 was detected in any microcosms serving as controls, and, therefore, results from controls are not considered further in our analyses.
FIG. 1.
(A) Abundance of V. cholerae O1 and O139 colonizing A. tonsa and E. affinis. Bar values represent mean counts of cells per mm2 of adult carapace surface area. (B) Total surface area of copepods per microcosm for A. tonsa and E. affinis. Bar indicates mean for each treatment. Error bars represent ±1 standard error of the mean.
A significant difference in total surface area between A. tonsa and E. affinis may account for the observed difference in attachment of total number of V. cholerae cells per mm2 between copepod species but not between serotypes. The total surface area of copepods was on average 38% greater in microcosms with E. affinis than A. tonsa (47.6 ± 2.44 mm2 and 29.5 ± 2.05 mm2, respectively; F1,3.18 = 32.75 and P = 0.0090) (Fig. 1B). There was no significant difference in V. cholerae attachment to A. tonsa versus E. affinis, when total cells attached per microcosm is the dependent variable (mixed-model ANOVA, F1,5.81 = 4.20 and P = 0.0877 and F1,6.28 = 2.99 and P = 0.1326 for V. cholerae O1 and O139, respectively).
Although initial analysis showed that surface area affected total V. cholerae densities, when A. tonsa was compared to E. affinis, surface area did not significantly contribute to differences between V. cholerae O1 and O139 cells attached per mm2 (F1,2.63 = 0.26 and P = 0.6502).
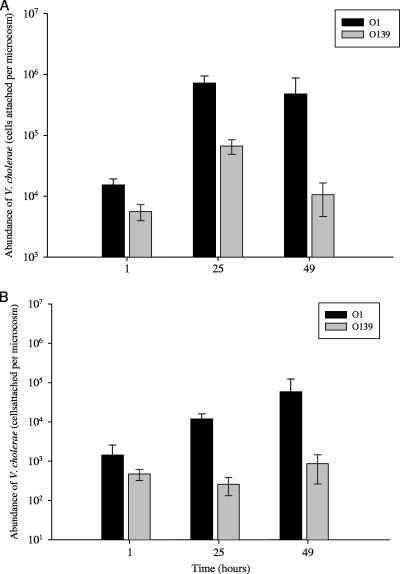
Colonization by V. cholerae O1 and O139 of adults and eggs of E. affinis over time.
For E. affinis adults, significant differences in the extent of colonization existed between serogroups and time periods (Fig. 2A). Overall, as in the previous experiment, a greater number of V. cholerae O1 than O139 cells attached to E. affinis (F1,6.98 = 35.01 and P = <0.0001). After 49 h of incubation, there were 4.79 × 105 ± 3.96 × 105 V. cholerae O1 cells per microcosm associated with adults and 1.06 × 104 ± 5.90 × 103 V. cholerae O139 cells. Colonization of both V. cholerae O1 and O139 on adult E. affinis copepods, although different in abundance, increased by greater than 1 order of magnitude within the first 24 h of incubation (1.54 × 104 ± 3.76 × 103 to 7.23 × 105 ± 2.28 × 105 cells per microcosm and 5.59 × 103 ± 1.64 × 103 to 6.70 × 104 ± 1.82 × 104 cells per microcosm, respectively). V. cholerae O1 remained at similar cell counts between 25 and 49 h, whereas V. cholerae O139 cell counts declined (Fig. 2A). There was no interaction between serogroup and time period (F2,0.38 = 1.91 and P = 0.1715).
FIG. 2.
Colonization of E. affinis by V. cholerae O1 and O139 over time for adults (A) and eggs (B). Data shown for both adults and eggs are means of total cells attached per microcosms ±1 SE as a function of time: 1 h (n = 5), 25 h (n = 5), and 49 h (n = 4).
For E. affinis eggs, a significant difference was observed in colonization between serogroups, with V. cholerae O1 counts exceeding those of O139 (Fig. 2B). In contrast to adults, there was not an effect of time on colonization (F2,0.43 = 0.54 and P = 0.5886).
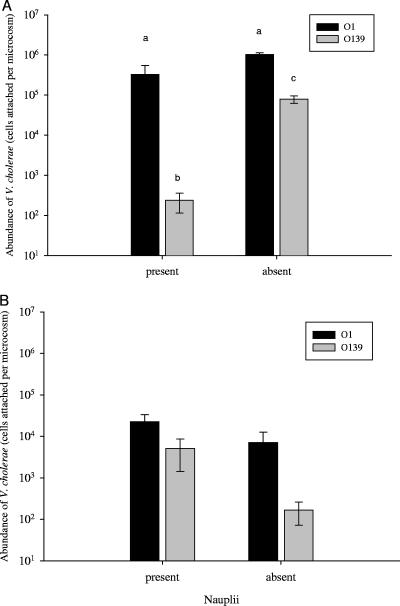
Effect of nauplii on colonization of adults and eggs of E. affinis.
Overall, there was a significant effect of nauplii presence on the numbers of V. cholerae attached to adults (F1,9.55 = 11.51; P = 0.0073), and there was a significant difference between serotypes in the number of attached cells; but the effect of nauplii was not the same for serogroups O1 and O139, as indicated by the significant interaction of main effects (F1,9.09 = 15.28; P = 0.0035). Tukey's honestly significant differences test, used for post hoc multiple comparisons of strain and nauplii, showed that the presence or absence of nauplii did not affect numbers of V. cholerae O1 that were attached (Fig. 3A). However, V. cholerae O139 attached in greater numbers to adults in the absence of nauplii (7.90 × 104 ± 1.63 × 104) compared to the presence of nauplii (2.38 × 102 ± 1.24 × 102).
FIG. 3.
Association of V. cholerae O1 and O139 with E. affinis adults (A) and eggs (B) in the presence or absence of hatched nauplii. Bar values are the means of total cells attached per microcosm ±1 SE (for adults and eggs with serotype O1, n = 5 for values where nauplii were present and n = 2 when absent; for adults and eggs with serotype O139, n = 3 for values where nauplii were present and n = 4 when absent). In panel A, means with different letters are significantly different.
As for the association of V. cholerae with eggs, there was a significant difference between V. cholerae O1 and O139 (F1,10 = 9.81; P = 0.011), with V. cholerae O1 showing significantly greater numbers than V. cholerae O139 (1.80 × 104 ± 8.15 × 103 and 2.26 × 103 ± 1.61 × 103, respectively) (Fig. 3B). Furthermore, the two V. cholerae strains attached to eggs in greater numbers when nauplii were present than in the absence of nauplii (Fig. 3B). Unlike attachment of V. cholerae to adults, there was no significant interaction between serogroups and presence of nauplii with respect to eggs (F1,10 = 1.90; P = 0.1983).
Colonization of adults and nauplii by V. cholerae O1 El Tor.
In separate microcosms, there was no significant difference in colonization of adults and nauplii by V. cholerae O1 (F1,1.32 = 2.29; P = 0.3263). Although there was a difference between the number of adults and nauplii, the total surface area present between the two treatment microcosms was not significantly different (F1,2.08 = 5.08; P = 0.1380). Therefore, total cell count was used as the dependent variable. A significant number of these nauplii were N6, the stage just prior to the copepodite stages, and much larger than newly hatched nauplii.
DISCUSSION
The Chesapeake Bay provides an example of zooplankton composition and species-level differences that may influence vibrio dynamics. Copepods comprised 98% of zooplankton in an analysis of the Chesapeake Bay (25) and 95% in Long Island Sound (13), two estuarine ecosystems in areas of historic cholera epidemics in the United States. A. tonsa and E. affinis are two of the predominant copepod species in both estuaries; these species occur commonly in estuaries throughout the northern hemisphere, and they have congeners in areas that experience modern epidemics of cholera, i.e., India, Bangladesh, Southeast Asia, and Peru.
Differences in physical characters and life history distinguish these copepods. Although A. tonsa and E. affinis are both considered pelagic, calanoid copepods, E. affinis is also known to be demersal (attaching to substrates such as rocks and pilings), whereas A. tonsa is largely planktonic. Egg production is another feature that differentiates these copepods. A. tonsa is a broadcast spawner, releasing eggs one at a time, essentially “scattering” them, and E. affinis copepods brood their eggs in an exterior assemblage (egg sac) near the anal pore.
Finally, these two species also differ in salinity distribution and seasonal abundance, as reported for the Patuxent River of Chesapeake Bay (24). A. tonsa is continuously present in the Patuxent River, where it is the most abundant copepod, comprising 55 to 60% of the total zooplankton and occurring at all salinities. Population densities are commonly 1 × 105 to 2 × 105 individuals per m3 and sometimes 106 individuals per m3 during the peak (24). E. affinis, however, is the second most abundant copepod (37%); it occurs in a range of salinities but usually dominates the lower-salinity waters. Its population density is similar to that of A. tonsa but at times exceeds 3 × 106 individuals per m3. Acartia makes up the largest fraction in the warmer months, whereas Eurytemora increases in abundance during the winter and occurs, commonly, until late spring. There is a seasonal shift in dominance between Acartia and Eurytemora which results in a period of sympatry, coinciding with a shift from the cooler, less saline months to warmer, more saline months (5).
Epidemic forms of V. cholerae in the aquatic environment are widely distributed, fluctuating temporally and seasonally. Annual shifts in the predominance of V. cholerae O1 El Tor and O139 Bengal in Bangladesh and the sympatric coexistence of the two epidemic serogroups raise many questions about their similarities and differences. V. cholerae O1 and O139 are similar in molecular characteristics (34), suggesting similar responses and utilization of available resources (i.e., broad overlap in their fundamental niches). Both serogroups cause epidemics of cholera in Southeast Asia (17), and their clinical presentation and general modes of transmission do not differ significantly (40). The two serogroups exist in the same environmental landscape, based on geographical information systems mapping and zooplankton sampling data (1, 30), but comparative aspects of the biology and ecology in the aquatic environment remain unexplored.
This study specifies numerical differences in colonization of plankton by V. cholerae O1 and O139. After incubation in river water microcosms for 24 h with copepods, V. cholerae O1 El Tor and O139 colonized both A. tonsa and E. affinis but to different extents. Specifically, when incubated separately in an experiment, V. cholerae O1 attached to each copepod species at higher abundances than V. cholerae O139. Chiavelli et al. (8) reported similar results in a comparison of the attachment of mannose-sensitive hemagglutinin mutants of V. cholerae O1 El Tor and O139 Bengal to Daphnia exoskeletons. Although it was unclear whether differences between serogroups were statistically significant for Daphnia, it appears that differences in colonization between these two serotypes may be robust across multiple taxa, given our results.
Chiavelli et al. (8) proposed that deficient colonization by serotype O139 relative to serotype O1 may be associated with exopolysaccharide that contributes to biofilm formation. Although exopolysaccharides provide serotype O139 with the ability to form biofilms, it may hinder initial attachment to plankton. The exopolysaccharide produced by V. cholerae O139, which is thicker than that of V. cholerae O1, may shield outer membrane components (membrane-associated chitin binding proteins) involved in zooplankton colonization. This hypothesis remains to be tested.
Studies of the emergence and periodic preeminence of V. cholerae O1 El Tor compared with V. cholerae O1 Classical and V. cholerae O139 Bengal strains suggest that genotypes responsible for the recent cholera epidemics are those that are better adapted to survival in a stochastic aquatic environment (15). Different efficiencies of resource use by the V. cholerae serogroups could greatly influence the character of an epidemic. For example, if particular serogroups are often unable to achieve concentrations or densities sufficient to allow successful transmission (i.e., colonizing copepods), they may be selected against. In this fashion, if a greater abundance of V. cholerae O1 El Tor than O139 Bengal attached to copepods, this may in part contribute to the dominance of V. cholerae O1 El Tor in cholera epidemics.
Despite consistent differences in colonization of copepods by V. cholerae O1 versus O139, there was no clear difference in the abundance of attached cells between A. tonsa or E. affinis when measured as total cells attached. Nor was there an association between the amount of surface area present and the total number of colonized cells. Taken together, these results suggest that factors other than species specificity and the surface area available for colonization are more important in initial colonization of zooplankton. For instance, multiple abiotic and biotic factors, e.g., salinity, temperature, and zooplankton exudates, may be more important influences on the number of V. cholerae cells colonizing zooplankton, Heidelberg et al. (23) showed that populations of V. cholerae and Vibrio mimicus in the Chesapeake Bay were most abundant in both the small- and large-size classes of plankton sampled during the summer months when A. tonsa was the predominant copepod. The average temperature and salinity in the Chesapeake Bay during the summer months when the survey was conducted were 25°C and 12‰, respectively, optimum growth conditions for V. cholerae (45). Furthermore, Heidelberg et al. (22) showed the fluctuating abundance of V. cholerae in relation to seasonal shifts in salinity and temperature in the Chesapeake Bay, with greater abundance during summer months. Huq et al. (29) reported similar seasonal patterns for V. cholerae during the cholera season in Bangladesh, an area of endemicity.
V. cholerae may not be restricted to only a few species of plankton in the aquatic environment, but the relative role or importance of different planktonic organisms in the dynamics of these bacteria or various serotypes remains poorly resolved. Differences may exist in colonization of various species, life stages, or particular habitats, perhaps coupled with abiotic factors. At present, very few comparative measures exist to evaluate this possibility, and these are restricted to only a few plankton species under a limited range of the possible environmental conditions.
Whether or not V. cholerae is a symbiont of plankton has yet to be shown. However, V. cholerae does occur in the gut and on the surface of zooplankters. For example, Huq et al. (26) reported that V. cholerae largely accumulated in areas of copepods where exudates were concentrated, e.g., around the mouthparts and on the egg sacs (which are located adjacent to the anal pore). Sochard et al. (46) also found Vibrio spp. in the guts of zooplankton. Similarly, results of earlier research revealed that vibrios, including V. cholerae, attached to and used the chitinaceous exoskeleton and eggs of aquatic invertebrates as nutrient substrates (6, 19, 20, 35, 38, 42, 51).
The association of V. cholerae O1 and O139 with E. affinis life stages exemplifies one of the benefits of epibiosis, the proximity of the epibiont to nutrients and newly emerging surfaces created by the host (49). The copepod host provides new, unoccupied habitat patches by molting, as well as by the production of eggs and nauplii. Results of this study showed that hatching nauplii can affect V. cholerae attachment to both adults and eggs, notably in the case of V. cholerae O139. The presence of hatched nauplii resulted in eggs with greater numbers of V. cholerae. It is unclear, however, whether this result was mechanistic in nature: chemoattractants released by the adult before or during hatching may attracted V. cholerae cells to the egg sac and hatching nauplii or the increase may a direct effect of the presence of nauplii.
Studies of other vibrio species have shown initial juvenile stages attracting and providing an important substrate on which vibrios can settle. Vibrio fischeri and its squid host, Euprymna scolopes, are a good example, whereby V. fischeri from adults colonize the crypt epithelia of squid juveniles. The crypt epithelia of the squid provide an array of amino acids, peptides, and proteins, supporting the proliferation of V. fischeri (18). Periodic proliferation of V. fischeri furnishes the adult host with a source of expelled cells that subsequently colonize newly hatched juveniles. Adult squid provide a source habitat of V. fischeri for juveniles, which, in time, also become source habitats and maintain the persistence of symbiotic V. fischeri (37).
Similarly, copepod eggs and hatching nauplii, which go though the multiple molt stages of juvenile development, may play a significant role in the regional dispersal and population dynamics of V. cholerae during epidemic-initiating and epidemic periods. Production of large numbers of nauplii occurs during copepod blooms, in which large numbers of eggs and juveniles are produced and often outnumber adults (39), increasing the overall available substrate as well as the density of plankters and the probability of bacterial-plankton contact. Increases in copepods and consequent offspring production follow phytoplankton blooms and precede the onset of cholera seasons in Bangladesh (11). A period of bacterial transmission, whereby cells are dispersed to nauplii, could facilitate a regional V. cholerae bloom. Plankton samples collected from the Bangladeshi aquatic environment demonstrate that at the times when nauplii are the majority of the copepod population, V. cholerae has continued to be associated with copepods through the production of offspring (A. Huq et al., unpublished data).
The availability of new surfaces provided by nauplii and eggs may be especially important to the population dynamics of V. cholerae, given the saturation (i.e., limited colonization) of adult copepods after 24 h (Fig. 2A). Saturation of attached V. cholerae O1 and O139 cells may not be associated explicitly with spatial limitation, as there were differences in surface area between the two copepods; yet the total numbers of attached cells was similar, suggesting that total area is not a limiting factor. Abundance of available nutrients and the optimal proximity to those nutrients may be more important. For example, Huq et al. (26) reported that V. cholerae largely accumulated on copepods where exudates were concentrated, e.g., around the mouthparts and on the egg sacs (which are located adjacent to the anal pore). Similarly, results of earlier research revealed that vibrios, including V. cholerae, attached to and used the chitinaceous exoskeleton and eggs of aquatic invertebrates as nutrient substrates (6, 19, 20, 35, 38, 42, 51). Thus, the ability to attach to live copepods in “hot spots,” in microcolonies or biofilms, may be crucial for efficient nutrient capture and may be an important factor in the persistence and/or ascendance of one genotype over another (50).
It is difficult to uncouple the many abiotic and biotic factors influencing the amplification and transmission of V. cholerae during cycles of cholera endemicity because abiotic factors, such as temperature and salinity, regulate many of the physiological changes that occur during the life cycle of copepods (such as reproduction, hatching, and molting) as well as V. cholerae. However, adult and juvenile stages of copepods may play different and critical roles in the transmission and epidemiology of V. cholerae O1 and O139 in a region like the Ganges Delta where cholera is endemic. We surmise that the interactive effects of plankton dynamics and environmental conditions on these two serogroups are likely to reveal much about the abundance and distribution of V. cholerae in the aquatic environment and in epidemics in humans.
Acknowledgments
Grateful acknowledgment is made to Bud Milsaps, Chesapeake Bay Laboratory, Solomons, MD, for providing E. affinis copepod stocks and to Tim Mullady, Jaime Lawshe, and the many interns of the Smithsonian Environmental Research Center, Edgewater, MD, for their excellent assistance in this study. Estelle Russek-Cohen and Mark Minton provided excellent statistical guidance, and Anwar Huq offered helpful discussions on the association of zooplankton and V. cholerae.
This research was supported, in part, by National Institutes of Health grant 1R01A139129-01, National Sea grant SA7528006-A, and National Oceanic and Atmospheric Administration/Oceans and Human Health Initiatives Distinguished Scholar subaward S06-60009. T. Rawlings gratefully acknowledges the support of The Robert D. Watkins Fellowship from the American Society of Microbiology, Washington, DC; a Smithsonian Institution Graduate Fellowship from the Smithsonian Institution, Washington, DC; and a UNCF-Merck Scientific Dissertation Fellowship from the United Negro College Fund, Arlington, VA.
Footnotes
Published ahead of print on 19 October 2007.
REFERENCES
- 1.Ali, M., M. Emch, M. Yunus, and R. B. Sack. 2001. Are the environmental niches of Vibrio cholerae O139 different from those of Vibrio cholerae O1 El Tor? Int. J. Infect. Dis. 5:214-219. [DOI] [PubMed] [Google Scholar]
- 2.Amako, K., S. Shimodori, T. Imoto, S. Miake, and A. Umeda. 1987. Effects of chitin and its soluble derivatives on survival of Vibrio cholerae O1 at low temperature. Appl. Environ. Microbiol. 53:603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu, A., P. Garg, S. Datta, S. Chakraborty, T. Bhattacharya, A. Khan, T. Ramamurthy, S. K. Bhattacharya, S. Yamasaki, Y. Takeda, and G. Balakrish Nair. 2000. Vibrio cholerae O139 in Calcutta, 1992-1998: incidence, antibiograms, and genotypes. Emerg. Infect. Dis. 6:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bik, E. M., A. E. Bunschoten, R. D. Gouw, and F. R. Mooi. 1995. Genesis of novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 14:209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownlee, D. C., and F. Jacobs. 1987. Mesozooplankton and microzooplankton in the Chesapeake Bay, p. 217-269. In S. K. Majumdar, L. W. Hall, Jr., and H. M. Austin (ed.), Contaminant problems and management of living Chesapeake Bay resources. The Pennsylvania Academy of Science, York, PA.
- 6.Broza, M., and M. Halpern. 2001. Chironomid egg masses and Vibrio cholerae. Nature 412:40. [DOI] [PubMed] [Google Scholar]
- 7.Carman, K. R. 1994. Stimulation of marine free-living and epibiotic bacterial activity by copepod excretions. FEMS Lett. Microbiol. Ecol. 14:255-262. [Google Scholar]
- 8.Chiavelli, D. A., J. W. Marsh, and R. K. Taylor. 2001. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl. Environ. Microbiol. 67:3220-3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cholera Working Group, International Centre of Diarrhoeal Disease Research, Bangladesh. 1993. Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae O139 synonym Bengal. Lancet 342:387-390. [PubMed] [Google Scholar]
- 10.Chowdhury, M. A. R., B. Xu, R. Montilla, J. A. K. Hasan, A. Huq, and R. R. Colwell. 1995. DFA-DVC: a simplified technique for detection of viable cells of V. cholerae O1 and O139. J. Microbiol. Methods 24:165-170. [Google Scholar]
- 11.Colwell, R. R., and A. Huq. 1994. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann. N. Y. Acad. Sci. 740:44-54.154. [DOI] [PubMed] [Google Scholar]
- 12.Comstock, L. E., D. Maneval, Jr., P. Panigrahi, A. Joseph, M. M. Levine, J. B. Kaper, J. G. Morris, Jr., and J. A. Johnson. 1995. The capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in V. cholerae O1. Infect. Immun. 63:317-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conover, R. J. 1956. Oceanography of Long Island Sound, 1952-1954. VI. Biology of Acartia clausi and A. tonsa. Bull. Bingham Oceanogr. Coll. 15:156-233. [Google Scholar]
- 14.Dawson, M. P., B. A. Humphrey, and K. C. Marshall. 1981. Adhesion: a tactic in the survival strategy of a marine vibrio during starvation. Curr. Microbiol. 6:195-199. [Google Scholar]
- 15.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faruque, S. M., A. R. M. A. Alim, S. K. Roy, F. Khan, G. B. Nair, R. B. Sack, and M. J. Albert. 1994. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strain of toxigenic Vibrio cholerae O139 synonym Bengal. J. Clin. Microbiol. 32:1050-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf, J., and E. G. Ruby. 1998. Host-derived amino acids support the proliferation of symbiotic bacteria. Microbiology 95:1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guthrie, R. K., and D. Q. Cofie. 1991. Culture of Vibrio cholerae in presence of shrimp and crab chitin. Int. Biodeterioration 27:39-48. [Google Scholar]
- 20.Halpern, M., H. Gancz, M. Broza, and Y. Kashi. 2003. Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl. Environ. Microbiol. 69:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasan, J. A. K., D. Bernstein, A. Huq, L. Loomis, M. L. Tamplin, and R. R. Colwell. 1994. Cholera DFA: an improved direct fluorescent monoclonal antibody staining kit for rapid detection and enumeration of V. cholerae O1. FEMS Microbiol. Lett. 120:143-148. [DOI] [PubMed] [Google Scholar]
- 22.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002a. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002b. Bacteria of the γ-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinle, D. R. 1969. Temperature and zooplankton. Chesapeake Sci. 10:186-209. [Google Scholar]
- 25.Herman, S., J. A. Mihursky, and A. J. McErlean. 1968. Zooplankton and environmental characteristics of the Patuxent River Estuary, 1963-1965. Chesapeake Sci. 9:67-82. [Google Scholar]
- 26.Huq, A., E. B. Small, P. A. West, I. Huq, R. Rahman, and R. R. Colwell. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl. Environ. Microbiol. 45:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huq, A., E. B. Small, P. A. West, and R. R. Colwell. 1984. Role of plankton copepods in the survival and multiplication of Vibrio cholerae in the aquatic environment, p. 521-534. In R. R. Colwell (ed.), Vibrios in the environment. John Wiley and Sons, New York, NY.
- 28.Huq, A., S. A. Huq, D. J. Grimes, M. O'Brien, K. H. Chu, J. M. Capuzzo, and R. R. Colwell. 1986. Colonization of the gut of the blue crab (Callinectes sapidus) by Vibrio cholerae. Appl. Environ. Microbiol. 52:586-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huq, A., P. A. West, E. B. Small, I. Huq, R. Rahman, and R. R. Colwell. 1994. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Appl. Environ. Microbiol. 48:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huq, A., R. R. Colwell, M. A. R. Chowdhury, B. Xu, S. M. Moniruzzaman, M. S. Islam, M. Yunus, and M. J. Albert. 1995. Co-existence of Vibrio cholerae O1 and O139 Bengal in plankton in Bangladesh. Lancet 345:1245. [DOI] [PubMed] [Google Scholar]
- 31.Huq, A., R. B. Sack, A. Nizam, I. M. Longini, G. B. Nair, A. Ali, J. G. Morris, Jr., M. N. Khan, A. K. Siddique, M. Yunus, M. J. Albert, D. A. Sack, and R. R. Colwell. 2005. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl. Environ. Microbiol. 71:4645-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Islam, M. S., B. S. Drasar, and D. J. Bradley. 1989. Attachment of toxigenic Vibrio cholerae O1 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J. Trop. Med. Hyg. 92:396-401. [PubMed] [Google Scholar]
- 33.Islam, M. S., B. S. Drasar, S. Mahmuda, M. G. Morshed, H. B. M. Bakht, M. N. H. Khan, R. B. Sack, and D. A. Sack. 2004. Role of cyanobacteria in the persistence of Vibrio cholerae O139. Can. J. Microbiol. 50:127-131. [DOI] [PubMed] [Google Scholar]
- 34.Johnson, J. A., C. A. Salles, P. Panigrahi, M. J. Albert, A. C. Wright, R. J. Johnson, and J. G. Morris, Jr. 1994. Vibrio cholerae O139 Synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect. Immun. 62:2108-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaneko, T., and R. R. Colwell. 1975. Adsorption of Vibrio parahaemolyticus onto chitin and zooplanktonic copepods. Appl. Microbiol. 29:269-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khetawat, G., R. K. Bhadra, S. Nandi, and J. Das. 1999. Resurgent Vibrio cholerae O139: rearrangement of cholera toxin genetic elements and amplification of rrn operon. Infect. Immun. 67:148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, K., and E. G. Ruby. 1994. Effect of the squid host on the abundance and distribution of symbiotic Vibrio fischeri in nature. Appl. Environ. Microbiol. 60:1565-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meibom, K. L., B. L. Xibing, A. T. Nielsen, C. Wu, S. Roseman, and G. K. Schoolnik. 2004. The Vibrio cholerae chitin utilization program. Proc. Natl. Acad. Sci. USA 101:2524-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller, C. B. 1983. The zooplankton of estuaries, p. 103-149. In B. H. Ketchum (ed.), Estuaries and enclosed seas. Elsevier Scientific, New York, NY.
- 40.Morris, J. G., Jr., G. E. Losonsky, J. A. Johnson, C. O. Tacket, J. P. Nataro, P. Panigrahi, and M. M. Levin. 1995. Clinical and immunologic characteristics of Vibrio cholerae O139 Bengal infection in North American volunteers. J. Infect. Dis. 71:903-908. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhyay, A. K., S. Garg, R. Mitra, A. Basu, K. Rajendran, D. Dutta, S. K. Bhattacharya, T. Shimada, T. Takeda, and Y. Takeda. 1996. Temporal shifts in traits of Vibrio cholerae strains isolated from hospitalized patients in Calcutta: a 3-year (1993 to 1995) analysis. J. Clin. Microbiol. 34:2537-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nalin, D. R., V. Daya, A. Reid, M. M. Levine, and H. C. Wu. 1979. Adsorption and growth of Vibrio cholerae on chitin. Infect. Immun. 25:768-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siddique, A. K., K. Akram, K. Zaman, P. Mutsuddy, A. Eusof, and R. B. Sack. 1996. Vibrio cholerae O139: how great is the threat of a pandemic? Trop. Med. Int. Health. 1:393-398. [DOI] [PubMed] [Google Scholar]
- 44.Simidu, U., and K. Tsukamoto. 1985. Habitat segregation and biochemical activities of marine members of the family Vibrionaceae. Appl. Environ. Microbiol. 50:781-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singleton, F. L., R. W. Attwell, M. S. Jangi, and R. R. Colwell. 1982. Influence of salinity and organic nutrient concentration on survival and growth of Vibrio cholerae in aquatic microcosms. Appl. Environ. Microbiol. 43:1080-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sochard, M. R., D. F. Wilson, B. Austin, and R. R. Colwell. 1979. Bacteria associated with the surface and gut of marine copepods. Appl. Environ. Microbiol. 37:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamplin, M. L., A. L. Gauzens, A. Huq, D. A. Sack, and R. R. Colwell. 1990. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl. Environ. Microbiol. 56:1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Threlkeld, S. T., and R. L. Willey. 1993. Colonization, interaction, and organization of cladoceran epibiont communities. Limnol. Oceanogr. 38:584-591. [Google Scholar]
- 49.Wahl, M. 1989. Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar. Ecol. Prog. Ser. 58:175-189. [Google Scholar]
- 50.Ward, J. P., J. R. King, A. J. Koerber, J. M. Croft, R. E. Sockett, and P. Williams. 2003. Early development and quorum sensing in bacterial biofilms. J. Math. Biol. 47:23-55. [DOI] [PubMed] [Google Scholar]
- 51.Yu, C., A. M. Lee, B. L. Bassler, and S. Roseman. 1991. Chitin utilization by marine bacteria. J. Biol. Chem. 25:24260-24267. [PubMed] [Google Scholar]