Abstract
The resurgence of dengue (DEN) virus infections in the last few decades coupled with the lack of a preventive vaccine and specific antiviral drugs has jointly contributed to making this a significant global public health problem. Currently, symptomatic supportive treatment and fluid replacement therapy are the only means available to minimize DEN-induced mortality. As the clinical symptoms associated with DEN virus infections are indistinguishable from those of many other viral, bacterial, and parasitic infections, specific diagnostic tests assume critical importance in the unequivocal identification of DEN virus infections. We have designed a novel chimeric antigen based on envelope domain III (EDIII), a critical antigenic region of the major structural protein of DEN viruses. We fused EDIIIs corresponding to each of the four DEN virus serotypes using pentaglycyl linkers, overexpressed the resultant tetravalent chimeric protein in Escherichia coli, and affinity purified it in high yields, obtaining ∼30 mg protein of >95% purity per liter of culture. We show that this tetravalent antigen could specifically recognize anti-DEN virus antibodies of both the immunoglobulin M (IgM) and IgG classes. Using a large panel of IgM antibody capture-enzyme-linked immunosorbent assay- and hemagglutination inhibition-confirmed DEN virus-infected and uninfected patient sera (n = 289), we demonstrate that this tetravalent antigen can function as a diagnostic tool of high sensitivity and specificity.
Dengue (DEN) fever (DF), for which there is neither a vaccine nor any therapeutic drug, is currently the most important arboviral disease that places approximately half the global population at risk (16, 35). While in the majority of cases, DF is clinically inapparent, it can progress in some cases to a severe hemorrhagic disease, dengue hemorrhagic fever (DHF), culminating in potentially fatal dengue shock syndrome (DSS) (11, 14, 17, 35, 43). DF and DHF/DSS are caused by infection with the mosquito-borne DEN viruses, of which there exist four antigenically distinct serotypes (DEN-1, -2, -3, and -4). These are members of the family Flaviviridae, which includes other flaviviruses such as yellow fever (YF), Japanese encephalitis (JE) and West Nile (WN) viruses (32). It is estimated that annually there are about 50 to 100 million cases of DF, of which ∼500,000 progress to DHF, leading to ∼25,000 deaths (12, 15-17). In untreated cases, mortality can be as high as 40 to 50% (20, 35). This can be significantly minimized by supportive care and symptomatic treatment through fluid replacement therapy (12). However, the combination of effective patient care and management rests on early definitive diagnosis of DEN virus infections. The clinical presentation of DF is often indistinguishable from those of other infectious diseases such as measles, influenza, typhoid, and other viral hemorrhagic fevers (11). In recent years, models have been developed to distinguish DF from other infections, based on clinical features and laboratory parameters such as white cell count, hemoglobin, prothrombin time, and creatinine and bilirubin levels (7) and DHF based on the use of an artificial neural network (19). However, these are predictive models and cannot serve to substitute for actual diagnostic tests.
Definitive diagnosis of DEN virus infection depends on the identification of infectious virus, its genomic RNA, virus-encoded antigens, or virus-induced antibodies (23, 39). Of these, the first three are direct markers of DEN virus infection (that depend on the presence of circulating virus) and can provide highly sensitive and reliable indicators of current infection. The traditional method of detection of infectious DEN virus in patient sera either by mosquito inoculation or cell culture is laborious, time-consuming, and not always successful. A recent report has described an ultrasensitive method with the potential to detect single virions in serum. Using this technique, the investigators demonstrated the detection of DEN virus through its association with an anti-DEN virus antibody using fluorescence cross-correlation spectroscopy (44). Viral RNA can be detected with a high degree of sensitivity by coupled reverse transcription-PCR (RT-PCR) but is subject to amplicon contamination and wide variability (30). To improve the sensitivity of the RT-PCR assay further, Lien and coworkers have devised an integrated microsystem in which the virus is captured using antibodies conjugated to magnetic beads and enriched in a magnetic field prior to RT-PCR (31). Both of these technologies are nascent and have to be evaluated extensively. The complexity of such methods, the cost, and the need for sophisticated equipment would preclude their routine use, especially in a peripheral laboratory setting. Importantly, the detection of DEN virus infections based on viral markers that are dependent on the presence of circulating virus is of limited practical utility as the infected individual is viremic only for a short period of about 4 to 5 days (41). Often, DF patients do not seek immediate medical care because the initial manifestations of infection may include mild fever or may be asymptomatic. Thus, in a majority of cases the only feasible diagnostic test would have to be based on the identification of anti-DEN virus antibodies, which appear after the viremic phase and persist much longer.
The qualitative nature of the antibody response depends upon the immunological status of the individual at the time of infection (21, 35, 39). While flavivirus-naïve individuals mount a primary antibody response, those experiencing a repeat infection manifest a secondary antibody response. A primary infection is characterized by the appearance of anti-DEN virus immunoglobulin M (IgM) antibodies usually in 3 to 5 days after onset of illness, peaking ∼2 weeks later and waning thereafter. IgG antibodies which appear shortly afterwards persist for several years. In contrast, in a secondary infection, high-titer anti-DEN virus IgG antibodies appear either before or along with IgM antibodies. Furthermore, IgM antibody titers tend to be significantly lower (21, 35, 39).
One of the earliest tests used to detect anti-DEN virus antibodies was the hemagglutination inhibition (HI) test (42, 43). The HI test, which measures the ability of serum antibody to inhibit virus glycoprotein-mediated agglutination of erythrocytes (goose or human type O), is the World Health Organization (WHO) standard test for the serologic confirmation of DEN virus infections. It utilizes whole-virus antigen prepared from infected suckling mouse brain or insect cells in culture and needs paired acute- and convalescent-phase sera. HI titers displaying a ≥4-fold difference between the paired sera are considered diagnostic of recent infection (42, 43). In contrast to the HI assay, the IgM antibody capture-enzyme-linked immunosorbent assay (MAC-ELISA) can identify recent DEN virus infections using a single serum specimen. In this assay, anti-human IgM is used to capture anti-DEN virus IgM antibodies, which in turn are revealed using a mixture of DEN virus antigens in conjunction with an antiflavivirus antibody-enzyme conjugate (14, 23, 42). Recently, several antibody detection-based DEN diagnostic tests, in several different formats, have become available commercially (10, 13, 42). Again, the source of antigen is often infected mouse brain or insect cell extracts. A major drawback stemming from the use of whole-virus antigens, aside from the inherent biohazard risk, is the inability of these tests to differentiate among the various flaviviruses. This is due to the existence of shared antigenic determinants among members of the family Flaviviridae (6). Replacement of the whole-virus antigens with a mixture of recombinant envelope (E) proteins of the four DEN virus serotypes eliminated the safety risk but not the cross-reactivity problem (10). In an attempt to eliminate this cross-reactivity, we have focused on a discrete domain of the E protein, known as domain III (EDIII).
In recent years, several studies have identified EDIII as a critical immunodominant region of the DEN virus E protein. EDIII, which spans amino acids (aa) 300 to 400 of the E protein, is a highly stable, independently folding domain (4) that lies exposed and accessible on the virion surface (27). Multiple type- and subtype-specific neutralizing epitopes of the E protein have been mapped to EDIII (9, 34, 37, 38). A variety of studies have implicated this domain in host receptor binding (4, 8, 9, 18). Using a mixture of TrpE-EDIII fusion proteins corresponding to the four DEN virus serotypes, Simmons et al. reported the successful detection of anti-DEN virus antibodies of both IgM and IgG classes (40). Importantly, they also found that sera from YF and JE virus IgG+ individuals who had not been infected with DEN virus did not manifest any ELISA reactivity towards the TrpE-DEN-EDIII mixture. Another group, which used an immunoblot format, showed that recombinant EDIII (rEDIII) proteins could be used to serotype DEN virus-infected patient sera (33). While this method did not differentiate anti-DEN virus IgM or IgG class antibodies, DEN virus-infected patient sera (all four serotypes) did not react with JE and WN virus EDIIIs. Also, anti-WN virus EDIII antiserum did not recognize any of the four DEN virus-specific rEDIII proteins.
In this study, we created an rEDIII-T protein. We reasoned that this rEDIII-T protein may not only retain the potential to pick up anti-DEN virus antibodies (of both IgM and IgG classes) specific to each of the four DEN virus serotypes but would also obviate the need to express and purify four separate proteins. We, therefore, decided to evaluate the diagnostic utility of the rEDIII-T antigen in detecting anti-DEN virus antibodies. Thus, in this report we describe the expression and purification of the rEDIII-T antigen and an evaluation of its potential as a diagnostic tool for the detection of anti-DEN virus IgM and IgG antibodies using a large panel (n = 289) of well-characterized patient sera.
MATERIALS AND METHODS
Materials.
Escherichia coli host strain DH5α was purchased from Invitrogen (Carlsbad, CA). E. coli expression strain SG13009 (pREP4 [Kanr]), the expression plasmid pQE30 (Ampr), Ni-nitrilotriacetic acid (NTA) Super-flow resin, and anti-His (penta-His) monoclonal antibody were from QIAGEN (Hilden, Germany). Anti-mouse IgG-alkaline phosphatase (AP) conjugate and the substrate 5-bromo-4-chloro-3-indolyl phosphate-nitroblue tetrazolium (BCIP/NBT) were from Calbiochem (EMD Chemicals, Inc., La Jolla, CA). Anti-human IgM- and IgG-AP and anti-human IgM- and IgG-horseradish peroxidase (HRPO) conjugates were also from Calbiochem. The HRPO substrate, 3,3′,5,5′-tetramethylbenzidine (TMB), was from Kirkegaard Perry laboratories, Gaithersburg, MD. The DEN MAC-ELISA (IgM capture ELISA) kit was from Focus Diagnostics, Cypress, CA.
Human sera.
We obtained serum specimens from febrile patients (n = 201) from areas of DEN endemicity in Sri Lanka after informed written consent. All patients were warded at the North Colombo Teaching Hospital, Sri Lanka. Ethical permission for the collection of blood samples was obtained from the Ethical Committee, Faculty of Medicine, University of Kelaniya, Sri Lanka. Acute-phase sera were drawn in the early symptomatic phase from each patient. From several of them (n = 88), convalescent-phase samples were obtained 1 to 2 weeks after the acute-phase sample. Thus, a total of 289 patient sera consisting of 80 paired acute- and convalescent-phase DEN virus infection-confirmed sera, 82 single acute-phase and DEN virus infection-confirmed sera, 8 paired acute- and convalescent-phase confirmed non-DEN patient sera, and 31 single acute-phase confirmed non-DEN patient sera were available for this study. These sera were stored immediately at −80°C for about a year before being used in this study. Sera were tested for the presence of DEN virus, DEN virus RNA, and anti-DEN virus IgM and IgG antibodies with established reference assays (29, 42, 43). A summary of the sera is presented in Table 1. More detailed information is provided in the supplemental material.
TABLE 1.
Summary of patient sera used in this studya
| Panel (n)b | Diagnosisc | No. of samples
|
|||
|---|---|---|---|---|---|
| Virus infectedd | Serology
|
All negativeg | |||
| IgMe | HIf | ||||
| 1 (80)h | Confirmed DEN | 30 | 42 | 43 | 8 |
| 2 (82) | Confirmed DEN | 18 | 60 | 54 | 0 |
| 3 (39)h | Confirmed non-DEN | 0 | 0 | 0 | 39 |
Both acute-phase (early symptomatic phase) as well as convalescent-phase (1 to 2 weeks later) samples were collected; the data shown are for the acute-phase sera. (For details, see the supplemental material.)
Eighty patients for whom paired acute- and convalescent-phase sera could be collected were placed in panel 1. Eighty-two DEN virus infection-confirmed patients for whom single acute-phase sera were available were assigned to panel 2. Panel 3 consisted of 39 acute-phase febrile patients confirmed to be non-DEN cases. Of these, paired sera were available from 8 patients, while single acute-phase samples were collected from the remaining 31.
Based on the presence of DEN virus RNA and/or a positive IgM and/or HI test in conjunction with clinical presentation.
DEN virus RNA in sera was detected by RT-PCR; infectious virus could be isolated successfully from several RT-PCR-positive sera.
Anti-DEN virus IgM antibodies were detected using a commercial MAC-ELISA kit (Focus Diagnostics).
Laboratory confirmation of DEN infection was defined by positive detection of a ≥4-fold rise in anti-DEN virus IgG antibody titers in paired sera; HI titers of <20 were identified as non-DEN cases.
Sera that tested negative by RT-PCR, virus isolation, MAC-ELISA, and HI assay.
Convalescent-phase sera were obtained (1 to 2 weeks after collection of acute-phase sera) from all 80 patients in panel 1 and from 8 patients in panel 3. All panel 1 convalescent-phase sera were IgM+ HI+, while all 8 convalescent-phase panel 3 sera were IgM− HI−.
In addition, a panel (n = 6) of WHO reference sera containing four sera, each with IgG antibodies specific to a single DEN virus serotype, a serum containing IgG antibodies to all four DEN virus serotypes, and a flavivirus-naïve serum were obtained from Aravinda de Silva, North Carolina State University. Four patient sera, confirmed to be JE virus seropositive by the method of Bundo and Igarashi (5), were also included in the study.
Expression and purification of rEDIII-T antigen.
A chimeric ∼1.5-kb rEDIII-T gene, codon optimized for E. coli expression, was obtained by chemical synthesis (GeneArt; Germany). This gene was designed to encode the EDIIIs (ranging from 115 to 119 aa residues) of all four DEN virus serotypes, with adjacent EDIII units joined by pentaglycyl peptide linkers. The gene was ligated into BamHI and HindIII restriction enzyme sites of the bacterial expression vector pQE30, in frame with the vector-provided ATG codon and six-His tag-encoding sequences, to generate the plasmid pQ-EDIII-T in E. coli DH5α. For expression, the plasmid was transformed into E. coli host strain SG13009 and screened in test tube cultures. One clone that expressed the recombinant protein maximally was sequence verified and used for recombinant protein purification. All procedures were performed as previously described (2).
Purification was performed using 1 liter of isopropyl-β-d-1-thiogalactopyranoside (IPTG)-induced culture of an E. coli SG13009 clone harboring the pQ-EDIII-T construct identified above. The entire purification was performed under denaturing conditions throughout, essentially as described earlier (2). Briefly, the cell pellet (∼2.5 g [wet weight]) was lysed by sonication in a denaturing buffer (6 M guanidine HCl, 100 mM sodium phosphate, 10 mM Tris-HCl, 300 mM NaCl [pH 8]), clarified and chromatographed on an Ni-NTA matrix (bed volume, 4 ml). After washing, the bound proteins were eluted at acidic pH. Column fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and peak fractions were pooled, flash-frozen in liquid nitrogen, and stored at −80°C until use.
Western blot analysis.
To characterize the purified rEDIII-T protein, it was subjected to SDS-PAGE, together with appropriate controls and prestained protein markers, electroblotted onto nitrocellulose, and probed with penta-His monoclonal antibody (MAb) and visualized using anti-mouse IgG-AP conjugate and BCIP/NBT substrate as described previously (1, 2). To assess the reactivity of rEDIII-T protein towards anti-DEN virus IgM and IgG antibodies, the purified protein was electrophoresed in a single wide well and blotted onto nitrocellulose, which was then cut into narrow strips. Each strip was then probed separately with DEN patient sera that were previously characterized to be IgM+ IgG+, IgM+ IgG−, IgM− IgG+, and IgM− IgG− (1). We also used murine anti-rEDIII-T antiserum and murine preimmune serum as positive and negative controls, respectively. The secondary antibody-enzyme conjugate differed depending on the probing serum used as the source of primary antibodies. For strips that were probed with IgM+ IgG− and IgM− IgG+ patient sera, the secondary antibody-enzyme conjugates used were anti-human IgM and anti-human IgG, respectively. For strips probed with IgM+ IgG+ and IgM− IgG− patient sera, either anti-human IgM- or IgG-enzyme conjugate was used. Finally, for strips probed with murine serum, we used anti-mouse IgG-enzyme conjugate. All other procedural details were as reported earlier (1, 2).
In-house IgM and IgG ELISAs.
Ninety-six-well flat-bottom ELISA plates (Nunc, Roskilde, Denmark) were coated with 100 μl of diluted rEDIII-T protein (10 μg/ml in 0.1 M carbonate buffer [pH 9.5]) and incubated at 37°C for 1 h. The wells were blocked with 200 μl of 5% skim milk in 1× phosphate-buffered saline at 37°C for 2 h and washed with 1× phosphate-buffered saline-0.5% Tween 20-0.1% 3-[(3-cholamidopropyl)dimethyl-ammonio]-1-propanesulfonate five times (1 min/wash). Washed wells were incubated with 100 μl of patient serum (diluted 1:100 in blocking buffer) at 37°C for 15 min. For IgG detection, wells were washed again as described above and incubated with 100 μl of anti-human IgG-HRPO conjugate (diluted 1:10,000 in blocking buffer) at 37°C for 30 min. Wells were washed again and incubated with 100 μl of TMB soluble substrate at 37°C for 15 min. The reaction was stopped by adding 100 μl of 1 M H2SO4. The optical density value of each sample was measured at 450 nm with 630 nm as the reference wavelength, using an ELx800UV microplate reader (Bio-Tek Instruments, Inc., VT). For IgM detection, we used essentially the same protocol, except that the secondary antibody-enzyme conjugate was anti-human IgM-HRPO, for the following reasons. We have found a 15-min incubation step to be adequate for serum IgM determinations (1). Furthermore, prior removal of serum IgG using Pansorbin cells (catalog no. 507861; Calbiochem) did not result in any significant improvement in IgM ELISA titers.
Sera were designated as either positive or negative for the presence of anti-DEN virus IgM and IgG antibodies using cutoff absorbance values of 0.54 and 0.34, respectively. These cutoff values were obtained from the mean ELISA absorbance of 47 confirmed non-DEN serum samples plus 3 standard deviations (SD). DEN virus infection was ruled out in these patients based on RT-PCR, virus isolation, HI assay, and a commercial MAC-ELISA kit (Focus Diagnostics). Pooled DEN virus-seropositive and -seronegative samples, identified in our earlier studies (1, 2), were included in each run to control for interassay variation.
Statistical analysis.
The SD of the mean was calculated using the SPSS 15.0 package. A chi-square test (Epi 6, version 6.04d, software; Centers for Disease Control and Prevention) was used for comparison of data obtained using rEDIII-T antigen-based in-house IgM and IgG ELISAs (test methods) with the corresponding reference methods, MAC-ELISA and HI assays, respectively. Two variables were analyzed at a 95% confidence interval, and P values of <0.05 were considered significant.
RESULTS
Design of the EDIII-T antigen.
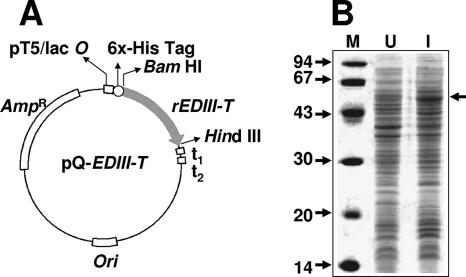
We chose EDIII as a precursor to create a tetravalent antigen, rEDIII-T, that would be potentially able to recognize antibodies specific to each of the four DEN virus serotypes. This chimeric antigen was designed to contain the EDIIIs of the four DEN virus serotypes, interlinked by pentaglycyl linkers. This antigen is predicted to have a molecular size of ∼55 kDa. A synthetic gene (EDIII-T gene) encoding this tetravalent antigen, codon optimized for expression in E. coli, was inserted in-frame with the initiator codon and a six-histidine tag-encoding sequence of the bacterial expression vector pQE30. The map of the resultant plasmid, pQ-EDIII-T, is shown in Fig. 1A. This plasmid was transformed into E. coli host strain SG13009 containing the pREP4 plasmid, which encodes the lacI repressor (required for regulated recombinant gene expression) and the kanamycin marker. The resultant double recombinants were analyzed by expression screening, wherein IPTG-induced cells were directly lysed in Laemmli sample buffer and analyzed by SDS-PAGE. Figure 1B depicts the induction profile of a typical clone. Induction of the EDIII-T gene resulted in the appearance of a new ∼55-kDa band (compare lanes I and U), consistent with the predicted size of the rEDIII-T protein. When induced cells were lysed by sonication in a native buffer, separated into supernatant and pellet fractions as described before (22), and analyzed by SDS-PAGE, the protein was found to be predominantly present in the insoluble fraction (data not shown).
FIG. 1.
Expression of r-EDIII-T protein in E. coli. (A) Map of the expression plasmid. The synthetic rEDIII-T gene was inserted into the BamHI and HindIII sites of plasmid pQE30, in-frame with the vector-provided ATG and six-His tag-encoding sequence. Abbreviations are as follows: pT5/lac O, prokaryotic promoter under lac operator control; rEDIII-T, synthetic gene encoding the tetravalent antigen; t1 and t2, transcriptional terminators; Ori and AmpR, plasmid replication origin and ampicillin resistance marker, respectively. (B) Coomassie-stained denaturing gel showing the polypeptide profiles of E. coli harboring the plasmid in panel A before (lane U [uninduced]) and after (lane I [induced]) IPTG induction. Protein molecular mass markers were run in lane M; their sizes in kDa are shown to the left. The arrow to the right indicates the position of the rEDIII-T protein.
Purification and characterization of the rEDIII-T antigen.
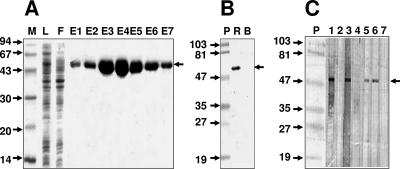
The rEDIII-T protein was purified by a single-step Ni-NTA affinity chromatography utilizing the six-His tag engineered at its N terminus. As the majority of the rEDIII-T protein was insoluble, the affinity chromatography was carried out under denaturing conditions throughout. An SDS-PAGE analysis of different fractions obtained during purification is presented in Fig. 2A. Under the experimental conditions, almost all of the recombinant protein bound to the column, as evident from a comparison of the polypeptide profiles of the initial lysate (lane L) and the flowthrough material (lane F). Elution of the bound proteins after extensive washing resulted in the emergence of highly purified protein (lanes E1 to E7). We estimate the purity to be at least ∼95% on the basis of comparison of the protein profiles of the eluted material (peak in lane E4) and the crude lysate (lane L). We obtained a yield of ∼30 mg rEDIII-T protein, corresponding to >95% recovery, starting from 1 liter of induced E. coli culture.
FIG. 2.
Purification and immunoblot analysis of the rEDIII-T protein. (A) SDS-PAGE analysis of Ni-NTA affinity column fractions obtained during the purification of rEDIII-T protein. The samples analyzed in the gel are load (lane L), flowthrough (lane F), and eluates (lanes E1 to E7). Protein molecular mass markers were run in lane M; their sizes in kDa are shown to the left. The arrow to the right indicates the position of the rEDIII-T protein. (B) Western blot analysis of the purified rEDIII-T protein using the penta-His MAb. An aliquot of the purified recombinant protein was run in lane R. A protein without His tag (bovine serum albumin) was run in lane B as a negative control. Prestained protein molecular mass markers were run in lane P; their sizes in kDa are shown to the left. The arrow to the right indicates the position of the rEDIII-T protein. (C) Western blot analysis performed with nitrocellulose strips onto which purified rEDIII-T protein had been transferred after SDS-PAGE. The test strips were probed with IgM+ IgG− (lanes 1 and 4) or IgM− IgG+ (lanes 2 and 3) confirmed DEN patient sera. The penta-His MAb (lane 6), murine anti-rEDIII-T polyclonal serum (lane 5), and murine preimmune serum (lane 7) were used to probe the control strips. The rEDIII-T protein was visualized using anti-human IgM (lanes 1 and 2)-, anti-human IgG (lanes 3 and 4)-, or anti-murine IgG (lanes 5 to 7)-enzyme conjugate. Prestained molecular mass markers were run in lane P; their sizes in kDa are indicated to the left. The arrow to the right indicates the position of the rEDIII-T antigen.
We tested the purified protein in a Western blot assay. An aliquot of pooled purified protein was probed with a commercially available murine penta-His MAb specific to the engineered six-His tag of the recombinant protein. It is evident from the data shown in Fig. 2B that the penta-His MAb specifically recognized the Ni-NTA-purified ∼55-kDa rEDIII-T protein.
The rEDIII-T antigen recognizes IgM and IgG antibodies in DEN patient sera.
Using the purified rEDIII-T antigen, we next sought to evaluate its ability to specifically recognize human anti-DEN virus antibodies of both IgM and IgG classes. To this end, we performed a Western analysis in which the rEDIII-T protein, transferred onto nitrocellulose strips, was probed separately with several different antisera known to either contain or lack anti-DEN virus antibodies. We used sera that were characterized in our earlier studies (1, 2). In this experiment, the binding of anti-DEN virus IgM and anti-DEN virus IgG antbodies in the sera to rEDIII-T was identified using anti-human IgM and anti-human IgG secondary antibody conjugates, respectively. The results of this immunoblot analysis are presented in Fig. 2C. As positive controls, we included the penta-His MAb as in the previous experiment (lane 6), as well as a murine anti-rEDIII-T polyclonal serum (lane 5). As expected, both of these specifically recognized the rEDIII-T protein. Preimmune murine serum, which served as the negative control, did not pick up the recombinant protein (lane 7). When the strips were probed with an IgM+ IgG− DEN patient serum, the rEDIII-T could be visualized if the secondary antibody was anti-human IgM (lane 1) but not anti-human IgG (lane 4). Similarly, when the primary serum used for probing was IgM− IgG+, the tetravalent protein was discernible in the blot if the secondary antibody was anti-human IgG (lane 3) but not anti-human IgM (lane 2). The rEDIII-T protein could also be detected in the strip blots when probed with a double-positive (IgM+ IgG+) but not double-negative (IgM− IgG−) serum, irrespective of the secondary antibody-enzyme conjugate used (data not shown). Taken together, these data strongly indicate that the rEDIII-T protein can specifically recognize and bind to both anti-DEN virus IgM and anti-DEN virus IgG antibodies in patient sera.
The rEDIII-T protein recognizes antibodies specific to multiple DEN serotypes.
The question that we sought to address at this point was whether the rEDIII-T protein would recognize human antibodies specific to each one of the four DEN virus serotypes. To address this question, we obtained a WHO reference serum panel (n = 6) with samples containing IgG antibodies to single DEN virus serotypes, IgG antibodies to all four DEN virus serotypes, and a sample lacking flaviviral antibodies. These reference sera were tested in an in-house ELISA using rEDIII-T antigen to capture the anti-DEN virus IgG antibodies, followed by detection of the bound antibodies using the anti-human IgG-HRPO conjugate. The results summarized in Table 2 lead to the conclusion that indeed the rEDIII-T protein manifests a high level of reactivity towards anti-DEN virus IgG antibodies of all four DEN virus serotypes. The unavailability of the corresponding anti-DEN virus IgM antibody-containing reference panel precluded the possibility of addressing the question of whether the rEDIII-T antigen would bind IgM antibodies specific to each of the four DEN virus serotypes. However, we found during the collection and characterization of patient sera for evaluation of the rEDIII-T protein (below) several samples that tested positive for DEN virus genomic RNA by RT-PCR (Table 1). Of these we could identify two DEN-2 virus-positive and 16 DEN-3 virus-positive sera, all of which manifested significant levels of reactivity in an rEDIII-T antigen-based in-house IgM ELISA, as shown in Table 3. While this assay was similar to the in-house IgG ELISA described above, in that we again utilized rEDIII-T protein as the coating antigen, it differed in one important respect, namely, the use of anti-human IgM in the detection phase, instead of anti-human IgG. Unfortunately, we could not identify DEN-1 and DEN-4 viruses in any of the DEN virus-confirmed sera used in this study. In regard to anti-DEN virus IgM antibody recognition, available data demonstrate specificity for DEN virus serotypes 2 and 3. Patient sera that were positive for JE virus-specific antibodies failed to manifest any significant reactivity towards the DEN virus-derived rEDIII-T protein. Mean (±SD) absorbance values of these JE virus+ sera (n = 4) in the in-house IgM and IgG ELISA were 0.17 (±0.08) and 0.31 (± 0.12), respectively.
TABLE 2.
Reactivity of WHO reference sera in the in-house IgG ELISA
| Sample | Virus serotype | Anti-DEN virus IgG status | In-house IgG ELISA A450 |
|---|---|---|---|
| Ref-1 | DEN-1 | Positive | 5.00 |
| Ref-2 | DEN-2 | Positive | 1.76 |
| Ref-3 | DEN-3 | Positive | 1.92 |
| Ref-4 | DEN-4 | Positive | 1.70 |
| Ref-5 | All 4 DEN virus serotypes | Positive | 2.63 |
| Ref-6 | Flavivirus naïve | Negative | 0.40 |
TABLE 3.
Reactivity of viremic sera in the in-house IgM ELISA
| Panel | Virus serotypea | No. of serum samples | In-house ELISA mean A450 (range)b |
|---|---|---|---|
| 1 | DEN-3 | 7 | 2.45 (1.47-5.00) |
| 2 | DEN-2 | 2 | 1.07 (0.92-1.22) |
| DEN-3 | 9 | 2.27 (0.83-5.00) |
Virus serotype was identified by RT-PCR (29). Infectious virus could be isolated from both DEN-2 virus- and 12 DEN-3 virus-infected sera.
The values in this column indicate the reactivity of anti-DEN virus IgM antibodies to rEDIII-T protein in terms of mean A450; the values in parentheses indicate the range of observed absorbance values.
Detection of anti-DEN virus IgM and IgG antibodies in patient sera.
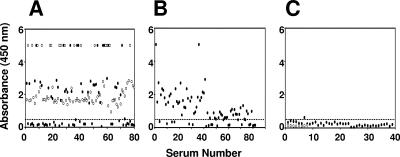
Based on these encouraging observations, we next proceeded to evaluate the utility of this tetravalent antigen as a diagnostic tool for the detection of both anti-DEN virus IgM and IgG antibodies using a large collection of confirmed DEN and non-DEN patient sera (Table 1). All of these sera (n = 289) were screened using the rEDIII-T antigen-based in-house IgM and IgG ELISAs described above (see the supplemental material). In parallel, each sample was also tested using the MAC-ELISA and HI assay as reference assays for anti-DEN virus IgM and IgG antibodies, respectively. The results of the in-house IgM ELISA are depicted in Fig. 3. With regard to acute-phase sera from DEN-confirmed patients, about half of panel 1 sera (Fig. 3A) and nearly three-quarters of panel 2 sera (Fig. 3B) were found to be IgM+ based on their reactivity towards the rEDIII-T protein in the in-house IgM ELISA. Interestingly, 100% of the panel 1 convalescent-phase sera were found to be IgM+ (Fig. 3A). The panel 3 acute-phase samples which tested negative using the MAC-ELISA failed to display any significant reactivity towards the rEDIII-T protein in the in-house IgM ELISA. This was true for the panel 3 convalescent-phase sera as well (Fig. 3C). It is to be pointed out that all panel 3 sera also tested negative in the virus culture, RT-PCR, and HI assays (Table 1). A comparison of the in-house IgM ELISA data with those of the reference MAC-ELISA is shown in Table 4. We found that 55% of the confirmed DEN patient sera tested positive for the presence of anti-DEN virus IgM antibodies with the in-house IgM ELISA. Significantly, each of these sera also tested positive with the reference MAC-ELISA. Likewise, 29% of the confirmed DEN patient sera which tested negative in the in-house IgM ELISA also tested negative in the reference MAC-ELISA.
FIG. 3.
Analysis of anti-DEN virus IgM antibodies in patient sera using rEDIII-T antigen-based in-house IgM ELISA. Anti-DEN virus IgM antibodies in serum panels 1 (A), 2 (B), and 3 (C) were tested in an ELISA using the rEDIII-T protein as the capture antigen. Bound IgM antibodies were detected using anti-human IgM-HRPO conjugate in conjunction with TMB substrate. The dotted line indicates the cutoff absorbance (A450 of 0.54). Acute- and convalescent-phase sera are indicated by the solid and open symbols, respectively.
TABLE 4.
Evaluation of rEDIII-T protein as a DEN diagnostic antigen
| Antibody statusa | No. of samples tested to determine the presence ofb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Anti-DEN virus IgM
|
Anti-DEN virus IgG
|
|||||||
| Panel 1 | Panel 2 | Panel 3 | Total | Panel 1 | Panel 2 | Panel 3 | Total | |
| Ref+ Test+ | 37 | 52 | 0 | 89 | 38 | 49 | 0 | 87 |
| Ref− Test+ | 7 | 6 | 0 | 13 | 2 | 7 | 0 | 9 |
| Ref+ Test− | 5 | 8 | 0 | 13 | 5 | 5 | 0 | 10 |
| Ref− Test− | 31 | 16 | 39 | 86 | 35 | 21 | 39 | 95 |
Ref+, antibody present as determined by the reference assay; Ref−, antibody absent as determined by the reference assay; Test+, antibody present as determined by the in-house ELISA; Test−, antibody absent as determined by the in-house ELISA. Anti-DEN virus IgM antibodies were determined using the commercial MAC-ELISA kit (Ref) and the in-house IgM ELISA (Test). Anti-DEN IgG antibodies were determined using the HI assay (Ref) and the in-house IgG ELISA (Test). The cutoff values (mean + 3 SD) used for the IgG and IgM in-house ELISAs were 0.34 and 0.54, respectively.
A total of 201 samples were tested. The number of samples in each panel was as follows: in panel 1, 80 samples; in panel 2, 82 samples; and in panel 3, 39 samples.
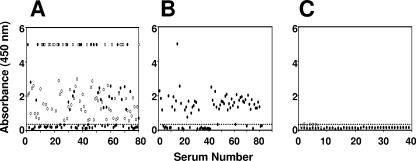
Next, we examined the capacity of the rEDIII-T antigen to detect anti-DEN virus IgG antibodies as well using the in-house IgG ELISA. For this experiment, we used the HI assay as the reference, which is the WHO “gold standard” assay for confirming DEN virus infections (43). The in-house IgG ELISA data for all 289 sera are presented in Fig. 4. With regard to acute-phase sera, the data showed that about half of panel 1 (Fig. 4A) and two-thirds of panel 2 (Fig. 4B) sera were IgG+. However, all panel 1 convalescent-phase sera were invariably IgG+ (Fig. 4A). Once again all of the panel 3 sera (both acute as well as convalescent phase), which tested negative in the reference assay (HI titers of <20), did not manifest any significant IgG ELISA reactivity (Fig. 4C). A comparison of the in-house IgG ELISA results with those of the HI assay is presented in Table 4. About 54% of the DEN virus-confirmed sera which manifested the presence of rEDIII-T antigen-reactive IgG antibodies were also HI+, with titers of >2,560 for the majority of these samples. Furthermore, ∼35% of these DEN virus-confirmed sera, which did not have any rEDIII-T antigen-reactive IgGs, also tested negative in the HI assay.
FIG. 4.
Analysis of anti-DEN virus IgG antibodies in patient sera using rEDIII-T antigen-based in-house IgG ELISA. Anti-DEN virus IgG antibodies in serum panels 1 (A), 2 (B), and 3 (C) were tested in an ELISA using the rEDIII-T protein as the capture antigen. Bound IgG antibodies were detected using anti-human IgG-HRPO/TMB. The dotted line indicates the cutoff absorbance (A450 of 0.34). Acute- and convalescent-phase sera are indicated by the solid and open symbols, respectively.
Discrepant sera.
It is evident that the results of the in-house IgM and IgG ELISAs agreed with those of the cognate reference assays in most but not all samples tested. With regard to anti-DEN virus IgM status, the in-house ELISA results of 84% of the DEN virus-confirmed sera agreed with those obtained using the reference MAC-ELISA (Table 4). Thirteen sera which were identified as IgM+ based on the reference MAC-ELISA were found to be IgM− based on the in-house ELISA. Similarly, another set of 13 sera were IgM− in the MAC-ELISA but IgM+ in the in-house ELISA. The in-house IgM ELISA data for the discrepant sera, which did not conform to the MAC-ELISA results, are presented in Fig. 5A. About 25% of the discrepant sera were borderline cases, with the rest resulting in absorbance values that fell clearly below or above the cutoff mark in the in-house IgM ELISA. Interestingly, such inconsistencies were not evident when the anti-DEN virus IgM status of convalescent-phase sera was tested by these two assays. A total of 88 convalescent-phase sera (80 from panel 1 and 8 from panel 3) gave identical results in both the test and reference assays for anti-DEN virus IgM antibodies. While all of the panel 1 convalescent-phase sera (n = 80) tested positive, all panel 3 convalescent-phase sera (n = 8) tested negative for anti-DEN virus IgM antibodies in both the MAC-ELISA as well as the in-house IgM ELISA (see the supplemental material).
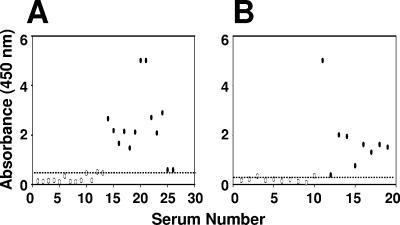
FIG. 5.
ELISA reactivities of the discrepant sera. (A) ELISA reactivities of patient sera that manifested inconsistent results in the in-house IgM ELISA (Test) with reference to the commercial MAC-ELISA (Ref). (B) ELISA reactivities of patient sera that manifested inconsistent results in the in-house IgG ELISA (Test) with reference to the HI assay (Ref). The in-house IgM and IgG ELISA cutoffs, indicated by the dotted lines, were 0.54 and 0.34, respectively. Ref+ Test− and Ref− Test+ sera are indicated by open and solid symbols, respectively.
Again as with the in-house IgM ELISA, we found that a small percentage of the sera analyzed by the in-house IgG ELISA yielded discrepant results with reference to the HI assay data, as shown in Fig. 5B. Ten HI+ sera, with titers ranging from 80 to >2,560, tested negative in the in-house IgG ELISA. Similarly, nine sera that were IgG+ in the in-house ELISA were HI− (HI titers of <20). Once again, the discrepancies between the test and reference assays were indiscernible for the convalescent-phase sera (see supplemental material). All panel 1 convalescent-phase sera (n = 80), which were designated as IgG+, were also HI+. Of these, 10 were primary infections (with HI titers ranging from 80 to 640) and the rest were secondary infections (HI titers of >2,560). Likewise, all panel 3 convalescent-phase sera (n = 8), which were IgG−, displayed HI titers of <20. Interestingly, the discrepant IgM and IgG serum groups were largely distinct from each other, with just two sera being common to both groups (see the supplemental material).
Performance evaluation of the diagnostic utility of the rEDIII-T antigen.
A summary of an analysis performed by comparing the rEDIII-T antigen-based in-house IgM and IgG ELISA data with data generated using the reference assays is presented in Table 5. An overall comparison of the data for the acute-phase sera in terms of sensitivity, specificity, and concordance suggests that there is excellent agreement between our in-house ELISAs and the currently accepted MAC-ELISA and HI reference assays (43). Interestingly, the use of the rEDIII-T antigen-based in-house ELISAs for the detection of anti-DEN IgM and IgG antibodies in convalescent-phase sera (n = 88) showed complete agreement of all three parameters with respect to the cognate reference assays. Statistical analysis revealed no significant difference between the results generated using the in-house IgM-ELISA and reference MAC-ELISA (χ2 = 187; P = 0.0); similarly, there was no significant difference between the in-house IgG-ELISA and the reference HI assay (χ2 = 214; P = 0.0).
TABLE 5.
Performance parameters assessed by comparison of the EDIII-T-based IgM and IgG ELISA results with those of the corresponding reference methods
| Sera (n) | IgM
|
IgG
|
||||
|---|---|---|---|---|---|---|
| % Sensitivitya | % Specificityb | % Concordancec | % Sensitivitya | % Specificityb | % Concordancec | |
| Acute phase (201) | 86 (89/104) | 89 (86/97) | 87 (175/201) | 90 (87/97) | 91 (95/104) | 91 (182/201) |
| Convalescent phase (88) | 100 (80/80) | 100 (8/8) | 100 (88/88) | 100 (80/80) | 100 (8/8) | 100 (88/88) |
Values in parentheses are numbers of sera antibody-positive by both tests/numbers of sera positive by only the reference assay.
Values in parentheses are numbers of sera antibody-negative by both tests/numbers of sera negative by only the reference assay.
Values in parentheses are numbers of sera with concordant reference and in-house test results/total number of sera.
DISCUSSION
As DEN virus infections are endemic to resource-poor nations and generally coprevalent with other flaviviral infections, an ideal DEN diagnostic test designed to detect DEN virus-induced antibodies would have to satisfactorily meet four essential criteria, namely, safety, high sensitivity, high specificity, and low cost. This work is based on the premise that such a diagnostic test would have to be based on an antigen which should (i) be a recombinant protein; (ii) incorporate immunodominant antigenic features unique to each one of the DEN virus serotypes, as multiple serotypes often cocirculate in many areas of endemicity; (iii) specifically recognize anti-DEN virus antibodies of both IgM and IgG classes, while at the same time avoiding cross-reactivity towards other flavivirus-induced antibodies; and (iv) be amenable to inexpensive production. We recently developed a novel multiepitope strategy to create antigens with customized specificity. Using this strategy, we designed two first generation multiepitope proteins, one specific for IgM (1) and the other specific for the IgG (2) class (2) of anti-DEN antibodies. In this work, we have developed a second generation antigen, rEDIII-T, with the potential to detect both anti-DEN virus IgM and IgG antibodies. This antigen was created by linking together the EDIIIs corresponding to the four DEN virus serotypes. Our choice of the EDIII as the precursor in creating this tetravalent molecule was dictated by several key properties, such as its emergence as a critical and unique antigenic region (4, 8, 9, 18, 24-26, 34, 37, 38, 40), its ability to recognize anti-DEN virus antibodies of both IgM and IgG classes (40), and its lack of significant cross-reactivity towards antibodies induced by heterologous flaviviruses (3, 33, 40).
To express the rEDIII-T antigen, we designed a gene encoding the EDIIIs of each of the four DEN virus serotypes linked by pentaglycyl linkers. It has been shown that in designing chimeric proteins, glycine is one of the preferred linker residues capable of conferring flexibility owing to its lack of a β-carbon (36). A 5′ six-His tag-encoding sequence was engineered into this gene, which was then overexpressed in E. coli using an IPTG-inducible promoter. A major proportion of the rEDIII-T antigen remained in the insoluble phase, as is often the case with several proteins overexpressed in E. coli. This necessitated its purification on Ni-NTA matrix under denaturing conditions. Analysis by SDS-PAGE revealed that this one-step affinity chromatography had resulted in >95% purity, with a yield of ∼30 mg/liter of E. coli culture. Consistent with its ability to bind the Ni-NTA matrix, the purified protein could be detected with the penta-His MAb in a Western blot. Importantly, it also manifested immunoreactivity towards both anti-DEN virus IgM and anti-DEN virus IgG in Western blot analysis. This observation is consistent with the report of Simmons et al. (40), led to the conclusion that the rEDIII-T antigen does indeed possess both IgM and IgG antibody-specific epitopes and suggested that this antigen has the potential to detect anti-DEN virus antibodies of both IgM and IgG classes in patient sera. Furthermore, our observation that JE virus+ sera did not manifest discernible reactivity to the rEDIII-T protein in the in-house ELISAs corroborated earlier reports that flavivirus antibodies do not cross-react with heterologous rEDIII proteins (3, 33, 40).
To ascertain if indeed the rEDIII-T antigen would recognize anti-DEN virus antibodies specific to each of the four DEN virus serotypes, we performed an ELISA experiment using a WHO reference serum panel. In this experiment, we tested the ability of the rEDIII-T antigen to interact separately with anti-DEN virus IgG antibodies specific to each DEN virus serotype. This experiment demonstrated clearly that anti-DEN virus IgG antibodies of each one of the four serotypes were indeed recognized efficiently by the rEDIII-T antigen. The lack of a corresponding reference panel (of serotype-specific anti-DEN virus IgM-containing sera) precluded experimental testing of the ability of the rEDIII-T antigen to recognize anti-DEN virus IgM antibodies of each of the four DEN virus serotypes as well. However, it seems reasonable to assume that rEDIII-T most likely possesses this ability. We base this on our observation that in our panel of 201 acute-phase patient sera, we did identify DEN-2 and DEN-3 virus-infected sera, which were IgM+ by the reference MAC-ELISA as well as our in-house IgM ELISA.
Next, we proceeded to evaluate the utility of the rEDIII-T protein as a DEN diagnostic antigen. A large panel of acute- and convalescent-phase sera (n = 289) from confirmed DEN and non-DEN patients was tested for anti-DEN virus antibodies using two well-established assays, the HI assay and the MAC-ELISA (43). The acute-phase sera were also tested for the presence of virus by mosquito cell culture and for viral RNA by RT-PCR (29). The performance of the rEDIII-T protein as a diagnostic antigen for the detection of anti-DEN virus antibodies was evaluated using these characterized sera. To this end, we designed in-house ELISAs using the rEDIII-T as the coating antigen to capture serum anti-DEN virus antibodies. Captured IgM and IgG antibodies were detected using anti-human IgM-HRPO and anti-human IgG-HRPO conjugates, respectively. The resultant data were compared to the data from the MAC-ELISA and HI assay. Of these two assays that served as our reference assays, the former is specific for anti-DEN virus IgM antibodies, whereas the latter measures both IgM and IgG classes of antibodies (42). Nevertheless, we have used the HI assay results as a reference for our in-house IgG ELISA results. This is because most of the sera were obtained from cases of secondary infection, in which it is well recognized that the anamnestic IgG response that occurs within a few days of infection (acute phase) results in high HI titers (≥2,560) in the convalescent phase. Furthermore, it has been demonstrated that anti-DEN virus IgG titers show a good correlation with HI titers (28).
We used panel 3 sera to define the cutoff absorbance values for our in-house ELISAs. Each serum in this panel was shown to be negative for the presence of DEN virus and anti-DEN virus antibodies by multiple criteria (Table 1). Furthermore, none of the 39 febrile patients from whom the panel 3 sera were collected clinically progressed to DF/DHF. Finally, convalescent-phase sera collected from these patients ∼2 weeks later were still seronegative for anti-DEN virus antibodies in the MAC-ELISA as well as HI assay. Clearly, this group constitutes confirmed non-DEN cases.
Out of the 162 DEN virus-confirmed sera, 136 tested identically in both the reference MAC-ELISA and the in-house IgM ELISA, with both assays scoring 89 sera as IgM+ and the rest as IgM− (n = 47). However, 26 of the DEN virus-confirmed sera gave discrepant results in the in-house IgM ELISA. The in-house IgM ELISA did not identify 13 sera that scored positive in the reference MAC-ELISA. This raises questions regarding the sensitivity of the rEDIII-T protein-based IgM ELISA. However, a closer examination of the ELISA data revealed that many of these sera represent borderline samples (Fig. 5A). It is likely that these discrepant sera, which presumably lack detectable levels of EDIII-specific anti-DEN virus IgM antibodies, apparently contain MAC-ELISA-reactive E antigen-specific anti-DEN virus IgM antibodies directed at epitopes outside domain III. Intriguingly, the remaining 13 discrepant sera, which were MAC-ELISA negative, turned out to be positive in the in-house IgM ELISA. But for a couple of borderline cases, the rest manifested significantly high ELISA reactivities (absorbance at 450 nm ranging from 1.47 to 5; n = 11) in the in-house IgM ELISA. It is unlikely that these are false positives. That the observed data for these samples do indeed reflect genuine DEN virus infection is apparent from the fact that a majority of these sera (9 of 11 samples) contained DEN virus RNA (see the supplemental material). Additionally, seven of these yielded infectious virus in mosquito cell culture. This indicated that these nine sera were indeed positive for anti-DEN virus IgM antibodies, leading to the suggestion that the reference MAC-ELISA kit had missed scoring these sera as IgM+. In contrast, convalescent-phase sera corresponding to these nine acute-phase samples scored positive in the MAC-ELISA (as well as by the in-house IgM ELISA). Clearly, the rEDIII-T antigen-based in-house IgM ELISA is superior to the commercial MAC-ELISA kit in detecting early seroconversion.
With regard to the utility of the rEDIII-T antigen in the detection of anti-DEN virus IgG antibodies, 87 of 162 DEN virus-confirmed sera displayed rEDIII-T antigen-specific anti-DEN virus IgG antibodies. This was corroborated for all of these sera by data from the HI assay (HI titers were predominantly >2,560). Fifty-six sera did not manifest the presence of anti-DEN virus IgG antibodies in the in-house assay, a result that was mirrored by insignificant HI titers (<20) for all of these 56 sera. As seen in the in-house IgM ELISA, we once again found a small number (n = 19) of sera that generated discrepant results in the in-house IgG ELISA with reference to the HI assay data. Ten discrepant sera, which were HI+, tested negative in the in-house IgG assay. These are borderline cases based on their low ELISA absorbance values (Fig. 5B). In contrast, eight of the remaining nine discrepant sera, which were HI−, displayed significant reactivity in the in-house IgG ELISA (with A450 values ranging from 0.75 to 5). One of these acute-phase sera, the only one out of this group for which a convalescent-phase serum was available, manifested the highest observed reactivity (Fig. 5B) in the in-house IgG ELISA but did not contain any anti-DEN virus IgM antibody as determined by both the reference and test assays. The corresponding convalescent-phase serum, however, manifested HI titers of >2,560, and this patient was designated as having a case of secondary DEN virus infection. Another sample from this group of nine discrepant sera, which was also IgM−, was also viremic. This is also presumably a case of secondary DEN virus infection. With regard to these two sera, it is likely that the presence of high anti-DEN virus IgG antibodies in the acute phase, as evidenced by significant in-house IgG ELISA reactivity, coupled to the lack of any discernible anti-DEN virus IgM antibodies (by both reference and in-house assays) perhaps represents the anamnestic IgG response, which had presumably preceded and overshadowed the IgM response. Interestingly, six of these nine sera were also IgM+ by both MAC-ELISA and the in-house IgM assay. The simultaneous detection of anti-DEN virus IgM and anti-DEN virus IgG antibodies in the acute phase indicates that all these sera represent secondary infections.
An overall assessment of the in-house ELISA data in relation to the reference assays shows that the rEDIII-T antigen possesses the potential for dual detection of both anti-DEN virus IgM and IgG antibodies in patient sera. While the sensitivity, specificity, and concordance were 86%, 89%, and 87%, respectively, for IgM detection, the corresponding values were 90%, 91%, and 91%, respectively, for IgG detection in acute-phase sera. Importantly, the rEDIII-T-based IgM antibody detection was more sensitive in identifying early seroconversion than the reference assay. In the convalescent phase, the performance of both of the in-house ELISAs was indistinguishable from that of the reference assays in terms of sensitivity, specificity, and concordance. Based on our data, efforts are under way to develop a rapid strip test for the simultaneous detection of both anti-DEN virus IgM and IgG antibodies. In conclusion, we believe that our approach of fusing all four EDIIIs to create a tetravalent recombinant antigen coupled to high-level expression and single-step purification has the potential to lead to the development of an ideal DEN diagnostic test suitable for use, particularly, in the resource-poor regions of DEN endemicity around the globe.
Supplementary Material
Acknowledgments
This work has been supported by institutional core funds. M.D.H. gratefully acknowledges the International Atomic Energy Agency for funding that enabled characterization of the sera in Table 1 (TC grant 06/024).
We thank Aravinda de Silva for the WHO DEN reference panel and Sunethra Gunesena, Medical Research Institute, Colombo, Sri Lanka, for help and guidance with the HI assay.
Footnotes
Published ahead of print on 26 September 2007.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.AnandaRao, R., S. Swaminathan, S. Fernando, A. M. Jana, and N. Khanna. 2006. A recombinant multiepitope protein for the early detection of dengue infections. Clin. Vaccine Immunol. 13:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AnandaRao, R., S. Swaminathan, S. Fernando, A. M. Jana, and N. Khanna. 2005. A custom-designed recombinant multiepitope protein as a dengue diagnostic reagent. Protein Expr. Purif. 41:136-141. [DOI] [PubMed] [Google Scholar]
- 3.Beasley, D. W. C., M. R. Holbrook, A. P. A. Travassos da Rosa, L. Coffey, A.-S. Carrara, K. Phillippi-Falkenstein, R. P. Bohm, Jr., M. S. Ratterree, K. M. Lillibridge, G. V. Ludwig, J. Estrada-Franco, S. C. Weaver, R. B. Tesh, R. E. Shope, and A. D. T. Barrett. 2004. Use of a recombinant envelope protein subunit antigen for specific serological diagnosis of West Nile virus infection. J. Clin. Microbiol. 42:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhardwaj, S., M. Holbrook, R. E. Shope, A. D. T. Barrett, and S. J. Watowich. 2001. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J. Virol. 75:4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundo, K., and A. Igarashi. 1985. Antibody capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J. Virol. Methods 11:15-22. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 7.Chadwick, D., B. Arch, A. Wilder-Smith, and N. Paton. 2006. Distinguishing dengue fever from other infections on the basis of simple clinical and laboratory features: application of logistic regression analysis. J. Clin. Virol. 35:147-153. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 9.Crill, W. D., and J. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuzzubbo, A. J., T. P. Endy, A. Nisalak, S. Kalyanarooj, D. W. Vaughn, S. A. Ogata, D. E. Clements, and P. L. Devine. 2001. Use of recombinant envelope proteins for serological diagnosis of dengue virus infection in an immunochromatographic assay. Clin. Diagn. Lab. Immunol. 8:1150-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George, R., and L. C. S. Lum. 1997. Clinical spectrum of dengue infection, p. 89-113. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 12.Gibbons, R. V., and D. W. Vaughn. 2002. Dengue: an escalating problem. Br. Med. J. 324:1563-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groen, J., P. Koraka, J. Velzing, C. Copra, and A. D. M. E. Osterhaus. 2000. Evaluation of six immunoassays for detection of dengue virus-specific immunoglobulin M and G antibodies. Clin. Diagn. Lab. Immunol. 7:867-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubler, D. J. 2004. Cities spawn epidemic dengue viruses. Nat. Med. 10:129-130. [DOI] [PubMed] [Google Scholar]
- 16.Guha-Sapir, D., and B. Schimmer. 2005. Dengue fever: new paradigms for a changing epidemiology. Emerg. Themes Epidemiol. 2:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guzmán, M. G., and G. Kouri. 2002. Dengue: an update. Lancet Infect. Dis. 2:33-42. [DOI] [PubMed] [Google Scholar]
- 18.Hung, J.-J., M.-T. Hsieh, M.-J. Young, C.-L. Kao, C.-C. King, and W. Chang. 2004. An external loop region of domain III of dengue virus type 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 78:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ibrahim, F., M. N. Taib, W. A. B. Wan Abas, C. C. Guan, and S. Sulaiman. 2005. A novel dengue fever (DF) and dengue haemorrhagic fever (DHF) analysis using artificial neural network (ANN). Comput. Methods Programs Biomed. 79:273-281. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi, A. 1997. Impact of dengue virus infection and its control. FEMS Immunol. Med. Microbiol. 18:291-300. [DOI] [PubMed] [Google Scholar]
- 21.Innis, B. L. 1997. Antibody responses to dengue virus infection, p. 221-243. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 22.Jaiswal, S., N. Khanna, and S. Swaminathan. 2004. High-level expression and one-step purification of recombinant dengue virus type 2 envelope domain III protein in Escherichia coli. Protein Expr. Purif. 33:80-91. [DOI] [PubMed] [Google Scholar]
- 23.Kao, C.-L., C.-C. King, D.-Y. Chao, H.-L. Wu, and G.-J. J. Chang. 2005. Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J. Microbiol. Immunol. Infect. 38:5-16. [PubMed] [Google Scholar]
- 24.Khanam, S., B. Etemad, N. Khanna, and S. Swaminathan. 2006. A bivalent antigen composed of linked envelope domains III of two dengue virus serotypes elicits neutralizing antibodies specific to both constituent serotypes. Am. J. Trop. Med. Hyg. 74:266-277. [PubMed] [Google Scholar]
- 25.Khanam, S., P. Rajendra, N. Khanna, and S. Swaminathan. 2007. An adenovirus prime/plasmid boost strategy for induction of equipotent immune responses to two dengue virus serotypes. BMC Biotechnol. 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanam, S., N. Khanna, and S. Swaminathan. 2006. Induction of antibodies and T cell responses by dengue virus type 2 envelope domain III encoded by plasmid and adenoviral vectors. Vaccine 24:6513-6525. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn, R. J., W. Zhang, M. G. Rossman, S. V. Pletnev, J. Corver, E. Lenches, C. T. Jones, S. Mukhopadhyay, P. R. Chipman, E. G. Strauss, T. S. Baker, and J. H. Strauss. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam, S. K., and P. L. Devine. 1998. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin. Diagn. Virol. 10:75-81. [DOI] [PubMed] [Google Scholar]
- 29.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G.-J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmer, K., O. D. Mantke, H. G. Bae, J. Groen, C. Drosten, and M. Niedrig. 2004. External quality control assessment in PCR diagnostics of dengue virus infections, J. Clin. Virol. 30:291-296. [DOI] [PubMed] [Google Scholar]
- 31.Lien, K.-Y., W.-C. Lee, H.-Y. Lei, and G.-B. Lee. 2007. Integrated reverse transcription polymerase chain reaction systems for virus detection. Biosens. Bioelectron. 22:1739-1748. [DOI] [PubMed] [Google Scholar]
- 32.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 33.Ludolfs, D., S. Schilling, J. Altenschmidt, and H. Schmitz. 2002. Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J. Clin. Microbiol. 40:4317-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mégret, F., J. P. Hugnot, A. Falconar, M. K. Gentry, D. M. Morens, J. M. Murray, J. J. Schlesinger, P. J. Wright, P. Young, M. H. V. van Regenmortel, and V. Deubel. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue envelope glycoprotein. Virology 187:480-491. [DOI] [PubMed] [Google Scholar]
- 35.Rigau-Perez, J. G., G. G. Clark, D. J. Gubler, P. Reiter, E. J. Sanders, and A. V. Vorndam. 1998. Dengue and dengue haemorrhagic fever. Lancet 352:971-977. [DOI] [PubMed] [Google Scholar]
- 36.Robinson, C. R., and R. T. Sauer. 1998. Optimizing the stability of single-chain proteins by linker length and composition mutagenesis. Proc. Natl. Acad. Sci. USA 95:5929-5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 38.Roehrig, J. T., A. J. Johnson, A. R. Hunt, R. A. Bolin, and M. C. Chu. 1990. Antibodies to dengue 2 virus E-glycoprotein synthetic peptides identify antigenic conformation. Virology 177:668-675. [DOI] [PubMed] [Google Scholar]
- 39.Shu, P.-Y., and J.-H. Huang. 2004. Current advances in dengue diagnosis. Clin. Diagn. Lab. Immunol. 11:642-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmons, M., K. R. Porter, J. Escamilla, R. Graham, D. M. Watts, K. H. Eckels, and C. G. Hayes. 1998. Evaluation of recombinant dengue viral envelope B domain protein antigens for the detection of dengue complex-specific antibodies. Am. J. Trop. Med. Hyg. 58:144-151. [DOI] [PubMed] [Google Scholar]
- 41.Vaughn, D. W., S. Greene, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, A. L. Rothman, F. A. Ennis, and A. Nisalak. 1997. Dengue in the early febrile phase: viremia and antibody responses. J. Infect. Dis. 176:322-330. [DOI] [PubMed] [Google Scholar]
- 42.Vorndam, V., and G. Kuno. 1997. Laboratory diagnosis of dengue virus infections, p. 313-333. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 43.World Health Organization. 1997. Dengue haemorrhagic fever: diagnosis, treatment, prevention and control, 2nd ed. World Health Organization, Geneva, Switzerland.
- 44.Zhang, Y., J. T. Bahns, Q. Jin, R. Divan, and L. Chen. 2006. Toward the detection of single virus particle in serum. Anal. Biochem. 356:161-170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.