Abstract
Leprosy is a chronic and debilitating human disease caused by infection with the Mycobacterium leprae bacillus. Despite the marked reduction in the number of registered worldwide leprosy cases as a result of the widespread use of multidrug therapy, the number of new cases detected each year remains relatively stable. This indicates that M. leprae is still being transmitted and that, without earlier diagnosis, M. leprae infection will continue to pose a health problem. Current diagnostic techniques, based on the appearance of clinical symptoms or of immunoglobulin M (IgM) antibodies that recognize the bacterial phenolic glycolipid I, are unable to reliably identify early-stage leprosy. In this study we examine the ability of IgG within leprosy patient sera to bind several M. leprae protein antigens. As expected, multibacillary leprosy patients provided stronger responses than paucibacillary leprosy patients. We demonstrate that the geographic locations of the patients can influence the antigens they recognize but that ML0405 and ML2331 are recognized by sera from diverse regions (the Philippines, coastal and central Brazil, and Japan). A fusion construct of these two proteins (designated leprosy IDRI diagnostic 1 [LID-1]) retained the diagnostic activity of the component antigens. Upon testing against a panel of prospective sera from individuals who developed leprosy, we determined that LID-1 was capable of diagnosing leprosy 6 to 8 months before the onset of clinical symptoms. A serological diagnostic test capable of identifying and allowing treatment of early-stage leprosy could reduce transmission, prevent functional disabilities and stigmatizing deformities, and facilitate leprosy eradication.
Cases in which Mycobacterium leprae infection manifests to cause leprosy present as a bacteriologic, clinical, immunologic, and pathological spectrum ranging from the extremes observed in paucibacillary (PB) and multibacillary (MB) patients (21, 24). PB patients have one or a few skin lesions and a low or absent bacterial index (BI; a measure of the number of acid-fast bacilli in the dermis, expressed on a logarithmic scale) and demonstrate specific cell-mediated immunity against M. leprae, but they have low or absent titers of M. leprae-specific antibodies and a granulomatous dermatopathology. In marked contrast, MB patients have multiple symmetric skin lesions and a high BI and demonstrate high titers of anti-M. leprae antibodies but an absence of specific cell-mediated immunity and a dermatopathology largely devoid of functional lymphocytes (21). Despite the implementation of a WHO-directed eradication program over the last 20 years, the worldwide annual rate of new case detection for leprosy remains stable at approximately 300,000 (17, 18, 26, 27). Earlier and objective diagnosis of leprosy could interrupt transmission and, in the long term, help further reduce the number of new cases and facilitate eradication.
There is no single diagnostic laboratory test for leprosy, and diagnosis remains essentially clinical. Clinical diagnosis of leprosy is dependent upon recognition of disease symptoms and is therefore only possible once the disease has manifested. WHO experts have listed diagnostic criteria as one or more of the following: hypopigmented or reddish skin patches with definite loss of sensation; thickened peripheral nerves; acid-fast bacilli on skin smears/biopsy specimens (WHO Expert Committee on Leprosy, 1998). Pure neuritic leprosy forms, however, present with no skin lesion. Confounding WHO's implementation of a global leprosy eradication strategy is that the number of trained leprologists has diminished. This is inadvertently increasing the likelihood that a clinical diagnosis is delayed or even missed, especially in regions where leprosy has been controlled (1, 13, 16, 25).
The presence of serum immunoglobulin M (IgM) antibody to phenolic glycolipid I (PGL-I) correlates with BI in leprosy patients and has been used to support disease symptoms as a means to categorize leprosy patients. Enzyme-linked immunosorbent assay (ELISA) and rapid lateral flow test formats have been developed for the detection of anti-PGL-I antibody (3, 4, 8, 19, 22, 23, 28). In one study, a lateral flow assay correctly diagnosed 97.4% of MB patients, with a specificity of 86.2% (4). Patients toward the PB end of the leprosy spectrum have low or no BI, however, and the majority of these patients are not identified by PGL-I-based tests (4, 7, 19). In addition, false-positive results in areas of endemicity are relatively high (>10%) (4, 7, 19). Consequently, none of these PGL-I-based tests has been widely implemented in field situations. In addition, many studies have demonstrated that MB patients have high titers of M. leprae-specific antibodies but PB patients have low or absent titers. For these reasons, the potential for serological diagnosis of low-BI patients, such as PB patients or MB patients who are developing disease, has not been thoroughly pursued.
In a recent small-scale study, we demonstrated that the ML0405 and ML2331 proteins were recognized by sera from MB leprosy patients presenting with high BI (20). In the current study we demonstrate that ML0405 and ML2331 are diagnostically relevant antigens by analyzing a large panel of MB leprosy patient sera from a variety of leprosy-affected regions (the Philippines, central and coastal Brazil, and Japan). We also examine the ability of M. leprae protein antigens to diagnose low-BI leprosy (PB patients and early MB patients) and show here the diagnostic potential of ML0405, ML2331, and a newly discovered M. leprae antigen, ML1556c. Based on the results, we construct and evaluate a fusion protein comprising ML0405 and ML2331 (designated leprosy IDRI diagnostic 1 [LID-1]) and demonstrate that this construct can be used to serologically diagnose leprosy patients among presymptomatic individuals, that is, before a clinical diagnosis is possible. Moreover, ML1556c may be a valuable adduct to LID-1 for the diagnosis of PB leprosy.
MATERIALS AND METHODS
Subjects and samples.
Sera were obtained from patients with leprosy (MB and PB) or tuberculosis (TB), healthy household contacts of MB leprosy patients (HHC), and endemic and nonendemic controls (EC and NEC). MB and PB leprosy patient sera used in this study were derived from recently diagnosed, previously untreated individuals who did not have signs of reversal reactions. Leprosy was classified in each case by bacterial, histological, and clinical observations carried out by qualified personnel, with the BI recorded at the time of diagnosis. HHC were defined as adults living in the same house as an MB index case for at least 6 months. TB patients were included to evaluate potential antigen cross-reactivity with other mycobacterial infection. Sera from TB patients were obtained after drawing blood from Mycobacterium tuberculosis sputum-positive, human immunodeficiency virus-negative individuals with clinically confirmed pulmonary TB who were undergoing treatment. Normal sera (EC and NEC) were obtained after blood draws from volunteers with no history of leprosy or TB infection. In all cases, drawing of blood was carried out with informed consent (with local institutional review board approval or local ethics committee approval in Brazil, Japan, the Philippines, Seattle, and St. Louis). The composition of each study population is summarized in Table 1.
TABLE 1.
Study populations
| Site | Sample categorization (total no.) | BI (mean) | Sex ratioa | Mean age (yr) (range) |
|---|---|---|---|---|
| Cebu City, | MB (17) | 2.8 | 2.4 | 30 (18-55) |
| Philippines | PB (54) | 0.5 | 0.4 | 31 (15-45) |
| TB (6) | 5 | 45 (35-53) | ||
| EC (8) | 1 | 26 (19-38) | ||
| HHC (10) | 0.4 | 38 (18-60) | ||
| Goiânia, Brazil | MB (28) | 2.4 | 1.5 | 44 (19-81) |
| PB (83) | 0 | 0.4 | 33 (7-76) | |
| TB (26) | 2.7 | 39 (17-66) | ||
| EC (30) | 0.1 | 20 (19-26) | ||
| HHC (11) | 0.5 | 28 (18-51) | ||
| Salvador, Brazil | MB (10) | NAb | 3.5 | 35.1 (20-70) |
| PB (6) | 0 | 5 | 31.6 (12-42) | |
| HHC (11) | 0.1 | 48.5 (25-57) | ||
| Kagawa, Japan | MB (30) | NA | NA | 60 (48-79) |
| PB (30) | 0 | NA | 70 (55-90) | |
| EC (26) | NA | 54 (48-62) |
Male/female ratio.
NA, not available.
In Cebu City, leprosy and TB patients were recruited at the Cebu skin clinic and Leonard Wood Memorial Research Center in Cebu City, Cebu (Philippines) from 2003 to 2006. Between 1985 and 1991, sera were collected prospectively from individuals who resided with MB patients (BI > 2) for at least 2 years and were free of leprosy as determined by clinical dermato-neurological examination at the inclusion point of the study. Some of these individuals developed MB leprosy as the study progressed, and these sera have previously been described (11).
In Goiânia, the state capital of Goiás State (western central Brazil), leprosy and TB patients were recruited at the main outpatient clinics of Centro de Referência em Diagnóstico e Terapêutica and Hospital Anuar Auad in 2006. PB leprosy patients were selected from a cohort of leprosy patients with a single skin lesion recruited at Brazilian sites of endemicity from 1999 to 2001, as previously described (9).
In Salvador, the state capital of Bahia State (northeast coastal Brazil), leprosy patients were recruited at Hospital Dom Rodrigo de Menezes in 2006.
In Japan, leprosy patients were recruited at the National Sanatorium Oshimaseishoen, Kagawa.
In St. Louis, sera were collected from U.S.-based individuals at a variety of times following Mycobacterium bovis BCG immunization.
All serum specimens were aliquoted and stored at −20°C or −80°C prior to assay.
Cloning and purification of target antigens.
DNA encoding selected M. leprae proteins was PCR amplified from M. leprae Thai-53 genomic DNA using Pfx DNA polymerase (Invitrogen, Carlsbad, CA). PCR primers were designed to incorporate specific restriction enzyme sites 5′ and 3′ of the gene of interest and excluded in the target gene for directional cloning into the expression vector pET28a (Novagen, Madison, WI). After PCR amplification, purified PCR products were digested, ligated with vector DNA, and used to transform Escherichia coli, and individual clones were induced to produce recombinant proteins, as previously described (20). Recombinant proteins were quantified using the bicinchoninic acid protein assay (Pierce, Rockford, IL), and quality was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The characteristics of each M. leprae protein evaluated are summarized in Table 2. The ML1556c protein was included because portions of the ML1556 protein were identified in four separate clones during serological expression screening with sera from PB leprosy patients (data not shown) (20). Recognition of the clones was derived from amino acids 58 to 256 of ML1556, which are only 47% identical to the M. tuberculosis protein Rv2839 (compared to 82% identity across the entire amino acid sequences of ML1556 and Rv2839).
TABLE 2.
Main characteristics of M. leprae antigens testeda
| Gene accession no. | Functional classificationb | Protein type | Length (bp) | Product size (kDa) | % Identityc with:
|
||||
|---|---|---|---|---|---|---|---|---|---|
| M. tuberculosis H37Rve | M. bovis AF2122/97e | M. avium 104d | M. marinum ATCC BAA-535e | M. smegmatis MC2 155d | |||||
| ML0091 | II.C.2 | 28-kDa antigen precursor | 711 | 23.7 | 53 | 53 | 54 | 54 | 48 |
| ML0405 | V | Conserved hypothetical | 765 | 25.3 | 62 | 62 | None | NA | None |
| ML1633 | II.C.2 | Possible secreted hydrolase | 1,608 | 57.0 | 25 | 25 | 35 | 81 | 62 |
| ML2055 | IV.A | Probable cell surface protein | 864 | 29.5 | 72 | 72 | 69 | 73 | 54 |
| ML2331 | II.C.2 | Possible secreted protein | 771 | 26.5 | 80 | 80 | 77 | 80 | 67 |
| ML2346 | VI | Hypothetical | 906 | 33.9 | None | None | None | None | None |
| ML1556 | II.A.6 | Translation initiation factor | 2,775 | 96.6 | 84 | 82 | 90 | ||
Annotations for gene accession number, functional classification, and protein type are according to the Sanger database.
Functional classifications: II.C.2, surface polysaccharides, lipopolysaccharides, proteins, and antigens; V, conserved hypotheticals; IV.A, virulence; VI, unknowns; II.A.6, protein translation and modification.
BLAST reports were performed in September 2006; tBLASTn was used for comparisons of proteins versus translated DNA. NA, not applicable.
From http://www.tigr.org.
Determining patient reactivity by ELISA.
ELISAs were conducted independently at IDRI, Seattle, WA (Cebu and St. Louis sera); UFG, Goiânia, and UFB, Salvador, Brazil; and NIID, Tokyo, Japan. Polysorp 96-well plates (Nunc, Rochester, NY) were coated with 1 μg/ml recombinant protein or 200 ng/ml of natural disaccharide with octyl linkage (NDO), the synthetically derived B-cell epitope of PGL-I, conjugated to bovine serum albumin (NDO-BSA; kindly supplied by John Spencer, Colorado State University, under NIH contract N01 AI-25469), in bicarbonate buffer overnight at 4°C and blocked for 1 h at room temperature with phosphate-buffered saline-Tween with 1% BSA on a plate shaker. Serum diluted appropriately in 0.1% BSA was added to each well, and plates were incubated at room temperature for 2 h with shaking. Plates were washed with buffer only, and horseradish peroxidase-conjugated IgG or IgM (Rockland Immunochemicals, Gilbertsville, PA), diluted in 0.1% BSA, was added to each well and incubated at room temperature for 1 h with shaking. After washing, plates were developed with peroxidase color substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the reaction was quenched by the addition of 1 N H2SO4. The optical density (OD) of each well was read at 450 nm. Positive responses were defined as an OD of >2× the mean OD of endemic control sera or an OD of >0.1, whichever was higher.
Statistics.
P values were determined using Student's t test.
RESULTS
Recognition of M. leprae proteins by Filipino leprosy patient sera.
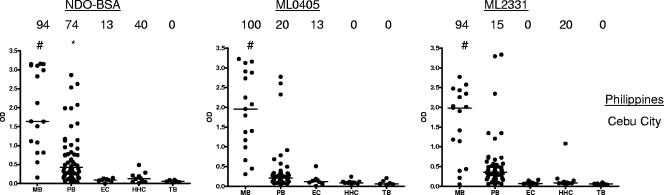
The majority of MB leprosy patients are readily identified by ELISA and lateral flow tests, which assess the capacity of patient IgM to bind M. leprae PGL-I or its synthetic analogue (NDO) conjugated to a carrier protein (BSA). In comparison with MB leprosy patients, PB leprosy patients have low or no anti-PGL-I responses and are more difficult to diagnose serologically. We therefore sought to determine whether PB sera recognized protein antigens, expanding our previous analyses and comparing the potential of NDO-BSA, ML0405, and ML2331 to diagnose leprosy, and found that the protein antigens have a similar profile for leprosy diagnosis as that for NDO-BSA; all three test antigens were readily detected by MB patient sera, by some PB patient sera, and by few, if any, EC, HHC, or TB sera (Fig. 1). Thus, similar to NDO-BSA, ML0405 and ML2331 demonstrate good potentials for the diagnosis of leprosy.
FIG. 1.
Sera from Filipino leprosy patients react with recombinant M. leprae antigens. Sera from clinically diagnosed MB and PB leprosy patients, EC individuals, and HHC of MB leprosy patients were assessed against NDO-BSA, ML0405, and ML2331. NDO-BSA reactivity was assessed by IgM binding, and protein reactivity was assessed by IgG binding. Sera were from Cebu City, Philippines. Each point represents an individual serum sample, and the median is represented by the line. The number above each data set is the percent positive responses. *, P < 0.05; #, P < 0.001 versus EC.
Recognition of MB leprosy patient sera with refined ML0405 antigen constructs.
To learn more regarding the seroreactivity of ML0405 and enhance recombinant ML0405 expression for purification, we expressed a variety of ML0405 polypeptide fragments and determined whether Filipino MB leprosy patient sera had similar binding capacities to these fragments and to full-length (ML0405FL) protein. All constructs were able to bind MB patient sera (Fig. 2) (P < 0.01 for MB versus EC). The reactivity of a truncated form (ML0405Tr) of the protein was equivalent to the reactivity of ML0405FL (P = 0.885 for MB patient sera), whereas the reactivity of the protein construct lacking the predicted membrane-spanning region (ML0405Tm) declined slightly (Fig. 2) (P = 0.047 and 0.060 for Tm versus FL and Tr forms, respectively, for MB). These data indicate that the majority, if not all, of the B-cell epitopes recognized by antibodies in patient sera are retained and accessible in the truncated form of the protein. Further testing was conducted using either ML0405FL or ML0405Tr.
FIG. 2.
ML0405 constructs react with MB leprosy patient sera. Different ML0405 constructs were created and expressed as recombinant proteins. The schematic diagram shows the sequence alignment of each of these constructs, with the deleted regions indicated by the line. Each construct was tested for IgG reactivity by ELISA with individual Filipino MB leprosy patient sera (n = 18) or EC sera (n = 6). *, P < 0.05; #, P < 0.001 versus EC.
Diagnosis of Filipino PB leprosy patients with M. leprae proteins.
We then went on to more closely investigate the potential of M. leprae antigens for diagnosing PB leprosy. Sera from Filipino patients clinically diagnosed with PB leprosy and with a low BI were tested for reactivity with potential diagnostic M. leprae antigens (ML0405Tr, ML2331, ML1556c, and NDO-BSA). NDO-BSA was capable of identifying 57% (26 of 46) of these Filipino PB leprosy patients, but a substantial number of samples provided weak positive responses (Fig. 3). ML0405 and ML2331 also reacted with sera from some PB patients (Fig. 3A and B). Most of these Filipino sera that reacted with these proteins also demonstrated strong NDO-BSA responses, however, and so the added benefit of using these antigens for leprosy diagnosis within the Filipino population appeared minimal. In contrast, 4 of 20 sera that were weak positive/negative by NDO-BSA ELISA testing demonstrated strong reactivity to ML1556c (Fig. 3C). This result suggests that ML1556c may be useful as an adjunct to PGL-I testing, or other tests, to improve the sensitivity and clarity of leprosy diagnosis.
FIG. 3.
M. leprae proteins react with PB leprosy patient sera. (A to C) Antibody reactivities of sera from a pool of clinically diagnosed MB leprosy patients, from a pool of negative control individuals, and from 46 clinically diagnosed PB leprosy patients were assessed against NDO-BSA and ML0405 (A), ML2331 (B), and ML1556c (C). NDO-BSA reactivity was assessed by IgM binding and, for reference, is shown in each plot. Recombinant protein reactivity was assessed by IgG binding. The first open circle represents the value obtained for pooled MB sera, while the next open circle represents the reactivity of pooled EC sera; individual PB sera are then arranged along the x axis according to their responsiveness versus NDO-BSA. The dashed line indicates the point at which diagnosis by NDO-BSA reactivity becomes unclear. ML1556c reacts with PB leprosy patient sera. (D) IgG reactivities of ML1556c with a small panel of individual sera from EC, leprosy patients (MB and PB), and TB patients were determined by ELISA using samples from Cebu City, Philippines. Each point represents an individual serum sample, and the median is represented by the line.
To test the specificity of ML1556c as a leprosy diagnostic reagent, we directly compared the reactivities of ML1556c with sera from PB leprosy patients, MB leprosy patients, TB patients, EC, and HHC of MB leprosy patients located in Cebu City, Philippines (Fig. 3D). Positive responses were observed in five of eight additional PB leprosy sera tested, with three of the sera yielding strong responses that could provide a clear diagnosis. Positive responses to ML1556c were also observed in two of seven MB leprosy sera tested in this experiment. ML1556c did not react with any of the Filipino TB patient sera tested, was recognized by only one of eight HHC sera, and reacted with only one of six EC sera. Negative results were obtained upon further testing involving another 45 TB sera and 23 NEC sera (data not shown). Taken together, these results generated from sera from the Philippines suggested the utility of ML1556c to improve the diagnosis of PB leprosy.
Identification of leprosy patients in Brazil.
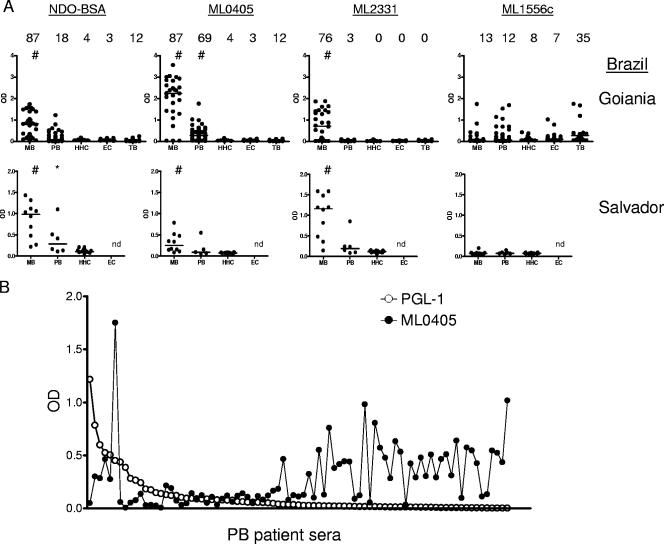
We also examined the ability of recombinant M. leprae antigens to identify leprosy patients located around Goiânia, Brazil, and Salvador, Brazil. Within the clinically diagnosed leprosy population, PGL-I/NDO-BSA was capable of identifying 87% (33 of 38) of the MB patients (Fig. 4). In agreement with the results obtained by analysis of Filipino leprosy patient sera, ML0405 and ML2331 reacted with large proportions of Brazilian MB patient sera (87% [33 of 38] and 76% [29 of 38], respectively), and ML1556c reacted with only some MB patient sera (13%, 5 of 38) (Fig. 4). In Goiânia, positive responses were also observed against antigens ML0091 (71%, 20 of 28), ML1633 (32%, 9 of 28), ML2055 (75%, 21 of 28), and ML2346 (29%, 8 of 28) (data not shown). The clarity of MB leprosy diagnosis (strength of signal in positive samples versus negative samples) in Goiânia was greater when using ML0405 rather than NDO-BSA, but in Salvador it was greater when using ML2331 rather than NDO-BSA.
FIG. 4.
Sera from Brazilian leprosy patients react with recombinant M. leprae antigens. Sera from clinically diagnosed MB and PB leprosy patients, EC individuals, and HHC of MB leprosy patients were assessed against NDO-BSA, ML0405, ML2331, and ML1556c. NDO-BSA reactivity was assessed by IgM binding, and protein reactivity was assessed by IgG binding. Sera were from Goiânia and Salvador (see Table 1). (A) Each point represents an individual serum sample, and the median is represented by the line. The number above each data set is the percent positive responses. *, P < 0.05; #, P < 0.001 versus EC. (B) To demonstrate complementarity, the individual PB sera from Goiânia are arranged along the x axis according to their responsiveness versus NDO-BSA and overlaid with the response of each serum to ML0405.
We also determined if these antigens were recognized by Brazilian PB patient sera. PGL-I/NDO-BSA was capable of identifying only 20% (18 of 89) of the PB patients, a level not appreciably higher than the proportion of positive responses observed with TB patients (12%, 3 of 26) (Fig. 4A). An IgG reactivity that permitted serologic diagnosis of an increased number of PB leprosy patients was observed for ML0405 (69%, 61 of 89), but responses to ML2331 were very weak, with very few positives (3%, 3 of 89) (Fig. 4A). The antigens ML0091 (6%, 5 of 83), ML1633 (17%, 14 of 83), ML2055 (13%, 11 of 83), and ML2346 (27%, 22 of 83) were recognized by some PB patient sera, but responses were generally weak (data not shown). Many of the PB patient sera that did not react with PGL-1 had a strong reactivity with ML0405 (Fig. 4B). ML1556c was recognized by only a minor subset of PB leprosy patient sera (12%, 11 of 89) and Brazilian EC individuals (6.7%, 2 of 30), but ML1556c reactivity was detected in a substantial number of Brazilian TB patients (35%, 9 of 26). These data indicate only a minor number of positive results in the Brazilian population if ML1556c is used for leprosy diagnosis, with a further complication of false-positive diagnosis in TB patients. Antigen ML0405, however, did not react with significant numbers of EC sera (3.3%, 1 of 30) or TB sera (12%, 3 of 26) (Fig. 4A). These results indicate that ML0405 can recognize some PB leprosy patients in the Brazilian population and could be used to augment leprosy diagnosis with PGL-1.
Construction of a fusion construct of ML0405-ML2331 (LID-1).
Having extended our earlier observation that the single antigens ML0405 and ML2331 have the potential to diagnose leprosy (20), and given the observations that ML0405 appeared better for diagnosis in Goiânia and Cebu City but ML2331 appeared better for diagnosis in Salvador, we constructed a single fusion molecule incorporating both proteins. ML0405Tr was expressed at the C terminus of the molecule and ML2331 in the N terminus. Following recombinant expression, we validated the reactivity of the construct by assaying LID-1 versus a small panel of sera from Salvador that had bound each single component. These sera readily detected LID-1, ML0405FL, ML0405Tr, and ML2331 (Fig. 5A). Importantly, construction of the fusion protein did not introduce false-positive results with NEC sera (Fig. 5A).
FIG. 5.
LID-1 retains reactivity with leprosy patient sera. (A) LID-1 (a fusion construct of ML0405 and ML2331), ML0405FL, ML0405Tr, and ML2331 reactivities were assessed by IgG binding in an ELISA with eight MB leprosy patient serum samples from Salvador and eight NEC serum samples. (B) Sera from clinically diagnosed Japanese MB and PB leprosy patients, and Japanese EC individuals, were assessed for IgG reactivities with LID-1, ML0405, and ML2331. Each point represents an individual serum sample, and the median is represented by the line. The number above each data set is the percent positive responses. *, P < 0.05; #, P < 0.001 versus EC.
We further extended our examination of sera from different geographic locations by assessing sera from Japanese leprosy patients for reactivity with ML0405, ML2331, and LID-1. Positive response were observed with MB patient sera (67% [20 of 30] for ML0405, 97% [29 of 30] for ML2331, and 87% [26 of 30] for LID-1) and PB patient sera (13% [4 of 30] for ML0405, 13% [4 of 30] for ML2331, and 20% [6 of 30] for LID-1), with few responses in EC sera (4% [1 of 26] for ML0405, 8% [2 of 26] for ML2331, and 4% [1 of 26] for LID-1) (Fig. 5B). Taken together, these data indicate that LID-1 is useful as a diagnostic antigen for leprosy.
LID-1 reactivity can diagnose leprosy before clinical symptoms.
Having demonstrated that the LID-1 fusion molecule retained the ability to diagnose leprosy patients but lacked responses to EC sera, we obtained sera from a prospective study conducted in Cebu City, Philippines, between 1985 and 1991 (11). In that study, household contacts of leprosy patients were monitored over a prolonged period of time, and some developed clinical MB leprosy. In sera from the individuals who developed MB leprosy, as previously reported, anti-PGL-I levels increased before leprosy was diagnosed by clinical exam (Fig. 6A). Our data also indicate that anti-LID-1 antibody levels began to increase markedly as soon as 1 year prior to clinical diagnosis (Fig. 6A). For many of the patients (7 of 11, 64%) the increase in the anti-LID-1 IgG response was strikingly more obvious than the increase in the anti-PGL-I IgM response (Fig. 6B). Those patients that developed clinical leprosy had anti-PGL-1 antibody levels not dissimilar to many individuals who did not develop leprosy (Fig. 6C). The difference in anti-LID-1 antibody levels was much clearer, with a much larger differentiation between the positive responses of patients who developed leprosy compared with the extremely low levels of anti-LID-1 antibody in individuals who did not develop leprosy (Fig. 6C). Taken together, these data indicate that LID-1 is capable of providing an early serological diagnosis of leprosy.
FIG. 6.
LID-1 reactivity can diagnose leprosy before clinical symptoms. (A) LID-1 and NDO-BSA reactivities within sera from a prospective study conducted in Cebu City, Philippines, were assessed by either IgG or IgM binding in an ELISA. Sera were collected at a variety of times prior to the clinical diagnosis of MB leprosy in 11 patients and at a variety of times after the commencement of treatment. (B) Representative plots for individual patients are shown. (C) Sera were collected from 57 household contacts that did not develop clinical leprosy and were compared with single serum samples from each individual contact that developed leprosy (serum samples were collected within 3 months of clinical diagnosis). #, P < 0.001. (D) LID-1 and NDO-BSA reactivities within sera from a prospective study using 10 U.S.-based individuals who were immunized with BCG were assessed. Sera were collected at regular intervals following BCG immunization. The solid circle at day zero designates the reactivity of a leprosy patient serum sample that was included as a positive control.
LID-1 does not react with sera from individuals recently exposed to BCG.
To examine in detail if leprosy diagnosis could be complicated by exposure to or infection with other mycobacteria, we also examined sera collected longitudinally from 10 U.S.-based individuals who were immunized with BCG. None of these BCG-immunized individuals developed positive serological responses against LID-1 or NDO-BSA (Fig. 6D). These data indicate that LID-1 can provide a clear diagnosis of M. leprae infection prior to the onset of signs that permit clinical leprosy diagnosis and that LID-1-based diagnostic tests could be used to expedite leprosy treatment.
DISCUSSION
Current diagnosis of leprosy is based on the appearance of clinical signs, and it is well established that the earlier a patient is identified the better their response to treatment. In addition, MB leprosy patient household contacts have a higher risk of developing clinical leprosy than contacts of PB leprosy patients (10, 12). This has been attributed to increased shedding and spreading of viable bacteria by MB leprosy patients (2). Accurate and early detection of M. leprae-infected individuals will open the possibility of earlier treatment that could both prevent disability and significantly reduce leprosy transmission.
We have evaluated the serological responses to a variety of M. leprae protein antigens in an attempt to discover antigens that can improve diagnosis of leprosy by detecting patients with a low BI (PB leprosy patients or early MB leprosy patients). We demonstrated that (i) ML0405 and ML2331 can be used to diagnose MB leprosy patients independently of geographic location; (ii) ML1556c can recognize some PB patients (although it is recognized by some TB sera as well); (iii) ML0405 and ML2331 can be used for diagnosis of some PB patients; (iv) a fusion construct of ML0405 and ML2331 (LID-1) retains diagnostic capability; and (v) LID-1 can provide a clear leprosy diagnosis before the onset of clinical symptoms. These findings will improve both leprosy diagnosis and patient care.
One approach for the early detection of M. leprae infection is through serological diagnosis. We have conducted screening to identify M. leprae antigens that have not previously been described, and we then evaluated the diagnostic potential of these antigens with leprosy patient sera. In this study, the diagnostic potential of select antigens was assessed in clinically disparate leprosy patient groups, ranging from MB patients who presented with large bacterial burdens and large skin lesions to PB patients who presented with low or absent bacterial burdens and a few, small skin lesions. As expected, MB leprosy patients were easier to identify by serological assays and typically yielded higher responses than PB patients. Unexpectedly, close examination of patients with a low BI from the Philippines indicated that some patients exhibited strong responses against the ML1556c protein. The responses of Filipino PB patients to ML1556c were often greater than those of MB patients. These results suggested the utility of this protein either as an adjunct to antigens that could identify MB patients to provide a cross-spectrum leprosy diagnosis or as a stand-alone protein for PB leprosy diagnosis. An objective and differential diagnosis of MB or PB leprosy could lead to better treatment of patients by guiding the multidrug therapy regimen provided to them.
We also analyzed the diagnostic potential of each antigen within geographically disparate groups of patients, from the Philippines and two sites in Brazil. In the Brazilian (Goiânia) PB leprosy patient group, ML1556c provided only a few positive responses; this dampened the enthusiasm for ML1556c to be a widely used diagnostic or prognostic leprosy antigen. Of interest, many PB leprosy patients in Brazil (both Goiânia and Salvador) could be diagnosed by ML0405 reactivity, and several PB patients (Salvador) could be diagnosed with ML2331 reactivity. It is unclear if the differences in the responses of patients from different geographic locations are related to differences in M. leprae strains or to regional variations in host genetics. These possibilities might be addressed by analysis of patient sera on fragments of ML1556c or by a survey of anti-ML1556c antibody on lysates of different M. leprae strains. Regardless, the observed differences indicate the importance of examining antigen-specific responses in several regions when considering their ability to diagnose leprosy globally.
Given that the ML0405Tr and ML2331 proteins could provide diagnosis of leprosy, we made a fusion protein (LID-1) of these individual components. After ensuring the fusion protein retained reactivity against leprosy sera from Salvador, Brazil, we tested the antigens against sera from Japan. As with results obtained using sera from Brazil, Japanese MB leprosy patient sera reacted as strongly with the fusion LID-1 as with the ML0405 and ML2331 components. In addition, some Japanese PB leprosy patient serum antibodies recognized these antigens.
Studies have argued that the presence of anti-PGL-I antibodies is an indicator of leprosy development, but this has been debated (5, 6, 14, 15). Many contacts of leprosy patients have anti-PGL-I antibodies but do not develop disease, limiting the capacity of PGL-I-based assays to predict disease development. Indeed, PGL-I-based tests are typically marketed as a support reagent to confirm clinical diagnosis and aid leprosy classification but are not recommended for use as a stand-alone for diagnosis (19). The differential in responses of sera from contacts that developed leprosy compared with contacts that did not develop leprosy was much greater for LID-1 than PGL-1. We demonstrated that LID-1 is capable of providing an early serological diagnosis of MB leprosy. A clear and early diagnosis was achieved in 7 of 11 contacts of leprosy patients who themselves went on to develop clinical leprosy. For the small panel of sera tested, the time benefit of a LID-1-based diagnosis over a clinical-based diagnosis was 6 to 8 months. Thus, screening for LID-1-reactive antibodies, either in the general population or within more focused at-risk populations, could significantly expedite treatment of leprosy patients and, also, affect transmission rates by reducing the number of individuals who develop large bacterial burdens. As another benefit, antibody levels against LID-1 dropped following the implementation of drug treatment in these individuals, and thus the reduction and disappearance of antibodies against LID-1 may be a useful measure of multidrug therapy efficacy.
We are currently evaluating additional antigens, diagnostic formats, and different geographic sources of patient sera with the objective of early and simple identification of leprosy patients regardless of incidence locality.
Acknowledgments
The manuscript is dedicated to John Dawson, without whom this work would not have been possible. We thank Randy Howard and Karen Cowgill for critical reading of the manuscript.
This work was supported by the American Leprosy Missions (S.R.) and the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (grant A20509) (M.M.A.S.).
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Anderson, H., B. Stryjewska, B. L. Boyanton, and M. R. Schwartz. 2007. Hansen disease in the United States in the 21st century: a review of the literature. Arch. Pathol. Lab. Med. 131:982-986. [DOI] [PubMed] [Google Scholar]
- 2.Bakker, M. I., M. Hatta, A. Kwenang, P. Van Mosseveld, W. R. Faber, P. R. Klatser, and L. Oskam. 2006. Risk factors for developing leprosy—a population-based cohort study in Indonesia. Lepr. Rev. 77:48-61. [PubMed] [Google Scholar]
- 3.Buhrer, S. S., H. L. Smits, G. C. Gussenhoven, C. W. van Ingen, and P. R. Klatser. 1998. A simple dipstick assay for the detection of antibodies to phenolic glycolipid-I of Mycobacterium leprae. Am. J. Trop. Med. Hyg. 58:133-136. [DOI] [PubMed] [Google Scholar]
- 4.Buhrer-Sekula, S., H. L. Smits, G. C. Gussenhoven, J. van Leeuwen, S. Amador, T. Fujiwara, P. R. Klatser, and L. Oskam. 2003. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J. Clin. Microbiol. 41:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cartel, J. L., S. Chanteau, J. P. Boutin, R. Plichart, P. Richez, J. F. Roux, and J. H. Grosset. 1990. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. Other Mycobact. Dis. 58:512-517. [PubMed] [Google Scholar]
- 6.Chanteau, S., P. Glaziou, C. Plichart, P. Luquiaud, R. Plichart, J. F. Faucher, and J. L. Cartel. 1993. Low predictive value of PGL-I serology for the early diagnosis of leprosy in family contacts: results of a 10-year prospective field study in French Polynesia. Int. J. Lepr. Other Mycobact. Dis. 61:533-541. [PubMed] [Google Scholar]
- 7.Cho, S. N., R. V. Cellona, L. G. Villahermosa, T. T. Fajardo, Jr., M. V. Balagon, R. M. Abalos, E. V. Tan, G. P. Walsh, J. D. Kim, and P. J. Brennan. 2001. Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin. Diagn. Lab. Immunol. 8:138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho, S. N., D. L. Yanagihara, S. W. Hunter, R. H. Gelber, and P. J. Brennan. 1983. Serological specificity of phenolic glycolipid I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41:1077-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa, M. B., P. F. Cavalcanti Neto, C. M. Martelli, M. M. Stefani, J. P. Maceira, M. K. Gomes, A. P. Schettini, P. F. Rebello, P. E. Pignataro, E. S. Ueda, K. Narahashi, and D. M. Scollard. 2001. Distinct histopathological patterns in single lesion leprosy patients treated with single dose therapy (ROM) in the Brazilian Multicentric Study. Int. J. Lepr. Other Mycobact. Dis. 69:177-186. [PubMed] [Google Scholar]
- 10.Deps, P. D., B. V. Guedes, J. Bucker Filho, M. K. Andreatta, R. S. Marcari, and L. C. Rodrigues. 2006. Characteristics of known leprosy contact in a high endemic area in Brazil. Lepr. Rev. 77:34-40. [PubMed] [Google Scholar]
- 11.Douglas, J. T., R. V. Cellona, T. T. Fajardo, Jr., R. M. Abalos, M. V. Balagon, and P. R. Klatser. 2004. Prospective study of serological conversion as a risk factor for development of leprosy among household contacts. Clin. Diagn. Lab. Immunol. 11:897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine, P. E., J. A. Sterne, J. M. Ponnighaus, L. Bliss, J. Saui, A. Chihana, M. Munthali, and D. K. Warndorff. 1997. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am. J. Epidemiol. 146:91-102. [DOI] [PubMed] [Google Scholar]
- 13.Flower, C., D. Gaskin, and S. Marquez. 2007. A case of recurrent rash and leg numbness mimicking systemic rheumatic disease: the occurrence of leprosy in a nonendemic area. J. Clin. Rheumatol. 13:143-145. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Abreu, E., J. A. Pon, P. Hernadez, J. Rodriguez, E. Mendoza, M. Hernandez, E. Cuevas, and A. B. Gonzalez. 1996. Serological reactivity to a synthetic analog of phenolic glycolipid I and early detection of leprosy in an area of low endemicity. Lepr. Rev. 67:4-12. [DOI] [PubMed] [Google Scholar]
- 15.Hussain, R., S. Jamil, A. Kifayet, F. Firdausi, H. M. Dockrell, S. Lucas, and R. Hasan. 1990. Quantitation of IgM antibodies to the M. leprae synthetic disaccharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. Other Mycobact. Dis. 58:491-502. [PubMed] [Google Scholar]
- 16.Lockwood, D. N., and A. J. Reid. 2001. The diagnosis of leprosy is delayed in the United Kingdom. QJM 94:207-212. [DOI] [PubMed] [Google Scholar]
- 17.Lockwood, D. N., and S. Suneetha. 2005. Leprosy: too complex a disease for a simple elimination paradigm. Bull. W. H. O. 83:230-235. [PMC free article] [PubMed] [Google Scholar]
- 18.Meima, A., J. H. Richardus, and J. D. Habbema. 2004. Trends in leprosy case detection worldwide since 1985. Lepr. Rev. 75:19-33. [PubMed] [Google Scholar]
- 19.Oskam, L., E. Slim, and S. Buhrer-Sekula. 2003. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr. Rev. 74:196-205. [PubMed] [Google Scholar]
- 20.Reece, S. T., G. Ireton, R. Mohamath, J. Guderian, W. Goto, R. Gelber, N. Groathouse, J. Spencer, P. Brennan, and S. G. Reed. 2006. ML0405 and ML2331 are antigens of Mycobacterium leprae with potential for diagnosis of leprosy. Clin. Vaccine Immunol. 13:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 22.Roche, P. W., W. J. Britton, S. S. Failbus, D. Williams, H. M. Pradhan, and W. J. Theuvenet. 1990. Operational value of serological measurements in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. Other Mycobact. Dis. 58:480-490. [PubMed] [Google Scholar]
- 23.Roche, P. W., S. S. Failbus, W. J. Britton, and R. Cole. 1999. Rapid method for diagnosis of leprosy by measurements of antibodies to the M. leprae 35-kDa protein: comparison with PGL-I antibodies detected by ELISA and “dipstick” methods. Int. J. Lepr. Other Mycobact. Dis. 67:279-286. [PubMed] [Google Scholar]
- 24.Scollard, D. M. 2004. Classification of leprosy: a full color spectrum, or black and white? Int. J. Lepr. Other Mycobact. Dis. 72:166-168. [DOI] [PubMed] [Google Scholar]
- 25.Van Buynder, P., J. Eccleston, J. Leese, and D. N. Lockwood. 1999. Leprosy in England and Wales. Commun. Dis. Public Health 2:119-121. [PubMed] [Google Scholar]
- 26.WHO. 2005. Global leprosy situation, 2005. Wkly. Epidemiol. Rec. 80:289-295. [PubMed] [Google Scholar]
- 27.WHO. 2007. Global leprosy situation, 2007. Wkly. Epidemiol. Rec. 82:225-232. [PubMed] [Google Scholar]
- 28.Young, D. B., and T. M. Buchanan. 1983. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221:1057-1059. [DOI] [PubMed] [Google Scholar]