Abstract
The development of new protein subunit vaccines has stimulated the search for improved adjuvants to replace traditional aluminum-containing products. We investigated the adjuvant effects of a synthetic Toll-like receptor 4 (TLR4) agonist on vaccine efficacy in an experimental model of toxic shock syndrome. The TLR4 agonist E6020 has a simplified structure consisting of a hexa-acylated acyclic backbone. The vaccine examined is a recombinantly attenuated form of staphylococcal enterotoxin B (STEBVax). Using cells stably transfected with TLRs, E6020 transduced signals only through TLR4, suggesting monospecificity, while Escherichia coli 055:B5 lipopolysaccharide activated both the TLR2/6 heterodimer and TLR4. Coadministration of E6020 with STEBVax, by the intramuscular or intranasal route, induced significant levels of immunoglobulin G (IgG) in BALB/c mice. Further, increased IgG production resulted from the combination of E6020 with aluminum hydroxide adjuvant (AH). The antibody response to the vaccine coadministered with E6020 was a mixed Th1/Th2 response, as opposed to the Th2-biased response obtained with AH. Mice vaccinated with STEBVax coadministered with AH, TLR4 agonists, or a combination of both adjuvants were protected from toxic shock. Our data demonstrate the effectiveness of the synthetic TLR4 agonist E6020 as an alternative adjuvant for protein subunit vaccines that may also be used in combination with traditional aluminum-containing adjuvants.
The development of new recombinant protein vaccines has produced a new requirement for the development of alternative adjuvants. Only adjuvants that contain aluminum are approved by the FDA for use with human vaccines. However, aluminum-containing adjuvants are not fully compatible with the storage conditions and the preferred biological effects of recombinant protein vaccines. For example, formulation with aluminum-containing adjuvants may lead to a loss in vaccine immunogenicity (3) following freezing or lyophilization, often requiring storage of vaccines as refrigerated suspensions in order to maintain stability. Further, aluminum-containing adjuvants are limited to stimulating Th2-biased immune responses, which may be inappropriate for the control of certain diseases.
It is likely that many biological effects of adjuvants are linked to the activity of Toll-like receptors (TLRs). The TLRs are primary components of the innate immune system that recognize pathogen-associated molecular patterns present on bacterial, fungal, or viral pathogens, but limited in host cells (14, 15). Signal transduction through TLRs is mediated either by sequential recruitment of MyD88 (myeloid differentiation factor 88), IRAK (interleukin-1 [IL-1] receptor-associated kinases), and TRAF6 (tumor necrosis factor alpha [TNF-α] receptor-associated factor 6) or by a MyD88-independent TRIF (TIR domain-containing adapter inducing beta interferon) pathway, followed by activation of NF-κβ and mitogen-activated protein kinases (18). Signal transduction pathways activated by TLR agonists regulate antigen-producing cell (APC) function and production of cytokines and chemokines (10, 12, 24). Thus, the characteristics of TLR agonists suggest that these activators of innate immunity may be exploited as potential adjuvants.
Cell wall lipopolysaccharides (LPS) of gram-negative bacteria are a class of pathogen-associated molecules that were first observed to be associated with acute inflammatory responses (4). Subsequent studies from a number of laboratories demonstrated that the inflammatory response to LPS directly involved TLR4 (2, 19). Although signaling can occur through TLR4 alone, the fully competent LPS receptor is composed of TLR4, CD14, and MD2 (7). While LPS is a potent TLR4 agonist, the toxicity profile of the natural product precludes its use in humans. To circumvent the undesirable features of LPS, we evaluated a novel synthetic TLR4 agonist, E6020, as a stand-alone adjuvant or in combination with aluminum-containing adjuvants. Unlike LPS, E6020 is chemically well defined, has a promising safety profile based on investigations with animal models (20), and has a single mechanism of action. Structurally, E6020 consists of a simple hexa-acylated acyclic backbone, which allows for a more direct preparation of high-purity material than other synthetic TLR4 agonists (8).
We performed these studies in a mouse model of toxic shock by using the vaccine STEBVax, a recombinant protein derivative of staphylococcal enterotoxin B (SEB). Previous reports indicate that aluminum-containing adjuvants significantly enhance the immune response to STEBVax (1). The vaccine incorporates three site-specific mutations in a hydrophobic binding loop, a polar binding pocket, and a disulfide loop of SEB, which collectively alter key protein surfaces, leading to loss of receptor binding (23). Staphylococcal enterotoxins (SEs) are superantigens expressed by most isolates of the common human pathogen Staphylococcus aureus (5, 21). These secreted proteins bind to T-cell receptors and major histocompatibility complex class II molecules, stabilizing interactions that lead to potent activation of T cells. All SEs share a common binding surface for interacting with major histocompatibility complex class II molecules, with additional stabilizing interactions that are unique to each toxin (22). In addition, each SE preferentially stimulates T cells bearing distinct Vβ subsets of antigen receptors, causing a generalized release of proinflammatory cytokines and disruption of innate and adaptive immunity. Life-threatening toxic shock syndrome results from the rapid release of high levels of gamma interferon, IL-6, TNF-α, and other cytokines in response to SEs. Due to the loss of receptor binding, STEBVax does not harbor any superantigen-like biological activity yet maintains protective immunologic epitopes (1).
MATERIALS AND METHODS
Vaccine components.
The recombinant SEB vaccine B899445C (STEBVax) was prepared under current GMP conditions, as described elsewhere (23). Solvent-extracted LPS (E. coli 0111:B4) that retains only TLR4 agonist activity was obtained from InvivoGen (San Diego, CA). The synthetically produced TLR4 agonist E6020 was obtained from the Eisai Research Institute (Wilmington, MA). E6020 is equivalent to ER-804057 due to the Eisai Research Institute's internal development numbering system.
In vitro evaluation of TLR4 agonists.
To determine whether E6020 and Escherichia coli 055:B5 LPS (Difco, Detroit, MI) could signal through TLR4 or TLR2-TLR6 (TLR2/6), HEK293 cells transfected with either TLR4, MD2/CD14, TLR4/MD2/CD14, or TLR2/6 were obtained from InvivoGen (San Diego, CA). The cells were thawed and allowed to grow for 3 days at 37°C in Dulbecco's modified Eagle medium with 10% fetal calf serum and PenStrep. Cells were then transfected with the pNIFTY plasmid containing the secreted embryonic alkaline phosphatase gene. The next day, the cells were treated for 3 days with twofold dilutions of agonist; the top concentration was 130 μg/ml. The amount of secreted embryonic alkaline phosphatase was determined using the Great EscAPe kit (Becton Dickinson, Franklin Lakes, NJ).
The abilities of E6020 and wild-type LPS to activate NF-κB and stimulate the production of TNF-α, IL-10, IL-4, and IL-5 in human CD14+ monocytes were compared. CD14+ monocytes were isolated by positive selection with magnetic beads (Miltenyi Biotec, Auburn, CA) from peripheral blood mononuclear cells separated from blood over Ficoll. Following the isolation of CD14+ monocytes, 7 × 106 cells were transferred in RPMI medium with 5% human AB sera to 15-ml conical polypropylene tubes and allowed to rest for 2 h at 37°C under 5% CO2. Cells were then treated for either 1 or 12 h with the medium, 10 ng of LPS, or 10 ng of E6020. NF-κB activation was measured by the TransAM assay (Active Motif, Carlsbad, CA). Cytokine levels were measured by a cytometric bead array (Becton Dickinson, Franklin Lakes, NJ).
Vaccine immunogenicity and efficacy.
Male BALB/c mice were obtained for this study. Mice were administered three doses of 20 μg STEBVax either alone or with an adjuvant. The adjuvant used was either an aluminum hydroxide adjuvant (AH) (140 μg/dose), solvent-extracted LPS (20 μg/dose), E6020 (20 μg/dose), AH with LPS, or AH with E6020. The vaccinations were given 3 weeks apart by intramuscular (i.m.) injection or intranasal (i.n.) instillation. For i.m. administration, mice were injected with 25 μl of vaccine in the thigh muscle of each hind leg. For i.n. administration, mice were first anesthetized with a mixture of ketamine, acepromazine, and xylazine. The vaccine was administered by instilling 15 μl into each nostril with a micropipette. Serum samples were collected 2 weeks after the first two vaccine administrations and 1 week after the final dose. Two weeks after the final dose, the mice were challenged intraperitoneally (i.p.) with 20 50% lethal doses (LD50) of SEB, followed 4 h later with 40 μg E. coli 055:B5 LPS to potentiate the response. The animals were observed for 72 h following challenge, and the number of survivors was recorded.
The immunogenicities of the various vaccine formulations were evaluated by determining the concentrations of immunoglobulin M (IgM), total IgG, IgG1, and IgG2a in serum by enzyme-linked immunosorbent assays. Plates were coated with 100 μl of serially diluted purified mouse IgM or IgG in phosphate-buffered saline (PBS) (pH 7.4) for the standard curves and with 100 μl of 2-μg/ml SEB in PBS (pH 7.4) for the unknowns. Plates were incubated for 1 h at 37°C and then washed three times with PBS-0.1% Tween 20. The plates were then blocked for 1 h at 37°C with 100 μl of 0.2% casein in PBS (pH 7.4). Following a wash as described above, 100 μl of each sample was added in triplicate to the appropriate wells, and the plates were incubated for 1 h at 37°C. The plates were washed as described above, and 100 μl of a detection antibody (anti-mouse IgM, IgG, IgG1, or IgG2a conjugated to horseradish peroxidase) was added to the plate and incubated for 1 h at 37°C. Next, the plates were washed as described above, 100 μl of tetramethylbenzidine working reagent was added to the wells, and the plates were incubated for 30 min at room temperature. The absorbance at 650 nm of each well was then measured with a plate reader. To determine the serum concentrations, the absorbance for the unknowns was compared to that for the standard curve.
RESULTS
TLR4 signaling and induction of cytokine release by E6020 are equivalent to those by native LPS.
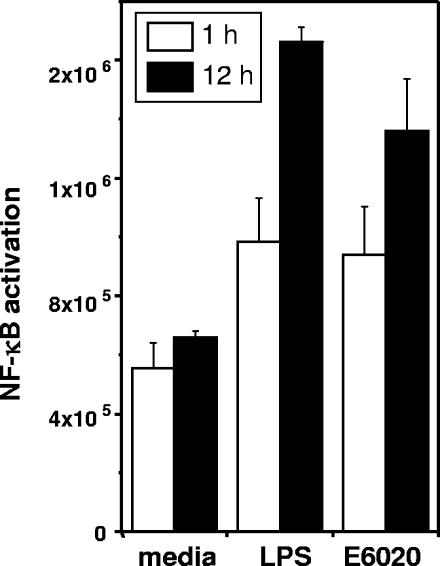
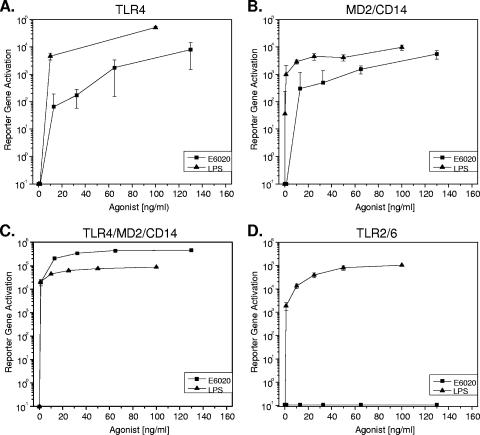
To compare the biological activity of E6020 to that of native LPS, we first examined the activation of the master regulatory gene NF-κB. E. coli 0111:B4 LPS was solvent extracted to remove TLR2-stimulatory factors (9). We isolated CD14+ monocytes from human blood and measured NF-κB activation following treatment with E6020 or LPS (Fig. 1). These results indicated that 1 or 12 h of treatment with E6020 or LPS equivalently stimulated NF-κB activation. To further investigate the minimal cell receptor requirements for signaling, we examined responses to E6020 and nonextracted E. coli 055:B5 LPS by HEK cells stably transfected with combinations of the LPS receptors TLR4, MD2, and CD14. Both wild-type LPS from E. coli 055:B5 and E6020 induced NF-κB reporter gene activation in cells transfected with TLR4, MD2/CD14, or the complete receptor complex comprising TLR4, MD2, and CD14 (Fig. 2A to C). In contrast, only the nonextracted E. coli 055:B5 LPS induced signaling in HEK cells transfected with the TLR2/6 heterodimer (Fig. 2D). These results suggested that E6020 required only TLR4 for signaling and did not exhibit cross-reactivity with TLR2/6, while the agonist activity of E. coli 055:B5 LPS was not specific to TLR4.
FIG. 1.
NF-κB activation in CD14+ monocytes is equivalently enhanced following treatment with a natural or a synthetic TLR4 agonist. Monocytes were isolated and treated either with solvent-extracted LPS, which stimulates only TLR4, or with E6020. Levels of NF-κB activation were measured at 1 and 12 h following treatment. Activation of NF-κB is expressed in relative light units, presented as arithmetic means. Error bars, standard deviations.
FIG. 2.
The synthetic TLR4 agonist E6020 exhibits dose-dependent signaling through the TLR4 receptor complex and does not cross-react with TLR2/6. HEK293 cells transfected with TLR4 (A), MD2/CD14 (B), TLR4/MD2/CD14 (C), or TLR2/6 (D) were treated with various concentrations of E. coli 055:B5 LPS or E6020, and secretion of the reporter gene (human placental alkaline phosphatase) was measured after 3 days. NF-κB-dependent activation of the reporter gene is expressed in relative light units.
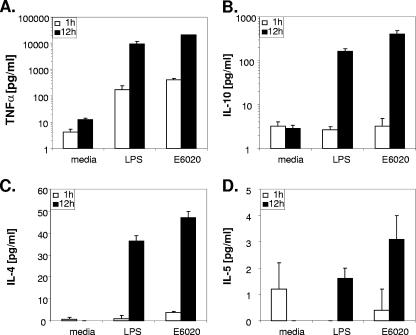
Because early TLR4 activation events with the natural versus the synthetic TLR4 agonist were indistinguishable, we next investigated cytokine production in order to detect potential differences in the induction of these downstream immunomodulators. Human CD14+ monocytes were incubated with the TLR4 agonists, and cytokines released into the cell culture medium were measured at multiple time points. Levels of TNF-α produced by CD14+ monocytes were elevated by 1 h (P < 0.05 for comparison to the untreated control) following treatment with either the natural or the synthetic TLR4 agonist and were further increased by 12 h of culture (Fig. 3A). Significant levels of IL-10 and IL-4 were observed for both treatments at 12 h (Fig. 3B and C). Elevated, but not significant, production of IL-5 was induced in CD14+ monocytes by either agonist following 12 h of culture (Fig. 3D). Activation levels of NF-κB (Fig. 1) corresponded closely with production of proinflammatory, inhibitory, and Th2-enhancing cytokines for both E6020 and LPS.
FIG. 3.
Production of TNF-α, IL-10, and IL-4 was stimulated in CD14+ monocytes following treatment with a natural or a synthetic TLR4 agonist. Monocytes were isolated and treated either with solvent-extracted LPS, which stimulates only TLR4, or with E6020. Levels of TNF-α (A), IL-10 (B), IL-4 (C), and IL-5 (D) were measured at 1 and 12 h following treatment. Data are presented as arithmetic means. Error bars, standard deviations. Significant increases (P < 0.05) in TNF-α, IL-10, and IL-4 levels were observed by 12 h (and by 1 h for TNF-α) with both treatments.
Enhancement of serum IgG and IgM levels by synthetic and natural TLR4 agonists.
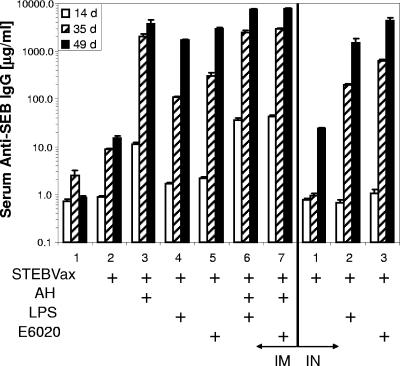
To determine the potential enhancing effects of TLR4 agonists on vaccine immunity, groups of 10 BALB/c mice were immunized (on days 0, 21, and 42) and sera were collected for measurement of antigen-specific antibodies. Administration of STEBVax with AH or a TLR4 agonist by the i.m. route resulted in a significant increase (P < 0.05) in SEB-specific IgG levels versus levels with the saline control at every time point of serum collection after vaccination (Fig. 4). Results with i.n. administration demonstrated significant increases in IgG levels following the first boost with STEBVax and either the synthetic or the natural TLR4 agonist (Fig. 4). By day 49, mice administered STEBVax with E6020 i.n. maintained significantly higher (P = 0.028) serum IgG concentrations than mice receiving the same vaccine i.m. Further, animals administered STEBVax with LPS by either the i.m. or the i.n. route displayed equivalent (P = 0.377) serum IgG concentrations, though these results may have been dose dependent. A combination of AH and a TLR4 agonist coadministered i.m. with the vaccine also resulted in significant IgG responses following primary vaccination, in comparison to those for the vaccine with no adjuvant or for saline-only controls. These results suggested that the inclusion of AH with the TLR4 agonist did not inhibit serological responses to the vaccine.
FIG. 4.
Levels of SEB-specific IgG in serum were enhanced following i.m. or i.n. administration of various formulations of STEBVax. BALB/c mice were administered three doses of vaccine at 3-week intervals. Seroconversion occurred by day 14 for i.m. administration and by day 35 for i.n. administration.
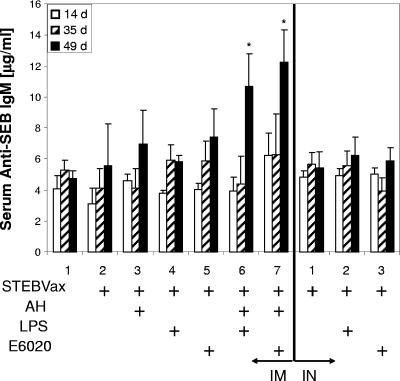
Although for most vaccines, IgM responses are likely to be significantly elevated only during the early phase of immunity, the pentavalent antibody may also contribute to protection from toxic shock. Therefore, we also examined serum IgM levels following coadministration of STEBVax with the synthetic or natural TLR4 agonist (Fig. 5). We noted that significant levels of antigen-specific IgM were evident following the secondary boost with the vaccine in combination with a mixture of AH and either TLR4 agonist. None of the other vaccine formulations exhibited significant increases in serum IgM levels at any of the time points regardless of the delivery route.
FIG. 5.
Mice administered STEBVax vaccine containing AH and a TLR4 agonist, either LPS or E6020, had significantly higher SEB-specific serum IgM levels on day 49, 1 week following the final vaccine administration. Asterisks indicate IgM concentrations significantly greater (P < 0.05) than those observed for the saline control.
Vaccination with STEBVax and a TLR4 agonist results in a mixed Th1/Th2 response.
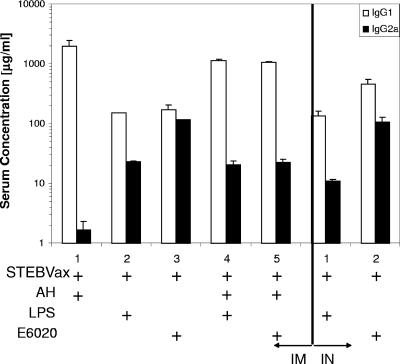
In general, development of a predominant Th1 or Th2 immune response may impact vaccine efficacy. Therefore, we analyzed serum antibody isotypes to determine the effects of TLR4 agonists on the type of immune response to STEBVax. Serum samples collected following the second boost with the vaccine were analyzed for levels of antigen-specific IgG1 and IgG2a. As expected, i.m. vaccination with STEBVax formulated with AH resulted in a strong Th2-biased response, and only IgG1 was produced at significant levels (Fig. 6). Vaccines that were coadministered with either LPS or E6020 stimulated mixed IgG1 and IgG2a production. However, the combination of AH with a TLR4 agonist resulted in an IgG1/IgG2a ratio that was skewed more toward a Th2 response than use of the TLR4 agonist alone. The IgG1/IgG2a ratio for vaccines containing LPS increased from 6.5 for LPS alone to 55 when LPS was combined with AH. Further, the IgG1/IgG2a ratio increased to 47 from a value of 1.5 for E6020 alone as a result of vaccination with a formulation containing E6020 in combination with AH. In contrast, the vaccine delivery route had only a minor effect on the ratio of IgG isotypes, regardless of the adjuvant used (Fig. 6).
FIG. 6.
Mice that received STEBVax vaccine containing a TLR4 agonist, either LPS or E6020, responded with a mixed Th1/Th2 response, as opposed to the Th2-biased response obtained with AH. Levels of SEB-specific IgG1 and IgG2a were determined by enzyme-linked immunosorbent assays for sera obtained on day 49, 1 week after the final vaccination.
TLR4 agonists and protection from SEB-induced toxic shock.
Previous studies established that the protection of vaccinated mice from SEB-induced toxic shock is antibody mediated. To determine if the antibody responses observed were protective against toxic shock, groups of 10 mice each were challenged with 20 LD50 of SEB 2 weeks after the final vaccine administration, and the number of survivors in each group was recorded 72 h postchallenge. All vaccinated animal groups had significantly greater (P < 0.05) numbers of survivors than the nonvaccinated control group and the group receiving no adjuvant (Table 1). Somewhat lower survival rates were observed for mice receiving the vaccine coadministered i.m. with LPS and AH and for mice administered vaccine i.n. with LPS. However, the survival rates for these groups were not significantly different from 100% (α = 0.05; P, 0.211 and 0.087, respectively).
TABLE 1.
TLR4 agonists and protection from SEB-induced toxic shocka
| Vaccination group | % Survivorsb with the following administration route:
|
|
|---|---|---|
| i.m. | i.n. | |
| Saline | 0 | NA |
| STEBVax only | 0 | 0 |
| STEBVax + AH | 90 | NA |
| STEBVax + LPS | 100 | 60 |
| STEBVax + E6020 | 100 | 90 |
| STEBVax + AH + LPS | 70 | NA |
| STEBVax + AH + E6020 | 100 | NA |
Vaccinated mice were protected from toxic shock following i.p. challenge with SEB. Groups of 10 BALB/c mice were immunized (on days 0, 21, and 42) by i.m. injection or i.n. instillation. Two weeks after the final dose, the mice were challenged i.p. with 20 LD50 of wild-type SEB, followed 4 h later by 40 μg of E. coli 055:B5 LPS to potentiate the response. The animals were observed for 72 h following challenge, and the numbers of survivors were recorded.
NA, not examined; the physical nature of AH precluded i.n. administration.
DISCUSSION
We investigated the adjuvant-like properties of the TLR4 agonists as potential substitutes for aluminum-containing products. Our results suggest that TLR4 agonists may be useful adjuvants for enhancing the immune response to protein subunit vaccines. While LPS is an excellent stimulator of the immune system, it is not suitable for vaccine formulations due to human toxicity and the variable potency that is associated with the heterogeneity of the natural product. The hexa-acylated, acyclic TLR4 agonist E6020 is a synthetic mimic of LPS that has many features in common with the naturally produced TLR4 agonist yet may circumvent the safety concerns associated with LPS (20). The biological activities of E6020 and LPS were nearly indistinguishable when they were used as adjuvants for STEBVax, though subtle differences in dose effects may be anticipated. Coadministration of either TLR4 agonist or AH with a vaccine by the i.m. or i.n. route stimulated robust immune responses that were protective against lethal toxic shock. In contrast to the Th2-biased response obtained with AH, the antibody response induced by E6020 and LPS was a mixed Th1/Th2 response. Vaccine formulations that favor Th1 or a mixed Th1/Th2 response may be useful for protection against certain diseases (6, 13, 17), suggesting that it may be advantageous to employ TLR4 agonists to preferentially direct the immune response.
In addition to the considerable adjuvant responses noted for the TLR4 agonists, combining E6020 or LPS with AH enhanced the antibody response to STEBVax above the levels obtained for vaccine formulations containing only a single adjuvant, and this effect was observed within 14 days from the initial vaccine administration. Although the precise mechanism is not presently known, in general the adsorption of proteins to AH facilitates the internalization of the complex by APCs. Because E6020 contains two phosphate groups, it is also capable of interacting with AH through a ligand exchange mechanism by which adsorption of phosphate groups displaces hydroxyls on the adjuvant surface to form a more stable complex with aluminum (11). Thus, adsorption concentrates the TLR4 agonist with the aluminum particles, resulting in enhanced uptake by APCs. Further, APCs that internalize the particles may receive a concentrated dose of the vaccine and TLR4 agonist, potentially resulting in more-efficient maturation of dendritic cells and enhanced presentation of antigen to T cells. The observed enhancement of immunity also suggests that dose sparing may be possible by development of vaccine formulations with combinations of adjuvants.
Immune adjuvants are required for optimal potency of most protein subunit vaccines, because individual proteins are generally poorly immunogenic. Though traditional aluminum-containing adjuvants are effective enhancers of immune responses, decreased potencies following freezing or lyophilization, Th2-biased responses, and incompatibility with i.n. administration have all driven the search for alternative adjuvants. The TLRs are expressed by many cells of the immune system and play an important role in linking innate and adaptive immunity (16, 25). We focused our adjuvant study on agonists to TLR4 because these receptors of the innate immune system signal through either MyD88-dependent or -independent pathways and appear to be especially robust immunopotentiators (10, 12). The synthetic agonist E6020 activated NF-κB and stimulated cytokine production in a time- and dose-dependent fashion that was similar to that for natural LPS (2). Cell signaling for both LPS and E6020 was transduced through TLR4 alone, through MD2/CD14, or TLR4/MD2/CD14 receptor complexes. In contrast, our data indicated that only E. coli 055:B5 LPS, not E6020, activated NF-κB through TLR2/6. The reduced toxicity of E6020 observed in previous reports (20) may be a consequence of this more-specific TLR signaling. Lipid stacking on the chemical scaffolding was noted to affect the molecular shape and hence the activity of the agonist by diminishing the receptor-binding affinity (20). Further, the lack of a sugar scaffold in the synthesis of E6020 did not adversely affect biological activity; the levels of cytokine production we observed were similar to those obtained with LPS.
We conclude that E6020, a chemically defined TLR4 agonist with a simplified structure, exhibits excellent potential to serve as an alternative to aluminum-containing adjuvants in the development of protein subunit vaccines. The synthetic TLR4 agonist has the advantage of homogeneity over natural LPS. Additional advantages of LPS and LPS-like agonists of TLR4 over AH formulations are the potential for delivery to nasal passages, stability during lyophilization, and stimulation of both Th1 and Th2 immunity. Our data also demonstrate the effectiveness of the synthetic TLR4 agonist E6020 in combination with traditional aluminum-containing adjuvants.
Acknowledgments
This research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals, and it adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). USAMRIID is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Garry L. Morefield was an associate of the National Research Council at the U.S. Army Medical Research Institute for Infectious Diseases.
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Boles, J. W., M. L. Pitt, R. D. LeClaire, P. H. Gibbs, E. Torres, B. Dyas, R. G. Ulrich, and S. Bavari. 2003. Generation of protective immunity by inactivated recombinant staphylococcal enterotoxin B vaccine in nonhuman primates and identification of correlates of immunity. Clin. Immunol. 108:51-59. [DOI] [PubMed] [Google Scholar]
- 2.Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. [DOI] [PubMed] [Google Scholar]
- 3.Corbel, M. J. 1996. Reasons for instability of bacterial vaccines. Dev. Biol. Stand. 87:113-124. [PubMed] [Google Scholar]
- 4.Fekety, F. R., Jr. 1969. Skin window studies of the effect of endotoxin upon the acute inflammatory response. Johns Hopkins Med. J. 124:291-295. [PubMed] [Google Scholar]
- 5.Fraser, J., V. Arcus, P. Kong, E. Baker, and T. Proft. 2000. Superantigens—powerful modifiers of the immune system. Mol. Med. Today 6:125-132. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh, A., W. W. Zhang, and G. Matlashewski. 2001. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 20:59-66. [DOI] [PubMed] [Google Scholar]
- 7.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins, L. D., S. T. Ishizaka, P. McGuinness, H. Zhang, W. Gavin, B. DeCosta, Z. Meng, H. Yang, M. Mullarkey, D. W. Young, H. Yang, D. P. Rossignol, A. Nault, J. Rose, M. Przetak, J. C. Chow, and F. Gusovsky. 2002. A novel class of endotoxin receptor agonists with simplified structure, toll-like receptor 4-dependent immunostimulatory action, and adjuvant activity. J. Pharmacol. Exp. Ther. 300:655-661. [DOI] [PubMed] [Google Scholar]
- 9.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 11.Jiang, D., G. L. Morefield, H. HogenEsch, and S. L. Hem. 2006. Relationship of adsorption mechanism of antigens by aluminum-containing adjuvants to in vitro elution in interstitial fluid. Vaccine 24:1665-1669. [DOI] [PubMed] [Google Scholar]
- 12.Kaisho, T., and S. Akira. 2003. Regulation of dendritic cell function through Toll-like receptors. Curr. Mol. Med. 3:373-385. [DOI] [PubMed] [Google Scholar]
- 13.Lagranderie, M., A. M. Balazuc, M. Abolhassani, P. Chavarot, M. A. Nahori, F. Thouron, G. Milon, and G. Marchal. 2002. Development of mixed Th1/Th2 type immune response and protection against Mycobacterium tuberculosis after rectal or subcutaneous immunization of newborn and adult mice with Mycobacterium bovis BCG. Scand. J. Immunol. 55:293-303. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 15.Medzhitov, R., and C. Janeway, Jr. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 8:452-456. [DOI] [PubMed] [Google Scholar]
- 16.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 17.Near, K. A., A. W. Stowers, D. Jankovic, and D. C. Kaslow. 2002. Improved immunogenicity and efficacy of the recombinant 19-kilodalton merozoite surface protein 1 by the addition of oligodeoxynucleotide and aluminum hydroxide gel in a murine malaria vaccine model. Infect. Immun. 70:692-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oda, K., and H. Kitano. 18 April 2006. A comprehensive map of the toll-like receptor signaling network. Mol. Syst. Biol. doi: 10.1038/msb4100057. [DOI] [PMC free article] [PubMed]
- 19.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 20.Przetak, M., J. Chow, H. Cheng, J. Rose, L. D. Hawkins, and S. T. Ishizaka. 2003. Novel synthetic LPS receptor agonists boost systemic and mucosal antibody responses in mice. Vaccine 21:961-970. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich, R. G., S. Bavari, and M. A. Olson. 1995. Bacterial superantigens in human disease: structure, function and diversity. Trends Microbiol. 3:463-468. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich, R. G., S. Bavari, and M. A. Olson. 1995. Staphylococcal enterotoxins A and B share a common structural motif for binding class II major histocompatibility complex molecules. Nat. Struct. Biol. 2:554-560. [DOI] [PubMed] [Google Scholar]
- 23.Ulrich, R. G., S. Bavari, and M. A. Olson. 1998. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine 16:1857-1864. [DOI] [PubMed] [Google Scholar]
- 24.Underhill, D. M. 2003. Toll-like receptors: networking for success. Eur. J. Immunol. 33:1767-1775. [DOI] [PubMed] [Google Scholar]
- 25.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]