Abstract
We used a severe challenge model that produces clinical West Nile virus (WNV) disease to test the efficacy of three commercially available equine WNV vaccines in horses. Twenty-four healthy, WNV-seronegative horses of varying ages and genders were placed, in random and blind manner, into three trial groups consisting of eight horses each; two horses in each group received (i) an inactivated WNV vaccine (K-WN), (ii) a modified-live vaccine (CP-WN) containing the WNV prM and E proteins expressed by a canarypox vector, (iii) a live-chimera vaccine (WN-FV) containing WNV prM and E proteins expressed in a YF17D vector, or (iv) a diluent control. Challenge by this model caused grave neurological signs, viremia, moderate to severe histopathologic lesions in the brain and spinal cord, and an outcome of 0% survivorship in all six control horses. In contrast, challenge in horses at between 28 days postvaccination with the chimera vaccine and 56 days postvaccination with the commercial inactivated or modified-live vaccine resulted in 100% survivorship (protection from the onset of WNV encephalitis and viremia). Horses vaccinated with the live-chimera vaccine showed significantly fewer clinical signs than did the control horses (P ≤ 0.01) and the horses vaccinated with inactivated vaccine (P = 0.035). Mild residual inflammatory lesions were seen in a few of the vaccinated horses.
West Nile virus (WNV) is a mosquito-borne flavivirus of the Japanese encephalitis virus serogroup that causes central nervous system (CNS) infection in horses and humans (11). Since its emergence in the United States in 1999 and subsequent spread throughout the country, WNV has caused clinical disease in 23,962 humans and 24,824 horses in the United States (1, 5), killing approximately 928 humans, over 6,000 horses, and an estimated hundreds of thousands of birds. Currently endemic throughout much of North America, WNV is an important infectious pathogen, with 4,085 clinical cases of disease reported for humans and 1,061 encephalomyelitis cases reported for horses in 2006 (1, 5).
Early epidemiological studies of the horse indicated that vaccines were the best strategy for preventing WNV infections (19). Since then, four WNV vaccines have been licensed for use in horses by the United States Department of Agriculture. The first licensed vaccine (K-WN) (West Nile-Innovator; Fort Dodge, Fort Dodge, IA) for horses has been available since 2001 and is composed of formalin-inactivated whole virus with adjuvant. A DNA plasmid vaccine was licensed in 2003, but it has not been available commercially (29, 31). In 2004, a recombinant vaccine (CP-WN) was licensed and consists of a canarypox virus vector with insertion and expression of the membrane (prM) and envelope (E) proteins of WNV genes (Recombitek equine WNV vaccine; Merial Limited, Athens, GA) (15). This preparation also contains an adjuvant. Several epidemiological studies investigating the outbreaks throughout the United States have justified the use of these commercially available vaccines as a preventative strategy (6, 8, 21, 23, 30).
The latest equine vaccine (granted licensure in September 2006) is a single-dose, attenuated West Nile virus, live flavivirus chimera vaccine (WN-FV) (PreveNile; Intervet, De Soto, KS) for horses and is marketed without an adjuvant. This vaccine was based on the technology used to create an attenuated human vaccine for Japanese encephalitis virus (ChimeriVax-JE) (10). The recombinant chimera expresses the E and prM proteins of WNV in a yellow fever vector (YF17D). The vaccine has been labeled for use in horses for the prevention of West Nile virus viremia and as an aid in the prevention of WNV disease and encephalitis.
Two previous WNV challenge models in horses (mosquito feeding and needle inoculation) were used to substantiate the primary efficacy studies and labeling claims of the K-WN and CP-WN vaccines. These two models mirror the asymptomatic-to-symptomatic field infection ratio of 11:1, and both models induce viremia, but neither is able to induce significant clinical signs of WNV in horses (4). Hence, in these models, protection is defined as “prevention of viremia due to WNV.” The development of a WNV challenge model of infection, where grave, reproducible encephalomyelitis occurred in all naïve horses when injected intrathecally with virulent WNV, has been described previously (3). This model was used to establish 28-day efficacy for the CP-WN vaccine and 12-month duration of immunity for the WNV-FV vaccine (14, 25). The current data report the use of the model to investigate the comparative efficacy of the three commercially available equine vaccines in a short-duration challenge trial.
MATERIALS AND METHODS
Animals.
All work with animals was performed under the approval and guidance of the University of Florida (Gainesville) Institutional Animal Care and Use Committee. Horses used were mixed breed male and female horses, with no previous WNV exposure, as indicated by WNV neutralization titers of ≤5. Standard practices for determinations of health status and for preventative medicine (diet monitoring, parasite and infectious disease prevention, and podiatry) were performed under the guidance of Animal Care Services, University of Florida. Values for rectal temperature, heart and respiratory rates, appetite, and attitude were recorded on a daily basis prior to the commencement of study protocols.
Efficacy trials.
Horses were placed, in a random and blind manner, into three trial groups consisting of eight horses, of which two horses in each group received (i) the inactivated K-WN vaccine, (ii) the canarypox virus vectored CP-WN vaccine, (iii) the chimera WN-FV vaccine, or (iv) a diluent. For K-WN and CP-WN, the horses received an initial primary intramuscular (IM) injection with a 1-ml volume of vaccine; this was followed in 28 days with a second injection according to the manufacturer's protocol. For the WN-FV, each horse received an initial IM injection of 1 ml diluent; this was followed in 28 days by a 1-ml IM injection of the vaccine. All work with live virulent WNV was performed in accordance with the guidelines of the Biosafety in Microbiological and Biomedical Laboratories (U.S. Department of Health and Human Services, Public Health Service/Centers for Disease Control and Prevention and National Institutes of Health 1999) under the approval and supervision of the University of Florida Environmental Health and Safety Office.
Virulent viral challenge.
The challenge virus, designated WNV NY99 (4132), was originally isolated from the brain of an infected crow (CDC, Ft. Collins, CO). This virus was passaged once in Vero cells, once in C6/36 mosquito cells, and one additional time in BHK-21 cells and cultured in 5% horse serum in order to avoid potential immune reactions to bovine serum proteins. All virus stocks were then stored in 1-ml aliquots at −80°C until use. On the day of challenge, the stock virus was thawed on ice and virus was diluted to the desired concentration in phosphate-buffered saline immediately prior to intrathecal inoculation of horses. The stock virus was prepared and aliquoted as stock in a single laboratory (by R. A. Bowen), and all challenges utilized the same material.
Horses were to be challenged with virulent virus more than 28 days (or day 56 [D56] of the study period) after the second vaccine injection according to the original study design. Trials with the three efficacy groups, CE1, CE2, and CE3, were conducted from July 2004 through April 2005, during which Gainesville, FL, was affected by three major hurricanes. Due to these circumstances, the actual day of challenge for CE1, CE2, and CE3 was D70, D56, and D70, respectively. Horses were anesthetized by the administration of xylazine (1.0 mg/kg of body weight, intravenously), and after 10 min, ketamine (2.2 mg/kg, intravenously) was administered for the induction of anesthesia. The atlanto-occipital space was penetrated with an 18-gauge, 20-cm spinal needle by using an aseptic technique and a 1-ml volume of virus.
Clinical scoring.
Physical evaluations were performed from days −1, 0, and 1 to study completion on D21 after challenge (D91, D77, and D91 of the study for CE1, CE2, and CE3, respectively). Daily parameters recorded included rectal temperature, pulse rate, and respiratory rate. Temperature was further categorized as a score (grade) of 0 if rectal temperature was >37.5 to 38.3°C (99.9 to 100.9°F), 1 if rectal temperature was >38.3 to 38.6°C (100.9 to 101.5°F), 2 if rectal temperature was >38.6 to 38.8°C (101.5 to 101.9°F), 3 if rectal temperature was >38.6 to 39.2°C (101.9 to 102.5°F), and 4 if rectal temperature was ≥39.2°C (≥102.5°F) or more. Changes in attitude, feed intake, hydration, and locomotion were scored as normal (0), mild (1), moderate (2), and severe (3). Injection sites were monitored daily throughout the study period. A full neurological examination was performed weekly after vaccination and daily after viral challenge until the end of the study. Signs of clinical neurological disease, consisting of changes in mentation, the development of ataxia and weakness, and the occurrence of fasciculations and cranial nerve paresis, were scored in severity as normal (0), mild (1), moderate (2), and severe (3). Normal was defined as no change from baseline examinations. Mild abnormalities were defined as detectable only with repeated movement or stimulation, depending on the site tested. Moderate abnormalities were defined as obvious, with limited movement or stimulation. Severe abnormalities were those that occurred spontaneously without movement or stimulation. The total daily clinical score was calculated with the sum of graded physical parameters and neurological signs.
Postmortem analysis.
At the end of the study (21 days postchallenge), an overdose of pentobarbital was administered to all remaining living horses to facilitate a full gross and histological postmortem examination.
A full gross postmortem evaluation was performed on all study horses. Tissue samples of brain (from the cerebrum, thalamus, midbrain, pons, and medulla), cervical spinal cord, lumbar spinal cord, left ventricular and right atrial heart muscle, liver, lung, spleen, kidney, mesenteric lymph node, adrenal gland, thymus, and injection site muscle were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin for histopathologic evaluation.
Histopathologic grading of neural tissue (from the cerebrum, thalamus, midbrain, pons, medulla, cervical cord, and lumbar cord) for evidence of viral encephalitis quantified both gliosis and perivascular cuffing. Tissues were evaluated by two independent and blinded evaluators. For scoring, 10 random views were examined at a magnification of ×100 for each location. The number of glial nodules at each site was documented and averaged, each section was reexamined, and the percentage of vessels with perivascular cuffing (vessels with accumulations of inflammatory cells) was estimated and averaged per site. Histopathologic scoring was based on methodology used by Monath et al (17). Grade 1 was defined as “minimal” change characterized by one to three small focal inflammatory infiltrates and a few glial nodules. Grade 2 was defined as “moderate” change, with more extensive focal inflammatory infiltrates and glial nodules centered around one-third of the neurons. Grade 3 was defined as “severe” change, with glial nodules affecting 33 to 90% of neurons and moderate focal or diffuse inflammatory changes. Grade 4 was defined as “overwhelming,” with more than 90% glial nodules and severe, widespread inflammatory infiltration.
Case criteria and outcome.
The normal range of rectal temperature in the horse was defined as 37.5 to 38.3°C (99.5 to 100.9°F), but a rectal temperature of ≥39.2°C (102.5°F) was the criterion defined for CNS disease caused by WNV in this model (14). Failure of a protective response against clinical disease (encephalomyelitis) caused by WNV was defined as moderate or severe signs of disease (a neurological score of ≥2) for two or more consecutive days, viremia, histopathologic changes consistent with encephalitis, and requirement for euthanasia. Case outcome was defined as either survival to the end of the study period or nonsurvival, which was euthanasia or sudden death. As defined by Institutional Animal Care and Use Committee protocols, euthanasia was performed on the horses when dictated by humane reasons: any overall severe health condition of the animal as a result of WNV infection or persistent or acute signs of West Nile virus disease coupled with recumbency and/or the inability to locomote without assistance.
Virus isolation.
One milliliter of plasma from heparinized blood was incubated per well in a 24-well plate format on Vero cells as described previously and incubated at 37°C in 5% CO2 for 5 to 7 days (14, 22). The plates were observed daily for toxicity, bacterial contamination, and cytopathic effects typical of WNV infection.
Antibody and virus neutralization testing.
A plaque reduction neutralization test was used to quantify neutralizing antibody titers in the horses, as previously described (4). Any sample that neutralized the challenge virus dose at a level of ≥70% was confirmed by testing in duplicate and titrated by serial twofold dilutions. Neutralization at a level of ≥90% was considered positive for each dilution. Antibody titers were defined as the reciprocal of the highest dilution showing 90% neutralization, and a titer of 5 or greater was considered positive for antibody to WNV.
Back titration of virulent West Nile virus.
For back titrations of material used to challenge horses, a PFU assay was performed using sample aliquots of challenge virus stock (prepared for intrathecal challenge) frozen at −80°C. Samples included challenge virus frozen immediately after preparation and replicate aliquots of virus that were maintained on ice until after actual intrathecal challenge. For each back titration assay, 300 μl of 10-fold dilutions of sample challenge material was added in triplicate to six-well cell plates containing Vero 76 monolayers. Plates were incubated for 1 h at 37°C and 5% CO2 for virus adsorption. The cells were overlaid with 3 ml per well of 0.5% agarose in minimal essential medium supplemented with 350 mg/liter of sodium bicarbonate, 29.2 mg/liter of l-glutamine, 5% heat-inactivated fetal bovine serum, and antibiotics. After 48 h of additional incubation, a second 3-ml 0.5% agarose overlay, containing 0.004% neutral red dye, was added for plaque visualization. The plaques were scored on D3, D4, and D5 of incubation. Dilution wells closest to 30 plaques per well were used to calculated the virus titer. Viral titer was calculated as PFU/ml = 1/dilution × number of plaques × amount of inoculum/well (2).
Statistics.
Clinical data from all three trials were combined as 2 by 2 contingency tables and analyzed using χ2 and Fisher's exact tests for case criteria, outcome, and viremia by using MedCalc software (version 8.1; Mariakerke, Belgium). The level of significance was set at a P value of <0.05. Clinical scores and histological data were analyzed using Kruskal-Wallis one-way analysis of variance on ranks with pairwise multiple comparison with Dunn's method. The level of significance was set at a P value of <0.05. Statistical analysis was performed by using a computer spreadsheet statistical analysis package (SigmaStat V; Systat Software, Inc., Point Richmond, CA).
RESULTS
Clinical scoring. (i) Postvaccination assessment.
No injection site reactions or systemic effects were observed in any of the horses postvaccination. Four of five horses infected with CP-WN (CP-WN horses) had increased rectal temperatures, ranging from 38.4 to 38.9°C (101.2 to 102°F), with durations of up to 5 days after the second vaccination booster. Three of six horses infected with K-WN (K-WN horses) had increased rectal temperatures of 38.4°C within 5 days after the second vaccination.
(ii) Postchallenge assessment.
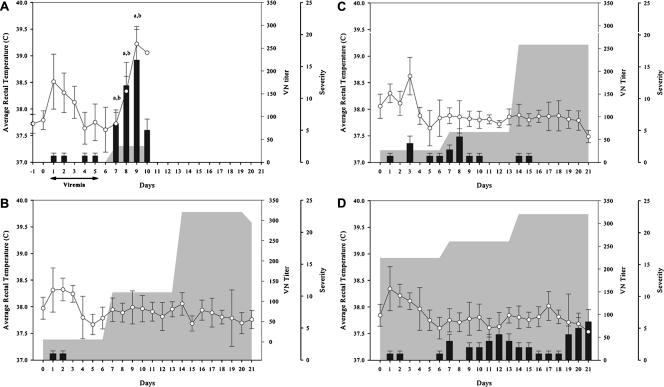
None of the horses infected with WN-FV (WN-FV horses) developed an increased rectal temperature of >39.2°C on any day postchallenge, while three of six control horses, one of five CP-WN horses, and one of six K-WN horses had increased rectal temperatures. The number of control horses with rectal temperatures of >39.2°C was significantly greater than that for all vaccinated horses (P < 0.0001), with no significant difference detected between vaccinated horses (Fig. 1A to D). Mild elevations in rectal temperature of 38.4 to 38.8°C were recorded for 2 to 5 days postchallenge in four of six WN-FV horses, three of five CP-WN horses, one K-WN horse, and three control horses. Mild elevations in rectal temperature between 38.5 and 38.6°C also occurred in two K-WN horses late in the challenge period on D17 and D18. One of the CP-WN horses also exhibited mild malaise and anorexia after challenge.
FIG. 1.
(A) Postchallenge clinical course controls. a, P < 0.0001 (temperature control versus vaccinates); b, P < 0.0001 (clinical score control versus vaccinates). (B) Postchallenge clinical course for WN-FV. (C) Postchallenge clinical course for CP-WN. (D) Postchallenge clinical course for K-WN. Error bars indicate standard deviations.
Postchallenge, all six control horses demonstrated moderate or severe (grade 2 or 3, respectively) signs of WNV for at least 1 day and five of six horses demonstrated moderate to severe signs of WNV for two or more consecutive days (Fig. 1A). None of six WN-FV horses developed neurological abnormalities postchallenge. For the CP-WN vaccinates, one of five horses had consistent grade 1 neurological signs in several categories. Four of six K-WN horses developed mild or moderate (grade 1 or 2, respectively) neurological signs that occurred late in the challenge period. The WN-FV and CP-WN horses had significantly fewer clinical signs than did K-WN horses (P = 0.035) or control animals (P < 0.01) (Table 1).
TABLE 1.
Summarization of case criteria for vaccinated and control horses after intrathecal WNV challenge
| Vaccine group | No. of horses with criteriona
|
||||
|---|---|---|---|---|---|
| Clinical signs | Fevere | Deathf | Virus isolation | Histopathic lesions | |
| WN-FV | 0/6 | 0/6 | 0/6 | 0/6 | 1/6h |
| CP-WN | 1/5b | 1/5 | 0/5 | 0/5 | 1/5h |
| K-WN | 4/6c | 1/6 | 0/6 | 0/6 | 3/6h |
| Controls | 6/6d | 3/6 | 6/6 | 6/6 | 6/6g |
Results are shown as number of horses with criterion/total number of horses.
Mild signs in several neurological categories (mentation, paresis, fasciculations, and ataxia) were noted for 1 day.
Mild to moderate signs in at least one of the following categories were noted for 1 to 2 days: mentation, paresis, fasciculations, and ataxia.
Moderate or severe signs in at least one of the following categories were noted for at least 2 days: mentation, paresis, fasciculations, and ataxia.
Fever was indicated by a body temperature of ≥39.2°C (102.5°F).
Death due to development of WNV disease severe enough to require euthanasia for humane reasons.
Encephalitic horses in the control group had moderate or severe encephalitis on histopathology.
Mild inflammatory histopathologic changes were seen in neural tissues of vaccinated horses.
Histopathology.
All control horses had moderate to severe (grade 3) microscopic changes in the midbrain, medulla, and thalamus, which were significantly more extensive (P < 0.05) than those for all of the vaccinated horses. Changes consisted of glial nodules and perivascular cuffing throughout the gray matter of the brain and spinal cord, associated primarily with the large neuronal cell bodies of the midbrain, pons, medulla, and thalamus (Tables 2 and 3). Mild inflammatory changes (grade 1), with little to no glial nodules, were found in one of six of the WN-FV and one of five of the CP-WN horses, although these horses did not exhibit any neurological signs. Four of six K-WN horses had mild inflammatory changes in the brain and spinal cord. Two of these horses exhibited no mild neurological signs, two exhibited mild neurological signs late in the postchallenge period, and one horse had an elevated temperature of 38.7°C on D4 without progression of clinical signs.
TABLE 2.
Summary of glial nodules in vaccinates and control horsesa
| Vaccine group | Avg no. of glial nodules for:
|
|||||
|---|---|---|---|---|---|---|
| Thalamus | Midbrain | Pons | Medulla | Cervical | Lumbar | |
| WN-FV | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.67 ± 1.63 | 0.58 ± 1.20 |
| CP-WN | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.10 ± 0.22 | 0.10 ± 0.22 |
| K-WN | 0.00 ± 0 | 0.00 ± 0 | 1.17 ± 1.83 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 |
| Controls | 4.67 ± 1.86* | 8.33 ± 5.79* | 4.17 ± 3.49 | 12.00 ± 2.76* | 1.67 ± 1.89 | 1.08 ± 2.18 |
*, P < 0.05.
TABLE 3.
Summary of perivascular cuffs in vaccinates and control horsesa
| Vaccine group | Avg % of perivascular cuffs for:
|
|||||
|---|---|---|---|---|---|---|
| Thalamus | Midbrain | Pons | Medulla | Cervical | Lumbar | |
| WN-FV | 0.00 ± 0 | 1.00 ± 2.45 | 0.17 ± 0.41 | 0.17 ± 0.41 | 0.42 ± 0.66 | 2.27 ± 5.55 |
| CP-WN | 0.10 ± 0.22 | 0.00 ± 0 | 2.40 ± 5.37 | 0.00 ± 0 | 0.10 ± 0.22 | 0.20 ± 0.45 |
| K-WN | 1.00 ± 2.00 | 0.50 ± 0.84 | 10.3 ± 24.8 | 0.33 ± 0.82 | 0.92 ± 2.25 | 1.42 ± 3.01 |
| Controls | 59.4 ± 23.3* | 60 ± 26.5* | 47.8 ± 28.6 | 67.7 ± 14.9* | 9.92 ± 12.87 | 5.33 ± 13.06 |
*, P < 0.05.
Case criteria/case outcome.
All vaccinated horses met the criteria for protection against WNV encephalitis. In all groups, irrespective of the vaccine product, the levels of protection were significantly different from those of the control horses (P < 0.0001). All six control horses met the case criteria for WNV encephalitis of grade 2 or more (moderate to severe), fever, viremia, and histopathologic evidence of encephalomyelitis, and accordingly, all were euthanized humanely before the end of the study period (Table 1). None of the vaccinated horses met the criteria for euthanasia, and all survived to the end of the observation period. Irrespective of product, the case outcome or survivorship was significantly higher in the vaccinated horses than in the control horses (P < 0.0001).
Virology.
West Nile virus was not recovered from the plasma of any vaccinated horse postchallenge, and the levels of recovery were significantly different from those of the control horses (P < 0.0001). West Nile virus was recovered from all six control horses during D1 through D5 postchallenge. The median duration of viremia was 1.5 days, and the duration of viremia ranged from 1 to 3 days.
Serology.
Prior to their arrival at the high-containment testing facility (University of Florida), all horses were screened for previous exposure to WNV based on negative immunoglobulin M (<400) and neutralizing antibodies (<5) to WNV by using the immunoglobulin M capture-enzyme-linked immunosorbent assay and plaque reduction neutralization test. One horse in the CP-WN group was found to be negative for a neutralizing antibody titer of <5 at screening, but the horse had a neutralizing antibody titer of >5 on study D0 and was dropped from the study.
At the time of the second immunization, low neutralizing titers, ranging from <5 to 20, were detected in the six K-WN horses. One of the five CP-WN horses had a titer of 10, and none of the six WN-FV horses had detectable neutralizing antibody titers at that second injection. At the time of challenge, the K-WN horses had a geometric mean titer (GMT) of 224 (range, <5 to 320), the CP-WN horses had a GMT of 26 (range, <5 to 80), and the WN-FV horses had a GMT of 5 (range, <5 to 10). All control horses remained negative for neutralizing antibody titers after vaccination and on the day of challenge (Table 4).
TABLE 4.
WNV neutralizing antibody (GMT) titers for vaccinated and control horses postvaccination and after WNV challengea
| No. or day of injection | GMT titer for vaccination group
|
|||
|---|---|---|---|---|
| WN-FV | CP-WN | K-WN | Controls | |
| Injection 1 | <5* | <5 | <5 | <5* |
| Injection 2 | <5 | 2 | 8 | <5* |
| PC D0 | 5 | 26 | 224 | <5 |
| PC D7 | 122 | 66 | 260 | <5 |
| PC D14 | 320 | 258 | 320 | <5 |
| PC D21 | 293 | 258 | 320 | 35 |
PC, postchallenge; *, horses received diluent only.
At 7 days after challenge, all six control horses developed low levels of neutralizing antibody titers to WNV (GMT, 35; range, 10 to 80). The GMT of all six K-WN horses increased moderately to 320 and remained the same through the end of the study. Four of five CP-WN horses had a slight increase in neutralizing antibody levels (GMT 66), and all five horses developed a mean titer of 258 by the second week postchallenge. All six WN-FV horses had rapidly and steadily increasing titers, increasing from a GMT of 122 at 7 days to a GMT of 320 at 14 days and remaining at 293 during the third week after challenge (Table 4 and Fig. 1A to D).
Virus challenge back titration.
The challenge material had back titration values of 2 × 106, 1 × 105, and 3 × 105 PFU/ml for trial groups CE1, CE2, and CE3, respectively.
DISCUSSION
This study supports the efficacy of all three commercially available equine WNV vaccines for the prevention of WNV-induced encephalitis in horses. Irrespective of the vaccine administered, all were protective, resulting in 100% survivorship against a severe challenge model of WNV encephalomyelitis, with 100% of the controls exhibiting clinical disease. Challenge of naïve horses by this model resulted in fever, viremia, and onset of grave neurological disease, with corresponding histopathologic lesions in the CNS and 100% mortality before the end of the study.
Protection against WNV was achieved with one dose of WN-FV, and a protective immune response was demonstrated as early as 7 days postinfection for the WN-FV vaccines, with the rapid rise of neutralizing antibody consistent with previous studies of this vaccine (14). A protective immune response was also seen in the CP-WN horses postinfection, with a more gradual increase of neutralizing antibody by 14 days postinfection. The CP-WN horses were vaccinated according to the manufacturer's protocol of two immunizations in this study. Whether one dose of vaccine would induce protective immunity with the severe WNV challenge model is important to demonstrate. One study has shown protection against viremia after one dose of this vaccine by utilizing the mosquito challenge model (26). In a field study, a marked anamnestic response occurred in horses that were vaccinated previously with K-WN and injected a year later with one dose of the CP-WN vaccine (9). As expected, a rapid increase in neutralizing antibody occurred after the second immunization in the K-WN horses in this study.
In this study, the level of neutralizing antibody titer at the time of challenge is not predictive of protective immunity, as evidenced by the range of GMTs (226 [KP-WN], 26 [CP-WN], and 5 [WN-FV]) among the three vaccinated groups on the day of challenge. These results mirror comparative vaccine studies performed with hamsters, wherein neutralizing antibody levels as low as 5 were found to be protective against WNV challenge (28). Modified live vaccines, such as CP-WN and WN-FV, should be capable of the induction of cell-mediated immunity in addition to humoral immunity. The importance of cell-mediated immunity (T-helper response and cytotoxic T cells) and memory T cells in the protection against WNV and other related flaviviruses has been demonstrated in experimental murine studies (7, 24) and field studies characterizing populations among whom flavivirus is endemic (Japanese encephalitis and dengue fever) (13, 27). Characterization of the protective immune responses to equine WNV vaccines has not been studied for horses due to the limitations of the previous mosquito and needle inoculation challenge models combined with the limited availability of equine-specific immunologic reagents.
Information regarding the duration of immunity of vaccines against clinical disease is extremely important for flavivirus vaccines, especially in climates with year-long mosquito activity. All three vaccines (K-WN, CP-WN, and WN-FV) are able to generate protection against WNV infection at 28 and 56 days postvaccination. The duration of immunity for 12 months against clinical disease, including encephalitis, and the prevention of viremia have been reported for the WN-FV (14). The duration of immunity for 12 months, with the development of neutralizing antibodies and the prevention of viremia, but not against the development of clinical WNV disease has been shown with the CP-WN and K-WN vaccines (15). Long-term immunity is not a feature of inactivated vaccines, and the K-WN-vaccinated horses in an epidemiological study of the Nebraska and Colorado outbreaks were found to have decreased neutralizing titers at 5 to 7 months (6). A recommendation for repeated vaccinations throughout the year was the frequent conclusion made in several epidemiological studies when using the K-WN product, particularly in less temperate areas in North America with prolonged mosquito activity (6, 30). Further studies of the efficacy of all three of these vaccines against WNV-induced disease at >56 days postvaccination are warranted.
The WN-FV is a novel vaccine developed from the technology used to create a live, attenuated Japanese encephalitis human vaccine that has been studied in phase 2 clinical trials and a human West Nile vaccine currently in phase 1 clinical trials (16, 18). The WN-FV vaccine does not require an adjuvant and, hence, would be less likely to induce vaccine reaction than would the adjuvanted vaccines. By utilizing an attenuated flavivirus (YF17D) genome backbone (16), the vaccine is essentially an attenuated modified live vaccine with enhanced immunogenicity and induction of cell-mediated immune responses. None of the six horses in the WN-FV group had any reaction postvaccination. While no systemic or injection site reactions were noted in any of the other vaccine groups, mildly increased rectal temperatures were noted in the first 5 days after the second vaccination in the other two groups and may likely be the result of adjuvant-induced inflammatory cytokines (interleukin-1, interleukin-6, and tumor necrosis factor alpha) and immune responses. These horses were obtained from farms that do not practice any immunoprophylaxis in their foals; thus, there had been no prior exposure to any vaccine components and this also likely decreased their overall reaction rates.
Mild elevations in rectal temperature, ranging from 101 to 102.5°F, were recorded for all four groups within the first 5 days postchallenge. These changes are suspected to be a response to intrathecal injection into the CNS. Whether from the actual trauma induced during the procedure, replication of the virus locally and/or more likely infiltration of inflammatory immune mediators (cytokines and immune cells) has yet to be elucidated. Interestingly, four of six horses in the WN-FV group had evidence of this mild elevation in temperature postchallenge and all six of these horses were completely protected from the onset of WNV and did not manifest any clinical signs during the study. Murine studies have shown the role of WNV antigen-specific CD8 and CD4 T cells in the clearance of WNV from the CNS, along with a interplay of cytokines, such as gamma interferon, tumor necrosis factor alpha, and beta interferon (7, 12, 24).
Mild to moderate neurological deficits of grade 1 or 2 and less than two consecutive days duration were observed in a few of the horses in the K-WN and CP-WN groups postchallenge. An interpretation of these findings should take into account whether the degree of clinical disease observed would correspond to field conditions. The observations may have been biased toward the detection of minimal clinical signs since the observers in this study were aware of the infection day and were presumably more able to detect neurological disease due to additional specialty training (a post-doctorate level of veterinary medical training). In addition, horses in this study were evaluated very closely and several times per day postchallenge for clinical signs, allowing for the detection of slight changes from original baseline values that would not have been detected by owners or field veterinarians not associated intimately with baseline values. Epidemiological studies characterizing the outbreaks have reported a percentage of horses with appropriate vaccination histories that developed WNV disease (6, 20). Although none of the vaccinated horses in this study fit the case criteria for West Nile virus encephalitis, the percentage of horses that developed mild clinical signs mirrors the findings of these outbreak studies. None of the WN-FV vaccinates manifested any clinical signs in this study, but the small sample size may be a factor, as previous studies conducted by the investigators resulted in 1 of 20 horses vaccinated with WN-FV developing mild clinical signs at 28 days postchallenge (14).
All six control horses developed clinical signs of encephalitis and had severe (grade 3) pathological changes in the brain and spinal cord. Complete agreement between severity of clinical signs and grading of histopathologic findings was not observed among the vaccinated horses. This finding is not unexpected, as only representative samples of the brain and spinal cord sections were taken from each horse and these may not have included the affected tissue. In each of the vaccine groups, a few horses had evidence of mild (grade 1) lesion changes in the brain and spinal cord. Similar residual mild focal inflammatory infiltrates were found at 30 days in the brains and spinal cords of immunized rhesus monkeys challenged intracerebrally with wild-type Japanese encephalitis virus but protected from clinical disease (17). Further investigation of the resolution of these inflammatory lesions beyond 21 days postinfection is likely warranted.
Several epidemiological outbreak studies have reported the justification for the use of vaccines as a preventative strategy against WNV disease in horses. Veterinarians and owners have the unique opportunity of selecting among three effective and commercially available equine WNV vaccines for implementing a suitable and personalized preventative program. Further study can be performed to elucidate the most effective protocol for each vaccine and risk group.
Acknowledgments
Funding was provided by Intervet, Inc. (Millsboro, DE).
Technical assistance was provided by T. Monath (Acambis, Cambridge, MA). In addition, we acknowledge the contributions of the following undergraduate and veterinary students: David Gosche, Brett Weldon, Jeremy Campfield, and Tamara Weber. E. P. J. Gibbs has been a consultant for Akzo Nobel.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Animal and Plant Health Inspection Service. USDA animal health monitoring and surveillance: equine West Nile virus surveillance data. Animal and Plant Health Inspection Service, U.S. Department of Agriculture, Washington, DC. www.aphis.usda.gov/vs/nahss/equine/wnv/. Accessed 7 March 2007.
- 2.Beaty, B. J., C. H. Calisher, and R. E. Shope. 1989. Arboviruses, p. 797-856. In N. H. Schmidt and R. W. Emmons, Diagnostic procedures for viral, rickettsial, and chlamydial infections, 6th ed. American Public Health Association, Washington, DC.
- 3.Bowen, R. A., P. Gordy, M. W. Mellencamp, and D. Baker. 2004. Efficacy of a live attenuated chimeric West Nile virus vaccine in horses against clinical disease following challenge with virulent West Nile virus, abstr. 2096. Suppl. Proc. 53rd Annu. Meet. Am. J. Trop. Med. Hyg., Miami Beach, FL.
- 4.Bunning, M. L., R. A. Bowen, C. B. Cropp, K. G. Sullivan, B. S. Davis, N. Komar, M. S. Godsey, D. Baker, D. L. Hettler, D. A. Holmes, B. J. Biggerstaff, and C. J. Mitchell. 2002. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 8:380-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. West Nile virus: statistics, surveillance, and control. Centers for Disease Control and Prevention, Atlanta, GA. www.cdc.gov/ncidod/dvbid/westnile/surv&controlCaseCount06_detailed.htm. Accessed 6 March 2007.
- 6.Davidson, A. H., J. L. Traub-Dargatz, R. M. Rodeheaver, E. N. Ostlund, D. D. Pedersen, R. G. Moorhead, J. B. Stricklin, R. D. Dewell, S. D. Roach, R. E. Long, S. J. Albers, R. J. Callan, and M. D. Salman. 2005. Immunologic responses to West Nile virus in vaccinated and clinically affected horses. J. Am. Vet. Med. Assoc. 226:240-245. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, M. S., B. Shrestha, E. Mehlhop, E. Sitati, and M. Engle. 2003. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 16:259-278. [DOI] [PubMed] [Google Scholar]
- 8.Gardner, I. A., S. J. Wong, G. L. Ferraro, U. B. Balasuriya, P. J. Hullinger, W. D. Wilson, P. Y. Shi, and N. J. MacLachlan. 2007. Incidence and effects of West Nile virus infection in vaccinated and unvaccinated horses in California. Vet. Res. 38:109-116. [DOI] [PubMed] [Google Scholar]
- 9.Grosenbaugh, D. A., C. S. Backus, K. Karaca, J. M. Minke, and R. M. Nordgren. 2004. The anamnestic serologic response to vaccination with a canarypox virus-vectored recombinant West Nile virus (WNV) vaccine in horses previously vaccinated with an inactivated WNV vaccine. Vet. Ther. 5:251-257. [PubMed] [Google Scholar]
- 10.Guirakhoo, F., Z. X. Zhang, T. J. Chambers, S. Delagrave, J. Arroyo, A. D. Barrett, and T. P. Monath. 1999. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology 257:363-372. [DOI] [PubMed] [Google Scholar]
- 11.Hayes, E. B. 2005. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg. Infect. Dis. 11:1174-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein, R. S., E. Lin, B. Zhang, A. D. Luster, J. Tollett, M. A. Samuel, M. Engle, and M. S. Diamond. 2005. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 79:11457-11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar, P., P. D. Uchil, P. Sulochana, G. Nirmala, R. Chandrashekar, M. Haridattatreya, and V. Satchidanandam. 2003. Screening for T cell-eliciting proteins of Japanese encephalitis virus in a healthy JE-endemic human cohort using recombinant baculovirus-infected insect cell preparations. Arch. Virol. 148:1569-1591. [DOI] [PubMed] [Google Scholar]
- 14.Long, M., E. Gibbs, M. Mellencamp, R. Bowen, K. Seino, S. Zhang, S. Beachboard, and P. Humphrey. Efficacy, duration, and onset of immunogenicity of a West Nile virus vaccine, live flavivirus chimera in horses with an experimentally induced clinical disease challenge model. Equine Vet. J., in press. [DOI] [PubMed]
- 15.Minke, J. M., L. Siger, K. Karaca, L. Austgen, P. Gordy, R. Bowen, R. W. Renshaw, S. Loosmore, J. C. Audonnet, and B. Nordgren. 2004. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch. Virol. Suppl. 18:221-230. [DOI] [PubMed] [Google Scholar]
- 16.Monath, T. P., F. Guirakhoo, R. Nichols, S. Yoksan, R. Schrader, C. Murphy, P. Blum, S. Woodward, K. McCarthy, D. Mathis, C. Johnson, and P. Bedford. 2003. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J. Infect. Dis. 188:1213-1230. [DOI] [PubMed] [Google Scholar]
- 17.Monath, T. P., I. Levenbook, K. Soike, Z. X. Zhang, M. Ratterree, K. Draper, A. D. Barrett, R. Nichols, R. Weltzin, J. Arroyo, and F. Guirakhoo. 2000. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J. Virol. 74:1742-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monath, T. P., J. Liu, N. Kanesa-Thasan, G. A. Myers, R. Nichols, A. Deary, K. McCarthy, C. Johnson, T. Ermak, S. Shin, J. Arroyo, F. Guirakhoo, J. S. Kennedy, F. A. Ennis, S. Green, and P. Bedford. 2006. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. USA 103:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostlund, E. N., R. L. Crom, D. D. Pedersen, D. J. Johnson, W. O. Williams, and B. J. Schmitt. 2001. Equine West Nile encephalitis, United States. Emerg. Infect. Dis. 7:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter, M. B., M. T. Long, L. M. Getman, S. Giguere, R. J. MacKay, G. D. Lester, A. R. Alleman, H. L. Wamsley, R. P. Franklin, S. Jacks, C. D. Buergelt, and C. J. Detrisac. 2003. West Nile virus encephalomyelitis in horses: 46 cases (2001). J. Am. Vet. Med. Assoc. 222:1241-1247. [DOI] [PubMed] [Google Scholar]
- 21.Salazar, P., J. L. Traub-Dargatz, P. S. Morley, D. D. Wilmot, D. J. Steffen, W. E. Cunningham, and M. D. Salman. 2004. Outcome of equids with clinical signs of West Nile virus infection and factors associated with death. J. Am. Vet. Med. Assoc. 225:267-274. [DOI] [PubMed] [Google Scholar]
- 22.Savage, H. M., C. Ceianu, G. Nicolescu, N. Karabatsos, R. Lanciotti, A. Vladimirescu, L. Laiv, A. Ungureanu, C. Romanca, and T. F. Tsai. 1999. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Am. J. Trop. Med. Hyg. 61:600-611. [DOI] [PubMed] [Google Scholar]
- 23.Schuler, L. A., M. L. Khaitsa, N. W. Dyer, and C. L. Stoltenow. 2004. Evaluation of an outbreak of West Nile virus infection in horses: 569 cases (2002). J. Am. Vet. Med. Assoc. 225:1084-1089. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha, B., and M. S. Diamond. 2004. Role of CD8+ T cells in control of West Nile virus infection. J. Virol. 78:8312-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siger, L., R. Bowen, K. Karaca, M. Murray, S. Jagannatha, B. Echols, R. Nordgren, and J. M. Minke. 2006. Evaluation of the efficacy provided by a recombinant canarypox-vectored equine West Nile virus vaccine against an experimental West Nile virus intrathecal challenge in horses. Vet. Ther. 7:249-256. [PubMed] [Google Scholar]
- 26.Siger, L., R. A. Bowen, K. Karaca, M. J. Murray, P. W. Gordy, S. M. Loosmore, J. C. Audonnet, R. M. Nordgren, and J. M. Minke. 2004. Assessment of the efficacy of a single dose of a recombinant vaccine against West Nile virus in response to natural challenge with West Nile virus-infected mosquitoes in horses. Am. J. Vet. Res. 65:1459-1462. [DOI] [PubMed] [Google Scholar]
- 27.Simmons, C. P., T. Dong, N. V. Chau, N. T. Dung, T. N. Chau, T. T. Thao le, N. T. Dung, T. T. Hien, S. Rowland-Jones, and J. Farrar. 2005. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 79:5665-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesh, R. B., J. Arroyo, A. P. Travassos da Rosa, H. Guzman, S. Y. Xiao, and T. P. Monath. 2002. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg. Infect. Dis. 8:1392-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turell, M. J., M. Bunning, G. V. Ludwig, B. Ortman, J. Chang, T. Speaker, A. Spielman, R. McLean, N. Komar, R. Gates, T. McNamara, T. Creekmore, L. Farley, and C. J. Mitchell. 2003. DNA vaccine for West Nile virus infection in fish crows (Corvus ossifragus). Emerg. Infect. Dis. 9:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward, M. P., M. Levy, H. L. Thacker, M. Ash, S. K. Norman, G. E. Moore, and P. W. Webb. 2004. Investigation of an outbreak of encephalomyelitis caused by West Nile virus in 136 horses. J. Am. Vet. Med. Assoc. 225:84-89. [DOI] [PubMed] [Google Scholar]
- 31.Yang, J. S., J. J. Kim, D. Hwang, A. Y. Choo, K. Dang, H. Maguire, S. Kudchodkar, M. P. Ramanathan, and D. B. Weiner. 2001. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999). J. Infect. Dis. 184:809-816. [DOI] [PubMed] [Google Scholar]