Abstract
In this study, we aimed to evaluate the validity of the conventional enzyme-linked immunosorbent assay (ELISA) and the Western blotting test for the diagnosis of anthroponotic cutaneous leishmaniasis (ACL) using serum samples obtained from 51 patients with parasitologically proven nontreated CL (NonT-CL patients) and 62 patients under treatment for CL (UT-CL patients). Additionally, 29 serum samples obtained from patients with parasitologically and serologically proven visceral leishmaniasis (VL) were also used as positive controls, and serum samples from 43 blood donors were used as negative controls. All sera were diluted to the same dilution (1/100). Leishmania infantum MON-1 was used as the antigen in the conventional ELISA. The sera of 27 (93.1%) of 29 VL patients were seropositive by ELISA, while the sera of 40 (78.4%) of 51 NonT-CL patients and 43 (69.3%) of 62 UT-CL patients were seropositive by the conventional ELISA. The absorbance values of the CL patients' sera were significantly lower than the absorbance values of the VL patients' sera. Bands between 15 and 118 kDa were detected in two groups of CL patients. Among all bands, the 63-kDa band was found to be more sensitive (88.5%). When we evaluated the Western blotting results for the presence of at least one of the diagnostic antigenic bands, the sensitivity was calculated to be 99.1%. By using serological tests, a measurable antibody response was detected in most of the CL patients in Sanliurfa, Turkey. It is also noted that this response can be changed according to the sizes, types, and numbers of lesions that the patient has. The Western blot test was found to be more sensitive and valid than the conventional ELISA for the serodiagnosis of ACL. In some instances, when it is very difficult to demonstrate the presence of parasites in the smears, immunodiagnosis can be a valuable alternative for the diagnosis of ACL.
Cutaneous leishmaniasis (CL) is highly endemic in the southeastern and east Mediterranean regions of Turkey, with more than 98% of all CL cases recorded from these two regions (http://www.saglik.gov.tr/extras/istatistikler/temel2004/index.htm). Anthroponotic CL (ACL), which is caused by Leishmania tropica MON-53, is highly endemic in the Sanliurfa Province of Turkey, where more than 4,100 and 2,500 cases were recorded in 2004 and 2005, respectively. Field studies consistently indicate that Phlebotomus sergenti is the primary vector associated with epidemic CL in Sanliurfa (1, 22, 25, 38).
In the 1950s, CL was endemic in the southeastern region of Turkey and was characterized by anthroponotic epidemics. After the implementation of a campaign against mosquitoes aimed at controlling malaria, the rate of CL declined considerably; however, at the beginning of the 1980s, its incidence increased in the city of Sanliurfa, and an epidemic of 1,741 cases occurred in 1983, after which the disease regained momentum in the Cukurova region. After 1997, a decrease in the number of CL cases was noted in Sanliurfa.
The number of CL cases reported during the 20-year period from 1981 to 2000 was 22,335 in Sanliurfa. The reported incidence reached a peak in 1994, with 4,185 cases. There was a considerable reduction in the number of reported cases from 1995 onwards, but the number of reported cases increased again in 2002 and continued for the next 4 years (2,800, 2,500, 4,100, and 2,600 cases in 2002, 2003, 2004, and 2005, respectively). The majority (70%) of CL cases have still been reported from the Sanliurfa Province in Turkey (http://www.saglik.gov.tr/extras/istatistikler/temel2004/index.htm).
The Cutaneous Leishmaniasis Diagnosis and Treatment Center was established in Sanliurfa in the early 1980s, and a standard treatment regimen (three intralesional injections per week for 3 weeks) with antimonials (Glucantime) was started for patients with parasitologically proven CL.
Although serological tests are very sensitive and specific for the diagnosis of visceral leishmaniasis (VL) (2, 17, 35), these tests have been considered of limited importance for the diagnosis of CL. However, several papers which described encouraging results by the use of alternative diagnostic methods have been reported from the New World (13, 16, 29, 32).
In the present study, we aimed to determine the antibody responses, using two conventional serological tests, enzyme-linked immunosorbent assay (ELISA) and Western blotting (WB), of sera obtained from two groups of ACL patients (patients receiving treatment and nontreated patients) with different, well-characterized clinical pictures (by lesion size, type, and number) in Sanliurfa Province of Turkey, where ACL is highly endemic, and to determine the effects of the clinical differences on the antibody responses.
MATERIALS AND METHODS
Study region.
Sanliurfa Province (37°9′4‴N, 38°47′34‴E; altitude, 477 m), located in southeastern Turkey along the border with Syria, comprises 18,500 km2 of the western part of southeastern Anatolia. The provincial capital city is Sanliurfa, where more than half of the province's 1,443,422 million inhabitants live (2000 census).
Human sera.
Four groups of subject serum samples were included in the study: (i) 51 serum samples obtained from patients with parasitologically proven nontreated ACL (NonT-CL patients); (ii) 62 serum samples obtained from patients with parasitologically proven ACL and under treatment for ACL (UT-CL patients; the average number of intralesional injections was four per patient); (iii) 29 serum samples obtained from patients with parasitologically and serologically proven VL admitted to the Ege University Medical School Department of Parasitology; and (iv) 43 serum samples obtained from blood donors admitted to Ege University Hospital in Izmir Province, which were used as negative controls.
The ACL patients included the study were admitted to the Cutaneous Leishmaniasis Diagnosis and Treatment Center in Sanliurfa.
The clinical characteristics of the CL patients were noted; and their lesions were clinically classified as nodular, papular, or ulcerative.
Antigen preparations.
Promastigotes from local Leishmania infantum MON-1 stocks obtained by mass cultivation in RPMI 1640 medium containing 10% fetal calf serum were used as the antigens for both serological tests. Promastigotes from up to 200 ml of stationary-phase cultures were harvested and washed five times in phosphate-buffered (10 mM) saline (100 mM) (PBS; pH 7.4) by centrifugation (3,000 × g, 10 min, 4°C). The washed promastigotes were suspended in PBS and submitted to eight freeze-thaw cycles, until the promastigotes were disrupted. The suspension was centrifuged (14,000 × g, 10 min, 4°C), and the supernatants were stored at −30°C. The parasite pellet was resuspended in 20% sodium dodecyl sulfate (SDS) for 30 min at room temperature and then centrifuged (14,000 × g, 10 min, 4°C). The supernatant was removed and was stored at −30°C. The amount of protein was determined by the Lowry method. The final antigen protein concentration was measured and was found to be 9.03 mg/dl by this method.
ELISA.
ELISA was carried out in flat-bottom 96-well microtiter plates (Maxi-Sorp; Nunc, Roskilde, Denmark). The plates were coated with 5 μg of crude antigen and incubated overnight at 4°C. The wells were loaded with 100 μl of serum (1:100 dilution in casein buffer) and incubated at 37°C for 1 h. The plates were washed four times with PBS (pH 7.4) and incubated with peroxidase-conjugated goat anti-human immunoglobulin G (diluted 1:5,000 dilution in casein buffer; Zymed, South San Francisco, CA) at 37°C for 1 h. After the plates were washed four times, they were incubated with the substrate 2,2′-azinobis(3-ethylbinzthiazoleinesulfonic acid) (KPL Inc., Gaithersburg, Md.) for 20 min at room temperature, and the optical density was measured at 450 nm. Each sample was assayed in duplicate. The cutoff point for anti-L. infantum immunoglobulin G was set as the mean plus 3 standard deviations of the log (unit + 1) values of the healthy control sera to obtain maximum sensitivity and specificity.
WB analysis.
SDS-polyacrylamide gel electrophoresis was performed by the method of Schagger and von Jagov (34). Briefly, the antigens were solubilized in sample buffer (250 mM Tris-HCl [pH 6.8], 10% glycerol, 4% SDS, 0.005% phenol red) in the presence of 5% (vol/vol) 2-mercaptoethanol (Sigma). Antigen concentrations ranging from 25 to 500 μg per minigel (8.3 cm wide) and polyacrylamide concentrations of 14% were used. The antigens were electrophoresed by using Mini-Protean III electrophoresis cells (Bio-Rad). Each gel included prestained protein molecular weight marker (for a range of low molecular weights) (SM0441; Fermentas). The antigens were transferred to nitrocellulose membranes (Schleicher & Schuell) by electroblotting (Mini Trans-Blot electrophoretic transfer cell; Bio-Rad) in transfer buffer (25 mM Tris-HCl, 193 mM glycine, 15% methanol [pH 8.3]) for 1 h at 300 mA. The blots were blocked with 0.25% casein in Tris-buffered saline (TBS) (10 mM Tris, 150 mM NaCl [pH 7.6]), washed with TBS, and then cut into 2-mm strips. The strips were incubated with 1:100 dilutions of patient sera in TBS for 45 min, washed with TBS three times, incubated with alkaline phosphatase-conjugated goat anti-human immunoglobulin G (Sigma), diluted 1:10,000 in TBS for 45 min, and washed three times with TBS. All incubations were performed at room temperature on a rotatory shaker. Antibody reactivity was visualized with 5-bromo-4-chloro-3-indolylphosphate and toluidinium-nitroblue tetrazolium substrate.
Statistical analysis.
For determination of the statistical significance between the clinical characteristics, both the ELISA and the immunoblotting tests results were compared by using the chi-square test and Fisher's exact test. The Kruskal-Wallis and Mann-Whitney U tests were used to compare the continuous nonnormally distributed data. The concordance between the ELISA and the WB test results was determined by using the kappa index measure of agreement. Evaluation of the WB results was based on the sensitivity, specificity, positive and negative predictive values, and kappa index agreement. The kappa index is a measure of intertest agreement, based on an evaluation of the differences between the observed concordance and the expected concordance, and its 95% confidence interval (CI) was calculated. The kappa index has a range from 0 to 1.00, with larger values indicating better reliability. Generally, a kappa index >0.70 is considered satisfactory. The data were analyzed with the SPSS statistical package (version 13.0; SPSS Inc., Chicago, IL). A two-tailed P value less than 0.05 was considered statistically significant.
RESULTS
Clinical characterizations of the CL patients.
Ulcerative and nodular lesions were found to be the predominant clinical type lesions, and papular lesions were seen very rarely. The characteristics of the patients with CL, including their clinical appearance, are shown in Table 1. Most of the patients had more than one lesion on their face and/or upper extremities. We observed that the majority of the patients were admitted to the Cutaneous Leishmaniasis Diagnosis and Treatment Center in the late phase of the disease.
TABLE 1.
Characteristics of the 113 patients with CL caused by L. tropica in Turkey
| Feature | Value |
|---|---|
| No. (%) of male patients | 43 (38) |
| Male/female ratio | 0.61 |
| Age (yr) | |
| Avg | 21 |
| Range | 1-77 |
| No. (%) of NonT-CL (new) cases | 51 (45.14) |
| No. (%) of UT-CL cases | 62 (54.86) |
| No. of lesions | |
| Avg | 2 |
| Range | 1-9a |
| No. (%) of ulcerative lesions | 63 (56) |
| No. (%) of nodular lesions | 43 (38) |
| No. (%) of papular leisons | 7 (6) |
| Diam (mm) of lesions | |
| Mean | 16.5 |
| Range | 3-80 |
Only one patient had nine lesions.
ELISA results.
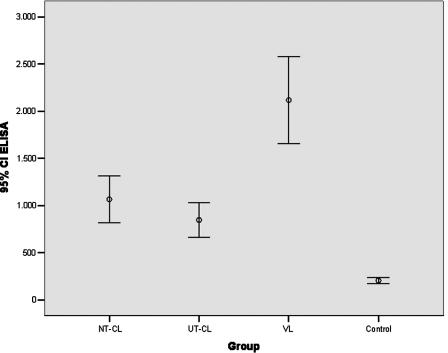
Forty-five (88.2%) of the 51 NonT-CL patients and 44 (71%) of 62 UT-CL patients were found to be seropositive by the ELISA. In total, 78.8% (89/113) were seropositive. However, 27 (93.1%) of 29 VL cases and 2 (4.6%) of 43 control serum samples were found to be seropositive. The absorbance values for the CL patient sera were significantly lower than the absorbance values for the VL patient sera (P = 0.000) (Fig. 1), although no difference in the absorbance values was found between the NonT-CL and UT-CL groups of patients.
FIG. 1.
Mean ELISA absorbance values with 95% confidence intervals observed for sera from different groups.
An important significance between seropositivity and lesion type and lesion size (>20 mm) (P = 0.006 [χ2 = 10.283] and P = 0.036, respectively) was also observed. The absorbance values detected for sera from patients with ulcerative lesions were significantly higher than those detected for sera from patients with other lesion types (Kruskal-Wallis P = 0.035). Other significantly higher absorbance values were detected for the patients who had more than one lesion (P = 0.004; Z = −2.855). In the meantime, a positive correlation between lesion number and the absorbance values was detected (P = 0.000; r = 0.323). No correlation between lesion size and the absorbance values was also detected.
Results of WB analysis.
Nearly all serum samples from CL patients (112 [99.1%] of 113) showed similar patterns of reactivity by WB. The soluble protein profiles of the parasites obtained by SDS-polyacrylamide gel electrophoresis showed at least 22 major protein bands to L. infantum, with the relative molecular masses ranging from 15 kDa to 118 kDa. The bands seen the most frequently in the sera of CL patients were 15 kDa (58.4%), 21 to 22 kDa (61.9%), 63 kDa (88.5%), 74 to 75 kDa (71.7%), 86 to 88 kDa (70.8%), 110 kDa (85.0%), and 118 kDa (85.8%) (Table 2).
TABLE 2.
Frequency of recognition of the diagnostic bands in human sera obtained from CL and VL patients
| Band size (kDa) | No. (%) of serum samples
|
||
|---|---|---|---|
| CL | VL | Control | |
| 15 | 66 (58.4) | 17 (53.1) | 0 |
| 21-22 | 70 (61.9) | 22 (68.8) | 0 |
| 28 | 9 (8.0) | 5 (15.6) | 0 |
| 29 | 17 (15.0) | 4 (12.5) | 0 |
| 30 | 17 (15.0) | 7 (21.9) | 0 |
| 34 | 50 (44.2) | 22 (68.8) | 0 |
| 35 | 49 (43.4) | 10 (31.3) | 0 |
| 36 | 44 (38.9) | 24 (75.0) | 0 |
| 38 | 40 (35.4) | 11 (34.4) | 0 |
| 40 | 15 (13.3) | 8 (25.0) | 0 |
| 42 | 45 (39.8) | 9 (28.1) | 0 |
| 47 | 31 (27.4) | 9 (28.1) | 1 (2.3) |
| 63 | 100 (88.5) | 28 (87.5) | 2 (4.7) |
| 74-75 | 81 (71.7) | 25 (78.1) | 2 (4.7) |
| 78 | 64 (56.6) | 23 (71.2) | 1 (2.3) |
| 86-88 | 80 (70.8) | 27 (84.4) | 3 (6.9) |
| 105 | 56 (49.6) | 24 (75.0) | 1 (2.3) |
| 110 | 96 (85.0) | 23 (71.9) | 2 (4.7) |
| 118 | 97 (85.8) | 24 (75.0) | 2 (4.7) |
The serum samples from patients with VL recognized 22 antigenic bands of L. infantum; and the most frequently recognized bands (diagnostic for VL) were 15 kDa (53.1%), 21 to 22 kDa (68.8%), 34 kDa (68.8%), 36 kDa (75.0%), 63 kDa (87.5%), 74 to 75 kDa (78.1%), 78 kDa (71.2%), 86 to 88 kDa (84.4%), 105 kDa (75.0%), 110 kDa (71.9%), and 118 kDa (75.0%).
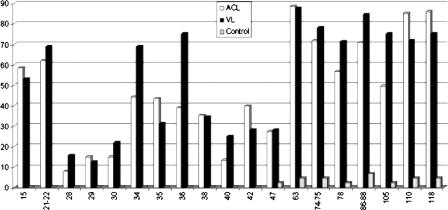
The most reactive bands (diagnostic for CL and VL) are shown in Fig. 2. More than 60% (70 patients) of the serum samples from the CL patients recognized at least eight reactive bands, and more than 78% (89 patients) of them recognized at least six reactive bands.
FIG. 2.
Frequency of recognition of the diagnostic bands in human sera obtained from ACL and VL patients and the control group.
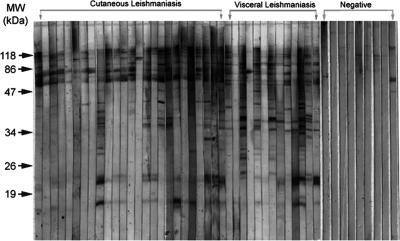
Only one serum sample among the CL patient serum samples and none of the serum samples from VL patients showed any band by WB (Fig. 3). The negative control sera recognized high-molecular-mass proteins (Fig. 2).
FIG. 3.
WB analysis of SDS-polyacrylamide gels of promastigote forms of the Leishmania infantum strain recognized by serum samples from patients with CL and VL and from negative controls. MW, molecular weight markers.
Although no band was found to be common in all CL and VL patient sera, a band for a 63-kDa protein in the sera of CL and VL patients was found to be more sensitive. When we evaluated the WB results for the presence of at least one diagnostic antigenic band, the sensitivity of the WB test was calculated to be 99.1% for CL patients.
Among all antigenic proteins, four proteins of 34 kDa, 36 kDa, 38 kDa, and 42 kDa were detected in a significantly higher number of NonT-CL patients than UT-CL patients (P = 0.022, P = 0.021, P = 0.010, and P = 0.034, respectively) (Table 3).
TABLE 3.
Results obtained by WB with the diagnostic antigenic bands and serum samples from NonT-CL and UT-CL patients
| Band size (kDa) | No. (%) of serum samples
|
Exact P value | |
|---|---|---|---|
| NonT-CL | UT-CL | ||
| 15 | 29 (56.9) | 37 (59.7) | 0.849 |
| 21-22 | 31 (60.8) | 39 (62.9) | 0.848 |
| 28 | 7 (13.7) | 2 (3.2) | 0.076 |
| 29 | 11 (21.6) | 6 (9.7) | 0.112 |
| 30 | 4 (7.8) | 13 (21.0) | 0.066 |
| 34 | 29 (56.9) | 21 (33.9) | 0.022 |
| 35 | 27 (52.9) | 22 (35.5) | 0.086 |
| 36 | 26 (51.0) | 18 (29.0) | 0.021 |
| 38 | 25 (49.0) | 15 (24.2) | 0.010 |
| 40 | 9 (17.6) | 6 (9.7) | 0.269 |
| 42 | 26 (51.0) | 19 (30.6) | 0.034 |
| 47 | 17 (33.3) | 14 (22.6) | 0.213 |
| 63 | 45 (88.2) | 55 (88.7) | 0.937 |
| 74-75 | 40 (78.4) | 41 (66.1) | 0.208 |
| 78 | 30 (58.8) | 34 (54.8) | 0.706 |
| 86-88 | 37 (72.5) | 43 (69.4) | 0.836 |
| 105 | 26 (51.0) | 30 (48.4) | 0.851 |
| 110 | 47 (92.2) | 49 (79.0) | 0.066 |
| 118 | 47 (92.2) | 50 (80.6) | 0.106 |
We found a correlation between some of the immunoblot patterns and the clinical characteristics of CL disease, such as the types, sizes, and numbers of lesions and treatment. Reactivity to the 21- to 22-kDa band was detected in 61.9% of the serum samples obtained from CL patients, including all patients with papular lesion types (P = 0.021; χ2 = 7.689). The 35-kDa and 36-kDa bands were detected significantly more often in most of the CL patients with larger lesions (>30 mm) than in the other CL patients (P = 0.035 and P = 0.003, respectively).
The 15-kDa, 36-kDa, and 47-kDa bands were detected more frequently in most of the CL patients with more than one lesion (exact P values, 0.035, 0.036, and 0.020, respectively) (Table 4).
TABLE 4.
Comparison of clinical characteristics of CL patients and antigenic bands showing statistical significance
| Band size (kDa) | P value for no. of lesions | Lesion type
|
Lesion size
|
||
|---|---|---|---|---|---|
| P value | Comment | P value | Comment | ||
| 15 | 0.035 (significant) | ||||
| 21 | 0.021 (significant) | More significant for papular lesions | |||
| 35 | 0.035 (significant) | More significant for lesions with diam that were >30 mm | |||
| 36 | 0.036 (significant) | 0.003 (significant) | More significant for lesions with diam that were >30 mm | ||
| 47 | 0.020 (significant) | ||||
The WB blot results for both VL and CL patients and the negative controls were found to be in agreement with the ELISA results. Although the agreement between the two serological tests for only CL patients was 76.9% (95% CI = 65.0 to 89.0%; kappa index = 0.492; P = 0.000), the overall agreement between the tests was 79.5% (95% CI = 67.9 to 91.0; kappa index = 0.503, P = 0.000).
Table 5 shows the sensitivities (95% CIs), specificities, and positive and negative predictive values of both tests for the CL and VL groups of patients.
TABLE 5.
Evaluation of results of two serologic methods for patients with CL and VLa
| Test and patient group (no. of patients) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Agreement (%) | Kappa index |
|---|---|---|---|---|---|---|
| ELISA | ||||||
| CL (113) | 78.0 (64.8-91.0)b | 95.3 (78.7-100) | 97.8 (84.0-100) | 63.1 (48.0-78.1) | 83.3 (71.2-95.4) | 0.63 |
| VL (29) | 93.1 (74.7-100) | 95.3 (78.7-100) | 93.1 (74.7-100) | 95.3 (78.7-100) | 94.4 (79.8-100) | 0.89 |
| WB | ||||||
| CL (113) | 99.1 (86.0-100) | 72.1 (55.4-88.8) | 90.3 (77.5-100) | 96.9 (78.9-100) | 91.7 (79.6-100) | 0.77 |
| VL (29) | 100 (81.6-100) | 72.1 (55.4-88.8) | 70.7 (53.8-87.6) | 100 (82.0-100) | 83.3 (68.7-98.0) | 0.67 |
P was 0.00 for all comparisons.
The values in parentheses represent the 95% CIs.
DISCUSSION
The use of serological tests for the diagnosis of CL is very limited in the Old World because of the easy and cheaper direct diagnosis and cross-reactivity in the areas of endemicity where more than one clinical form of leishmaniasis is seen. PCR techniques for the diagnosis of CL have also been performed in some countries where leishmaniasis is endemic when clinically suspected lesions are negative by the conventional parasitological methods. It is reported that the kinetoplast DNA PCR showed a higher sensitivity than the ITS1 PCR for patients with CL caused by L. tropica (4, 18).
In our study area, ACL is highly endemic, and no other clinical types of leishmaniasis have been reported so far. The inhabitants of the area have known of the disease for centuries, and lesions with different types of clinical characteristics are often seen.
To determine the validity of both the ELISA and the WB serological tests used for diagnosis, we carried out a detailed study using sera obtained from CL patients in the area. In the overall evaluation, the WB test was found to be more sensitive than the conventional ELISA for the diagnosis of CL.
The main line of defense against infection in CL is the cellular immune response, which is responsible for the clinical spectrum of leishmaniasis, which ranges from localized CL to its progressive form, diffuse CL (8, 36). The humoral immune response occurs only during the active phase of infection, with the appearance of low titers of antibodies that disappear some months after the end of treatment, representing a temporary response (3, 24). Several techniques have been developed for the serological diagnosis of leishmaniasis. These include an immunofluorescence assay, an ELISA, and the WB assay (11, 16, 28, 39). The WB technique is highly sensitive and specific and provides more information about the parasite's antigenic profile (15, 17). Therefore, it is very useful for the standardization of more sensitive and specific diagnostic procedures.
Serological tests for the diagnosis of VL are generally highly sensitive (>90%) (2, 35). On the other hand, serological tests are rarely performed for the diagnosis CL, as the sensitivities and specificities of these tests have been disappointingly low (11, 33). By the use of serological tests, a measurable antibody response was detected in most of the sera of CL patients in Sanliurfa. Importantly, this response can be changed according to the lesion size, the lesion type, and the numbers of lesions that the patient has. This finding showed that the humoral immune response has been changed according to the clinical characteristics of the lesions.
In the ELISA, the seropositivity ratios were calculated to be 88% and 71% for NonT-CL and UT-CL patients, respectively. We think that this expected result is because of the loss of the antigen burden in treated patients. The absorbance values for the sera of CL patients were significantly lower than the absorbance values for the sera of VL patients, and no difference in absorbance values between the NonT-CL and UT-CL patients was observed in both ELISAs. A positive correlation between seropositivity by the conventional ELISA and clinical properties, like lesion size, lesion location, the number of lesions in the patient, and lesion type, was also detected.
Bands between 15 and 118 kDa were detected in all serum samples from the two groups of CL patients, and the 63-kDa band was found to be more sensitive (88.5%) but was one of the less specific (4.7%) bands among all the bands. When we evaluated the WB results for the presence of at least one of the diagnostic antigenic bands, the sensitivity was calculated to be 99.1%. The 63-kDa protein was identified as a glycoprotein by staining with periodic acid-Schiff, indicating that it could be the main promastigote surface molecule (molecular mass, 60 to 65 kDa) common to all Leishmania species (5, 7, 10, 12, 21). Gp63 has been described to be important in cell-cell (host-parasite) interaction (30), cell infectivity (19, 40), specific diagnosis (14), and immunoprotection (31, 41). This molecule was also identified in the sera from patients with CL, mucocutaneous leishmaniasis, or VL (7, 9, 14, 20, 21, 26).
In acute clinical VL, Marty et al. (23) reported the simultaneous presence of four major bands of 18, 21, 23, and 31 kDa in all patients with acute clinical VL. It is possible that slight modifications in the WB technique could result in the different migrations of diagnostic bands. Therefore, it is possible that the 15-, 21- to 22-, and 34-kDa bands correspond to the diagnostic bands identified by Marty et al. (23). These bands were less often detected in both VL and CL patients, possibly because all VL patients did not have acute CL or because of the severity of the infection.
Isaza et al. found reactivities with high-molecular-mass bands (120, 123 and 129, 138, and 141 kDa) when a soluble Leishmania panamensis strain was used as the antigen with sera from 86 CL patients The reactivity of sera with the 120-kDa fraction was 76.7%, and this fraction was detected in 85.5% of the CL patients in the present study (16). Goncalves et al. (13) reported that bands between 13 kDa and 150 kDa were detected when they used different Leishmania species as the antigen sources. Most of the polypeptide fractions (93%) were determined in the serum samples (42).
Dos Santos et al. (9) reported on the importance of the 32- and 35-kDa antigenic bands; and Valli et al. (37) determined that the cutaneous sera generally reacted weakly to antigens of 45, 66, and 75 kDa and intensely to 48- to 50-kDa antigens.
Four 34-kDa, 36-kDa, 38-kDa, and 42-kDa proteins fractions were detected in a significantly higher number of NonT-CL patients than in UT-CL patients, and the differences between these two groups were significant (P = 0.022, P = 0.021, P = 0.010, and P = 0.034, respectively). The antibody response to these antigens was significantly lower in patients with UT-CL, suggesting that it may be useful to monitor sera for these antigens to determine clinical cure. However, interestingly, the 30-kDa antigen was, in fact, more frequently recognized in the UT-CL patients, suggesting that this antigen may play a role in protective immunity against CL caused by L. tropica. Brito et al. (6) reported that the 27- and 30-kDa proteins are important for serodiagnosis and that the 19-kDa protein is important for monitoring of the treatment in patients with ACL.
In the present study, proteins of 15, 21 to 22, 63, 74 to 75, 110, and 118 kDa were found to be useful for serodiagnosis; and we found that the 63-kDa protein has a specific importance in the serodiagnosis of CL patients. Cross-reactivity was detected with sera from patients with VL, but these antigens can be good markers for the serodiagnosis of CL in patients inhabiting only this specific area of endemicity, Sanliurfa, because VL is not reported in that region. Two other important findings were that proteins with higher molecular masses (>118 kDa) reacted with negative control sera and that proteins other than those of 15, 21 to 22, 63, 74 to 75, 110, and 118 kDa reacted with the antibodies in the sera of very few patients with CL and VL. However, Reiche et al. (27) reported that the detection of proteins of 18, 30, 39, 45, 52, 64, 70, and 82 kDa in CL patient sera is not enough to establish a positive result in Brazilian CL patients.
The direct parasitological method, the smear method, has always been considered the procedure of first choice for the diagnosis of ACL. Its specificity can be accepted to be 100%, but its sensitivity can change according to the people taking samples from the lesion, the people checking the smear, and amount of parasites in the lesion aspirate. In some instances, when it is very difficult to demonstrate the presence of parasites and there is no possibility for the application of molecular diagnostic techniques, immunodiagnosis becomes an important alternative for the diagnosis of CL. At this point, our results suggest that the WB test is more sensitive than the conventional ELISA for the diagnosis of ACL.
In conclusion, our results demonstrate that ELISA and WB can be used for the reliable and sensitive diagnosis of ACL in Sanliurfa, Turkey. These observations are sufficiently promising to warrant further validation of the methods to determine the potential of the use of WB for the diagnosis of CL.
Acknowledgments
We gratefully acknowledge Cumhur Gunduz for statistical analysis. We also thank Seray Ozensoy Toz for providing the L. infantum strain.
Footnotes
Published ahead of print on 29 August 2007.
REFERENCES
- 1.Alptekin, D., M. Kasap, U. Luleyap, et al. 1999. Sandflies (Diptera: Psychodidae) associated with epidemic cutaneous leishmaniasis in Sanliurfa, Turkey. J. Med. Entomol. 36:277-281. [PubMed] [Google Scholar]
- 2.Bagchi, A. K., S. Tiwari, S. Gupta, and J. C. Katiyar. 1998. The latex agglutination test: standardization and comparison with direct agglutination and dot-ELISA in the diagnosis of visceral leishmaniasis in India. Ann. Trop. Med. Parasitol. 92:159-163. [DOI] [PubMed] [Google Scholar]
- 3.Behin, R., and L. Jacques. 1984. Immune response to leishmania. Crit. Rev. Trop. Med. 2:141-188. [Google Scholar]
- 4.Bensoussan, E., A. Nasereddin, F. Jonas, L. F. Schnur, and C. L. Jaffe. 2006. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J. Clin. Microbiol. 44:1435-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouvier, J., R. Etges, and C. Bordier. 1987. Identification of promastigote surface protease in seven species of Leishmania. Mol. Biochem. Parasitol. 24:73-79. [DOI] [PubMed] [Google Scholar]
- 6.Brito, M. E. F., M. G. Mendonqa, Y. M. Gomes, M. L. Jardim, and F. G. C. Abath. 2001. Dynamics of the antibody response in patients with therapeutic or spontaneous cure of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 9:203-206. [DOI] [PubMed] [Google Scholar]
- 7.Colomer-Gould, V., L. Galvao Quintao, J. Keithly, and N. Nogueira. 1985. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J. Exp. Med. 162:902-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Convit, J., M. Ulrich, C. T. Fernández, F. J. Tapia, G. Cáceres-Dittmar, M. Castes, and A. J. Rondón. 1993. The clinical and immunological spectrum of American cutaneous leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 87:444-448. [DOI] [PubMed] [Google Scholar]
- 9.Dos Santos, J. I., M. G. Morgado, and B. Galvao-Castro. 1987. Human visceral leishmaniasis: analysis of the specificity of the humoral immune response to polypeptides of Leishmania donovani chagasi. Am. J. Trop. Med. Hyg. 37:263-270. [DOI] [PubMed] [Google Scholar]
- 10.Edges, R. J., J. Bouvier, R. Hoffman, and C. Bordier. 1985. Evidence that the major surface protein of three Leishmania species are structurally related. Mol. Biochem. Parasitol. 14:141-149. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Miss, M. R., F. J. Andrade-Narvaez, R. E. Esquivel-Vinas, E. B. Simmonds-Diaz, S. B. Canto-Lara, and A. L. Cruz-Ruiz. 1990. Localized cutaneous leishmaniasis (chiclero's ulcer) in Mexico: sensitivity and specificity of ELISA for IgG antibodies to Leishmania mexicana mexicana. Trans. R. Soc. Trop. Med. Hyg. 84:356-358. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner, P. R., C. L. Jaffe, and D. M. Dwyer. 1984. Identification of some cross-reactive promastigote cell surface antigens of some leishmanial stocks by 125I labeling and immunoprecipitation. Infect. Immun. 43:637-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goncalves, C. C. M., E. M. V. Reiche, B. C. A. De Abreu Filho, T. G. V. Silveira, T. N. C. Felizardo, K. Rocha Maria, R. Costacurta, E. J. Padovesi, B. P. Dias Filho, S. I. Jankevicius, and J. V. Jankevicius. 2002. Evaluation of antigens from various Leishmania species in a Western blot for diagnosis of American tegumentary leishmaniasis. Am. J. Trop. Med. Hyg. 66:91-102. [DOI] [PubMed] [Google Scholar]
- 14.Heath, S., M. L. Chance, M. Hommel, and J. M. Crampton. 1987. Cloning of a gene encoding the immunodominant surface antigen of Leishmania donovani. Mol. Biochem. Parasitol. 23:211-222. [DOI] [PubMed] [Google Scholar]
- 15.Hermann, K. L., and D. D. Erdman. 1995. Diagnos by serological assays, p. 129-134. In E. H. Lennette, D. A. Lennette, and E. T. Lennette (ed.), Diagnostic procedures for viral, rickettsial and clamydial infections, vol. 6. American Public Health Association, Washington, DC. [Google Scholar]
- 16.Isaza, D. M., M. Restrepo, and W. Mosca. 1997. Immunoblot analysis of Leishmania panamensis antigens in sera of patients with American cutaneous leishmaniasis. J. Clin. Microbiol. 35:3043-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kar, K. 1995. Serodiagnosis of leishmaniasis. Crit. Rev. Microbiol. 21:123-152. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, R., R. A. Bumb, N. A. Ansari, R. D. Mehta, and P. Salotra. 2007. Cutaneous leishmaniasis caused by Leishmania tropica in Bikaner, India: parasite identification and characterization using molecular and immunologic tools. Am. J. Trop. Med. Hyg. 76:896-901. [PubMed] [Google Scholar]
- 19.Kweider, M., J. L. Lemesre, F. Santoro, J. P. Kusnierz, M. Sadigursky, and A. Capron. 1989. Development of metacyclic Leishmania is associated with the increasing of GP65, the major surface antigen. Parasite Immunol. 11:197-209. [DOI] [PubMed] [Google Scholar]
- 20.Lemesre, J. L., F. S. Rizvi, D. Afchain, M. Sadigursky, A. Capron, and F. Santoro. 1985. Subspecies-specific surface antigens of promastigotes of the Leishmania complex. Infect. Immun. 50:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lepay, D. A., N. Nogueira, and Z. Cohn. 1983. Surface antigens of Leishmania donovani promastigotes. J. Exp. Med. 157:1562-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Pont, P. F., Y. Bayazit, M. Konyar, et al. 1996. Cutaneous leishmaniasis in the urban area of Sanliurfa (Turkey). Bull. Soc. Pathol. Exot. 89:274-275. [PubMed] [Google Scholar]
- 23.Marty, P., A. Lelievre, J. F. Quaranta, I. Suffia, M. Eulalio, M. Gari-Toussaint, Y. Le Fichoux, and J. Kubar. 1995. Detection by Western blot of four antigens characterizing acute clinical leishmaniasis due to Leishmania infantum. Trans. R. Soc. Trop. Med. Hyg. 89:690-691. [DOI] [PubMed] [Google Scholar]
- 24.Masuda, A., S. F. Nascimento, C. S. Guerra, G. S. Paranhos, and A. W. Ferreira. 1989. Analysis of the specificity of human antibodies to antigens of Leishmania braziliensis braziliensis. Rev. Inst. Med. Trop. Sao Paulo 31:228-234. [DOI] [PubMed] [Google Scholar]
- 25.Ozcel, M., Y. Ozbel, S. Ozensoy, et al. 1999. The current status of leishmaniasis in Turkey, p. 27-30. In Y. Matsumoto (ed.), Epidemiology and control of leishmaniasis in central Eurasia. Research report series no. 1 (1996-1998). International Press Editing Centre Incorporation, Tokyo, Japan.
- 26.Reed, S. G., R. Badaro, and R. M. C. Lloyd. 1987. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J. Immunol. 138:1596-1601. [PubMed] [Google Scholar]
- 27.Reiche, E. M., M Cavazzana, Jr., H. Okamura, E. C. Tagata, S. I. Jankevicius, and J. V. Jankevicius. 1998. Evaluation of the Western blot in the confirmatory serologic diagnosis of Chagas' disease. Am. J. Trop. Med. Hyg. 59:750-756. [DOI] [PubMed] [Google Scholar]
- 28.Rolland-Burger, L., X. Rolland, C. W. Grieve, and L. Monjour. 1991. Immunoblot analysis of the humoral immune response to Leishmania donovani infantum polypeptides in human visceral leishmaniasis. J. Clin. Microbiol. 29:1429-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero, G. A., M. de la Gloria Orge Orge, M. V. de Farias Guerra, M. G. Paes, V. de Oliveira Macedo, and E. M. de Carvalho. 2005. Antibody response in patients with cutaneous leishmaniasis infected by Leishmania (Viannia) braziliensis or Leishmania (Viannia) guyanensis in Brazil. Acta Trop. 93:49-56. [DOI] [PubMed] [Google Scholar]
- 30.Russell, D. G., and H. Wilhelm. 1986. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J. Immunol. 136:2613-2620. [PubMed] [Google Scholar]
- 31.Russell, D. J., and J. Alexander. 1988. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J. Immunol. 140:1274-1279. [PubMed] [Google Scholar]
- 32.Ryan, J. R., A. M. Smithyman, G.-H. Rajasekariah, L. Hochberg, J. M. Stiteler, and S. K. Martin. 2002. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J. Clin. Microbiol. 40:1037-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez, J. L., B. M. Diniega, J. W. Small, R. N. Miller, J. M. Andujar, P. J. Weina, P. Lawyer, W. R. Ballou, and J. K. Lovelace. 1992. Epidemiologic investigation of an outbreak of cutaneous leishmaniasis in a defined geographic focus of transmission. Am. J. Trop. Med. Hyg. 47:47-54. [DOI] [PubMed] [Google Scholar]
- 34.Schagger, H., and G. von Jagov. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 35.Senaldi, G., H. Xiao-Su, D. C. Hoessli, and C. Bordier. 1996. Serological diagnosis of visceral leishmaniasis by a dot-enzyme immunoassay for the detection of Leishmania donovani-related circulating antigen. J. Immunol. Methods 193:9-15. [DOI] [PubMed] [Google Scholar]
- 36.Tapia, F. J., G. Caceres-Dittmar, and M. A. Sanchez. 1994. Inadequate epidermal homing leads to tissue damage in human cutaneous leishmaniasis. Immunol. Today 15:160-165. [DOI] [PubMed] [Google Scholar]
- 37.Valli, L. C., V. M. Passos, R. Dietze, H. L. Callahan, J. D. Berman, and M. Grogl. 1999. Humoral immune responses among mucosal and cutaneous leishmaniasis patients caused by Leishmania braziliensis. J. Parasitol. 85:1076-1083. [PubMed] [Google Scholar]
- 38.Volf, P., Y. Ozbel, F. Akkafa, M. Svobodová, J. Votýpka, and K.-P. Chang. 2002. Sand flies (Diptera: Phlebotominae) in Sanliurfa, Turkey. Relationship of Phlebotomus sergenti with the epidemic of Anthroponotic cutaneous leishmaniasis. J. Med. Entomol. 39:12-15. [DOI] [PubMed] [Google Scholar]
- 39.Walton, B. C., W. H. Brooks, and I. Arjona. 1972. Serodiagnosis of American leishmaniasis by indirect fluorescent antibody test. Am. J. Trop. Med. Hyg. 21:296-299. [DOI] [PubMed] [Google Scholar]
- 40.Wilson, M. E., K. K. Hardin, and J. E. Donelson. 1989. Expression of the major surface glycoprotein of Leishmania donovani chagasi in virulent and attenuated promastigotes. J. Immunol. 143:678-684. [PubMed] [Google Scholar]
- 41.Yang, D. M., N. Fairweather, L. L. Button, W. R. McMaster, L. P. Kahl, and F. Y. Liew. 1990. Oral Salmonella typhimurium (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J. Immunol. 145:2281-2285. [PubMed] [Google Scholar]
- 42.Zalis, M. G., A. Schubach, M. Oliveira Neto, D. A. Costa, C. Jaffe, V. G. Lopes, and M. A. Barcinski. 1988. The cell immune response of patients with cutaneous leishmaniasis to leishmanial antigen with different molecular weights. Mem. Inst. Oswaldo Cruz 83(Suppl I):117. [Google Scholar]