Abstract
New Zealand (NZ) has experienced a Neisseria meningitidis serogroup B epidemic since 1991. MeNZB, a strain-specific outer membrane vesicle vaccine made using an NZ epidemic strain isolate, NZ98/254 (B:4:P1.7b,4), from two manufacturing sites, the Norwegian Institute of Public Health (NIPH) and Chiron Vaccines (CV; now Novartis), was evaluated for safety, immunogenicity, and reactogenicity in this observer-blind trial with 8- to 12-year-old children. In year 1, cohort A (n = 302) was randomized 4:1 for receipt of NIPH-MeNZB or MenBvac (Norwegian parent vaccine strain 44/76; B:15:P1.7,16). In year 2, cohort B (n = 313) was randomized 4:1 for receipt of CV-MeNZB or NIPH-MeNZB. Participants all received three vaccinations 6 weeks apart. Local and systemic reactions were monitored for 7 days. Seroresponse was defined as a fourfold or greater rise in the serum bactericidal antibody titer from the baseline titer as measured by a serum bactericidal assay. Those with baseline titers of <1:4 required titers of ≥1:8 to serorespond. Intention-to-treat (ITT) and per protocol (PP) analyses are presented. In cohort A, 74% (ITT) and 73% (PP) of NIPH-MeNZB recipients demonstrated seroresponses against NZ98/254 after three doses, versus 32% (ITT and PP) of MenBvac recipients. In cohort B, seroresponses against NZ98/254 after three doses occurred in 79% (ITT and PP) of CV-MeNZB versus 75% (ITT) and 76% (PP) of NIPH-MeNZB recipients. Vaccines were tolerable, with no vaccine-related serious adverse events. In conclusion, the NZ strain meningococcal B vaccine (MeNZB) from either manufacturing site was immunogenic against New Zealand epidemic vaccine strain meningococci with no safety concerns when given in three doses to these 8- to 12-year-old children.
Meningitis, sepsis, and other serious infections caused by Neisseria meningitidis continue to occur in both developed and developing countries (17). Since 1991, New Zealand (NZ) has experienced an epidemic of serogroup B meningococcal disease, with the B:4:P1.7b,4 strain accounting for 86% of isolates from 1990 to 2003 (10). The incidence peaked in 2001 (17.4 per 100,000). Approximately 80% of diseases occur in those under 20 years old. The highest-risk age group is infants under 1 year old (124.4 per 100,000 in 2003) (10).
Development of a meningococcal serogroup B vaccine has been protracted due to the immunologic cross-reactivity of B polysaccharide with human neural tissue and other concerns (13). Control of serogroup B disease by outer membrane vesicle (OMV) vaccines has been limited. Strain specificity reduces the application of these vaccines. The efficacy in randomized controlled trials of three serogroup B meningococcal OMV vaccines, different from each other in manufacturing process and strain, has been shown in older children and adolescents (3, 7, 26). Strain-specific immunogenicity to these OMV vaccines has been demonstrated in all age groups (27). Limited safety information is available from randomized controlled trials (7, 22, 26). However, serogroup B meningococcal OMV vaccines have received widespread use in Latin America (21).
To address the NZ meningococcal epidemic, the Norwegian Institute of Public Health (NIPH) produced a strain-specific meningococcal B OMV vaccine (NIPH-MeNZB), in collaboration with Chiron Vaccines (CV; now Novartis). The NZ vaccine was manufactured using a selected NZ epidemic strain (B:4:P1.7b,4; NZ98/254) and produced in a manner similar to that for MenBvac, the Norwegian strain parent OMV vaccine (14). Demonstrated physicochemical similarities between MenBvac and MeNZB (19) supported the bridging of preexisting safety information from the parent vaccine. The NZ vaccine was subsequently produced at CV (CV-MeNZB).
The technique of a serum bactericidal-activity assay has been used to measure the serum bactericidal antibody activity responses to serogroup B vaccines and was the primary test of immunogenicity for this trial (7, 18, 20, 21, 23, 24, 27). Correlation between serum bactericidal antibody activity and protection against meningococcal disease was reported in 1969 (15). Subsequently, age groups with comparatively high levels of serum bactericidal antibody activity were found to have higher efficacy after mass vaccination with serogroup B OMV vaccines in Brazil and Chile (7, 21).
Trials were planned in NZ to assess the immunogenicity, reactogenicity, and safety of MeNZB in different age groups prior to application for regulatory approval for widespread use (25). Two phase I/II studies with adults, demonstrating the tolerability and safety of NIPH-MeNZB (28) and CV-MeNZB (unpublished data), were conducted prior to the use of vaccines in this trial. This study was the first to use either vaccine in children and evaluated the immunogenicity, reactogenicity, and safety of three doses of NIPH-MeNZB and CV-MeNZB in two cohorts of healthy 8- to 12-year-old children. In year 1, cohort A evaluated NIPH-MeNZB, with MenBvac (a licensed, NIPH-produced OMV vaccine of a different strain) used as a comparison providing necessary bridging data. In year 2, cohort B evaluated and compared vaccines from two manufacturing sites, namely, CV-MeNZB and NIPH-MeNZB. Data from this and other studies contributed to licensure of CV-MeNZB as MeNZB with provisional consent in 2004 for use in a mass vaccination program.
(This study was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 30 October to 2 November 2004.)
MATERIALS AND METHODS
This was a phase II, randomized, controlled, observer-blind trial involving two cohorts of participants. The Auckland Ethics Committee approved this study.
Population.
Children 8 to 12 years old from schools in Manurewa and Papakura, Auckland, NZ, were eligible to participate. These were areas with high meningococcal-disease incidence. Forty-two schools were invited to take part, with 39 accepting. Prior to the cohort A trial, 10,500 flyers inviting participation were distributed in these schools, with a further 4,500 distributed prior to the cohort B trial.
For the first year of the study, for cohort A, 1,182 respondents were contacted by telephone; those from schools with the most expressions of interest were approached first. Of those contacted, 337 attended an enrollment appointment, but 35 of them did not satisfy the eligibility criteria; thus, 302 participants were enrolled during October and November 2002. For the second year of the study, for cohort B, 1,518 respondents from schools not involved in cohort A were contacted. Of these, 350 attended an enrollment appointment; 37 of these did not satisfy the eligibility criteria, and thus, 313 participants were enrolled during August and September 2003.
Written informed consent was obtained from parents/legal guardians and written assent from children at the first visit. Children were not eligible if they had previously had a meningococcal disease or recent household contact with a meningococcal disease, had recently received other vaccines, had a history of vaccine hypersensitivity, had had a fever within the past 3 days, had taken systemic antibiotics within the past 14 days, had received blood products in the past 12 weeks, or had a serious chronic disease.
Vaccination, specimen collection, and laboratory methods.
Three vaccines were used, prepared as previously described (28). Each vaccine was from a single lot and contained 25 μg of OMV protein, 1 to 3 μg lipopolysaccharide, 1.65 mg aluminum hydroxide, and 2.5 to 10 μg deoxycholate per 0.5-ml dose. MenBvac OMV was from strain B:15:P1.7,16 (44/76). NIPH-MeNZB and CV-MeNZB OMV were from strain B:4:P1.7b,4 (NZ98/254). MenBvac and NIPH-MeNZB were produced at the NIPH in Oslo, Norway, and CV-MeNZB was produced at CV in Siena, Italy. All vaccines were approved for use by the NZ Standing Committee on Therapeutic Trials.
In each cohort, children were randomized at a 4:1 ratio by using a computer-generated list. An unblinded team member and vaccinator ensured that vaccines were given by intramuscular injection into the deltoid muscle. Children received three vaccine doses 6 weeks apart and were observed for 30 min after each vaccination.
Blood samples were obtained immediately prior to the first vaccination and at 4 to 6 weeks after doses two and three. Samples were handled and delivered to the Institute of Environmental Science and Research as previously described (28), where sera from each individual child were tested concurrently as described by Wong et al. (29).
Outcome measures.
For 7 days following vaccination, parents recorded local and systemic reactions daily on a standardized diary card as described previously (28). Absence from school as a result of vaccination was also recorded. Active surveillance for reactions was undertaken, including all events requiring a physician visit (28).
The primary immunogenicity outcome measure was the proportion of participants who were seroresponders, defined as those participants demonstrating a fourfold or greater rise in serum bactericidal antibody titer compared to the prevaccination baseline titer. Children with baseline titers of <1:4, however, were considered seroresponders only if their serum bactericidal antibody titers increased to ≥1:8 (20).
Statistical methods.
Percentages are quoted with 95% confidence intervals, calculated using the adjusted Wald method (1). Serum bactericidal antibody titers of <2 were assigned values of 1 for calculation of geometric mean bactericidal antibody titers (GMTs). GMTs are reported with 95% confidence intervals of the GMT. Immunogenicity results were analyzed using an intention-to-treat (ITT) population, including children who received at least one vaccination and provided evaluable data before and at least once after vaccination, and a per protocol (PP) population, judged to have no major protocol violations. Major protocol violations were receipt of an immunization within 21 days prior to enrollment, past history of an immunization reaction, antibiotic use within 2 weeks prior to the first blood draw, and receipt of the study vaccine outside a specified window. Reactogenicity was analyzed for all children receiving vaccination.
A post hoc analysis was conducted to investigate whether there was any likely explanation for some observed variation in the patterns of response to the NIPH-MeNZB vaccine between the two cohorts. A linear mixed model was performed, using the log bactericidal antibody titer following vaccinations 2 and 3 as the outcome. Age, gender, ethnicity, and baseline bactericidal titer were included as time-constant variables. Time between vaccination, time between vaccination and blood draw, and vaccination number were included as time-dependent covariates. Interaction of vaccination number and cohort was also included. Another analysis, identical except that the outcome was based on whether or not the subject was a responder, was performed using a nonlinear mixed model.
RESULTS
In 2002, 302 children were enrolled in cohort A, and 313 participants were enrolled in cohort B in 2003 (Table 1).
TABLE 1.
Baseline participant characteristics for the randomized population
| Cohort and vaccine | No. of participants | Mean age (yrs) | No. (%) of female participants | No. (%) of participants of indicated ethnicity
|
|||
|---|---|---|---|---|---|---|---|
| NZ European | Māori | Pacific | Other | ||||
| A | |||||||
| NIPH-MeNZB | 241 | 9.5 | 120 (50) | 88 (37) | 57 (24) | 86 (36) | 10 (3) |
| MenBvac | 61 | 9.5 | 22 (36) | 20 (33) | 13 (21) | 22 (36) | 6 (10) |
| B | |||||||
| CV-MeNZB | 250 | 10.0 | 136 (54) | 97 (39) | 80 (32) | 55 (22) | 18 (7) |
| NIPH-MeNZB | 63 | 10.0 | 39 (62) | 29 (46) | 19 (30) | 10 (16) | 5 (8) |
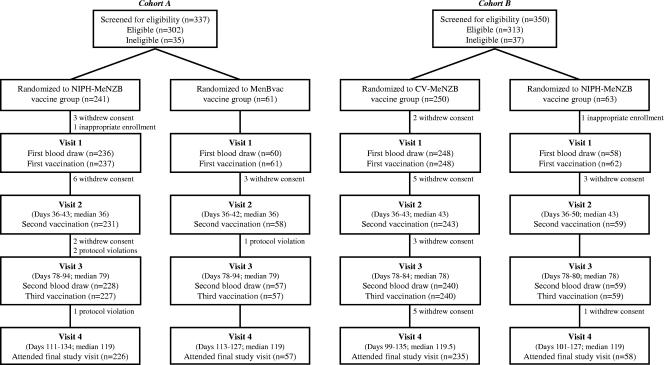
Vaccines were tolerable for 94% of participants completing the study protocol in both cohorts (Fig. 1). The reasons for withdrawal were withdrawal of consent (n = 33), protocol violation (n = 4), and inappropriate enrollment (n = 2). Common reasons for withdrawal of consent included pain at injection site and dislike of phlebotomy.
FIG. 1.
Participant flowcharts.
Immunogenicity.
Baseline serum bactericidal antibody titers of ≥1:4 against the NZ vaccine strain (NZ98/254, B:4:P1.7b,4) were demonstrated in 14% of cohort A children and 11% of cohort B children. Baseline serum bactericidal antibody titers of ≥1:4 against the Norwegian vaccine strain (44/76, B:15:P1.7,16) were demonstrated in 9% of children in cohort A and not evaluated in cohort B.
Cohort A: comparing NIPH-MeNZB (NZ strain) and MenBvac (Norwegian strain). (i) NIPH-MeNZB recipients.
Seroresponse to the homologous NZ vaccine strain (B:4:P1.7b,4, NZ98/254) was seen in over 70% of MeNZB recipients after three doses. Only 16% showed a seroresponse to the heterologous Norwegian parent vaccine strain (B:15:P1.7,16, 44/76) (Table 2). GMTs against the NZ vaccine strain (NZ98/254) (ITT and PP) were 9 (range, 7 to 11) after two doses and 22 (range, 18 to 27) after three doses, with greater than 90% of recipients achieving serum bactericidal antibody titers of ≥1:4 after three doses (Table 3).
TABLE 2.
Preteens classified as seroresponders to meningococcal group B strains following administration of serogroup B OMV vaccinesa
| Cohort and vaccine | Dose no.b | Type of analysis | No. of participants | No. (%) of seroresponders for indicated strainc
|
|
|---|---|---|---|---|---|
| B:4:P1.7b,4 (NZ98/254) | B:15:P1.7,16 (44/76) | ||||
| A | |||||
| NIPH-MeNZB | 2 | ITT | 221 | 44 (38-50) | 10 (7-15) |
| PP | 217 | 43 (36-50) | 10 (7-15) | ||
| 3 | ITT | 220 | 74 (67-79) | 16 (12-21) | |
| PP | 214 | 73 (67-79) | 16 (12-21) | ||
| MenBvac | 2 | ITT | 54 | 28 (18-41) | 44 (32-58) |
| PP | 53 | 28 (18-42) | 45 (33-59) | ||
| 3 | ITT | 56 | 32 (21-45) | 82 (70-90) | |
| PP | 56 | 32 (21-45) | 82 (70-90) | ||
| B | |||||
| CV-MeNZB | 2 | ITT | 230 | 73 (67-78) | ND |
| PP | 230 | 73 (67-78) | ND | ||
| 3 | ITT | 230 | 79 (73-84) | ND | |
| PP | 217 | 79 (73-84) | ND | ||
| NIPH-MeNZB | 2 | ITT | 55 | 71 (58-81) | ND |
| PP | 55 | 71 (58-81) | ND | ||
| 3 | ITT | 55 | 75 (62-85) | ND | |
| PP | 54 | 76 (63-85) | ND | ||
Seroresponders are defined as those showing at least a fourfold increase in serum bactericidal antibody titer compared to the baseline (prevaccination) titer, using interpolated titers. A baseline titer of <1:4 was required to reach a titer of ≥1:8 to be considered a seroresponse.
Serum bactericidal antibody titers were measured following administration of the indicated dose.
Values in parentheses are 95% confidence intervals. ND, test not performed.
TABLE 3.
Preteens with serum bactericidal antibody titers (≥1:4) against New Zealand vaccine strain NZ98/254 (B:4:P1.7b,4) after administration of serogroup B OMV meningococcal vaccinesa
| Cohort and vaccine | Type of analysis | Prevaccinationb
|
Postvaccinationc
|
||
|---|---|---|---|---|---|
| % of children with serum bactericidal antibody titers of ≥1:4d | No. of children | % of children with serum bactericidal antibody titers of ≥1:4d | No. of children | ||
| A | |||||
| NIPH-MeNZB | ITT | 11 (8-16) | 224 | 92 (88-95) | 224 |
| PP | 11 (8-16) | 221 | 93 (88-95) | 214 | |
| MenBvac | ITT | 23 (14-36) | 56 | 54 (41-66) | 56 |
| PP | 23 (14-36) | 56 | 54 (41-66) | 56 | |
| B | |||||
| CV-MeNZB | ITT | 11 (7-15) | 236 | 95 (91-97) | 230 |
| PP | 11 (7-15) | 236 | 94 (90-97) | 217 | |
| NIPH-MeNZB | ITT | 11 (5-22) | 57 | 89 (78-95) | 56 |
| PP | 11 (5-22) | 57 | 89 (77-95) | 54 | |
Children (age range, 8 to 12 years) received three doses of each vaccine, and serum bactericidal antibody titers were measured before and after vaccination.
Prevaccination titers are considered baseline values.
Postvaccination titers were measured 4 to 6 weeks after administration of the third vaccine dose.
A titer of ≥1:4 has been suggested to be a correlate of protection (5, 6, 18).
(ii) MenBvac recipients.
Seroresponse to the homologous Norwegian parent vaccine strain (B:15:P1.7,16, 44/76) occurred in 82% of participants following three vaccine doses. In contrast, seroresponse to the heterologous NZ vaccine strain (B:4:P1.7b,4, NZ98/254) was 32% after three doses (Table 2).
GMTs against the Norwegian parent strain (44/76) were 8 (range, 5 to 12) after two doses and 24 (range, 16 to 36) after three doses (ITT and PP). After three doses of MenBvac, serum bactericidal antibody titers of ≥1:4 occurred in 91% (80 to 96%) against the parent strain (44/76) (ITT and PP). Serum bactericidal antibody titers of ≥1:4 against the heterologous NZ strain (NZ98/254) were demonstrated in 54% after three doses (Table 3).
Cohort B: comparison of NIPH-MeNZB (NZ strain) and CV-MeNZB (NZ strain). (i) CV-MeNZB recipients.
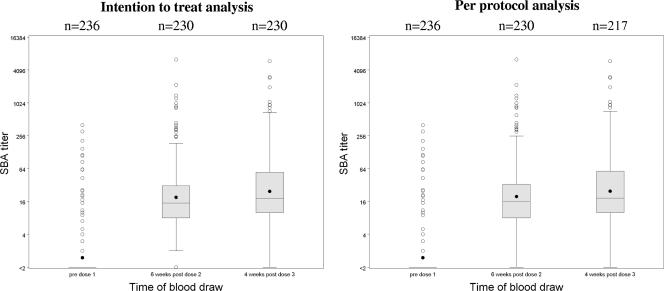
Seroresponse to the homologous NZ vaccine strain (B:4:P1.7b,4, NZ98/254) occurred in 79% of children following three vaccine doses (Table 2). GMTs against the NZ vaccine strain (NZ98/254) were 20 (range, 16 to 24) (ITT and PP) after two doses and 25 (range, 20 to 30) by ITT analysis (24 [range, 20 to 30] by PP) after three doses. The distribution of serum bactericidal antibody titers is shown in Fig. 2. More than 90% demonstrated serum bactericidal antibody titers against NZ98/254 of ≥1:4 after three doses (Table 3).
FIG. 2.
Interpolated serum bactericidal antibody titers against NZ98/254 (B:4:P1.7b,4) in 8- to 12-year-old children who received the CV-MeNZB NZ strain serogroup B OMV meningococcal vaccine. Serum bactericidal antibody (SBA) titers are plotted on the log2 scale. The box represents the interquartile range, the center line the median, the black dot the mean, and circles individual values beyond the whiskers, which extend to the last value within 1.5 times the interquartile range.
(ii) NIPH-MeNZB recipients.
Seroresponse to the homologous NZ vaccine strain (B:4:P1.7b,4, NZ98/254) occurred in at least 75% following three vaccine doses (Table 2). GMTs against the NZ vaccine strain (NZ98/254) were 20 (range, 13 to 30) (ITT and PP) after two doses and 26 (ranges of 17 to 39 by ITT and 17 to 40 by PP) after three doses. Serum bactericidal antibody titers against NZ98/254 of ≥1:4 after three doses were achieved by 89% of children (Table 3).
Cohort A and cohort B NIPH-MeNZB recipients (NZ strain).
The observed difference in response to NIPH-MeNZB between the two cohorts could not be explained by any differences in demographics (sex, ethnicity, age), baseline serum bactericidal titer, or timing of vaccinations or blood draws (Table 2). However, there was a tendency for those with blood draws longer after the time of vaccination to have lower bactericidal titers (P = 0.02).
Reactogenicity and safety.
All vaccines were well tolerated. Reactogenicity results were comparable for all four groups. The only reaction showing a statistical difference was swelling in cohort A; however, this could have occurred by chance, given the number of reactions investigated (Table 4). Analgesic use was lower in cohort B than in cohort A. Acetaminophen was provided on request at enrollment in cohort A and only after contacting the study team in cohort B.
TABLE 4.
Local and systemic reactions: percentages for listed reactions occurring after at least one vaccine dose among 8- to 12-year-old children receiving serogroup B OMV meningococcal vaccines
| Category and reaction | % of children who displayed reaction after receiving vaccine
|
|||
|---|---|---|---|---|
| Cohort Ad
|
Cohort Be
|
|||
| NIPH-MeNZB | MenBvac | MenBvac | CV-MeNZB | |
| Local | ||||
| Pain at injection site | 96 | 92 | 95 | 98 |
| Erythemaa | 25 | 16 | 25 | 24 |
| Swellinga | 17 | 3 | 13 | 15 |
| Indurationa | 22 | 18 | 20 | 18 |
| Systemic | ||||
| Nausea | 22 | 26 | 23 | 19 |
| Malaise | 35 | 31 | 42 | 39 |
| Myalgia | 24 | 18 | 22 | 15 |
| Arthralgia | 18 | 16 | 13 | 15 |
| Headache | 39 | 33 | 44 | 55 |
| Rash | 7 | 7 | 9 | 10 |
| Feverb | 7 | 10 | 7 | 11 |
| Any | 67 | 56 | 64 | 71 |
| Other | ||||
| Stayed home due to vaccinationc | 19 | 15 | 22 | 16 |
| Analgesic use | 77 | 75 | 35 | 48 |
Diameter of the affected area was ≥10 mm.
A fever was defined as a temperature of ≥38.5°C.
The background school absenteeism rate for this age group in New Zealand was 7.2% in 2002 (8).
In cohort A, 237 children received the NIPH-MeNZB vaccine, and 61 children received the MenBvac vaccine.
In cohort B, 248 children received the CV-MeNZB vaccine, and 62 children received the NIPH-MeNZB vaccine.
More-detailed results are presented for the CV-MeNZB vaccine, which was later used in the mass vaccination program (Table 5). Pain at injection site, most commonly mild, was the most frequent adverse event (Table 4 and Table 5). Other local reactions following CV-MeNZB treatment (≥10-mm diameter) were erythema (25%), swelling (13%), and induration (20%) after at least one of the three vaccine doses (Table 4). Headache was the most common systemic reaction (Table 4). In the CV-MeNZB group, the frequency was most commonly mild (Table 5). Systemic reactions were most frequent on the day following vaccination, mostly lasted less than 3 days, and were most frequently mild. The observed local and systemic reactions either remained similar or decreased slightly across doses. Nine serious adverse events (cohort A, n = 4, and cohort B, n = 5) were reported, none of which were judged to be related to the study vaccines.
TABLE 5.
Local and systemic reactions of 8- to 12-year-old children to the CV-MeNZB New Zealand strain serogroup B OMV meningococcal vaccine, arranged by dose number and severity of reaction
| Dose no. (no. of children) | Reaction category | Reaction | % of participants with reaction of indicated severitya
|
||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| 1 (248) | Local | Pain | 44 | 36 | 12 |
| Erythema | 14 | 2 | 1 | ||
| Swelling | 5 | 4 | <1 | ||
| Induration | 6 | 5 | 1 | ||
| Systemic | Nausea | 6 | 2 | 2 | |
| Malaise | 15 | 4 | 3 | ||
| Myalgia | 6 | 2 | 1 | ||
| Arthralgia | 4 | 2 | 0 | ||
| Headache | 17 | 8 | 2 | ||
| Rash | 4 | ||||
| Fever | 2 | ||||
| 2 (243) | Local | Pain | 40 | 28 | 7 |
| Erythema | 8 | 2 | 2 | ||
| Swelling | 3 | 1 | 1 | ||
| Induration | 7 | 2 | <1 | ||
| Systemic | Nausea | 5 | 3 | 2 | |
| Malaise | 11 | 6 | 3 | ||
| Myalgia | 9 | 2 | <1 | ||
| Arthralgia | 4 | 2 | 0 | ||
| Headache | 11 | 6 | 5 | ||
| Rash | 3 | ||||
| Fever | 3 | ||||
| 3 (240) | Local | Pain | 35 | 23 | 7 |
| Erythema | 3 | <1 | <1 | ||
| Swelling | <1 | <1 | <1 | ||
| Induration | 3 | 3 | 0 | ||
| Systemic | Nausea | 5 | 3 | 3 | |
| Malaise | 8 | 6 | 3 | ||
| Myalgia | 5 | 2 | 1 | ||
| Arthralgia | 2 | 1 | <1 | ||
| Headache | 13 | 4 | 3 | ||
| Rash | 3 | ||||
| Fever | 3 | ||||
Mild, no limitation of normal daily activities; moderate, some limitation of normal daily activities; severe, inability to perform normal daily activities. For erythema, swelling, and induration, affected areas were measured and classified as follows: <10 mm, no reaction; 10 to 25 mm, mild reaction; 26 to 50 mm, moderate reaction; and >50 mm, severe reaction. Fever was defined as a body temperature of ≥38.5°C. Rash and fever were not categorized by severity.
DISCUSSION
In this study, the NZ strain vaccine (MeNZB) from both manufacturing sites was immunogenic, safe, and tolerable in 8- to 12-year-old children. In the cohort A trial, the first meningococcal B vaccine trial performed with children in NZ, seroresponses against the NZ vaccine strain (B:4:P1.7b,4, NZ98/254) were demonstrated by 74% (ITT) and 73% (PP) of NIPH-MeNZB vaccine recipients 4 to 6 weeks after dose three. CV-MeNZB and NIPH-MeNZB vaccine recipients in cohort B demonstrated similar seroresponse rates after three doses. Significant but tolerable reactogenicity occurred in both candidate and parent vaccine recipients, with comparable results seen in all groups. Almost all participants experienced pain at the injection site, which is similar to previous experience with the parent vaccine (22).
It is difficult to compare the proportion of participants in this study experiencing fourfold rises in serum bactericidal antibody titers with results reported elsewhere in the literature, as there are differences in the definitions of a fourfold rise from a low or undetectable baseline (20). An interlaboratory study involving laboratories from four countries testing the same sera by serum bactericidal assays showed consistency in the percentages attaining fourfold rises in antibody titer, despite variation in the absolute titers achieved (4, 20).
Previous studies have correlated the presence of serum bactericidal antibody activity with protection from meningococcal disease (15, 16) and have correlated a fourfold rise in serum bactericidal antibody titer postvaccination with vaccine efficacy for serogroup B meningococcal disease in particular (7, 21). Norwegian vaccine efficacy (strain B:15:P1.7,16) after 29 months was 57% in a randomized controlled trial of two doses with youth aged 14 to 16 years (3), while efficacy for the first 10 months of the trial was estimated at 87% (23). Given the suggested correlation between the level of seroresponse and vaccine efficacy, MeNZB is likely to be protective against the NZ epidemic strain of serogroup B meningococcus. For serogroup B meningococcal vaccines, unlike for group C vaccines, the correlate of protection is less secure. A titer of ≥4 and/or a ≥4-fold rise demonstrated by a serum bactericidal-activity assay using human complement has been suggested (5, 6). The decline, at a population level, of serum bactericidal antibody titers below 4 was thought to correlate with breakthrough cases in Norway (18).
Seroresponse after dose two was significantly lower for NIPH-MeNZB vaccine recipients in cohort A than for those in cohort B. The findings of a relationship between time to blood draw and bactericidal titer, while plausible, resulted from a post hoc analysis, so the result needs to be viewed with caution and confirmed in other studies designed to investigate the dynamics of response to this vaccine.
Bactericidal antibody activity against serogroup B strains other than the strain from which the OMV vaccine is derived (heterologous strains) is usually less vigorous than response to the vaccine strain (12, 23, 27, 28). Bactericidal activity to heterologous strains appears to be age dependent, with higher levels seen in older children and adults than in infants (7, 23, 27). A similar trend has been observed in the NZ trials, with 42% of adult recipients (28), 32% of 8- to 12-year-old recipients (cohort A), and less than 5% of 16- to 24-month-old recipients (29) of MenBvac (Norwegian strain vaccine) demonstrating heterologous seroresponses against the NZ vaccine strain after three doses. This finding suggests that there may be limited applications for strain-specific meningococcal vaccines in situations other than epidemics dominated by the strain of the vaccine in question. However, there is some evidence from a case control efficacy study in Brazil that a strain-specific OMV vaccine can provide protection against some serogroup B meningococcal strains other than the vaccine strain (9).
Bactericidal antibody activity decay has been seen after two, three, and four doses of the MenBvac Norwegian parent vaccine (11, 18). MenBvac has been shown to elicit fourfold rises in serum bactericidal antibody titer in 61% of adolescents after three doses and in 90% after a fourth dose 1 year later (11). Further investigation of the decay in bactericidal activity after MeNZB will help to predict the potential duration of protection, and ongoing disease surveillance following mass vaccination will indicate whether a booster dose is necessary for ongoing epidemic control.
This study contributed to licensure with provisional consent for CV-MeNZB (as MeNZB produced by CV) for this age group by Medsafe, the NZ government pharmaceutical regulatory authority. Preliminary safety data from this study enabled further studies of younger age groups to proceed.
The development of an NZ meningococcal B OMV vaccine from the existing parent Norwegian OMV vaccine, utilizing a change in strain, has allowed NZ to respond to a highly strain-specific serogroup B meningococcal epidemic (18, 25). The resulting vaccine, MeNZB, has been shown to be immunogenic and tolerable in this study population of 8- to 12-year-old children, with no safety concerns observed during the trial. After licensure (in 2004), MeNZB was delivered to children aged 6 weeks to 19 years by region based on this and trials with younger age groups (25, 29). Evaluation of vaccine effectiveness by using descriptive methods will be important in the absence of a phase III efficacy trial (2). Postmarketing safety surveillance for rare adverse events was undertaken, accompanying the mass vaccination program (25).
Acknowledgments
We thank the families who participated in this study. We also thank Sarah Douglas and Robyn Beckerleg (research coordinators), Grace Hinder and Frances Ward (study nurse managers), Elizabeth Farrell (public health nurse coordinator), the public health nurses and other nurses involved in the study, principals and staff from the schools involved, members of the Community Advisory Board for the project, staff at ESR, Mark Wakefield and other staff at CV, and the NIPH.
The NZ Ministry of Health and CV (now Novartis Vaccines) funded this study.
D.L., as principal investigator, had access to the data presented in this paper and takes responsibility for the integrity of the data and the accuracy of the data analysis. Other specific author contributions are as follows: study concept and design, D.L., J.S., D.M., P.O., J.H., K.R., J.O., E.Y., S.R., I.A., and S.C.; acquisition of data, J.H., K.R., F.C.M., C.J., D.L., D.M., and P.O.; analysis and interpretation of data, J.H., K.R., F.C.M., C.J., D.L., J.S., D.M., P.O., E.Y., S.R., and I.A.; drafting of the manuscript, J.H., C.J., K.R., F.C.M., J.S., and D.L.; critical revision of the manuscript for important intellectual content, J.H., K.R., F.C.M., C.J., D.L., J.S., J.O., P.O., D.M., E.Y., S.R., I.A., and S.C.; statistical analysis, J.S., C.J., and E.Y.; administrative, technical, or material support, P.O. and D.M.; and study supervision, D.L., P.O., and D.M.
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Agresti, A., and B. Coull. 1998. Approximation is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52:119-126. [Google Scholar]
- 2.Ameratunga, S. N., A. Macmillan, J. Stewart, D. Scott, K. Mulholland, and S. Crengle. 2005. Evaluating the post-licensure effectiveness of a group B meningococcal vaccine in New Zealand: a multi-faceted strategy. Vaccine 23:2231-2234. [DOI] [PubMed] [Google Scholar]
- 3.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, et al. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, R., I. S. Aaberge, G. F. Santos, et al. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab Immunol. 12:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow, R., P. Balmer, and E. Miller. 2005. Meningococcal surrogates of protection—serum bactericidal antibody activity. Vaccine 23:2222-2227. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, R., G. M. Carlone, N. Rosenstein, M. Blake, I. Feavers, D. Martin, W. Zollinger, J. Robbins, I. Aaberge, D. M. Granoff, E. Miller, B. Plikaytis, L. van Alphen, J. Poolman, R. Rappuoli, L. Danzig, J. Hackell, B. Danve, M. Caulfield, S. Lambert, and D. Stephens. 2006. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 24:5093-5107. [DOI] [PubMed] [Google Scholar]
- 7.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, et al. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrave, R., F. Bishop, and N. Bennie. 2003. Attendance and absence in New Zealand schools. New Zealand Ministry of Education, Wellington, New Zealand.
- 9.de Moraes, J. C., M. C. Camargo, H. Barbosa, I. M. Gral, H. Vasconcelos, J. D. Wenger, B. A. Perkins, N. T. Hidalgo, C. T. Sacchi, V. L. Grattas, B. D. Plikaytis, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 10.Dyet, K., A. Devoy, R. McDowell, and D. Martin. 2005. New Zealand's epidemic of meningococcal disease described using molecular analysis: implications for vaccine delivery. Vaccine 23:2228-2230. [DOI] [PubMed] [Google Scholar]
- 11.Feiring, B., J. Fuglesang, P. Oster, L. M. Naess, O. S. Helland, S. Tilman, E. Rosenqvist, M. A. Bergsaker, H. Nokleby, and I. S. Aaberge. 2006. Persisting immune responses indicating long-term protection after booster dose with meningococcal group B outer membrane vesicle vaccine. Clin. Vaccine Immunol. 13:790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findlow, J., S. Taylor, A. Aase, R. Horton, R. Heyderman, J. Southern, N. Andrews, R. Barchha, E. Harrison, A. Lowe, E. Boxer, C. Heaton, P. Balmer, E. Kaczmarski, P. Oster, A. Gorringe, R. Borrow, and E. Miller. 2006. Comparison and correlation of Neisseria meningitidis serogroup B immunologic assay results and human antibody responses following three doses of the Norwegian meningococcal outer membrane vesicle vaccine MenBvac. Infect. Immun. 74:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 14.Fredriksen, J., E. Rosenqvist, and E. Wedege. 1991. Production, characterization and control of MenB-vaccine: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 15.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. II. Development of natural immunity. J. Exp. Med. 129:1327-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison, L. 2006. Vaccine prevention of meningococcal disease: making slow progress. Clin. Infect. Dis. 43:1395-1397. [DOI] [PubMed] [Google Scholar]
- 18.Holst, J., B. Feiring, J. E. Fuglesang, E. A. Hoiby, H. Nokleby, I. S. Aaberge, and E. Rosenqvist. 2003. Serum bactericidal activity correlates with the vaccine efficacy of outer membrane vesicle vaccines against Neisseria meningitidis serogroup B disease. Vaccine 21:734-737. [DOI] [PubMed] [Google Scholar]
- 19.Holst, J., B. Feiring, L. M. Naess, G. Norheim, P. Kristiansen, E. A. Hoiby, K. Bryn, P. Oster, P. Costantino, M. K. Taha, J. M. Alonso, D. A. Caugant, E. Wedege, I. S. Aaberge, R. Rappuoli, and E. Rosenqvist. 2005. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine 23:2202-2205. [DOI] [PubMed] [Google Scholar]
- 20.Martin, D., L. McCallum, A. Glennie, N. Ruijne, P. Blatchford, J. O'Hallahan, and P. Oster. 2005. Validation of the serum bactericidal assay for measurement of functional antibodies against group B meningococci associated with vaccine trials. Vaccine 23:2218-2221. [DOI] [PubMed] [Google Scholar]
- 21.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. Melles, V. S. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nokleby, H., and B. Feiring. 1991. The Norwegian meningococcal group B outer membrane vesicle vaccine: side effects in phase II trials. NIPH Ann. 14:95-101. [PubMed] [Google Scholar]
- 23.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 24.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sexton, K., D. Lennon, P. Oster, S. Crengle, D. Martin, K. Mulholland, T. Percival, S. Reid, J. Stewart, and J. O'Hallahan. 2004. The New Zealand Meningococcal Vaccine Strategy: a tailor-made vaccine to combat a devastating epidemic. N. Z. Med. J. 117:U1015. [PubMed] [Google Scholar]
- 26.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-210. [PubMed] [Google Scholar]
- 27.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Hoiby, J. Holst, H. Nokleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 28.Thornton, V., D. Lennon, K. Rasanathan, J. O'Hallahan, P. Oster, J. Stewart, S. Tilman, I. Aaberge, B. Feiring, H. Nokleby, E. Rosenqvist, K. White, S. Reid, K. Mulholland, M. J. Wakefield, and D. Martin. 2006. Safety and immunogenicity of New Zealand strain meningococcal serogroup B OMV vaccine in healthy adults: beginning of epidemic control. Vaccine 24:1395-1400. [DOI] [PubMed] [Google Scholar]
- 29.Wong, S., D. Lennon, C. Jackson, J. Stewart, S. Reid, S. Crengle, S. Tilman, I. S. Aaberge, J. O'Hallahan, P. Oster, K. Mulholland, and D. Martin. 2007. New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16-24 months. Pediatr. Infect. Dis. J. 26:345-350. [DOI] [PubMed] [Google Scholar]