Abstract
To add new insight to our previous work on the molecular epidemiology of Bordetella pertussis in Argentina, the prn and ptxS1 gene sequences and pulsed-field gel electrophoresis (PFGE) profiles of 57 clinical isolates obtained during two periods, 1969 to 1989 and 1997 to 2006, were analyzed. Non-vaccine-type ptxS1A was detected in isolates obtained since 1969. From 1989 on, a shift of predominance from the vaccine prn1 type to the nonvaccine prn2 type was observed. This was also reflected in a transition of PFGE group IV to group VI. These results show that nonvaccine B. pertussis strains are currently circulating. To analyze whether the observed genomic divergences between vaccine strains and clinical isolates have functional implications, protection assays using the intranasal mouse challenge model were performed. For such experiments, the clinical isolate B. pertussis 106 was selected as representative of circulating bacteria, since it came from the major group of the PFGE dendrogram (PFGE group VI). Groups of mice were immunized either with diphtheria-tetanus-whole-cell pertussis vaccine (ptxS1B prn1) or a vaccine prepared by us containing B. pertussis 106. Immunized mice were then challenged with a B. pertussis vaccine strain (Tohama, harboring ptxS1B and prn1) or the clinical isolate B. pertussis 106 (ptxS1A prn2). An adequate bacterial-elimination rate was observed only when mice were immunized and challenged with the same kind of strain. For further characterization, comparative proteomic profiling of enriched membrane proteins was done using three vaccine strains and the selected B. pertussis 106 clinical isolate. By matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis, a total of 54 proteins were identified. This methodology allowed us to detect differing proteins among the four strains studied and, in particular, to distinguish the three vaccine strains from each other, as well as the vaccine strains from the clinical isolate. The differing proteins observed have cellular roles associated with amino acid and carbohydrate transport and metabolism. Some of them have been proposed as novel vaccine candidate proteins for other pathogens. Overall, the global strategy described here is presented as a good tool for the development of next-generation acellular vaccines.
Pertussis is an infectious respiratory disease caused by the bacterium Bordetella pertussis, an exclusively human pathogen. This disease, though preventable by vaccination, remains one of the top 10 causes of death worldwide in childhood. Moreover, in countries with highly vaccinated populations, a pertussis resurgence has been reported (2, 13, 14, 19, 36, 40, 43, 50). Several explanations have been suggested for this resurgence in such populations: low efficacy of the vaccine used, waning immunity in the absence of both natural and vaccinal boosters after 18 months of age, and adaptation of the circulating B. pertussis population (24, 27, 31, 39, 41, 46, 51). One explanation is based on studies of B. pertussis strains, first from The Netherlands and then from other countries, that focused on genomic analysis by pulsed-field gel electrophoresis (PFGE) and on sequences of the genes that code for two immunogens, pertactin (prn) and the S1 subunit of pertussis toxin (ptxS1) (8, 19, 21, 26, 37, 40, 41). Data from these studies have been interpreted to suggest that vaccination selects for strains that have different genotypes from those used to make the vaccine, allowing the spread of escape mutants (15, 34, 40). This vaccine-driven antigenic evasion seems not to be exclusive to pertussis, since a growing body of evidence indicates it exists for at least three other diseases: hepatitis B virus, Streptococcus pneumoniae, and Haemophilus influenzae infections (5, 7, 10, 11, 44, 47, 48).
In Argentina during previous years, an increase in the pertussis incidence was registered by the Public Health Ministry, even before improvements in diagnostic methodology were introduced. In that country, whole-cell pertussis vaccine was introduced in the 1960s, and it is still the only kind of pertussis vaccine being used. Since 1985, the vaccination schedule has been 2, 4, 6, and 18 months of age, with a school entry booster at 5 to 6 years of age. Molecular characterization of B. pertussis isolates (n = 28) obtained in Argentina from 1997 to 2003 was done (19). The isolates appeared to be genotypically different from the vaccine strain in use. The isolates were comprised of ptxS1A (100%) and prn2 (93%) or prn1 or prn7 (7%) variants. These allelic variants are different from those present in the vaccine strain (ptxS1B or ptxS1D and prn1) but identical to those found in European and North American countries.
Though more epidemiological and molecular data should be collected in order to describe more precisely the pertussis disease scenario, it is clear that the control of pertussis requires improvement. Currently used pertussis vaccines in many countries are based on strains, or proteins obtained from strains, isolated before 1957, which are different from the currently circulating bacteria.
Thus, novel proteins from contemporary isolates should be considered in the design of new acellular vaccines. Surface proteins play a fundamental role in the interaction between the bacterial cell and its environment; hence, these bacterial constituents may become components of such vaccines. Comparison of multiple exposed proteins in B. pertussis strains used at present in vaccine production with contemporary circulating strains may allow the identification of new immunogens that can be incorporated in a new, broadly protective vaccine.
In this study, a procedure that allows the identification of surface-accessible proteins in B. pertussis is described for the first time. This methodology was applied to compare enriched membrane samples obtained from well-known laboratory-adapted B. pertussis strains widely used for vaccine production and a B. pertussis local isolate. The results obtained revealed that genomic divergence that seems to have a role in pertussis protection, as demonstrated here using the accepted animal model, could be extended to the bacterial surface proteome. Thus, this new insight into proteins located on the surfaces of B. pertussis strains may assist in the identification of potential vaccine candidates. Overall, this report presents an attractive approach for comparison of the surfaces of Bordetella strains and provides a framework for future proteomic analyses.
MATERIALS AND METHODS
Clinical isolates.
In this study, we characterized 57 B. pertussis isolates, mostly collected from patients residing in Buenos Aires (Argentina) from 1997 to 2006. Isolates from 1969, 1971, 1982, and 1989 were also included. Nasopharyngeal aspirate and Dacron swab samples were obtained from patients with a clinical diagnosis of pertussis, and Bordet-Gengou agar (BGA) supplemented with defibrinated sheep blood was used for primary culture. Inoculated plates incubated at 36°C were monitored daily for 10 days. Suspected colonies were Gram stained and tested by agglutination test with antiserum for B. pertussis (Murex Diagnostic, Dartfort, England) and specific B. pertussis PCR (22, 32). Biochemical typing using the API 20 NE system (bioMérieux, Marcy l'Etoile, France) was also performed on the clinical isolates. The ages of patients ranged from 1 month to 5 years, with the median age being 4 months. Thirty-three patients from whom bacteria were isolated were less than 18 months old, and only one was 5 years old. For the other positive culture patients, no data on age were reported.
PCR, sequencing, and PFGE.
PCR, sequencing, and PFGE were performed according to standardized recommendations for B. pertussis typing (38). Bacteria were cultivated on BGA containing defibrinated sheep blood at 36°C for 72 h. For PFGE, B. pertussis strains 134 (United States), 509 (The Netherlands and Mexico), 10536, Tohama (Japan), and 18323 (United States) were used as reference strains.
Briefly, DNA sequencing of relevant regions of the prn and ptxS1 genes was performed on PCR fragments as described by Mooi et al. (38). For sequencing of region 1, the primer pair AF/AR (5′-CAATGTCACGGTCCAA-3′/5′-GCAAGGTGATCGACAGGG-3′) was used, and for sequencing of region 2, the primer pair BF/BR (5′-AGCTGGGCGGTTCAAGGT-3′/5′-CCGGATTCAGGCGCAACTC-3′) was used. For pertussis toxin, primers S1-F2 (5′-CCCCTGCCATGGTGTGATC-3′) and S1-R2 (5′-AGAGCGTCTTGCGGTCGATC-3′) were used to amplify a 930-bp product that contained the complete ptxS1 gene. S1-F2/S1-R2 were also used for sequencing (18). Mutations were confirmed by sequencing using two additional primers, S1-MF/S1-MR (5′-ACAATGCCGGCCGTATCCTC-3′/5′-TTCGAAGTACGAGCTGGCGG-3′).
All clinical isolates and reference strains were genotyped by PFGE using the restriction enzyme XbaI (Promega) with a CHEF-DR III apparatus (Bio-Rad Laboratories) at 6 V/cm with a 120° angle and pulses of 5 to 6 s for 19 h at 14°C.
Gels were stained for 40 min in 500 ml deionized water containing 50 μl of a 10-mg/ml solution of ethidium bromide. Unbound ethidium bromide was removed by washing the gels in deionized water for 60 min. DNA fragments were visualized on a UV transilluminator, and the gels were photographed with a Gel Doc 2000 system.
The Tohama strain was used in each gel as an external quality control to assess whether the reproducibility of its banding pattern was consistent with those previously published and between runs.
BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium) version 3.5 software was used to normalize the DNA fragment migration distances relative to those of Salmonella enterica serotype Braenderup strain H9812. DNA fragments were analyzed, with at least one band discrepancy as the basis for discriminating among profiles.
The unweighted pair group method using arithmetic averages was used as the clustering method, with a 1% band tolerance and 1% optimization settings with the Dice coefficient. All profiles were verified by visual comparison.
Murine respiratory-infection model.
Female BALB/c mice at 3 to 4 weeks of age from Biol SAIC, Argentina, were used as a model of in vivo respiratory infection by B. pertussis. Bacteria grown on BGA were resuspended and adjusted to approximately 108 CFU ml−1 in phosphate-buffered saline (PBS). Fifty microliters of a bacterial suspension of each strain studied was delivered intranasally to each mouse via an air displacement pipette. At different times postinoculation, three mice from each group were sacrificed, and the lungs were removed aseptically. The lungs were homogenized in PBS, and appropriate dilutions were plated onto BGA to determine the number of viable bacteria present in the lungs. All the experiments were performed two times, and they gave consistent results.
Active immunization and intranasal challenge.
Female BALB/c mice were obtained at 4 weeks of age from Biol SAIC, Argentina. The animal protocol was performed according to the study by Guiso et al. (23). Groups of BALB/c mice were immunized intraperitoneally with 1/10 of the human dose of diphtheria- tetanus-whole-cell pertussis vaccine (ptxS1B prn1), following a two-dose schedule over a period of 2 weeks. The mice were challenged 2 weeks after the second immunization by nasal challenge with a B. pertussis vaccine strain (Tohama, harboring ptxS1B and prn1) or the clinical isolate B. pertussis 106 (ptxS1A prn2) delivered from a culture containing 108 bacteria per ml. We also evaluated the potential for bacterial clearance of a vaccine prepared by us containing a B. pertussis 106 clinical isolate (wPBp106).
The lungs of infected animals were aseptically removed, homogenized in sterile PBS, serially diluted, and then plated on BGA plates to determine bacterial recovery at different time points during the course of infection. The outcome of the challenge was followed by performing CFU counts on lung homogenates from individual mice.
All the experiments were performed three times, and they gave consistent results.
Statistical analysis.
Means and standard deviations were calculated from log10-transformed CFU numbers. Differences among means were assessed by two-tailed Student's t tests, with significance accepted as a P value of <0.05.
Membrane protein enrichment.
The membrane fraction of B. pertussis was prepared as described previously (6) with minor modifications. B. pertussis cells were harvested by centrifugation (10,000 × g; 30 min; 4°C) and washed twice with low-salt washing buffer containing 3 mM KCl, 68 mM NaCl, 1.5 mM KH2PO4, and 9 mM NaH2PO4. The cells were suspended in 10 mM Tris-HCl (pH 8.5) supplemented with phenylmethylsulfonyl fluoride and protease inhibitor cocktail tablets (Roche Applied Science) and then disrupted with an ultrasonicator (Sonics & Materials, Inc., Danbury, CT). DNase and RNase (20 μg/ml each) were added to the cell suspension, and the mixture was incubated at 37°C for 1 h. The unbroken cells were removed by centrifugation (12,000 × g; 30 min; 4°C), and the supernatant was retained. Total membrane proteins were then collected by centrifugation (30,000 × g; 1 h; 4°C) and resuspended in 7 M urea, 2 M thiourea, 10% isopropanol, and 2% Triton X-100. Membrane proteins were divided into aliquots and stored at −20°C until they were used.
For each strain, sample preparation, two-dimensional electrophoresis (2-DE), and protein identification were repeated at least four times to establish reproducibility.
Protein quantification.
Protein concentrations were determined by the Bradford method (4), with bovine serum albumin as a standard.
2-DE.
Isoelectric focusing (IEF) was performed by using immobilized pH gradient strips (Immobiline DryStrip; Amersham Biosciences). Portions (200 μg) of the membrane proteins were applied to 7-cm strips in a pH range from 4.0 to 7.0. The immobilized pH gradient strips were rehydrated overnight at room temperature with 125 μl of rehydration buffer (7 M urea, 2 M thiourea, 10% isopropanol, and 2% Triton X-100) plus 1.25 μl 28% dithiothreitol (DTT), 0.62 μl 0.5% ampholyte (pH 4.0 to 7.0 [Amersham]), and 0.01% bromophenol blue containing 200 μg of proteins. Three preset programs were executed with slight modifications so that the focusing conditions consisted of the conditioning step, voltage ramping, and final focusing.
The purpose of the conditioning step (500 V for 30 min) was to remove salt ions and charged contaminants, and this was followed by linear voltage ramping for 30 min at 1,000 V. In the final focusing step, the maximum voltage of the ramp step was maintained (up to 5,000 V·h) and the current did not exceed 50 μA/strip. After IEF, the strips were equilibrated in 50 mM Tris buffer (pH 8.8) containing 6 M urea, 2% sodium dodecyl sulfate, 30% glycerol, and 1% DTT, followed by another 1-h equilibration step with the same buffer supplemented with 4.5% iodoacetamide. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed according to the Laemmli method (35) with a 12.5% resolving polyacrylamide gel without a stacking gel. Separation in the second dimension was carried out at 40 V at 4°C until the running dye reached the bottom.
Coomassie staining and gel drying.
Proteins resolved on gels were visualized by using a colloidal Coomassie staining method (http://prospector.ucsf.edu) with slight modifications. Briefly, the gel was fixed in a solution containing 30% ethanol-2% phosphoric acid with double-distilled water (ddH2O) three times for 30 min each time. All incubations were performed with gentle shaking. After a fixing step, the gel was washed with a solution of 2% phosphoric acid in ddH2O three times for 20 min each time. The wash solution was discharged, and the gels were fixed again with a solution containing 2% phosphoric acid (vol/vol), 18% ethanol (vol/vol), and 15% ammonium sulfate (wt/vol) in ddH2O for 30 min. To this solution, a 1.5% volume of a suspension of 2% colloidal Coomassie (G-250) in ddH2O was added. The duration of this step was 24 to 72 h.
Image analysis.
A gel image was obtained by scanning Coomassie-stained gels with the Fluor-S MultiImager (Bio-Rad). The image was documented through the PDQUEST 2-D gel analysis software (version 6; Bio-Rad).
In-gel tryptic digestion of proteins.
Coomassie-stained spots were excised from 2-DE gels and transferred into microcentrifuge tubes. Plugs were washed with 100 μl 25 mM ammonium bicarbonate for 10 min and incubated for 30 min at 60°C in 20 μl of 5 mM DTT in 25 mM ammonium bicarbonate solution. This step was followed by another incubation for 15 min at room temperature in darkness with 20 μl 55 mM iodoacetamide in 25 mM ammonium bicarbonate. The solution was discharged, and a new wash with 25 mM ammonium bicarbonate was performed, followed by another wash with 100 μl acetonitrile. Then, the spots were subjected to a dehydration step three times with 25 μl acetonitrile for 10 min at room temperature. When the gel spots became completely white, the acetonitrile was eliminated by evaporation. Spot rehydration was performed with small volumes (up to 20 μl) of 50 mM ammonium bicarbonate containing trypsin (20 μg/ml) and incubation for 45 min at 4°C. The tryptic digestion was carried out overnight at 37°C. After digestion, the spots were washed twice with 20 μl acetonitrile-0.1% trifluoroacetic acid (33:66) for 10 min, and the supernatants were collected. Sample concentration was performed in a Speedvac.
Peptide mass fingerprinting.
A matrix solution composed of α-cyano-4-hydroxy cinnamic acid (0.2 g/liter) in 50% acetonitrile and 0.25% trifluoroacetic acid was prepared for peptide mass fingerprinting. A 0.4-μl mixture of matrix and sample solutions was applied to the target well. The solution mixture was dried for 10 min at room temperature and subjected to a matrix-assisted laser desorption ionization-time of flight mass spectrometry operation by using an Ultraflex (Bruker) with the following parameters: 20-kV accelerating voltage, 75% grid voltage, 0.02% guide wire voltage, 70-ns delay, and a mass gate of 800 to 3,000.
Identification of proteins.
Searches for and identification of peptides were performed with a licensed version of MASCOT software (Matrix Science) in a database containing the 3,436 accession number entries derived from the complete B. pertussis genome sequence (downloaded from http://www.ncbi.nlm.nih.gov/). The MASCOT search parameters were as follows: (i) species, bacteria (eubacteria); (ii) allowed number of missed cleavages (only for trypsin digestion), one; (iii) variable posttranslational modification, methionine oxidation; (iv) fixed modification, carbamidomethylation; (v) peptide tolerance, ±50 ppm; (vi) peptide charge, positive; and (vii) monoisotopic peptide masses were used to search the database, allowing a molecular mass range for 2-DE analyses of ±15%. Only significant hits as defined by MASCOT probability analysis were considered.
Prediction of protein localization was carried out using a PSORT algorithm available at http://psort.nibb.ac.jp.
RESULTS AND DISCUSSION
Genotypic analysis.
Polymorphism and variation in virulence factors and strains have been observed in different countries that use whole-cell pertussis vaccines where nonvaccine strains are circulating (9, 19, 21, 25, 30, 36, 49, 50). In this study, we used PFGE and prn and ptxS1 typing to add new insight into the characterization of B. pertussis isolates obtained in Argentina in two periods: 1969 to 1989 and 1997 to 2006.
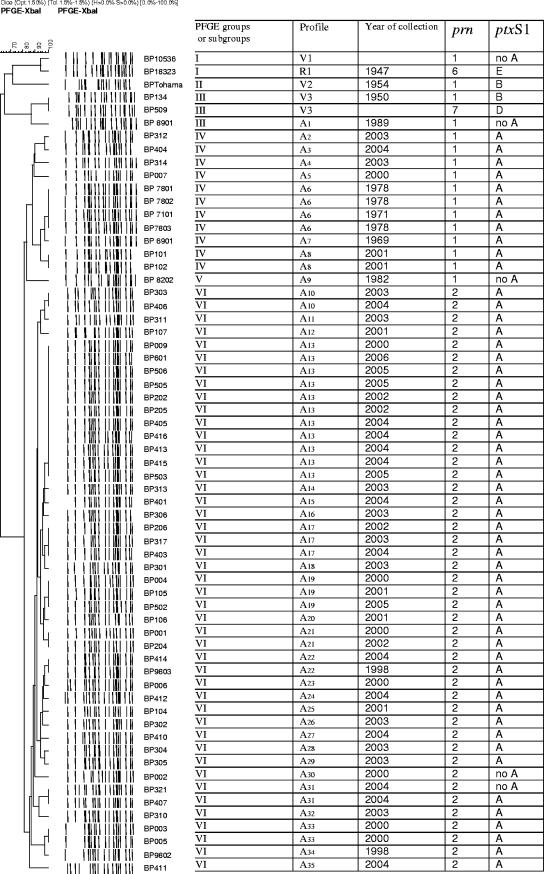
Since it was previously reported that PFGE analysis with XbaI gave optimal discrimination (1, 28), we used this enzyme to classify the vaccine strains and local isolates into PFGE profile groups. Figure 1 shows the dendrogram of DNA profile relatedness calculated by the unweighted pair group method using arithmetic averages clustering of Dice coefficients. A total of 39 distinct PFGE profiles, named V1 to V3 for vaccine strains, R1 for the reference strain, and A1 to A35 for clinical isolates, were observed. These profiles were classified into six major groups (I to VI) based on a criterion of similarity higher than 0.70. The three groups corresponding to the vaccine strains B. pertussis 10536 (group I, which also includes the WHO reference strain 18323), Tohama (group II), and B. pertussis 134 and B. pertussis 509 (group III, which also includes the clinical isolate B. pertussis 8901) had low similarity with all Argentinean clinical isolates tested, with relatedness of 67%. Most Argentinean isolates fell in a small number of PFGE groups. Eleven isolates were segregated into PFGE group IV, one into PFGE group V, and 44 into PFGE group VI. PFGE group IV was the only one detected among the strains from earlier than 1982, while only a single 1982 isolate corresponded to PFGE group V. While 6 contemporary B. pertussis isolates (12%) were clustered in PFGE group IV, 44 (88%) were grouped in PFGE group VI. PFGE group IV seems to have been dominant till at least 1982, and group VI has been dominant from 1998/2000 to 2006 (Fig. 1).
FIG. 1.
Genomic analysis of Argentinean B. pertussis isolates. On the left are shown chromosomal DNA profiles obtained after digestion with XbaI.
This lower-than-expected level of B. pertussis population diversity may be a consequence of sequential bottlenecks or fitness differences between the strains that favored only those variants able to evade the immunity induced by vaccination or with increased virulence. That is, circulating bacteria could have overcome the strong bottleneck of the immunity induced by the vaccine strain by accumulating mutations that allowed them to evade host responses.
Differences between circulating bacteria and vaccine strains were also observed when the sequences of ptxS1 and prn alleles (38) were analyzed. Studies of ptxS1 subunit polymorphism showed that 53 B. pertussis isolates contained the ptxS1A allele and only 4 had non-A ptxS1. By contrast, the vaccine strains used in Argentina contain ptxS1B or ptxS1D alleles. For pertactin, a significant correlation was observed between the PFGE groups and prn alleles. All 11 of the isolates from group IV contained prn1, and all isolates from group VI had the prn2 allele. It seems that prn2 has replaced the prn1 present in old isolates.
In the United Kingdom and Finland, a change from prn1 to prn2 was observed in isolates from as early as 1982, in The Netherlands from 1990, and in Taiwan from 1997 (16, 21, 36, 41). These same variations seem to support a possible explanation that the impact of the vaccination-induced immune responses to these epitopes that have been recorded were strong enough to structure the population into discrete combinations of epitopes or antigenic types. By contrast, under weak immune selection, a greater antigenic and genetic variability of the bacterial population is expected, as occurs, for example, with the poorly immunogenic meningococcus serogroup B capsular polysaccharide, which shows high genetic variability (3).
Though the genotypic divergences between the vaccine strains and the circulating bacterial population are clear, important questions remain. Among them is whether the B. pertussis population genotypic variation has affected vaccine efficacy and contributed to resurgence. Though it seems to be too early to predict this, since the shift in virulent B. pertussis alleles has continued over the years, it is important to begin to analyze the consequence of the structural genomic variation in pertussis protection using the accepted animal model.
Animal studies.
To analyze the implications of divergence between vaccine strains and clinical isolates for protection, we used an intranasal mouse challenge model. Briefly, this model consists of two immunizations of mice (3- to 4-week-old BALB/c mice) with either current pertussis whole-cell vaccine or a vaccine prepared by us containing the representative B. pertussis 106 clinical isolate. This isolate was selected to represent the circulating bacterial population because it comes from the most differentiated and major PFGE group (PFGE group VI). The immunized mice were then intranasally challenged with sublethal doses of either a B. pertussis vaccine strain or the representative clinical isolate, B. pertussis 106. Control animals were vaccinated with PBS. Two hours later and on days 5 and 8 after the challenge, the lungs were collected for bacterial counts. As expected, significant differences between immunized animals and the control group were observed (P < 0.001) (Table 1). Adequate elimination rates were observed in mice immunized and challenged with the same kind of strain (immunization and challenge with a vaccine strain). However, a lower potential of protective immunity induced by whole-cell vaccination was observed in elimination rates of the clinical B. pertussis isolate B. pertussis 106 (prn2 ptxS1A) from the lungs than for the vaccine strain Tohama (prn1 ptxS1B) (P < 0.001) (Table 1). Furthermore, when mice were immunized with a vaccine containing a clinical isolate and challenged with a vaccine strain or antigenically divergent clinical isolates, lower clearance efficiency for the vaccine strain was induced (P < 0.002) as early as day 5 after challenge (Table 1).
TABLE 1.
Lung clearance efficiency of diphtheria-tetanus- whole-cell pertussis vaccine and wPBp106 in intranasal-challenge model experiments
| Immunization | B. pertussis challenge strain/isolate | Bacteria recovered after challenge (log10 CFU/lung ± SD) on daya:
|
||
|---|---|---|---|---|
| 0 | 5 | 8 | ||
| PBS | Tohama | 6.70 ± 0.13 | 6.45 ± 0.30 | 6.05 ± 0.28 |
| DTwP | Tohama | 6.45 ± 0.73 | 5.20 ± 0.04 | −b |
| wPBp106 | Tohama | 6.90 ± 0.8 | 3.20 ± 0.19 | ND |
| PBS | 106 | 6.79 ± 0.13 | 6.51 ± 0.30 | 5.86 ± 0.28 |
| DTwP | 106 | 6.45 ± 0.02 | 5.41 ± 0.26 | 5.34 ± 0.05 |
| wPBp106 | 106 | 6.80 ± 0.04 | 1.99 ± 0.74 | ND |
Values shown are means of three or four animals sacrificed at each time point. ND, not determined.
CFU were not detected in the lung samples.
These results could not be attributed to differences in colonization abilities between the vaccine strain and the clinical isolate, since the number of colonies recovered at each time in infection experiments were the same for both strains (not shown). Overall, these results clearly demonstrate that the efficacies of whole-cell vaccines depend on the relatedness between the vaccine strains and the circulating strains. In addition, the present observations support the hypothesis of the role of divergence of circulating strains in pertussis resurgence.
Proteomic analysis.
For further characterization of bacterial divergence, we compared the bacterial surfaceomes of three known B. pertussis strains that are widely used for vaccine production and that clustered in different PFGE groups: B. pertussis 10536 (PFGE group I), Tohama I (PFGE group II), and 509 (PFGE group III), and extended this analysis to the representative local isolate, B. pertussis 106 (PFGE group VI). Several protocols have been developed for the preparation and analysis of membrane proteins, but none of them have yet been used for Bordetella. The procedure employed here allowed us to obtain an enriched fraction of surface proteins as established by PSORT analysis. Of the 54 proteins successfully identified, 9 were predicted to be associated with the external membrane, 6 had periplasmic localization, 6 had cytoplasmic/membrane localization (5 harbored signal peptide), 16 had an unknown origin (3 had signal peptide), and 17 had a cytoplasmic localization (Table 2). In the last group, some of the proteins, such as EF-Tu, ET TS, enolase, 60-kDa chaperonin, and some dehydrogenases, may be associated with membranes, as was observed in Neisseria meningitidis (17).
TABLE 2.
Surface proteome of B. pertussis vaccine strains Tohama, 10536, and 509 and Argentinean clinical isolate 106
| Order | Protein name/function | Gene locusa | Mass (kDa) | pI | Protein localizationb | Presence of gene in strainc:
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Tohama | 509 | 10536 | 106 | ||||||
| 1 | Competence lipoprotein precursor | BP1146 | 29.8 | 5.0 | Unknown | Yes | Yes | Yes | Yes |
| 2 | Conserved hypothetical protein | BP3441 | 19.8 | 5.1 | Cytoplasmic/membranec | Yes | Yes | Yes | Yes |
| 3 | Adhesin | BP2667 | 263.6 | 9.7 | Outer membrane | Yes | Yes | Yes | Yes |
| 4 | Putative lipoprotein | BP1296 | 30.6 | 7.4 | Unknown | Yes | Yes | Yes | Yes |
| 5 | Succinate dehydrogenase catalytic subunit | BP2360 | 27.2 | 6.2 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 6 | Outer membrane protein OMPQ | BP3405 | 39.1 | 5.7 | Outer membranec | Yes | Yes | Yes | Yes |
| 7 | Outer membrane porin protein precursor | BP0840 | 41 | 5.4 | Outer membrane | Yes | Yes | Yes | Yes |
| 8 | Putative ABC transport ATP binding protein | BP3757 | 29.6 | 5.1 | Unknown | Yese | Yes | Yes | Yes |
| 9 | Putative periplasmic solute binding protein | BP1487 | 40 | 7.8 | Unknownc | Yes | Yes | Yes | Yes |
| 10 | Putative binding protein-dependent transport protein | BP3322 | 40.9 | 6.94 | Periplasmic | Yes | No | Yes | Yes |
| 11 | Putative membrane protein | BP1440 | 33.4 | 5.3 | Unknownc | Yese | Yes | Yes | Yese |
| 12 | Antioxidant protein | BP0965 | 23.7 | 5.7 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 13 | Serin protease | BP2434 | 52.1 | 8.8 | Periplasmic | Yes | Yes | Yes | Yes |
| 14 | Chaperonin, 60 kDa | BP3495 | 57.4 | 4.9 | Cytoplasmicd | Yes | Yes | Yes | Yes |
| 15 | Serum resistance protein | BP3494 | 103.3 | 7.1 | Outer membrane | Yes | Yes | Yes | Yes |
| 16 | EF Tu | BP0007 | 42.9 | 5.1 | Cytoplasmicd | Yes | Yes | Yes | Yes |
| 17 | Leu-Ile-Val protein precursor | BP1285 | 39.6 | 6.8 | Periplasmic | Yes | Yes | No | No |
| 18 | DNA direct RNA α subunit polymerase | BP3642 | 36.1 | 5.7 | Cytoplasmic | Yes | Yes | No | Yes |
| 19 | Pertactin | BP1054 | 93.4 | 10 | Outer membrane | Yes | Yes | Yes | Yes |
| 20 | Putative quino protein | BP2196 | 40 | 8.7 | Unknown | Yes | Yese | Yes | Yes |
| 21 | Putative extracellular solute binding protein | BP3862 | 57.3 | 9.7 | Cytoplasmic/membranec | Yes | Yes | Yes | No |
| 22 | Putative molibdopterin oxidoreductase | BB0468 | 121.6 | 7.3 | Periplasmic | No | No | Yes | No |
| 23 | Outer membrane protein A precursor | BP0943 | 20.9 | 9.2 | Outer membranec | Yes | Yes | Yes | Yes |
| 24 | Superoxide dismutase | BP2761 | 21.2 | 6.5 | Unknown | Yes | Yes | Yes | Yes |
| 25 | Putative exported protein | BP2513 | 34.9 | 10.2 | Outer membrane | Yes | Yes | Yes | Yes |
| 26 | Ubiquinol cytochrome c reductase iron sulfur subunit | BP0277 | 22.8 | 5.2 | Cytoplasmic/membranec | Yes | Yes | Yes | Yes |
| 27 | Putative exported protein | BP2755 | 189 | 6.2 | Cytoplasmic/membranec | Yes | Yes | Yes | No |
| 28 | 3-Oxoacyl-(acyl carrier protein) synthase | BP2439 | 43.6 | 5.7 | Cytoplasmatic | No | No | No | Yes |
| 29 | Putative bacterial secretion system protein | BP3794 | 29.4 | 6.8 | Unknownc | Yes | Yes | Yes | Yes |
| 30 | Enolase | BP2386 | 45.9 | 4.5 | Cytoplasmicd | Yes | Yes | Yes | Yes |
| 31 | Two-component sensor protein | BP2483 | 97.4 | 8.7 | Cytoplasmic/membrane | Yes | Yes | No | No |
| 32 | Serum resistence protein | BP3494 | 103.3 | 7.1 | Outer membrane | Yes | Yes | Yes | Yes |
| 33 | Succinate dehydrogenase flavo subunit | BP2361 | 64.8 | 6.5 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 34 | Putative ABC transport solute binding protein | BP2747 | 40.6 | 6.5 | Cytoplasmic/membranec | Yes | Yes | Yes | Yes |
| 35 | ATP synthase subunit B | BP3288 | 50.5 | 4.7 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 36 | EF Ts | BP1420 | 30.9 | 5.1 | Cytoplasmicd | Yes | Yes | Yes | Yes |
| 37 | Ribose phosphate pyrophosphokinase | BP3125 | 34.1 | 5.1 | Unknown | No | No | No | Yes |
| 38 | Dihydrolipoamide dehydrogenase | BP0995 | 62.3 | 5.8 | Cytoplasmic | No | No | No | Yes |
| 39 | Hypothetical protein | BP3128 | 68.5 | 6.1 | Unknown | No | Yes | Yes | Yes |
| 40 | Hypothetical protein | BP3515 | 35.9 | 6.6 | Unknown | Yes | Yes | Yes | Yes |
| 41 | Polysaccharide biosynthesis protein | BP3150 | 46.7 | 5.6 | Cytoplasmic | No | No | No | Yes |
| 42 | Capsular polysaccharide biosynthesis protein | BP1630 | 37.3 | 5.7 | Unknown | No | No | No | Yes |
| 43 | Putative l-lactate dehydrogenase | BP0379 | 37.2 | 5.8 | Unknown | No | No | No | Yes |
| 44 | Hypothetical protein | BP2964 | 48.5 | 6.2 | Cytoplasmic | Yes | Yes | No | Yese |
| 45 | Hypothetical protein | BP1203 | 42.7 | 6 | Unknown | Yes | Yes | Yes | Yes |
| 46 | Putative sigma factor regulatory protein | BP2435 | 39.2 | 9.6 | Periplasmic | No | No | No | Yes |
| 47 | Enoyl-acyl carrier protein | BP3215 | 27.6 | 5.8 | Unknown | Yes | Yes | Yes | Yes |
| 48 | Lipoprotein | BP2750 | 23.1 | 7.7 | Unknown | Yes | Yes | Yes | Yes |
| 49 | Alkyl hydroperoxide reductase | BP3552 | 20.1 | 4.9 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 50 | Putative penicillin binding protein precursor | BP0102 | 44.8 | 7.9 | Periplasmic | No | Yes | Yes | Yes |
| 51 | Dihydrolipoamide dehydogenase | BP1126 | 50.1 | 6.7 | Cytoplasmic | No | No | No | Yes |
| 52 | Trigger factor | BP1774 | 47.5 | 4.9 | Cytoplasmic | Yes | Yes | Yes | Yes |
| 53 | Putative type III secretion protein | BP2235 | 63.3 | 5.9 | Outer membrane | No | No | No | Yes |
| 54 | Hypothetical protein | BP3559 | 37.9 | 4.7 | Cytoplasmic | Yes | Yes | Yes | Yes |
Gene loci are named according to NCBI (http://www.ncbi.nlm.nih.gov/).
Protein localization is as predicted by PSORT (http://psort.nibb.ac.jp).
Signal peptide present.
Membrane associated in other bacteria.
Greater quantity.
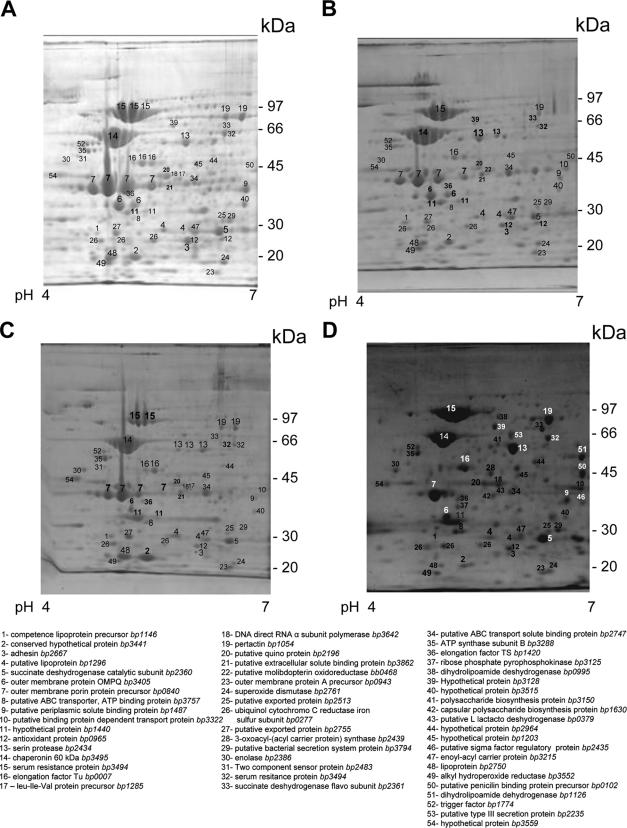
Some of the proteins separated by 2-DE were present as multiple spots exhibiting variability in pI values (horizontal spot patterns) (Fig. 2). Charge variants included EF-Tu, 60-kDa chaperonin, outer membrane porin protein precursor, serum resistance protein, and serine protease. These may represent natural isoforms or an artifact caused by sample preparation or 2-DE. Similarly, serum resistance protein resolved in multiple spots of differing masses and pIs, suggesting possible protein processing, degradation, and/or modifications (Fig. 2).
FIG. 2.
Separation and identification of membrane-related proteins in B. pertussis enriched membrane samples using two dimensional polyacrylamide gel electrophoresis, followed by matrix-assisted laser desorption ionization-time of flight mass spectrometry. (A) B. pertussis 509. (B) B. pertussis 10536. (C) B. pertussis Tohama. (D) B. pertussis 106. All samples were resolved by IEF (pH 4 to 7). Protein spots were visualized by colloidal Coomassie staining.
Few proteins could be detected as differential among the three analyzed vaccine strains. Leu-Ile-Val protein precursor (spot 17; BP1285), DNA direct RNA α subunit polymerase (spot 18; BP3642), and a two-component sensor protein (spot 31; BP2483) could be detected in Tohama and B. pertussis 509; however, they were not detected in strain 10536. Putative penicillin binding protein precursor (spot 50; BP0102) and hypothetical protein BP3128 (spot 39; BP3128) were detected in B. pertussis 509 and 10536 but were not evident in Tohama, and putative binding protein-dependent transport protein (spot 10; BP3322) was not observed in B. pertussis 509, whereas putative molibdopterin oxidoreductase (spot 22; BB0468) was clearly detected in B. pertussis 10536. Furthermore, putative ABC transport ATP binding protein (spot 8; BP3757) and putative membrane protein (spot 11; BP1440) were detected in strain Tohama in smaller quantities than in strains 509 and 10536, and putative quinoprotein (spot 20; BP2196) was detected at a high level in B. pertussis 509 but in small quantities in B. pertussis 10536 and Tohama.
While a number of protein spots were common among the vaccine strains tested and the clinical isolate B. pertussis 106, there were multiple spots unique to B. pertussis 106, i.e., ribose phosphate pyrophosphokinase (spot 37; BP3125), dihydrolipoamide dehydrogenase (spot 38; BP0995), polysaccharide biosynthesis protein (spot 41; BP3150), capsular polysaccharide biosynthesis protein (spot 42; BP1630), putative l-lactacte dehydrogenase (spot 43; BP0379), a hypothetical protein (spot 44; BP2964, which is present in greater quantity than was observed in Tohama and 10536), putative sigma factor regulatory protein (spot 46; BP2435), dihydrolipoamide dehydrogenase (spot 51; BP1126), and putative type III secretion protein (spot 53; BP2235). The presence of the last protein is an interesting result, since it was previously reported that the type III secretion system is expressed only in Bordetella bronchiseptica. This result is in agreement with the work presented by K. Mills at the Eighth International Symposium: Saga of Genus Bordetella, 1906 to 2006, at the Institut Pasteur, Paris, France. He described an effector of the type III secretion system that was found solely in clinical isolates and not in laboratory-adapted B. pertussis strains.
Though most of the proteins identified in B. pertussis 106 are involved in carbohydrate metabolism, some of them were recently described in other gram-negative bacteria as having roles in pathogenicity (12) and immunogenicity (33).
One of these enzymes is the dihydrolipoamide dehydrogenase (Lpd), which is an essential functional subunit of the multienzyme pyruvate dehydrogenase complex, with a predicted molecular mass of 50.37 kDa. The pyruvate deshydrogenase complex is comprised of multiple copies of the ternary complex of Lpd, dihydrolipoamide transacetylase, and pyruvate dehydrogenase enzymes and is involved in the oxidation of pyruvate. The dihydrolipoamide dehydrogenase enzyme allows the continued function of the pathway by maintaining the correct redox state of the enzymes involved in the bioconversion. For Mycobacterium, it was reported that Lpd has an important role in host colonization and pathogenesis, and in H. influenzae type B, a transposon insertion in lpdA, a dihydrolipoamide dehydrogenase gene homolog, converts the virulent bacteria into attenuated bacteria. When the H. influenzae LpdA mutant was used to challenge 5-day-old infant rats intraperitoneally, it was unrecoverable compared to the wild-type recovery rate (29). These evidences point out that the Lpd identified in B. pertussis 106 may have a role in pathogenicity that needs to be investigated.
In B. pertussis 106, two other differing spots were detected: polysaccharide biosynthesis protein (spot 41; BP3150) and capsular polysaccharide biosynthesis protein (spot 42; BP1630). Since capsules are often key contributors to the abilities of pathogens to withstand host defense mechanisms (42), the expression of these proteins in B. pertussis 106 may be a consequence of its need to evade the host immune response induced by vaccination. A locus that is likely to encode a type II polysaccharide capsule (20) comprising three regions that are involved in export/modification, biosynthesis, and transport was previously reported to be present in the Bordetella genome sequence (45). In B. pertussis strain Tohama, it was reported that the central part of the locus is intact but that the 3′ region of one gene has undergone an inversion event and, at the 5′ end of the locus, an insertion element-mediated rearrangement has deleted part of region 1 (which contains genes that are involved in export/modification) and moved two of the export genes to a different part of the chromosome. It will be interesting to analyze the structure of this locus in B. pertussis 106.
This study not only shows that the previously observed genomic divergences between the Argentinean circulating B. pertussis population and vaccine strains is now registered (by the Public Health Ministry), but more importantly, that these genomic differences have implications for pertussis protection and could also be extended to the protein expression patterns.
Acknowledgments
This work was supported by ANCPyT and CICBA (Argentina) grants to D.H. and a Malbrán-UNLP grant to N.B., M.R., and D.H. D.H. is a member of the Scientific Career of CICBA. J.F. and F.S. are members of the Scientific Career of CONICET. M.F., D.B., and M.E.G. have fellowships from CONICET. A.G. has a fellowship from ANCPyT, and R.R. is supported by an INCO (EU) grant.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Advani, A., D. Donnelly, and H. Hallander. 2004. Reference system for characterization of Bordetella pertussis pulse field gel electrophoresis profiles. J. Clin. Microbiol. 42:2890-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, R., A. Herceg, and C. Roberts. 1997. Pertussis notifications in Australia, 1991 to 1997. Commun. Dis. Intell. 21:145-148. [DOI] [PubMed] [Google Scholar]
- 3.Artenstein, M. S., B. L. Brandt, E. C. Tramont, W. C. Branche, Jr., H. D. Fleet, and R. L. Cohen. 1971. Serologic studies of meningococcal infection and polysaccharide vaccination. J. Infect. Dis. 124:277-288. [DOI] [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brook, I., P. A. Foote, and J. N. Hausfeld. 2006. Frequency of recovery of pathogens causing acute maxillary sinusitis in adults before and after the introduction of vaccination of children with the 7-valent pneumococcal vaccine. J. Med. Microbiol. 55:943-947. [DOI] [PubMed] [Google Scholar]
- 6.Bunai, K., and K. Yamane. 2005. Effectiveness and limitation of two-dimensional gel electrophoresis in bacterial membrane protein proteomics and perspectives. J. Chromatogr. 815:227-236. [DOI] [PubMed] [Google Scholar]
- 7.Carman, W. F., A. A. Zanetti, P. Karayiannis, J. Waters, G. Manzillo, E. Tanzi, A. J. Zuckerman, and H. C. Thomas. 1990. Vaccine induced escape mutant of hepatitis B virus. Lancet 336:325-329. [DOI] [PubMed] [Google Scholar]
- 8.Caro, V., V. Bouchez, N. Guiso, B. Gatti, M. R. Agosti, and S. E. Ayala. 2006. Pertussis in Argentina and France. Vaccine doi: 10.1016/j.vaccine.2006.08.024. [DOI] [PubMed]
- 9.Cassiday, P., G. Sanden, K. Heuvelman, F. R. Mooi, K. M. Bisgard, and T. Popovic. 2000. Polymorphism in Bordetella pertussis pertactin and pertussis toxin virulence factors in the United States, 1935-1999. J. Infect. Dis. 182:1402-1408. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. N., and C. J. Oon. 2000. Hepatitis B virus surface antigen (HBsAg) mutants in Singapore adults and vaccinated children with anti-hepatitis B virus antibody levels but negative for HBsAg. J. Clin. Microbiol. 38:2793-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Almeida, A. E. C. C., I. de Filippis, A. O. de Abreu, D. G. Ferreira, A. L. Gemal, and K. B. F. Marzochi. 2005. Occurrence of Haemophilus influenzae strains in three Brazilian states since the introduction of a conjugate Haemophilus influenzae type b vaccine. Braz. J. Med. Biol. Res. 38:777-781. [DOI] [PubMed] [Google Scholar]
- 12.Delvecchio, V. G., J. P. Connolly, T. G. Alefantis, A. Walz, M. A. Quan, G. Patra, J. M. Ashton, J. T. Whittington, R. D. Chafin, X. Liang, P. Grewal, A. S. Khan, and C. V. Mujer. 2006. Proteomic profiling and identification of immunodominant spore antigens of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Appl. Environ. Microbiol. 72:6355-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Melker, H. E., M. A. E. Conyn-van Spaendonck, H. C. Rumke, J. K. van Wijngaarden, F. R. Mooi, and J. F. P. Schellekens. 1997. Pertussis in the Netherlands: an outbreak despite high levels of immunization with whole cell vaccine. Emerg. Infect. Dis. 3:175-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Melker, H. E., J. F. P. Schellekens, S. E. Neppelenbroek, F. R. Mooi, H. C. Rumke, and M. A. E. Conyn-van Spaendonck. 2000. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg. Infect. Dis. 6:348-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diavatopoulos, D. A., M. Hijnen, and F. R. Mooi. 2006. Adaptive evolution of the Bordetella autotransporter pertactin. J. Evol. Biol. 19:1931-1938. [DOI] [PubMed] [Google Scholar]
- 16.Elomaa, A., A. Advani, D. Donnelly, M. Antila, J. Mertsola, H. Hallander, and Q. He. 2005. Strain variation among Bordetella pertussis isolates in Finland, where the whole-cell pertussis vaccine has been used for 50 years. J. Clin. Microbiol. 43:3681-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari, G., I. Garaguso, J. Adu-Bobie, F. Doro, A. R. Taddei, A. Biolchi, B. Brunelli, M. Giulani, M. Pizza, N. Norais, and G. Guido. 2006. Outer membrane vesicles from group B Neisseria meningitidis Δgna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856-1866. [DOI] [PubMed] [Google Scholar]
- 18.Fiett, J., I. Letowska, M. Gniadkowski, and W. Hryniewicz. 2003. The new strategy for allele identification of the genes coding for pertussis subunit S1 (ptxS1) and pertactin (prn) in Bordetella pertussis. J. Microbiol. Methods 55:651-666. [DOI] [PubMed] [Google Scholar]
- 19.Fingermann, M., J. Fernandez, F. Sisti, M. E. Rodriguez, B. Gatti, D. Bottero, A. Graieb, M. E. Gaillard, S. G. Ayala, F. R. Mooi, H. Lopardo, and D. Hozbor. 2006. Differences of circulating Bordetella pertussis populations in Argentina from the strain used in vaccine production. Vaccine 24:3513-3521. [DOI] [PubMed] [Google Scholar]
- 20.Frosch, M., U. Edwards, K. Bousset, B. Krausse, and C. Weisgerber. 1991. Evidence for a common molecular origin of the capsule gene loci in gram-negative bacteria expressing group II capsular polysaccharides. Mol. Microbiol. 5:1251-1263. [DOI] [PubMed] [Google Scholar]
- 21.Fry, N. K., S. Neal, T. G. Harrison, E. Miller, R. Matthews, and R. G. George. 2001. Genotypic variation in the Bordetella pertussis virulence factors pertactin and pertussis toxin in historical and recent clinical isolates in the United Kingdom. Infect. Immun. 69:5520-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimprel, E., P. Begue, I. Anjak, F. Betsou, and N. Guiso. 1993. Comparison of polymerase chain reaction, culture and Western immunoblot serology for diagnosis of Bordetella pertussis infection. J. Clin. Microbiol. 31:2745-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guiso, N., C. Capiau, G. Carletti, J. Poolman, and P. Hauser. 1999. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine 17:2366-2376. [DOI] [PubMed] [Google Scholar]
- 24.Guris, D., P. M. Strebel, B. Bardenheier, M. Brennan, R. Tachdjian, E. Finch, M. Wharton, and J. R. Livengood. 1999. Changing epidemiology of pertussis in the United States: increasing reported incidence among adolescents and adults, 1990-1996. Clin. Infect. Dis. 28:1230-1237. [DOI] [PubMed] [Google Scholar]
- 25.Gzyl, A., E. Augustynowicz, G. Gnladek, D. Rabczenko, G. Dulny, and J. Slusarczyk. 2004. Sequence variation in pertussis S1 subunit toxin and pertussis genes in Bordetella pertussis strains used for the whole-cell pertussis vaccine produced in Poland since 1960: efficiency of the DTwP vaccine-induced immunity against currently circulating B. pertussis isolates. Vaccine 22:2122-2128. [DOI] [PubMed] [Google Scholar]
- 26.Gzyl, A., E. Augustynowicz, I. van Loo, and J. Slusarczyk. 2001. Temporal nucleotide changes in pertactin and pertussis toxin genes in Bordetella pertussis strains isolated from clinical cases in Poland. Vaccine 20:299-303. [DOI] [PubMed] [Google Scholar]
- 27.Hardwick, T. H., P. Cassiday, R. S. Weyant, K. M. Bisgard, and G. N. Sanden. 2002. Changes in predominance and diversity of genomic subtypes of Bordetella pertussis isolated in the United States, 1935 to 1999. Emerg. Infect. Dis. 8:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardwick, T. H., B. Plikaytis, P. Cassiday, G. Cage, M. Peppler, D. Shea, D. Boxrud, and G. Sanden. 2002. Reproducibility of Bordetella pertussis genomic DNA fragments generated by XbaI restriction and resolved by pulse-field gel electrophoresis. J. Clin. Microbiol. 40:811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbert, M., A. Kraiss, A. K. Hilpert, S. Schlor, and J. Reidl. 2003. Aerobic growth deficient Haemophilus influenzae mutants are non-virulent: implications on metabolism. Int. J. Med. Microbiol. 293:145-152. [DOI] [PubMed] [Google Scholar]
- 30.Hoebrekx, N., G. Muyldermans, O. Soetens, I. De Schutter, A. Malfroot, D. Pierard, and S. Lauwers. 2000. Molecular typing of Bordetella pertussis isolates in Belgium from 1987 to 2000, p. 279. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 31.Hoey, J. 2003. Pertussis in adults. CMAJ 168:453-454. [PMC free article] [PubMed] [Google Scholar]
- 32.Hozbor, D., F. Fouque, and N. Guiso. 1999. Detection of Bordetella bronchiseptica by the polymerase chain reaction. Res. Microbiol. 150:333-341. [DOI] [PubMed] [Google Scholar]
- 33.Hudson, P., T. S. Gorton, L. Papazisi, K. Cecchini, S. Frasca, Jr., and S. Geary. 2006. Identification of a virulence-associated determinant, dihydrolipoamide dehydrogenase (lpd), in Mycoplasma gallisepticum through in vivo screening of transposon mutants. J. Infect. Immun. 74:931-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King, A. J., G. Berbers, H. F. van Oirschot, P. Hoogerhout, K. Knipping, and F. R. Mooi. 2001. Role of the polymorphic region 1 of the Bordetella pertussis protein pertactin in immunity. Microbiology 147:2885-2895. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1971. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Lin, Y. C., S. M. Yao, J. J. Yan, Y. Y. Chen, M. J. Hsiao, C. Y. Chou, H. P. Su, H. S. Wu, and S. Y. Li. 2006. Molecular epidemiology of Bordetella pertussis in Taiwan, 1993-2004: suggests one possible explanation for the outbreak of pertussis in 1997. Microbes Infect. 8:2082-2087. [DOI] [PubMed] [Google Scholar]
- 37.Mastrantonio, P., P. Spigaglia, H. van Oirschot, H. J. G. van der Heide, K. Heuvelman, P. Stefanelli, and F. R. Mooi. 1999. Antigenic variants in Bordetella pertussis strains isolated from vaccinated and unvaccinated children. Microbiology 145:2069-2075. [DOI] [PubMed] [Google Scholar]
- 38.Mooi, F. R., H. Hallander, C. H. Wirsing von Köning, B. Hoet, and N. Guiso. 2000. Epidemiological typing of Bordetella pertussis isolates: recommendations for a standard methodology. Eur. Clin. Infect. Dis. J. 19:174-181. [DOI] [PubMed] [Google Scholar]
- 39.Mooi, F. R., Q. He, H. van Oirschot, and J. Mertsola. 1999. Variation in the Bordetella pertussis virulence factors pertussis toxin and pertactin in vaccine strains and clinical isolates in Finland. Infect. Immun. 67:3133-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mooi, F. R., I. H. M. van Loo, and A. J. King. 2001. Adaptation of Bordetella pertussis to vaccination: a cause for its reemergence? Emerg. Infect. Dis. 7:526-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mooi, F. R., H. van Oirschot, K. Heuvelman, H. G. J. van der Heide, W. R. Gaastra, and J. L. Willems. 1998. Polymorphism in the Bordetella pertussis virulence factors P.69/pertactin and pertussis toxin in The Netherlands: temporal trends and evidence for vaccine driven evolution. Infect. Immun. 66:670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moxon, E. R., and J. S. Kroll. 1990. The role of bacterial polysaccharide capsules as virulence factors. Curr. Top. Microbiol. Immunol. 150:65-85. [DOI] [PubMed] [Google Scholar]
- 43.Ntezayabo, B., G. De Serres, and B. Duval. 2003. Pertussis resurgence in Canada largely caused by a cohort effect. Pediatr. Infect. Dis. J. 22:22-27. [DOI] [PubMed] [Google Scholar]
- 44.Obaro, S. K., R. A. Adegbola, W. A. S. Banya, and B. M. Greenwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 45.Preston, A., J. Parkhill, and D. Maskell. 2004. The Bordetelleae: lessons from genomics. Nat. Rev. Microbiol. 2:379-390. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, M., C. R. Vitek, F. B. Pascual, K. M. Bisgrad, J. E. Tate, and T. V. Murphy. 2003. Trends in pertussis among infants in the United States, 1980-1999. JAMA 290:2968-2975. [DOI] [PubMed] [Google Scholar]
- 47.Tsang, R. S. W., S. Mubareka, M. I. Sill, J. Wylie, S. Skinner, and D. K. S. Law. 2006. Invasive Haemophilus influenzae in Manitoba, Canada, in the post-vaccination era. J. Clin. Microbiol. 44:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turyilmaz, A. R., C. Yurdaydin, and A. M. Bozdayi. 2006. The first identified hepatitis B virus vaccine escape mutation in Turkey. J. Clin. Virol. 35:201-202. [DOI] [PubMed] [Google Scholar]
- 49.van Loo, I. H., and F. R. Mooi. 2002. Changes in the Dutch Bordetella pertussis population in the first 20 years after the introduction of whole-cell vaccines. Microbiology 148:2011-2018. [DOI] [PubMed] [Google Scholar]
- 50.van Loo, I. H. M., H. G. J. van der Heide, N. J. D. Nagelkerke, J. Verhoef, and F. R. Mooi. 1999. Temporal trends in the population structure of Bordetella pertussis during 1949-1996 in a highly vaccinated population. J. Infect. Dis. 179:915-923. [DOI] [PubMed] [Google Scholar]
- 51.Wirsing von Koning, C. H., S. Halperin, M. Riffelmann, and N. Guiso. 2002. Pertussis of adults and infants. Lancet Infect. Dis. 2:744-750. [DOI] [PubMed] [Google Scholar]