Abstract
Bacillus cereus ATCC 14579 can respond to nutrient changes by adopting different forms of surface translocation. The B. cereus ATCC 14579 ΔplcR mutant, but not the wild type, formed dendritic (branched) patterns on EPS [a low-nutrient medium that contains 7.0 g K2HPO4, 3.0 g KH2PO4, 0.1 g MgSO4·7H2O, 0.1 g (NH4)2SO4, 0.01 g CaCl2, 0.001 g FeSO4, 0.1 g NaCl, 1.0 g glucose, and 125 mg yeast extract per liter] containing 0.7% agar. The dendritic patterns formed by sliding translocation of nonflagellated cells are enhanced under low-nutrient conditions and require sufficient production of a biosurfactant, which appears to be repressed by PlcR. The wild-type and complemented strains failed to slide on the surface of EPS agar because of the production of low levels of biosurfactant. Precoating EPS agar surfaces with surfactin (a biosurfactant produced by Bacillus subtilis) or biosurfactant purified from the ΔplcR mutant rescued the ability of the wild-type and complemented strains to slide. When grown on a nutrient-rich medium like Luria-Bertani agar, both the wild-type and ΔplcR mutant strains produced flagella. The wild type was hyperflagellated and elongated and exhibited swarming behavior, while the ΔplcR mutant was multiflagellated and the cells often formed long chains but did not swarm. Thin-layer chromatography and mass spectrometry analyses suggested that the biosurfactant purified from the ΔplcR mutant was a lipopeptide and had a mass of 1,278.1722 (m/z). This biosurfactant has hemolytic activity and inhibited the growth of several gram-positive bacteria.
Bacteria can display six different forms of translocation, which are classified as swimming, swarming, twitching, gliding, sliding, and darting (15, 16). Swarming motility is flagellum dependent and has been studied in many organisms, including Bacillus cereus (38) and Bacillus subtilis (7, 20-22). Swimmer cells of B. cereus are short oligoflagellated rods, while swarmer cells are typically hyperflagellated and elongated and move over the surface in a coordinate manner, which may exhibit layered consolidation phases. Sliding or spreading is a type of surface translocation caused by the expansive forces of a growing colony and is often associated with the production of extracellular or cell surface biosurfactants such as lipopeptides or lipopolysaccharides (15, 16). In B. subtilis, sliding is flagellum independent and associated with the production of the biosurfactant surfactin (25, 26), while in nonflagellated Mycobacterium smegmatis, acetylation of glycopeptidolipids in the outer layer of the cell envelope is required (8, 34). For Serratia marcescens, mutants unable to produce the biosurfactant serrawettin are deficient in sliding. This deficiency was rescued by supplementation with serrawettin W1, an extracellular cyclic lipopeptide (30). Nonflagellated mutants of S. marcescens could spread rapidly on a surface of semisolid agar (17, 30). Vibrio cholerae and Escherichia coli also demonstrated flagellum-independent surface translocation in the absence of growth (5), while lipopolysaccharide synthesis mutants of V. cholerae lost this ability. Sliding motility in B. cereus has not been reported or addressed.
Biosurfactants are produced by many bacteria and fungi and can have a variety of structures, including neutral lipids, phospholipids, glycolipids, and lipopeptides (32). They have the ability to reduce surface and interfacial tension, and some also have antimicrobial activity. Biosurfactant production has been reported in several Bacillus species. Surfactin, a cyclic lipopeptide produced by B. subtilis, is the most studied and potent biosurfactant and possesses antimicrobial activity (39). Many biosurfactants, including the majority of those from B. subtilis, are produced by nonribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) (23), large multifunctional proteins with a modular organization. Information on biosurfactant production in B. cereus is limited. A strain of B. cereus was reported to produce plipastatins, a family of lipopeptides with antifungal activity (33, 41). Whether they have surfactant activity has not been determined.
B. cereus is a soil bacterium that is widespread in nature. It is also an opportunistic human pathogen (37). PlcR, a pleiotropic regulator in B. cereus, positively regulates many genes, including those that encode extracellular virulence factors at the onset of stationary phase (1, 13, 27). In a previous study to determine the effect of plcR on biofilm development, we reported that B. cereus ATCC 14579 and its ΔplcR mutant formed a delimiting ring on plates containing EPS [a low-nutrient medium that contains 7.0 g K2HPO4, 3.0 g KH2PO4, 0.1 g MgSO4·7H2O, 0.1 g (NH4)2SO4, 0.01 g CaCl2, 0.001 g FeSO4, 0.1 g NaCl, 1.0 g glucose, and 125 mg yeast extract per liter] and 0.7% agar (18). The ΔplcR mutant formed the delimiting ring earlier, and the ring diameter was larger than that of the wild type. The ring was attributed to the production of a biosurfactant, which appeared to be negatively regulated by PlcR. The ΔplcR mutant produced about 10 times more biosurfactant than the wild type (18). Here we showed that subsequent to the appearance of the delimiting ring, the ΔplcR mutant, but not the wild type, formed a dendritic colony morphology on EPS-0.7% agar, suggesting that the production of sufficient amounts of a biosurfactant may be involved in the formation of this dendrite pattern. The effect of nutrient conditions and PlcR on the correlation between dendrite pattern formation and biosurfactant production was investigated. Formation of the dendrite pattern is nutrient dependent and is enhanced under low-nutrient conditions. Dendrite formation is caused by a surface translocation that does not require flagella, similar to the sliding motility described in other organisms. A heretofore unidentified biosurfactant produced by B. cereus that facilitates flagellum-independent translocation and dendrite growth is characterized. The ability to translocate more efficiently under nutrient-stressed conditions may enhance the survival of the organism in the environment.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains used in this study were B. cereus type strain ATCC 14579, its ΔplcR mutant (36), a complemented strain of the ΔplcR mutant [ΔplcR(pHT304ΩB1)], and surfactin-producing B. subtilis strain NCIB 3610 (Bacillus Genetic Stock Center). The complemented strain was generated by transforming the ΔplcR mutant with the plasmid pHT304ΩB1, which carries both the plcR and papR genes (27). Plasmid DNA was prepared from Escherichia coli strain ET12467 and transformed into the B. cereus ΔplcR mutant by electroporation as previously described (4, 29). Strains were grown in Luria-Bertani (LB; Difco/Becton Dickinson, Sparks, MD) broth at 32°C and 200 rpm overnight to generate inoculum cultures. For the ΔplcR mutant, 150 μg kanamycin/ml of medium was added. For the complemented strain, 5 μg erythromycin/ml of medium was added.
Surface motility assay and nutrient conditions.
Bacterial cells from stationary-phase cultures (∼106 CFU in 10 μl) were spotted onto the center of 0.7% Bacto Agar plates (60 by 15 mm; Falcon/Becton Dickinson, Franklin Lakes, NJ) containing different media and incubated at 32°C for up to 96 h. When B. subtilis strain NCIB 3610 was tested, the plate size was increased to 100 by 15 mm (Fisher Scientific, Pittsburgh, PA). EPS, EPS supplemented with five times the yeast extract concentration (5×YE EPS), and LB broth were used to represent increasing nutrient conditions. Colony images were captured with an AlphaImager 2000 imaging system (Alpha Innotech, San Leandro, CA).
Flagellar staining.
Bacteria were lifted from the colony margins with a toothpick and then touched onto a drop of water on a microscope slide. Flagella were stained as described originally by Mayfield and Inniss (31) and modified by Kearns and Losick (22) and were observed with an Olympus BH-2 phase-contrast microscope (Olympus, Melville, NY). Microscopic images were captured with an Olympus DH70 digital camera.
Partial purification of the biosurfactant(s).
A 100-μl volume of an overnight culture of B. cereus ATCC 14579 or its ΔplcR mutant was inoculated into each of 10 1-liter flasks containing 100 ml EPS and incubated at 32°C and 100 rpm for 48 h. The optical density at 620 nm of the culture was measured. Under these conditions, the two strains grew similarly. The culture was centrifuged at 8,000 × g for 40 min at 4°C. The supernatant was filtered through a 0.22-μm-pore-size CA membrane bottle top filtration unit (Corning Incorporated, Corning, NY) and precipitated by the addition of 3 N HCl. Biosurfactant(s) was partially purified as described by Kim et al. (24). The partially purified biosurfactant was dissolved in 2 ml methanol, air dried overnight, and dissolved in 200 μl methanol or 50 mM Tris-HCl buffer (pH 7.4) as needed.
Thin-layer chromatography (TLC) analysis of the biosurfactant(s).
A 40-μl volume of the partially purified biosurfactant in methanol or 40 μl of surfactin (1 μg/μl; Sigma, St. Louis, MO) in methanol was spotted onto TLC plates (silica gel 60 Å, 20 by 20 cm, 250-μm thickness; Whatman, Clifton, NJ) and developed in chloroform-methanol-5 M NH4OH (80:25:4, vol/vol/vol). For detection of peptides, the plates were air dried, sprayed with ninhydrin (ACROS Organics, Geel, Belgium), air dried, and heated at 110°C for color development. For detection of lipids, the plates were air dried and sprayed with bromthymol blue (0.1% in 10% aqueous ethanol). Migration distances of sample spots relative to the mobile phase (Rf values) were calculated. TLC images were captured with an AlphaImager 2000 imaging system.
Matrix-assisted laser desorption-time of flight mass spectrometry (MALDI-TOF MS) analysis.
An unstained spot corresponding to the biosurfactant from the ΔplcR mutant or to surfactin was scraped from the TLC plate, dissolved in 1 ml methanol, and centrifuged at 10,000 × g for 3 min to remove the silica gel. The supernatant was removed and air dried overnight. The dried pellet was dissolved in 50 μl methanol and centrifuged as described above, and the supernatant (analyte) was analyzed by MALDI-TOF MS in the positive-ion reflectron mode with a 4800 MALDI-TOF/TOF analyzer (Applied Biosystems, Foster City, CA) at the University of Wisconsin Biotechnology Center. For tandem MS (MS-MS) analysis, air was used as the collision gas. 2,5-Dihydroxybenzoic acid or α-cyano-4-methoxycinnamic acid in acetonitrile (10 mg/ml)-water-trifluoroacetic acid (70:30:0.2, vol/vol/vol) was used as the matrix. The dried analyte was reconstituted in methanol and mixed 1:1 with the matrix solution, and 0.4 μl of the mixture was spotted onto a steel MALDI target plate. Mass spectra were accumulated to 1,000 individual laser shots in total at a final reflectron voltage of 20.36 kV.
Hemolysis assay.
A 50-μl volume of the purified biosurfactant from TLC plates was dissolved in methanol, air dried, dissolved in 100 μl Tris-HCl buffer, added to wells of sheep blood agar plates (Remel, Lenexa, KS), and then incubated at 37°C for 48 h. Five micrograms of surfactin in 100 μl of Tris-HCl buffer was used as a positive control. Hemolytic patterns were captured with an AlphaImager 2000 imaging system.
Antimicrobial assay.
A 50-μl volume of the purified biosurfactant from TLC plates was dissolved in methanol and spotted onto brain heart infusion agar plates. The same volume of methanol was spotted onto plates as a control. Fifteen different bacteria (Table 1) (∼106 CFU in 10 μl) were individually seeded onto the biosurfactant-coated area of the plates, which were incubated at 37°C for 48 h. The presence or absence of growth on the seeded areas was scored.
TABLE 1.
Inhibition of indicator organisms by the biosurfactant purified from the ΔplcR mutant
| Indicator organism | Inhibition of test straina |
|---|---|
| Gram-positive bacteria | |
| Enterococcus faecalis 4029 | − |
| Bacillus cereus ATCC 14579 | − |
| B. cereus ATCC 10987 | − |
| Bacillus subtilis NCIB 3610 | − |
| B. subtilis 168 | + |
| Listeria innocua M2055 | + |
| Listeria monocytogenes Scott A | + |
| Listeria welshimeri M2059 | + |
| Staphylococcus aureus FRI 913 | + |
| Staphylococcus epidermidis ATCC 35984 | + |
| Gram-negative bacteria | |
| Escherichia coliO157:H7 ATCC 43895 | − |
| Klebsiella pneumoniae ATCC 4359 | − |
| Pseudomonas aeruginosa PAO1 | − |
| Serratia marcescens | − |
| Salmonella enterica serovar Typhimurium AWS9-1 | − |
+, inhibited; −, not inhibited. Strains were used in triplicate in the independent experiments.
Precoating of agar plates with surfactin or B. cereus biosurfactant.
Ten-microliter volumes of increasing concentrations of surfactin or 40 μl of the purified biosurfactant from the ΔplcR mutant were spotted onto EPS agar and air dried for 2 h. Ten microliters of an overnight culture of the wild-type or complemented strain was spotted onto the biosurfactant-coated area, and the plates were incubated at 32°C for 40 h.
RESULTS
Effect of nutrient condition on surface motility.
We have observed previously that B. cereus ATCC 14579 and its ΔplcR mutant formed a delimiting ring on EPS-0.7% agar plates (18), while this phenomenon was not detected on LB agar (data not shown). The ΔplcR mutant formed the delimiting ring earlier, and the ring diameter was much larger than that of the wild type. The ring was attributed to the production of a biosurfactant, which appeared to be negatively regulated by PlcR. Subsequent to the appearance of the delimiting ring, the ΔplcR mutant formed a dendritic colony on EPS agar (Fig. 1A), suggesting that the production of a biosurfactant may be involved in the process. The dendrite pattern is similar to those noted for B. subtilis, where medium composition and the presence of surfactin were reported to play a role (19, 20, 25, 26). Dendrite formation in B. cereus is not an aberrant behavior that occurs only in the absence of plcR. We tested 11 other wild-type B. cereus strains isolated from different sources, and they were all able to produce a delimiting ring on EPS agar, followed by the formation of dendrite colonies of different sizes (data not shown).
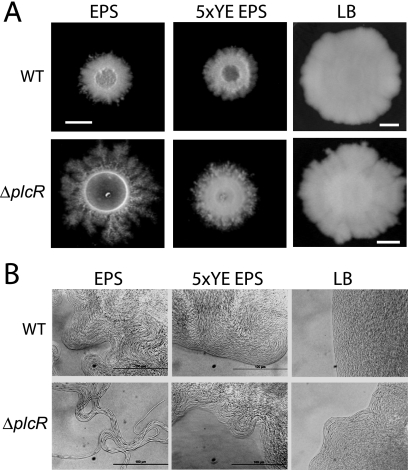
FIG. 1.
Effect of nutrient conditions on colony morphology. Overnight cultures of B. cereus ATCC 14579 (WT [wild type]) and the ΔplcR mutant were spotted onto EPS, 5×YE EPS, and LB agar plates. (A) Colony morphology was observed after incubation at 32°C for 96 h. Bars, 0.5 cm. (B) Microscopic images of the colony margins of the wild type and the ΔplcR mutant grown on EPS, 5×YE EPS, and LB agars at 32°C for 96 h. Bars, 100 μm.
Colonies of both B. cereus ATCC 14579 and its ΔplcR mutant on LB agar were larger than those on EPS and 5×YE EPS agars at 24 h (data not shown) and 96 h (Fig. 1A). There was very little difference in appearance between the wild type and the ΔplcR mutant on EPS and 5×YE EPS agars at 24 h. However, at 96 h, the ΔplcR mutant had spread out from the colony and formed a highly branched dendrite pattern on EPS agar (Fig. 1A) that was absent on the other two media.
The leading edges of the colonies formed by the wild type and the ΔplcR mutant on all three agars at 96 h were examined by microscopy (Fig. 1B). Under low-nutrient conditions (EPS), the cells at the leading edge of the ΔplcR mutant colony were linked in long chains and formed swirls that extended some distance from the colony edge. As the nutrient level increased, the cells at colony edges did not migrate outward to form swirls. Individual cells moved within the colony edge on the LB agar surface, but not in a coordinated manner (see movie S1 in the supplemental material). The wild-type colony morphology was also affected by the nutrient level but not to the same extent as that of the ΔplcR mutant. The colony edges on EPS agar were similar to those of the ΔplcR mutant on 5×YE EPS agar. As the nutrient level increased, the cells at the colony edges became more organized; on LB agar, cells moved in a coordinated manner typical of swarming (see movie S2 in the supplemental material) and as shown previously in B. cereus by Senesi et al. (38).
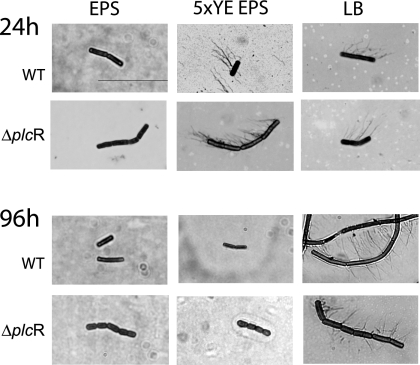
Cells of both strains lifted from the edge of colonies grown on EPS agar were nonflagellated and not elongated at 24 and 96 h (Fig. 2). On 5×YE EPS agar, the cells became multiflagellated but not elongated at 24 h; however, by 96 h, both strains had stopped producing flagella. On LB agar, which had the highest nutrient level, the wild-type cells were hyperflagellated and elongated at both 24 and 96 h, a phenotype typical of swarm cells. On the other hand, the ΔplcR mutant was multiflagellated and often formed long chains but was never elongated, even at 96 h (Fig. 2). The formation of dendrite colonies following biosurfactant production by nonflagellated cells of the ΔplcR mutant on EPS agar is similar to the flagellum-independent sliding motility described by Kinsinger et al. (26) in B. subtilis.
FIG. 2.
Effect of nutrient conditions on production of flagella. Overnight cultures of the wild type (WT) and the ΔplcR mutant were spotted onto EPS, 5×YE EPS, and LB agars. After incubation at 32°C for 24 and 96 h, cells were lifted from the colony margins, stained, and observed by microscopy for the production of flagella. Bar, 10 μm.
Dendrite pattern formation with surfactin- or B. cereus biosurfactant-precoated plates.
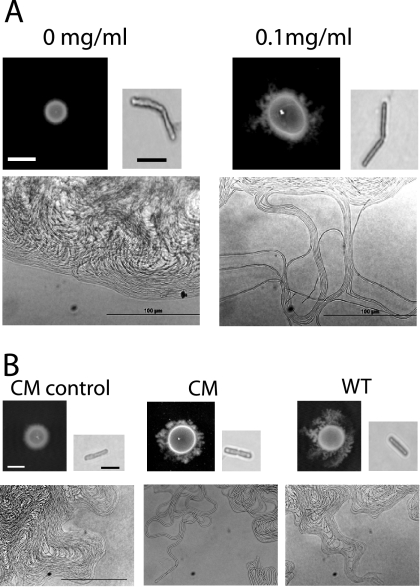
To test whether the absence of dendrite formation by the wild type was due to the lack of or decreased production of biosurfactant, different concentrations of surfactin were spotted onto EPS agar. Plates were observed for dendritic growth on agar precoated with 10 μl of surfactin at 0.01, 0.1, 1, or 10 mg/ml for up to 40 h. Dendrite patterns were formed by the wild type on agar precoated with the three higher concentrations of surfactin (a representative colony with 0.1 mg/ml is shown in Fig. 3A) but not with 0.01 mg/ml (data not shown). The dendrite cells were nonflagellated, and the colony margins were similar to that formed by the ΔplcR mutant on EPS agar (Fig. 1B). When the ΔplcR mutant was grown on agar precoated with these concentrations of surfactin, dendrite formation was accelerated compared to that on agar without precoating; in addition, the dendrite patterns were much more extensive than those developed by the wild type (data not shown).
FIG. 3.
(A) Effect of precoating with surfactin on colony morphology. An overnight wild-type (WT) culture was spot inoculated onto EPS agar plates after the plates were coated with 10 μl of 0 or 0.1 mg of surfactin/ml. Colony morphology was observed after 40 h at 32°C (bar, 0.5 cm). Colony margins were observed by microscopy (bar, 100 μm). Cells were lifted from the colony margins, stained, and observed by microscopy for the production of flagella (bar, 10 μm). (B) Effect of precoating with purified biosurfactant from the ΔplcR mutant on colony morphology. Overnight cultures of the wild type and the complemented ΔplcR mutant strain (CM) were spot inoculated onto EPS plates after the plates were coated with 40 μl of Tris-HCl or the purified biosurfactant from the ΔplcR mutant. Colony morphology was observed after 40 h at 32°C (bar, 0.5 cm). Colony margins were observed by microscopy (bar, 100 μm). Cells were lifted from the colony margins, stained, and observed by microscopy for the production of flagella (bar, 10 μm). The results presented are representative of triplicate experiments.
EPS agar precoated with 40 μl of purified biosurfactant from the ΔplcR mutant was inoculated with the wild-type and complemented strains. By 40 h, both strains formed dendrite patterns (Fig. 3B). The colony margins of the dendrite patterns were similar to that formed by the ΔplcR mutant on EPS agar, and cells were nonflagellated. As expected, the complemented strain behaved like the wild type and did not form a dendritic pattern on agar without precoating. These results showed that the presence of biosurfactant had a direct effect on dendrite formation.
Analysis by TLC.
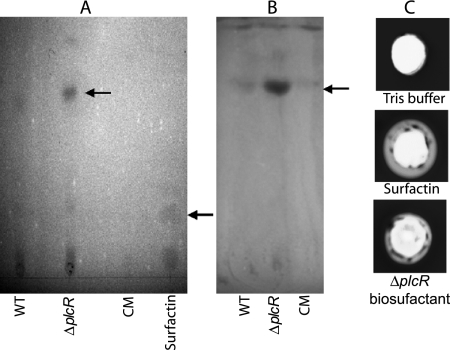
Partially purified biosurfactants from the wild-type, ΔplcR mutant, and complemented strains were analyzed by TLC. To characterize the chemical nature of the biosurfactant that may be regulated by PlcR, differential TLC staining was used. After spraying with ninhydrin, which stains amino acids, the spot observed with the ΔplcR mutant was larger and more intense than those observed with the wild-type and complemented strains (Fig. 4A), indicating a greater amount of biosurfactant production. This is consistent with our previous finding that about 10 times more biosurfactant (6 ng/ml) was isolated from the ΔplcR mutant than from the wild type (0.5 ng/ml) (18). Surfactin had an Rf value of 0.24, compared to 0.69 for the B. cereus biosurfactant. The results obtained with bromthymol blue, which stains lipid moieties, were similar to those obtained with ninhydrin (Fig. 4B). All three B. cereus strains had spots with the same Rf value (0.69). Our results suggest that the biosurfactant is a lipopeptide and is negatively regulated by PlcR. Alternatively, the biosurfactant might be degraded in the wild type through the action of degradative enzymes encoded by PlcR-regulated genes (1).
FIG. 4.
TLC analysis of the biosurfactant(s). Partially purified biosurfactant from the wild type (WT), the ΔplcR mutant, or the complemented ΔplcR mutant strain (CM) was run on a silica gel TLC plate and stained with ninhydrin (A) or bromthymol blue (B). The biosurfactant(s) is indicated by the upper arrow. Surfactin (40 μg) was run as a positive control (indicated by the lower arrow). (C) Hemolytic assay. One-hundred-microliter volumes of Tris-HCl buffer, surfactin (5 μg/100 μl buffer), and the biosurfactant of the ΔplcR mutant purified from TLC plates (50 μl/100 μl of buffer) were added to wells of sheep blood agar plates and incubated at 37°C for 48 h. The hemolysis results presented are representative of duplicate experiments.
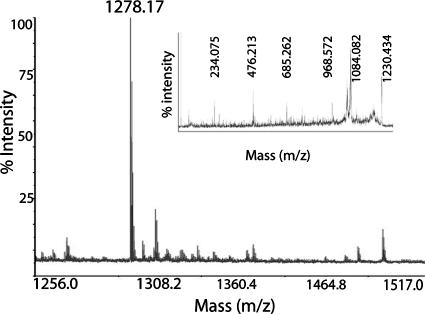
MALDI-TOF analysis.
To further characterize the biosurfactant, a MALDI-TOF MS analysis was conducted. The biosurfactant extracted from TLC plates showed a single [M+H]+ peak with an m/z ratio of 1,278.1722 (Fig. 5). As a control, analysis of surfactin showed a single [M+H]+ peak with the expected m/z ratio of 1,036.66 (data not shown). MS-MS analysis showed that both surfactin (data not shown) and the biosurfactant extracted from the ΔplcR mutant could be fragmented (Fig. 5, inset). However, an amino acid sequence could not be predicted from either spectrum, suggesting that the lipid moiety of both lipopeptides may have affected fragmentation. In addition, isoforms present in the surfactin and B. cereus biosurfactant preparations could have prevented definitive identification of the amino acid sequences.
FIG. 5.
MALDI-TOF MS analysis of the biosurfactant from the ΔplcR mutant, showing an [M+H]+ peak at 1,278.1722. Inset, MS-MS fragmentation spectrum of the 1,278.1722 peak.
Hemolysis and antimicrobial assay.
Biosurfactants can act as detergents on biological membranes, and their presence in culture supernatant often can be detected by the ability to lyse red blood cells (42). B. cereus produces many hemolysins (3); therefore, supernatants cannot be used directly on red blood cells to screen for biosurfactant activity. We showed that the biosurfactant separated on TLC plates, similar to surfactin, had the ability to lyse sheep red blood cells (Fig. 4C).
We tested whether the biosurfactant had antimicrobial activity. Growth was inhibited by the biosurfactant in 6 of the 10 gram-positive bacteria tested, while none of the 5 gram-negative bacteria was affected (Table 1). B. cereus ATCC 14579 and B. cereus ATCC 10987 were not inhibited.
Unlike B. cereus, B. subtilis NCIB 3610 can swarm under low-nutrient conditions.
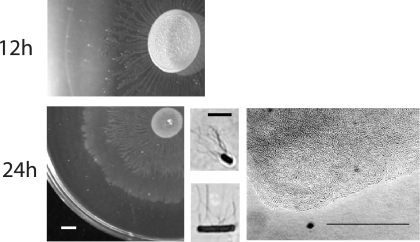
The dendrite colonies formed by the B. cereus ΔplcR mutant on EPS agar are similar to those developed by B. subtilis NCIB 3610 in a relatively rich casein digest-based medium supplemented with K+ ion, and surfactin was required (12, 26). To determine whether B. subtilis would also slide like B. cereus under the low-nutrient conditions used in this study, strain NCIB 3610 was inoculated onto EPS and 5×YE EPS agars and incubated for up to 24 h. It produced a visible delimiting ring on both agar surfaces as early as 2 h (data not shown). By 12 h, migrating cells had formed branched patterns on both agars (EPS agar is shown in Fig. 6); the pattern on 5×YE EPS agar was considerably more extensive (data not shown). At 24 h, a second concentric zone of spreading was observed (Fig. 6). This zone was larger on 5×YE EPS agar, and migration had occurred almost to the edge of the plate (data not shown). This pattern is similar to that of swarm colonies of B. subtilis 3610 observed on B medium agar by Julkowska et al. (19), who showed that a transparent zone produced by surfactin and bound by a delimiting ring preceded the edge of the swarm zone.
FIG. 6.
Effect of nutrient conditions on the colony morphology of B. subtilis NCIB 3610. An overnight culture was spotted onto EPS agar and incubated at 32°C. Colony morphology was observed at 12 and 24 h (bar, 0.5 cm). Colony margins were observed by microscopy at 24 h (bar, 100 μm). Cells were lifted from the colony margins, stained, and observed by microscopy for the production of flagella (bar, 10 μm).
Cells from the edge of the outer zone on EPS agar at 24 h were hyperflagellated and elongated, whereas cells from the edge of the central branched pattern colony were short flagellated rods (Fig. 6). This is consistent with the pattern of migration and consolidation of swarmer cells. Microscopic observation showed that the cells at the edge of swarm colonies on EPS agar were aligned in rafts and moved in a coordinated manner. Similar results were observed on 5×YE EPS agar (data not shown). These results showed that unlike B. cereus ATCC 14579 ΔplcR, B. subtilis 3610 is still able to swarm under low-nutrient conditions.
DISCUSSION
Surface translocation by B. cereus ATCC 14579 is nutrient dependent and negatively affected by PlcR under low-nutrient conditions. On EPS-0.7% agar, dendrite growth patterns are preceded by a delimiting ring due to biosurfactant production. The dendritic pattern is formed by nonflagellated cells, requires sufficient production of a biosurfactant, and resembles sliding translocation. In contrast to growth on EPS agar, a delimiting ring was not observed on 5× YE EPS or LB agar. B. cereus ATCC 14579 and its ΔplcR mutant produced flagella on rich medium. However, unlike the wild type, the ΔplcR mutant could not swarm. The lack of swarming ability is associated with the inability of mutant cells to hyperflagellate, which is probably related to the reduced expression of flagellin. PlcR was shown to indirectly affect flagellin expression and swimming motility in B. cereus (6, 13). Gohar et al. (13) demonstrated by two-dimensional electrophoresis analysis of the PlcR regulon that PlcR positively regulated flagellin production by B. cereus ATCC 14579. Flagellin production by the ΔplcR mutant was decreased threefold but not completely abolished.
Nutrient availability is an important factor for bacterial surface translocation. Swarming is a high-energy-consuming process that requires the synthesis of large numbers of flagella and therefore may not be a viable option when nutrients are scarce. Many bacteria do not swarm under low-nutrient conditions because of the increased metabolic cost of enhanced flagellum synthesis (15). We found that B. subtilis NCIB 3610 responded to nutrient levels quite differently. Unlike many bacteria, including B. cereus, B. subtilis can swarm under low-nutrient conditions.
In a low-nutrient environment like EPS, B. cereus adopts a different strategy for surface translocation. Sliding provides an alternative mode of translocation that requires much less energy, as flagella are not necessary. PlcR is affected by nutrient concentration, and its expression decreased when cells were grown in a low-nutrient medium used for sporulation (28). In addition, we have shown that in the ΔplcR mutant biosurfactant production is enhanced. The biosurfactant lowers surface tension and facilitates flagellum-independent sliding, which allows the organism a means of surface translocation with a minimal output of energy. In the nonflagellated strain Serratia marcescens SS-1, SpnR, a LuxR homologue, repressed biosurfactant production and inhibited sliding (17). The ΔspnR mutant produced more biosurfactant than the wild type and had increased sliding. PlcR in B. cereus may play a role similar to that of SpnR. However, the negative effect of PlcR on biosurfactant production or stability may be indirect, via the activity of degradative enzymes encoded by PlcR-regulated genes (1).
Under the conditions used in this study, the amount of biosurfactant produced by the wild type was insufficient to induce sliding; however, when surfactin or biosurfactant isolated from the ΔplcR mutant was added, it formed dendritic patterns identical to those of the mutant. The role of biosurfactant in sliding has been demonstrated in other organisms. In B. subtilis, sliding motility is flagellum independent and associated with the production of surfactin (25, 26). Our observation that flagella are not needed for sliding motility in B. cereus agrees with what has been reported for B. subtilis strains 6051, NCIB 3610, and 168, where dendrite formation is dependent on the production of surfactin and the presence of sufficient K+ concentrations in a relatively rich medium.
We tested 11 other wild-type B. cereus strains isolated from different sources, and they were all able to produce a delimiting ring on EPS agar, followed by the formation of dendrite colonies of different sizes, indicating that sliding motility in B. cereus is not an aberrant behavior that occurs only in the absence of plcR. B. cereus ATCC 14579 can also produce a delimiting ring, but the amount of biosurfactant generated is insufficient to facilitate sliding. In addition, we found that B. thuringiensis 407 Cry−, a close relative of B. cereus, was able to form a delimiting ring followed by sliding motility. Its ΔplcR mutant formed a larger delimiting ring sooner than the wild type and developed a more extensive dendritic pattern (data not shown).
The biosurfactant from the ΔplcR mutant was purified by TLC and shown to have a mass of 1,278.1722 (m/z), and it is most likely a lipopeptide. It can lyse red blood cells and inhibits the growth of several gram-positive bacteria. Unlike the bacteriocin-like inhibitory substance (3.4 kDa) that Risøen et al. (35) isolated from strain ATCC 14579, it does not inhibit the growth of B. cereus ATCC 10987. As nutrient levels become scarce, the enhanced production of a biosurfactant by B. cereus not only permits it to translocate more efficiently in search of nutrients but may also help it colonize and survive in a new environment by inactivating some competing microorganisms. We showed that biosurfactant production was required for biofilm production by B. cereus ATCC 14579 (18). It was demonstrated that surfactin production played a role in stable biofilm formation by B. subtilis strain 6051 on root surfaces and protected plants against attack by pathogenic bacteria (2).
Many biosurfactants are synthesized by NRPS and PKS, but information on biosurfactant synthesis by B. cereus is limited. With the universal primers TDG and LGG (10, 40), designed to detect a conserved domain of a peptide synthetase gene, we obtained a PCR amplicon from ATCC 14579 with the expected band size of 500 bp (10) (data not shown). In silico analysis of the ATCC 14579 genome showed a cluster of genes (BC2452 to BC2458) with sequences homologous to those of NRPS genes; however, some of these genes appear to be truncated. The antibiotic zwittermicin A produced by B. cereus UW101C was shown to be synthesized by an NRPS/PKS pathway (11). The emetic toxin cereulide is synthesized by an NRPS cluster that resides on a plasmid (9).
In conclusion, we showed that dendrite pattern formation by B. cereus is promoted by low-nutrient conditions and is related to the production of a biosurfactant that is negatively affected in a strain with an active PlcR regulon. Dendritic patterns are formed by sliding translocation of nonflagellated cells and provide an alternative mode of translocation that requires much less energy, as flagella are not necessary. The antibacterial activity of the biosurfactant may also aid in the survival of the organism in the environment. Interestingly, sliding was observed in Bacillus anthracis, a nonmotile member of the B. cereus group, as early as 1910 by Graham-Smith (14) and later by Henrichsen (16). To our knowledge, this is the first report of flagellum-independent surface translocation in B. cereus. How biosurfactant production or stability is reduced by PlcR remains to be determined.
Supplementary Material
Acknowledgments
This research was supported by Hatch funds (WIS04799) and by the College of Agricultural and Life Sciences, University of Wisconsin—Madison.
We thank Laurent Bouillaut for construction of the complemented strain B. cereus ATCC 14579 [ΔplcR(pHT304ΩB1)].
Footnotes
Published ahead of print on 5 October 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Agaisse, H., M. Gominet, O. A. Økstad, A. B. Kolstø, and D. Lereclus. 1999. PlcR is a pleiotropic regulator of extracellular virulence factor gene expression in Bacillus thuringiensis. Mol. Microbiol. 32:1043-1053. [DOI] [PubMed] [Google Scholar]
- 2.Bais, H. P., R. Fall, and J. M. Vivanco. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beecher, D. J., and A. C. L. Wong. 2000. Cooperative, synergistic and antagonistic haemolytic interactions between haemolysin BL, phosphatidylcholine phospholipase C and sphingomyelinase from Bacillus cereus. Microbiology 146:3033-3039. [DOI] [PubMed] [Google Scholar]
- 4.Bouillaut, L., N. Ramarao, C. Buisson, N. Gilois, M. Gohar, D. Lereclus, and C. Nielsen-Leroux. 2005. FlhA influences Bacillus thuringiensis PlcR-regulated gene transcription, protein production, and virulence. Appl. Environ. Microbiol. 71:8903-8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, I. I., and C. C. Häse. 2001. Flagellum-independent surface migration of Vibrio cholerae and Escherichia coli. J. Bacteriol. 183:3784-3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callegan, M. C., S. T. Kane, D. C. Cochran, M. S. Gilmore, M. Gominet, and D. Lereclus. 2003. Relationship of PlcR-regulated factors to Bacillus endophthalmitis virulence. Infect. Immun. 71:3116-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvio, C., F. Celandroni, E. Ghelardi, G. Amati, S. Salvetti, F. Ceciliani, A. Galizzi, and S. Senesi. 2005. Swarming differentiation and swimming motility in Bacillus subtilis are controlled by swrA, a newly identified dicistronic operon. J. Bacteriol. 187:5356-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshayes, C., F. Laval, H. Montrozier, M. Daffe, G. Etienne, and J. M. Reyrat. 2005. A glycosyltransferase involved in biosynthesis of triglycosylated glycopeptidolipids in Mycobacterium smegmatis: impact on surface properties. J. Bacteriol. 187:7283-7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehling-Schulz, M., M. Fricker, H. Grallert, P. Rieck, M. Wagner, and S. Scherer. 2006. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehling-Schulz, M., N. Vukov, A. Schulz, R. Shaheen, M. Andersson, E. Märtlbauer, and S. Scherer. 2005. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl. Environ. Microbiol. 71:105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmert, E. A. B., A. K. Klimowicz, M. G. Thomas, and J. Handelsman. 2004. Genetics of zwittermicin A production by Bacillus cereus. Appl. Environ. Microbiol. 70:104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fall, R., D. B. Kearns, and T. Nguyen. 2006. A defined medium to investigate sliding motility in a Bacillus subtilis flagella-less mutant. BMC Microbiol. 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gohar, M., O. A. Økstad, N. Gilois, V. Sanchis, A. B. Kolstø, and D. Lereclus. 2002. Two-dimensional electrophoresis analysis of the extracellular proteome of Bacillus cereus reveals the importance of the PlcR regulon. Proteomics 2:784-791. [DOI] [PubMed] [Google Scholar]
- 14.Graham-Smith, G. S. 1910. The division and post-fission movements of bacilli when grown on solid media. Parasitology 3:17-53. [Google Scholar]
- 15.Harshey, R. M. 2003. Bacterial motility on a surface: many ways to a common goal. Annu. Rev. Microbiol. 57:249-273. [DOI] [PubMed] [Google Scholar]
- 16.Henrichsen, J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horng, Y. T., S. C. Deng, M. Daykin, P. C. Soo, J. R. Wei, K. T. Luh, S. W. Ho, S. Swift, H. C. Lai, and P. Williams. 2002. The LuxR family protein SpnR functions as a negative regulator of N-acylhomoserine lactone-dependent quorum sensing in Serratia marcescens. Mol. Microbiol. 45:1655-1671. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh, Y. H., E. B. Somers, D. Lereclus, and A. C. L. Wong. 2006. Biofilm formation by Bacillus cereus is influenced by PlcR, a pleiotropic regulator. Appl. Environ. Microbiol. 72:5089-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julkowska, D., M. Obuchowski, I. B. Holland, and S. J. Séror. 2004. Branched swarming patterns on a synthetic medium formed by wild-type Bacillus subtilis strain 3610: detection of different cellular morphologies and constellations of cells as the complex architecture develops. Microbiology 150:1839-1849. [DOI] [PubMed] [Google Scholar]
- 20.Julkowska, D., M. Obuchowski, I. B. Holland, and S. J. Séror. 2005. Comparative analysis of the development of swarming communities of Bacillus subtilis 168 and a natural wild type: critical effects of surfactin and the composition of the medium. J. Bacteriol. 187:65-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearns, D. B., F. Chu, R. Rudner, and R. Losick. 2004. Genes governing swarming in Bacillus subtilis and evidence for a phase variation mechanism controlling surface motility. Mol. Microbiol. 52:357-369. [DOI] [PubMed] [Google Scholar]
- 22.Kearns, D. B., and R. Losick. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581-590. [DOI] [PubMed] [Google Scholar]
- 23.Keating, T. A., and C. T. Walsh. 1999. Initiation, elongation, and termination strategies in polyketide and polypeptide antibiotic biosynthesis. Curr. Opin. Chem. Biol. 3:598-606. [DOI] [PubMed] [Google Scholar]
- 24.Kim, P. I., H. Bai, D. Bai, H. Chae, S. Chung, Y. Kim, R. Park, and Y. T. Chi. 2004. Purification and characterization of a lipopeptide produced by Bacillus thuringiensis CMB26. J. Appl. Microbiol. 97:942-949. [DOI] [PubMed] [Google Scholar]
- 25.Kinsinger, R. F., D. B. Kearns, M. Hale, and R. Fall. 2005. Genetic requirements for potassium ion-dependent colony spreading in Bacillus subtilis. J. Bacteriol. 187:8462-8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinsinger, R. F., M. C. Shirk, and R. Fall. 2003. Rapid surface motility in Bacillus subtilis is dependent on extracellular surfactin and potassium ion. J. Bacteriol. 185:5627-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lereclus, D., H. Agaisse, M. Gominet, S. Salamitou, and V. Sanchis. 1996. Identification of a Bacillus thuringiensis gene that positively regulates transcription of the phosphatidylinositol-specific phospholipase C gene at the onset of the stationary phase. J. Bacteriol. 178:2749-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lereclus, D., H. Agaisse, C. Grandvalet, S. Salamitou, and M. Gominet. 2000. Regulation of toxin and virulence gene transcription in Bacillus thuringiensis. Int. J. Med. Microbiol. 290:295-299. [DOI] [PubMed] [Google Scholar]
- 29.Lereclus, D., O. Arantes, J. Chaufaux, and M. Lecadet. 1989. Transformation and expression of a cloned delta-endotoxin gene in Bacillus thuringiensis. FEMS Microbiol. Lett. 51:211-217. [DOI] [PubMed] [Google Scholar]
- 30.Matsuyama, T., A. Bhasin, and R. M. Harshey. 1995. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium. J. Bacteriol. 177:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayfield, C. I., and W. E. Inniss. 1977. A rapid, simple method for staining bacterial flagella. Can. J. Microbiol. 23:1311-1313. [DOI] [PubMed] [Google Scholar]
- 32.Mukherjee, S., P. Das, and R. Sen. 2006. Towards commercial production of microbial surfactants. Trends Biotechnol. 24:509-515. [DOI] [PubMed] [Google Scholar]
- 33.Nishikiori, T., H. Naganawa, Y. Muraoka, T. Aoyagi, and H. Umezawa. 1986. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. II. Structure of fatty acid residue and amino acid sequence. J. Antibiot. (Tokyo) 39:745-754. [DOI] [PubMed] [Google Scholar]
- 34.Recht, J., and R. Kolter. 2001. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J. Bacteriol. 183:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risøen, P. A., P. Ronning, I. K. Hegna, and A. B. Kolstø. 2004. Characterization of a broad range antimicrobial substance from Bacillus cereus. J. Appl. Microbiol. 96:648-655. [DOI] [PubMed] [Google Scholar]
- 36.Salamitou, S., F. Ramisse, M. Brehelin, D. Bourguet, N. Gilois, M. Gominet, E. Hernandez, and D. Lereclus. 2000. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology 146:2825-2832. [DOI] [PubMed] [Google Scholar]
- 37.Schoeni, J. L., and A. C. L. Wong. 2005. Bacillus cereus food poisoning and its toxins. J. Food Prot. 68:636-648. [DOI] [PubMed] [Google Scholar]
- 38.Senesi, S., F. Celandroni, S. Salvetti, D. J. Beecher, A. C. L. Wong, and E. Ghelardi. 2002. Swarming motility in Bacillus cereus and characterization of a fliY mutant impaired in swarm cell differentiation. Microbiology 148:1785-1794. [DOI] [PubMed] [Google Scholar]
- 39.Singh, P., and S. S. Cameotra. 2004. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 22:142-146. [DOI] [PubMed] [Google Scholar]
- 40.Turgay, K., and M. A. Marahiel. 1994. A general approach for identifying and cloning peptide synthetase genes. Pept. Res. 7:238-241. [PubMed] [Google Scholar]
- 41.Umezawa, H., T. Aoyagi, T. Nishikiori, A. Okuyama, Y. Yamagishi, M. Hamada, and T. Takeuchi. 1986. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG302-fF67. I. Taxonomy, production, isolation and preliminary characterization. J. Antibiot. (Tokyo) 39:737-744. [DOI] [PubMed] [Google Scholar]
- 42.Youssef, N. H., K. E. Duncan, D. P. Nagle, K. N. Savage, R. M. Knapp, and M. J. McInerney. 2004. Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods 56:339-347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.