Abstract
The grass parasites Claviceps purpurea and Claviceps fusiformis produce ergot alkaloids (EA) in planta and in submerged culture. Whereas EA synthesis (EAS) in C. purpurea proceeds via clavine intermediates to lysergic acid and the complex ergopeptines, C. fusiformis produces only agroclavine and elymoclavine. In C. purpurea the EAS gene (EAS) cluster includes dmaW (encoding the first pathway step), cloA (elymoclavine oxidation to lysergic acid), and the lpsA/lpsB genes (ergopeptine formation). We analyzed the corresponding C. fusiformis EAS cluster to investigate the evolutionary basis for chemotypic differences between the Claviceps species. Other than three peptide synthetase genes (lpsC and the tandem paralogues lpsA1 and lpsA2), homologues of all C. purpurea EAS genes were identified in C. fusiformis, including homologues of lpsB and cloA, which in C. purpurea encode enzymes for steps after clavine synthesis. Rearrangement of the cluster was evident around lpsB, which is truncated in C. fusiformis. This and several frameshift mutations render CflpsB a pseudogene (CflpsBΨ). No obvious inactivating mutation was identified in CfcloA. All C. fusiformis EAS genes, including CflpsBΨ and CfcloA, were expressed in culture. Cross-complementation analyses demonstrated that CfcloA and CflpsBΨ were expressed in C. purpurea but did not encode functional enzymes. In contrast, CpcloA catalyzed lysergic acid biosynthesis in C. fusiformis, indicating that C. fusiformis terminates its EAS pathway at elymoclavine because the cloA gene product is inactive. We propose that the C. fusiformis EAS cluster evolved from a more complete cluster by loss of some lps genes and by rearrangements and mutations inactivating lpsB and cloA.
Numerous phytopathogenic fungi belonging to the genus Claviceps produce ergot alkaloids (EA), which are tri- or tetracyclic derivatives of prenylated tryptophan. Some EA show structural similarity to the neurotransmitters serotonin and dopamine and have affinity to the cognate receptors in the central nervous system (1, 12). Therefore, EA have long been used for treatment of a variety of disorders of the central and peripheral nervous systems (22).
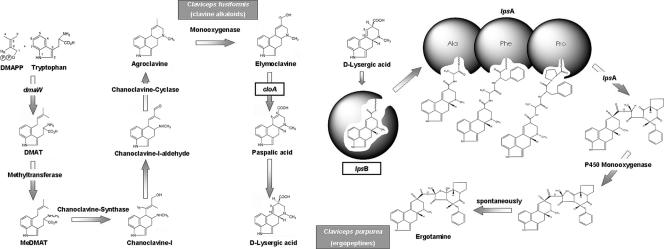
EA for pharmaceutical use are produced using Claviceps purpurea, a ubiquitous fungus that infects nearly 600 grasses and cereals and causes ergot disease (2). These alkaloids accumulate in sclerotia (“ergots”), the dense fungal resting structures that replace host seeds. The biochemistry of EA formation in C. purpurea, Claviceps fusiformis, and other fungi has been studied in detail (for reviews, see references 6 and 17). The first reaction of the pathway is prenylation of tryptophan with formation of dimethylallyltryptophan (Fig. 1). Biosynthesis proceeds to production of clavine alkaloids such as agroclavine and elymoclavine, the end product in C. fusiformis. In C. purpurea the pathway continues to lysergic acid, and the end products of the pathway are lysergic acid amides, including the complex ergopeptines.
FIG. 1.
Diagrams of EA biosynthesis in C. fusiformis (end product, elymoclavine) and C. purpurea (ergopeptides). Genes of interest are enclosed in boxes. DMAPP, dimethylallylpyrophosphate; DMAT, dimethylallyltryptophan; MeDMAT, methyl dimethylallyltryptophan. (Modified from reference 7 with permission.)
Roles for several genes of the EA synthesis (EAS) gene cluster (EAS cluster) in C. purpurea have been characterized to date. The gene encoding the enzyme catalyzing the first step (tryptophan aromatic prenylation) is dmaW. The cloA gene product converts elymoclavine to paspalic acid, which undergoes a spontaneous isomerization to lysergic acid. Lysergyl peptide synthetase 1 (a nonribosomal peptide synthetase [NRPS]) is a complex consisting of the products of lpsB and one of the two lpsA paralogues, lpsA1 or lpsA2. For example, the complex consisting of the lpsA1 and lpsB gene products catalyzes formation of ergotamam, which is then oxidized (presumably by another enzyme) to ergotamine (3). These genes have been functionally analyzed by heterologous expression or gene replacement approaches (3, 8, 21). The cluster also contains genes predicted to encode oxidoreductases, a catalase, and an additional NRPS, LpsC, the function of which is still not known (7, 23).
Whereas C. purpurea produces lysergic acid and its complex derivatives, the EAS pathway in C. fusiformis terminates at elymoclavine. These two fungi have similar life cycles, but the host range of C. fusiformis is limited to pearl millet (Pennisetum glaucum) and buffel grass (Pennisetum ciliare), whereas C. purpurea infects temperate grasses (subfamily Pooideae). Here we describe a comparative sequence analysis of EAS cluster genes in these two fungi, as well as expression studies, to determine the genetic basis for differences in their EA profiles.
MATERIALS AND METHODS
Strains and culture conditions.
C. purpurea strain P1 (= ATCC 20102), which produces mainly ergotamine along with low levels of ergocryptine, was described previously (10, 23), as were the standard media and culture conditions (23). C. fusiformis strain SD58 (= ATCC 26245), which produces mainly agroclavine and elymoclavine, and its culture growth conditions have been described previously (21). For alkaloid production and for RNA expression studies, C. purpurea was cultivated in T25N medium (23) and C. fusiformis was cultivated in T2 medium [100 g/liter saccharose, 10 g/liter l-asparagine, 1 g/liter Ca(NO3)2, 0.1 g/liter yeast extract, 0.25 g/liter MgSO4·7H2O, 0.15 g/liter ZnSO4·7H2O] with low (0.5 g/liter KH2PO4) and high (2.0 g/liter KH2PO4) levels of phosphates.
Molecular biology techniques.
Standard cloning and DNA analysis techniques were performed as described by Sambrook et al. (16). The Escherichia coli strain used for cloning (by using plasmids pUC19 [Fermentas, St. Leon-Rot, Germany] and pCR2.1-TOPO [Invitrogen, Karlsruhe, Germany]) and propagation of clones was TOP10F′ (Invitrogen, Karlsruhe, Germany). Extraction of genomic DNA, Southern and Northern blot analyses, and DNA sequencing were performed as described previously (13). Genomic Southern analyses of dmaW and lpsA were conducted as described by Wang et al. (24) and Panaccione et al. (14), respectively. For sequence comparisons and multiple-sequence alignment DNA STAR was used. For PCR analysis, BioTherm (Genecraft, Lüdinghausen, Germany) polymerase was used according to the manufacturer's instructions.
Cosmid clones, transposon insertion library, and sequencing.
A cosmid clone of C. fusiformis, designated Cf26E11, was previously identified as a clone containing dmaW (21). A library of transposon insertions in Cf26E11 was created using a HyperMu <KAN1> insertion kit (EPICENTRE Biotechnologies, Madison, WI) according to the manufacturer's instructions. Positive clones were selected on LB agar with kanamycin and ampicillin. The cosmid DNAs were isolated from bacterial cultures with Perfectprep BAC 96 (Eppendorf, Westbury, NY). DNA sequencing from both ends of each HyperMu insert was performed at the University of Kentucky Advanced Genetic Technologies Center, using a BigDye Terminator v3.1 cycle sequencing kit, a GeneAmp 9700 PCR system thermocycler, and a 3730XL DNA analyzer (Applied Biosystems, Foster City, CA). Sequencing was conducted on eight 96-well plates containing cosmids with independent HyperMu insertions.
Sequences were viewed and assembled using the Phred-Phrap package and were viewed with Consed for Linux Systems (D. Gordon, University of Washington). Coding sequences of C. fusiformis EAS genes were identified using the BLASTX resource of NCBI (http://www.ncbi.nlm.nih.gov/BLAST/). Exons were determined by combining results of three NCBI BLAST alignments (BLASTX, TBLASTX, and BLASTN) and also by using gene predictions obtained with Fgenesh (http://sun1.softberry.com/berry.phtml?topic=fgenesh&group=programs&subgroup=gfind) using Aspergillus spp., Magnaporthe oryzae (Magnaporthe grisea), and Neurospora crassa as reference fungi. Protein family signatures were identified with InterProScan (http://www.ebi.ac.uk/InterProScan/) and Prosite (http://www.expasy.ch/prosite/).
RNA isolation and reverse transcription PCR (RT-PCR).
Total RNA extraction from 7- to 14-day-old mycelia and DNase treatment were performed as described previously (18) using the RNAgents total RNA isolation system (Promega, Mannheim, Germany). Concentrations of purified RNA were determined using a BioPhotometer (Eppendorf, Hamburg, Germany), and RNA integrity was examined by electrophoresis in 1% agarose gels. For gene expression studies, total RNA was isolated from EA-inducing and -suppressing cultures, and the derived cDNA was synthesized and amplified with the SuperScript-II reverse transcriptase (Invitrogen, Karlsruhe, Germany) as described previously (20). The primers used for sequencing reactions are summarized in Table 1. For expression studies, sequencing of EAS genes from C. fusiformis cDNA was performed with primers to obtain products that span at least one intron of each gene. Splicing of the eighth intron of CfcloA was examined by PCR and sequencing with primers MO3 and MO2.
TABLE 1.
Primers used for RT-PCR and sequencing reactions
| Use | Forward primer
|
Reverse primer
|
||
|---|---|---|---|---|
| Designation | Sequence (5′-3′) | Designation | Sequence (5′-3′) | |
| C. fusiformis EAS gene expression | CfdmaW_1d | ACACGGATGGTATGAGATGGC | Cf_dmaW_3u | CTACGTCAGGGACAAGTCAC |
| Cf_easG_d | GTTTGCACGCACGGGAATAC | Cf_easG_u | AGGCAGTGGCAAGACTGCTG | |
| Cf_easF_d | AACGAAGGACTGGACCACTG | Cf_easF_u | GTGTTTCGCTGATCCATCGT | |
| Cf_easE_d | AAGTATATGCTCATGGTGTC | Cf_easE_u | CATATCATTGAAGTGCTGCA | |
| Cf_easD_d | TTGGCTCGCGGTACAGCTTC | Cf_easD_u | GACGATTCTCGAGGAAGACG | |
| Cf_easC_d | GTCAACTTGCCTACACATCG | Cf_easC_u | GGACCTCGTACATGCTCAAC | |
| Cf_cloA_d | AATCATGGCGTCCAGTCCAC | Cf_cloA_u | CTCTGGCTATACCAAGCTCT | |
| Cf_lpsB_d | CTGTTCACGAGAGCATCGCG | Cf_lpsB_u | CGAAACTACCATCTCAGAGC | |
| MO3 | TCAGGTCCATCGATCAGCCAGTAT | MO2 | TTTTCATCCTCTTCGCAACATTCC | |
| C. fusiformis lpsB integration test | Cf_lpsB_f | TGCGGCGAGGAACGAAGAATGG | Cf_lpsB_r | GAGACTGCGGCGGGAAGGCTAATC |
| C. fusiformis cloA integration test | Cf_cloA_f | CCTCCGAGCAGCCTTACTTTT | Cf_cloA_r | CTAGGCGCGTTGACCGACTTGT |
| HI-LF-P450-1 | CCCCTAGGACATTGATCGGAGA | HI-WT-P450-1 | AAGGGGCATTACTATAGGTTCTTTC | |
| UR450 | ATCGATCTGCCCTTCGCTTTCTCGTT | HI-RF-P450-1 | CGCTTGAACCCGCTATTACGAG | |
Construction of complementation vectors and transformation of C. purpurea and C. fusiformis.
Clones for complementation of C. purpurea deletion mutants were constructed by ligating restriction fragments from Cf26E11 into pUC19 (Fermentas, St. Leon-Rot, Germany). One clone contained CfcloA in a 5.8-kb XbaI fragment, and another clone contained CfcloA in a 3.9-kb EcoRI fragment. Transformation of protoplasts was performed as described previously (3), using the vector pAN7.1_UM, which includes a hygromycin resistance gene, as the covector for transformant selection. Plasmid cP450-1 (8), containing CpcloA and a hygromycin resistance gene, was used for transformation of C. fusiformis.
PCR.
To test for integration of the CflpsB pseudogene (CflpsBΨ), PCR was performed with primers cflpsB_f and cflpsB_r. To test for integration of CfcloA, PCR was performed with primers cfcloA_f and cfcloA_r.
The primers used to test for integration of the complete DNA segment, including the cloA promoter and the left portion of the gene, were HI-LF-P450-1 and HI-WT-P450-1. Integration of the right portion of the gene and its terminator was tested by PCR with primers HI-RF-P450-1 and UR450.
For details concerning the sequences of the primers see Table 1.
Extraction and analyses of ergot alkaloids.
For alkaloid extraction and determination, the pH of cultures was adjusted to 11 with concentrated aqueous ammonium hydroxide, the cultures were extracted three times with chloroform, and, after concentration, the resulting liquid was applied to thin-layer chromatography (TLC) plates (Silica Gel 60; Merck, Darmstadt, Germany). Alkaloids were identified by comparison with corresponding standards. After separation in CH3CN-isopropanol-H2O (125:28:15, vol/vol/vol), TLC plates were sprayed with van Urk's reagent for alkaloid visualization. The standards used were 1 mg/ml ergotamine (Sigma, Taufkirchen, Germany), 1 mg/ml ergocryptine (Sigma, Taufkirchen, Germany), 1 mg/ml ergocristine (Sigma, Taufkirchen, Germany), 1 mg/ml agroclavine, and 1 mg/ml d-lysergic acid (Sandoz AG, Basel, Switzerland).
High-performance liquid chromatography (HPLC) separation was carried out using a LiChrospher 100 RP-18 column (5 ml; 250 by 4 mm; Merck/Hitachi, Darmstadt, Germany) operated at 25°C and a flow rate of 1 ml/min. Compounds were eluted using a 1:1 mixture of 10 mM (NH4)2CO3 and CH3CN for 20 to 40 min and were detected with an L-7400 UV detector. The EA standards used were those described above.
Mass spectroscopy analyses.
The HPLC-electrospray ionization-mass spectroscopy system consisted of an analytical liquid chromatography system (1100 series; Agilent Technologies Deutschland GmbH, Böblingen, Germany) coupled to a QTrap2000 with a TurboIonSpray source (Applied Biosystems, Darmstadt, Germany). Separation was performed on a Luna 5m C18 (2) column (100A; 100 by 4.6 mm; Phenomenex, Aschaffenburg, Germany) with a flow rate of 1,500 μl/min, and 400 μl/min was directed into a mass spectrometer.
RESULTS AND DISCUSSION
Comparison of the EAS cluster sequences of C. purpurea and C. fusiformis.
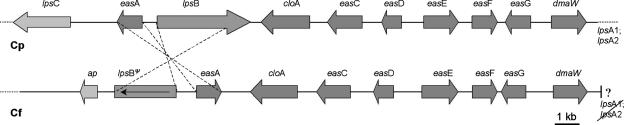
The EAS cluster sequence of C. purpurea P1 comprises 68.5 kb and includes 13 genes that are coordinately induced in EA production conditions (7). A clone (cosmid Cf26E11) of the homologous genomic region in C. fusiformis SD58 was previously obtained by screening a cosmid library with dmaW cDNA as a probe (21). We sequenced this cosmid and obtained a 35.4-kb contig, in which a 19.6-kb region contains nine homologues of the C. purpurea EAS cluster genes (Fig. 2). Corresponding to the orientation of the C. purpurea cluster, dmaW represents the right border of the sequenced C. fusiformis EAS cluster; the left border was flanked by a predicted aminopeptidase family gene (Fig. 2), to the left of which 15 hypothetical protein genes were identified. The corresponding location of the C. purpurea cluster was not homologous to the aminopeptidase gene or these hypothetical protein genes and instead contained (from right to left) another putative NRPS gene (lpsC), a gene encoding another hypothetical protein (orfD), and a putative amino acid biosynthesis gene (imd) (7).
FIG. 2.
Schematic comparison of the EAS clusters of C. purpurea (top) and C. fusiformis (bottom). Homologous genes in the two species are indicated by dark gray arrows, whereas genes identified in only one species are indicated by light gray arrows. The arrows indicate the orientation of transcription. The genes, as well as the intergenic regions, are drawn to scale. Rearrangement in the locus, indicated by dashed lines, is associated with truncation of lpsB in C. fusiformis. ap is a putative aminopeptidase gene adjacent to the C. fusiformis EAS cluster. The accession no. for the C. fusiformis cluster sequence is EU006773.
RT-PCR indicated that all genes of the C. fusiformis cluster were expressed in EA-producing cultures (data not shown). The expressed genes included the homologue of CpcloA, which encodes a cytochrome P450 monooxygenase necessary for synthesis of lysergic acid from elymoclavine. Also expressed was the homologue of CplpsB, which encodes the smaller subunit of the large NRPS, lysergyl peptide synthetase (23).
Considering the fact that C. fusiformis accumulates elymoclavine and not lysergic acid or its amides, there is no obvious role for cloA or lpsB in this species, and the possibility was considered that these genes are pseudogenes. A comparison of the amino acid sequences predicted for the cloA homologues in C. purpurea and C. fusiformis revealed 66.4% identity. This level of divergence was comparable to that between the functional homologues CfdmaW and CpdmaW. Within the heme-binding domain the level of identity was 80%. Therefore, there was no reason to expect that the gene has been inactive for a long evolutionary time. Furthermore, it was not apparent that any of the differences in CfcloA might affect the function of the protein product. Also, a comparison with the amino acid sequence derived from the putative orthologue from Epichloë festucae did not give any hint of a functional defect (data not shown). Therefore, we investigated whether CfcloA mRNA may be incorrectly processed. The gene sequence indicated the same exon-intron structure as CpcloA, although a potentially significant change was observed in the last intron, where CfcloA had a predicted 5′ GC splice junction, whereas CpcloA had the more common GT junction. RT-PCR analysis and sequence analysis of the products indicated that the expressed CfcloA transcript was nevertheless properly spliced (data not shown).
In the case of CflpsBΨ, numerous indel mutations shifted the reading frame throughout the coding sequence (Fig. 3). An in silico correction of these frameshifts gave a predicted product with 61.9% identity to the product of CplpsB (Fig. 3). However, this region of similarity extends only to codon 1084 of the 1,308 codons of CplpsB, and there is no recognizable match between the remaining 3′ portion of the C. purpurea gene in the C. fusiformis locus. Thus, CflpsBΨ lacks coding sequence for most of the last (condensation) domain of LPS2.
FIG. 3.
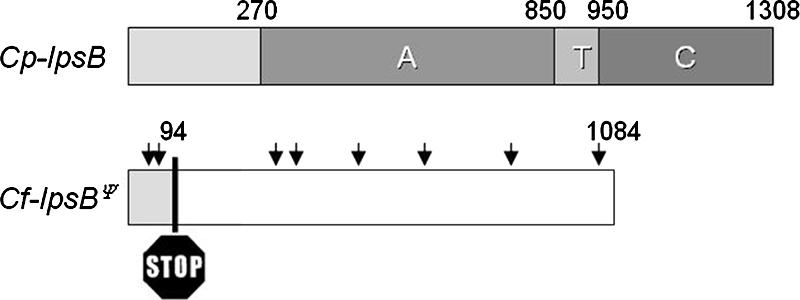
Schematic comparison of CplpsB and its homologue CflpsBΨ. Adenylation (A), thiolation (T), and condensation (C) domains are indicated by shaded boxes, and the numbers indicate the domain borders (amino acids) for CplpsB. The arrows indicate frameshift mutations in CflpsBΨ resulting in a premature stop codon at position 94. The open box indicates the portion of CflpsBΨ downstream of the stop codon that aligns with CplpsB.
The critical changes found in the first exon of CflpsBΨ included two deletions, 4 bp each, which shifted the reading frame to a reading frame ending with a stop at codon position 94 (Fig. 3). Another stop codon in this reading frame was at position 117. Therefore, only a short, nonfunctional peptide was expected as a product of this pseudogene. The insert in cosmid clone Cf26E11 contained no apparent fungal genes downstream of dmaW, whereas the corresponding region of the known EAS cluster in C. purpurea has genes for a putative nonheme iron oxygenase (easH) and the paralogous lpsA1 and lpsA2 genes (7, 17). We failed to extend the C. fusiformis sequence information in this direction by genome walking using thermal asymmetric interlaced PCR with genomic DNA of C. fusiformis as the template as well as identification of any cosmid that would extend this region. Apparently, the region downstream of CfdmaW is refractory to PCR or perhaps is telomere associated.
Southern blot analysis (Fig. 4), as well as PCR approaches (data not shown), revealed no lpsA homologues in the C. fusiformis genome. The CplpsA1 probe (14) hybridized to at least two EcoRI fragments from the genome of C. purpurea P1, but there was no detectable signal from C. fusiformis genomic DNA. In contrast, the CpdmaW probe cross-hybridized with CfdmaW (as well as with a paralogue in the C. purpurea genome), but not with the homologues in Neotyphodium species or Balansia obtecta. This observation is in keeping with previously published phylogenetic relationships, which group the Claviceps species closer to each other than to other genera in the family. Therefore, the observation that the CplpsA probe hybridized detectably to the lpsA genes in the Neotyphodium species and B. obtecta but not to DNA from C. fusiformis indicates that the latter species is devoid of detectable lpsA homologues. It has been reported that deletion of lpsA in a Neotyphodium species eliminates production of the ergopeptine ergovaline, as well as lysergic acid amide (14). The lack of functional lysergyl peptide synthetase genes in C. fusiformis is not surprising because this species does not synthesize the enzyme substrate, lysergic acid, or its derivatives. In all, we identified one pseudogene (CflpsBΨ) and eight potentially functional genes in the C. fusiformis EAS cluster that were homologues of genes in the C. purpurea EAS cluster. These genes included dmaW and cloA, as well as genes potentially encoding a flavin mononucleotide-containing oxidoreductase (easA), a catalase (easC), a dehydrogenase (easD), a flavin adenine dinucleotide-containing monooxygenase (easE), a methyltransferase (easF), and an oxidoreductase/epimerase (easG). However, the absence of paspalic acid and lysergic acid in C. fusiformis raised the question of whether CfcloA was functional.
FIG. 4.
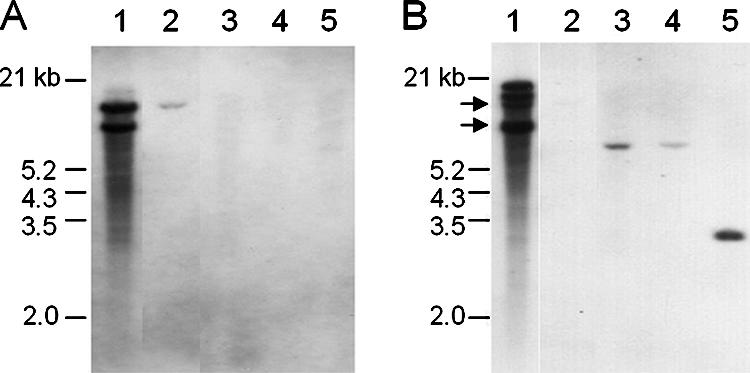
Southern blot analysis of genomic DNA from several EA-producing fungi. The membrane was first hybridized to a probe from CpdmaW (A) and then stripped and hybridized to probe from CplpsA (B). Samples (4 μg) of total fungal DNA were digested with EcoRI to completion, fractionated by electrophoresis in a 0.9% agarose gel, and transferred to a nylon membrane. The lanes contained DNA from C. purpurea P1 (lane 1), C. fusiformis SD58 (lane 2), Neotyphodium coenophialum ATCC 90664 (lane 3), N. lolii × Epichloë typhina Lp1 (lane 4), and B. obtecta B249 (lane 5). The arrows in panel B indicate the positions of a residual signal from the CpdmaW probe; the lower band (8.3 kb) was close to the position of an authentic lpsA signal.
Functional characterization of the cloA and lpsB homologues from C. fusiformis.
To test their functionality, the cloA and lpsB genes of C. fusiformis were used in an attempt to complement the corresponding deletion mutants of C. purpurea. A ΔlpsB deletion mutant (3) was transformed with the entire CflpsBΨ pseudogene, and its integration (including upstream and downstream regions) into the C. purpurea genome was confirmed by PCR and Southern blot analysis (data not shown). The transformed mutants were then cultivated under EA-producing conditions, and the expression of the EAS cluster genes was examined by Northern analysis. CflpsBΨ was expressed in transformed C. purpurea, but the transcript was much smaller than the C. purpurea homologue (data not shown). This was expected because of the apparent 3′ truncation of the coding sequence, as mentioned above. Extraction of EA from the cultivation medium and HPLC analysis showed the same alkaloid spectrum in the transformants (n = 6) as in the deletion mutant (data not shown), confirming that the gene was nonfunctional.
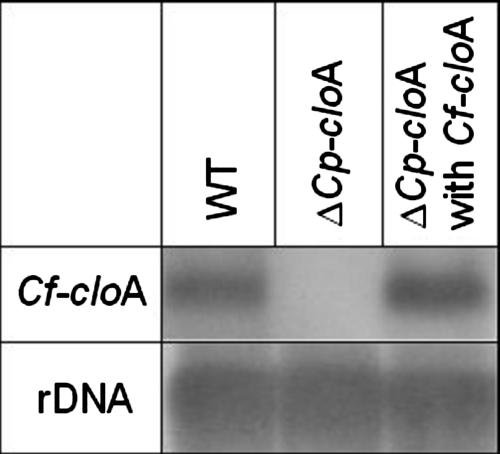
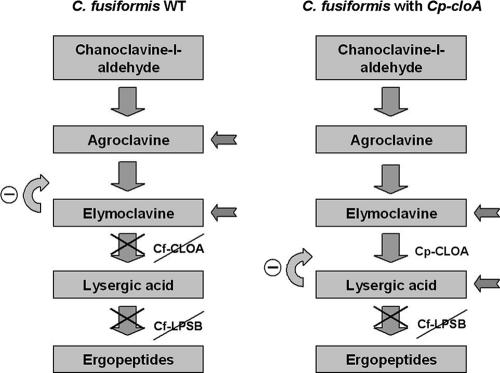
The same approach was used in an attempt to complement the ΔcloA mutant of C. purpurea (8) with CfcloA. Integration of CfcloA in C. purpurea genomic DNA was also confirmed by PCR and Southern blot analysis (data not shown). Northern analysis showed that CfcloA mRNA was expressed in the transformant (Fig. 5). However, alkaloid profiles showed no differences between the deletion mutant and the transformants (n = 10) (data not shown), indicating that CfcloA does not encode a functional homologue of CpcloA. These analyses show that the two C. fusiformis genes are expressed in the C. purpurea background (as they are in C. fusiformis); i.e., regulation of their transcription is normal, but the products that they encode seem to be inactive. This explains why the biosynthetic pathway terminates at elymoclavine in C. fusiformis (Fig. 1).
FIG. 5.
Heterologous expression of CfcloA in C. purpurea strains, including the wild type (P1) (WT), a ΔCpcloA mutant, and a transformant carrying the full-length CfcloA gene.
Modification of the alkaloid spectrum of C. fusiformis by a C. purpurea gene.
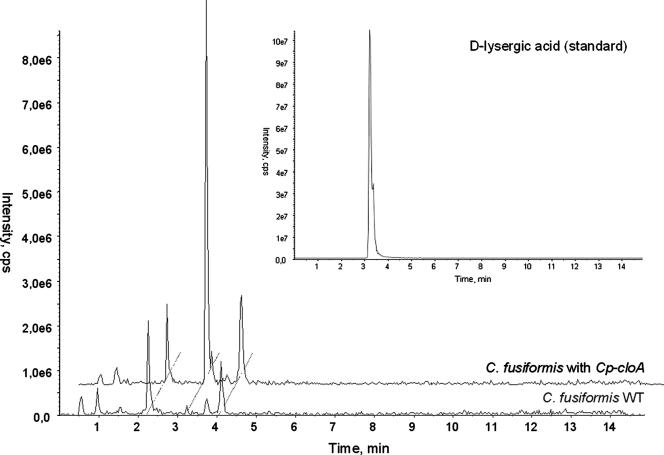
If the production of more complex EA by C. fusiformis is limited by the lack of a functional cloA, then the introduction of the functional cloA gene from C. purpurea should enable C. fusiformis to synthesize paspalic acid and lysergic acid. The construct used for complementation of C. fusiformis was previously used for restoration of the wild-type profile of the ΔcloA mutant of C. purpurea (8). Integration of CpcloA into the C. fusiformis genome was confirmed by PCR and Southern blot analysis, and expression of the gene was verified by RT-PCR (data not shown). Five of the transformants containing the C. purpurea gene were analyzed under EA-inducing conditions. Since the expected intermediates (paspalic acid and lysergic acid) are normally not secreted, mycelial extracts were also analyzed. As shown in Fig. 6, mycelial extracts of transformants indeed contained paspalic acid and lysergic acid, which were completely undetectable in the untransformed C. fusiformis recipient strain. The identity of the TLC peaks was confirmed by liquid chromatography-mass spectrometry analyses (Fig. 7). These results demonstrated that CloA converts elymoclavine to paspalic acid and confirmed that this step is missing in the C. fusiformis wild type.
FIG. 6.
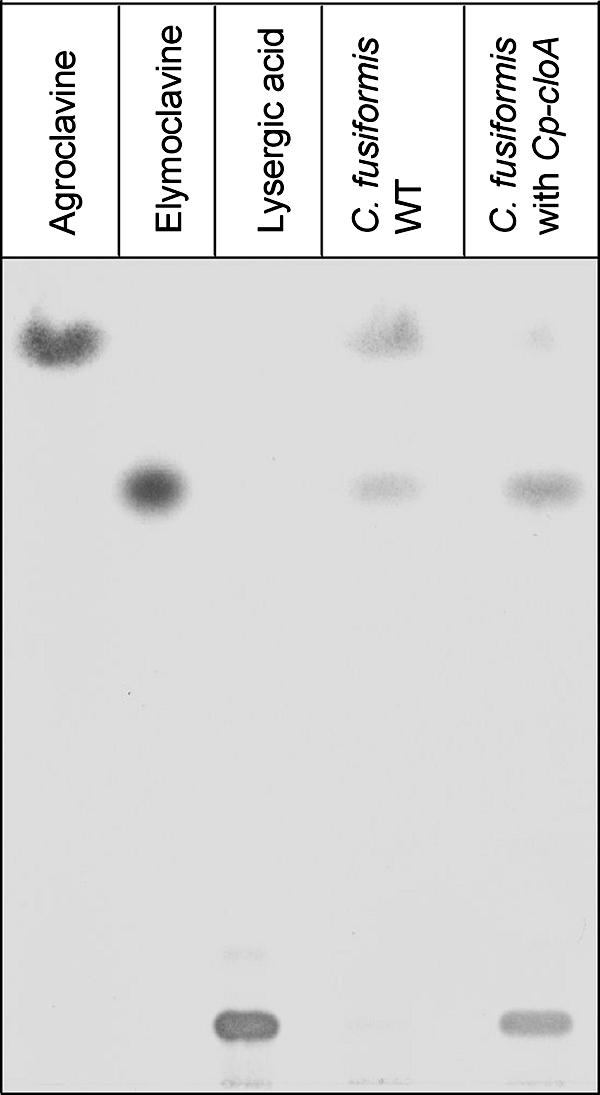
TLC analyses of ergot alkaloids from the C. fusiformis wild type (WT) and a transformant carrying the CpcloA gene. Culture extracts were chromatographed on TLC plates, visualized by using van Urk's reagent, and identified by comparison with pure standards.
FIG. 7.
Liquid chromatography-mass spectroscopy analyses of ergot alkaloids from the C. fusiformis wild type (WT) and a transformant carrying the CpcloA gene. The inset shows the migration of the lysergic acid standard.
The relatively small amount of lysergic acid present in the extracts could result from end product inhibition, as has been postulated to explain the alkaloid spectrum of the CpcloA mutant (8). As in this mutant, in C. fusiformis the end product of alkaloid biosynthesis, elymoclavine, is present at low levels compared to the levels of its immediate precursor, agroclavine, probably due to feedback inhibition of the agroclavine-oxidizing enzyme. In the complemented strain, elymoclavine accumulated to higher levels than lysergic acid, conceivably due to feedback inhibition by lysergic acid (Fig. 8).
FIG. 8.
Diagram of ergot alkaloid biosynthesis in the C. fusiformis wild type (WT) and a transformant carrying the CpcloA gene. In the C. fusiformis wild type the pathway ends at elymoclavine due to a nonfunctional CfcloA gene. In addition to elymoclavine, agroclavine accumulates, perhaps due to feedback inhibition. In the C. fusiformis transformant carrying a functional CpcloA gene, lysergic acid and elymoclavine accumulate. The absence of functional lpsA and lpsB genes in C. fusiformis apparently precludes the synthesis of ergopeptines in this transformant.
Evolution of EAS clusters in Claviceps species.
Here we show that C. fusiformis has homologues of nine of the C. purpurea EAS genes previously identified but that two of these genes (CfcloA and CflpsBΨ) apparently encode nonfunctional products. It seems likely that the other seven CfEAS genes are functional; they are most likely involved in clavine biosynthesis, since homologues of easA, easC, easD, easE, easF, easG, and dmaW are also present in the fumigaclavine biosynthesis gene cluster of Aspergillus fumigatus (4, 11). The EA profile of A. fumigatus (order Eurotiales) includes festuclavine and fumigaclavines but no lysergic acid or lysergic acid amides. Furthermore, A. fumigatus lacks identifiable homologues of cloA, lpsA, lpsB, or lpsC, suggesting that an ancestral gene cluster acquired cloA and the NRPS genes on the evolutionary lineage to the plant-associated Clavicipitaceae. In contrast, the presence of an lpsB pseudogene in C. fusiformis suggests that the common ancestor of C. purpurea and C. fusiformis synthesized amides of lysergic acid and that the trait was lost in the C. fusiformis lineage. An lpsA homologue has also been found in Epichloë (Neotyphodium) species, where it is required for formation of ergovaline (14). Furthermore, deletion of an lpsB homologue in Epichloë festucae led to accumulation of lysergic acid, as well as accumulation of the clavine compound 6,7-secolysergine, whereas the end product ergovaline was completely missing, as were lysergyl alanine and ergine, both of which are derivatives of lysergic acid (5). Interestingly, Fleetwood et al. also identified several long terminal repeat retrotransposons and nonautonomous transposable elements in the intergenic regions of the alkaloid cluster of Neotyphodium lolii, which could be the reason for rearrangement events in the overall structure of the alkaloid cluster (5). Also, Balansia species and clavicipitaceous symbionts of Ipomoea species (plant family Convolvulaceae) produce the ergopeptine ergobalansine, which, like ergotamine and ergovaline, comprise lysergic acid and three l-amino acids in a cyclol-lactone ring system (9, 15, 19). Therefore, it appears that the common ancestor of most or all plant-associated Clavicipitaceae species had one or more lpsA homologues, which were lost in the C. fusiformis lineage.
It is interesting to speculate about the order in which functions of cloA and lpsB were lost in the C. fusiformis lineage. The sequence divergence of the lpsB genes in the two species is slightly greater than that of the cloA genes. This may be because of an earlier loss of lpsB function or, alternatively, because functional lpsB is less conserved. If we speculate that lpsB function was lost first, a reasonable scenario would start with the rearrangement of the EAS cluster segment containing it, with concomitant deletion of most of the third domain. This might have provided selection for the frameshift mutations that eliminate the potential for production of anything other than a small peptide product from this gene. Furthermore, the loss of functional lpsB could have led to accumulation of lysergic acid as the end product. Interestingly, typical EA-producing Clavicipitaceae species tend to produce either lysergyl amides and ergopeptines or clavines, but they accumulate little lysergic acid. Perhaps the latter compound is detrimental to the producing fungi, or perhaps it is simply less beneficial as a protectant than either the clavines or lysergyl amides and ergopeptines. In either case, loss of lpsB would lead to selection for loss of functional cloA.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft within SPP “Evolution of metabolic diversity” (Tu 50/12) and by U.S. Department of Agriculture NRI grant 2005-35318-16184.
We thank Thomas Haarmann, Miao (“Mindy”) Liu, Ingo Ortel, Jinghong Wang, Daniel G. Panaccione, and Ullrich Keller for expert assistance and helpful discussions.
Footnotes
Published ahead of print on 24 August 2007.
REFERENCES
- 1.Berde, B., and E. Stürmer. 1978. Introduction to the pharmacology of ergot alkaloids and related compounds, p. 1-28. In W. H. Aellig, B. Berde, and H. O. Schild (ed.), Ergot alkaloids and related compounds. Springer, Berlin, Germany.
- 2.Bové, F. J. 1970. The story of ergot. S. Karger, Basel, Switzerland.
- 3.Correia, T., N. Grammel, I. Ortel, U. Keller, and P. Tudzynski. 2003. Molecular cloning and analysis of the ergopeptine assembly system in the ergot fungus Claviceps purpurea. Chem. Biol. 10:1281-1292. [DOI] [PubMed] [Google Scholar]
- 4.Coyle, C. M., and D. G. Panaccione. 2005. An ergot alkaloid biosynthesis gene and clustered hypothetical genes from Aspergillus fumigatus. Appl. Environ. Microbiol. 71:3112-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleetwood, D. J., B. Scott, G. A. Lane, A. Tanaka, and R. D. Johnson. 2007. A complex ergovaline gene cluster in epichloe endophytes of grasses. Appl. Environ. Microbiol. 73:2571-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gröger, D., and H. G. Floss. 1998. Biochemistry of ergot alkaloids—achievements and challenges, p. 171-218. In G. A. Cordell (ed.), The alkaloids, vol. 50. Academic Press, New York, NY. [Google Scholar]
- 7.Haarmann, T., C. Machado, Y. Lubbe, T. Correia, C. L. Schardl, D. G. Panaccione, and P. Tudzynski. 2005. The ergot alkaloid gene cluster in Claviceps purpurea: extension of the cluster sequence and intra species evolution. Phytochemistry 66:1312-1320. [DOI] [PubMed] [Google Scholar]
- 8.Haarmann, T., I. Ortel, P. Tudzynski, and U. Keller. 2006. Identification of the cytochrome P450 monooxygenase that bridges the clavine and ergoline alkaloid pathways. Chembiochem 7:645-652. [DOI] [PubMed] [Google Scholar]
- 9.Jenett-Siems, K., M. Kaloga, and E. Eich. 1994. Ergobalansine/ergobalansinine, a proline-free peptide-type alkaloid of the fungal genus Balansia, is a constituent of Ipomoea piurensis. J. Nat. Prod. 57:1304-1306. [Google Scholar]
- 10.Keller, U., M. Han, and M. Stoffler-Meilicke. 1988. d-Lysergic acid activation and cell-free synthesis of d-lysergyl peptides in enzyme fractions from the ergot fungus Claviceps purpurea. Biochemistry 27:6164-6170. [DOI] [PubMed] [Google Scholar]
- 11.Li, S. M., and I. A. Unsold. 2006. Post-genome research on the biosynthesis of ergot alkaloids. Planta Med. 10:1117-1120. [DOI] [PubMed] [Google Scholar]
- 12.Mantegani, S., E. Brambilla, and M. Varasi. 1999. Ergoline derivatives: receptor affinity and selectivity. Farmaco 54:288-296. [DOI] [PubMed] [Google Scholar]
- 13.Mey, G., K. Held, J. Scheffer, K. B. Tenberge, and P. Tudzynski. 2002. CPMK2, an SLT2-homologous mitogen-activated protein (MAP) kinase, is essential for pathogenesis of Claviceps purpurea on rye: evidence for a second conserved pathogenesis-related MAP kinase cascade in phytopathogenic fungi. Mol. Microbiol. 46:305-318. [DOI] [PubMed] [Google Scholar]
- 14.Panaccione, D. G., R. D. Johnson, J. H. Wang, C. A. Young, P. Damrongkool, B. Scott, and C. L. Schardl. 2001. Elimination of ergovaline from a grass-Neotyphodium endophyte symbiosis by genetic modification of the endophyte. Proc. Natl. Acad. Sci. USA 98:12820-12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell, R. G., R. D. Plattner, S. G. Yates, K. Clay, and A. Leuchtmann. 1990. Ergobalansine, a new ergot-type peptide alkaloid isolated from Cenchrus echinatus (sandbur grass) infected with Balansia obtecta, and produced in liquid cultures of B. obrecta and Balansia cyperi. J. Nat. Prod. (Lloydia) 53:1272-1279. [Google Scholar]
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Schardl, C. L., D. Panaccione, and P. Tudzynski. 2006. Ergot alkaloids—biology and molecular biology, p. 47-88. In G. A. Cordell (ed.), The alkaloids, vol. 63. Elsevier, Boston, MA. [DOI] [PubMed] [Google Scholar]
- 18.Spiering, M. J., H. H. Wilkinson, J. D. Blankenship, and C. L. Schardl. 2002. Expressed sequence tags and genes associated with loline alkaloid expression by the fungal endophyte Neotyphodium uncinatum. Fungal Genet. Biol. 36:242-254. [DOI] [PubMed] [Google Scholar]
- 19.Steiner, U., M. A. Ahimsa-Muller, A. Markert, S. Kucht, J. Gross, N. Kauf, M. Kuzma, M. Zych, M. Lamshoft, M. Furmanowa, V. Knoop, C. Drewke, and E. Leistner. 2006. Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae). Planta 224:533-544. [DOI] [PubMed] [Google Scholar]
- 20.Tenberge, K. B., V. Homann, B. Oeser, and P. Tudzynski. 1996. Structure and expression of two polygalacturonase genes of Claviceps purpurea orientated in tandem and cytological evidence for pectinolytic enzyme activity during infection of rye. Phytopathology 86:1084-1097. [Google Scholar]
- 21.Tsai, H. F., H. Wang, J. C. Gebler, C. D. Poulter, and C. L. Schardl. 1995. The Claviceps purpurea gene encoding dimethylallyltryptophan synthase, the committed step for ergot alkaloid biosynthesis. Biochem. Biophys. Res. Commun. 216:119-125. [DOI] [PubMed] [Google Scholar]
- 22.Tudzynski, P., T. Correia, and U. Keller. 2001. Biotechnology and genetics of ergot alkaloids. Appl. Microbiol. Biotechnol. 57:593-605. Review. [DOI] [PubMed] [Google Scholar]
- 23.Tudzynski, P., K. Hölter, T. Correia, C. Arntz, N. Grammel, and U. Keller. 1999. Evidence for an ergot alkaloid gene cluster in Claviceps purpurea. Mol. Gen. Genet. 261:133-141. [DOI] [PubMed] [Google Scholar]
- 24.Wang, J., C. Machado, D. G. Panaccione, H. F. Tsai, and C. L. Schardl. 2004. The determinant step in ergot alkaloid biosynthesis by an endophyte of perennial ryegrass. Fungal Genet. Biol. 41:189-198. [DOI] [PubMed] [Google Scholar]