Abstract
The concentrations of γ-aminobutyric acid (GABA) in 22 Italian cheese varieties that differ in several technological traits markedly varied from 0.26 to 391 mg kg−1. Presumptive lactic acid bacteria were isolated from each cheese variety (total of 440 isolates) and screened for the capacity to synthesize GABA. Only 61 isolates showed this activity and were identified by partial sequencing of the 16S rRNA gene. Twelve species were found. Lactobacillus paracasei PF6, Lactobacillus delbrueckii subsp. bulgaricus PR1, Lactococcus lactis PU1, Lactobacillus plantarum C48, and Lactobacillus brevis PM17 were the best GABA-producing strains during fermentation of reconstituted skimmed milk. Except for L. plantarum C48, all these strains were isolated from cheeses with the highest concentrations of GABA. A core fragment of glutamate decarboxylase (GAD) DNA was isolated from L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48 by using primers based on two highly conserved regions of GAD. A PCR product of ca. 540 bp was found for all the strains. The amino acid sequences deduced from nucleotide sequence analysis showed 98, 99, 90, and 85% identity to GadB of L. plantarum WCFS1 for L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48, respectively. Except for L. lactis PU1, the three lactobacillus strains survived and synthesized GABA under simulated gastrointestinal conditions. The findings of this study provide a potential basis for exploiting selected cheese-related lactobacilli to develop health-promoting dairy products enriched in GABA.
During the last decade, fundamental studies have opened a new field of research dealing with bioactive or biogenic substances derived from foods. Numerous definitions have been given for bioactive compounds, and one of the most appropriate could be the following: components of consumption-ready foods which may exert a regulatory activity in the human organism, irrespective of their nutritive functions (12). γ-Aminobutyric acid (GABA), a nonprotein amino acid, possesses well-known physiological functions such as neurotransmission, induction of hypotension, and diuretic and tranquilizer effects (24, 53). Treatments for sleeplessness, depression, and autonomic disorders (39); treatment for chronic alcohol-related symptoms (37); and stimulation of immune cells (36) have also been related to the administration of GABA. Recently, GABA has been considered as a strong secretagogue of insulin from the pancreas (1) that may prevent diabetic conditions (16). Owing to these physiological functions, several functional foods are manufactured: GABA-enriched green tea by anaerobic or cyclic treatments of tea leaves or shoots (38); GABA-enriched rice germ by soaking in water (46); GABA-enriched brown rice by high-pressure treatment and germination (26, 35); GABA-enriched germinated wheat through the activity of endogenous enzymes (30); and GABA-enriched fermented beverages such as tempeh-like beverage (3), dairy products (18, 20, 39), and red-mold rice containing the Monascus fungus (44).
GABA is synthesized by glutamate decarboxylase (GAD) (EC 4.1.1.15), a pyridoxal 5′-phosphate-dependent enzyme that catalyzes the irreversible α-decarboxylation of l-glutamate to GABA. GAD is widely distributed among eukaryotes and prokaryotes (51). Several reports (27, 32-34, 40, 42, 52) have shown the presence of GAD in lactic acid bacteria also. Overall, GABA may confer resistance to bacterial cells under acidic conditions (for a review see reference 5), and the GAD decarboxylation process has also been coupled with energy synthesis in Lactobacillus sp. strain E1 (19).
Lactic acid bacteria are largely used in a variety of fermented foods, especially for the manufacture of dairy products with functional and probiotic properties (28). The screening of lactic acid bacteria based on their capacity for synthesizing GABA may open new perspectives on production of GABA-enriched dairy products. During milk fermentation and proteolysis, a high level of l-glutamate may be theoretically liberated, since native caseins contain a high proportion of this amino acid. To our knowledge, just a few reports have considered cheeses as a potential vehicle for GABA (32-34). Overall, the protocol of manufacture, type of primary starters, and, especially, the autochthonous microbiota and ripening conditions may affect the concentration of GABA in cheese.
The aims of this study were to (i) screen 22 Italian cheese varieties, which differ for several technological traits, for their concentrations of GABA; (ii) select cheese-related GABA-producing lactic acid bacteria to be used as starters; (iii) determine the partial sequence of the GAD genes from selected GABA-producing strains; and (iv) assay the synthesis of GABA under simulated gastrointestinal (GI) conditions.
MATERIALS AND METHODS
Cheeses.
Twenty-two Italian cheese varieties were considered in this study. Cheese varieties were manufactured by using milk from different cows (Parmigiano Reggiano, Barricato San Martino, Vento d'Estate, Ubriaco di Raboso, Caciocavallo, Gorgonzola, and Crescenza), buffaloes (Mozzarella), sheep (Pecorino Piemontese, Pecorino Marchigiano, Pecorino Umbro, Pecorino del Reatino, Pecorino Sardo, Pecorino di Filiano, Pecorino del Tarantino, and Pecorino Leccese), sheep and cows in combination (Canestrato Pugliese and Caciotta di Urbino), or goats (Caprino di Valsassina, Caprino di Cavalese, Flor di Capra, and Capritilla) which was used raw (Parmigiano Reggiano, Barricato San Martino, Vento d'Estate, Ubriaco di Raboso, Canestrato Pugliese, Casciotta di Urbino, Pecorino Piemontese, Pecorino Marchigiano, Pecorino del Reatino, Pecorino Sardo, Pecorino di Filiano, Pecorino del Tarantino, and Pecorino Leccese) or pasteurized (all other cheeses). Other main technological traits were as follows: (i) use of primary natural starters (Parmigiano Reggiano, Caciocavallo, Mozzarella, Canestrato Pugliese, Pecorino Sardo, and all goat cheeses) or commercial starters (Gorgonzola and Pecorino Umbro); (ii) use of calf powder or liquid (Parmigiano Reggiano, Gorgonzola, Crescenza, Mozzarella, Canestrato Pugliese, Casciotta di Urbino, and all goat cheeses) or lamb (Caciocavallo) or calf (all other cheeses) paste; (iii) cooking (Parmigiano Reggiano) or stretching (Caciocavallo and Mozzarella); and (iv) very long ripening (18 months; Parmigiano Reggiano), long ripening (8 months; Canestrato Pugliese), medium ripening (2 to 5 months; Barricato San Martino, Vento d'Estate, Ubriaco di Raboso, Caciotta di Urbino, Caciocavallo, and all sheep and goat cheeses), or no ripening to short ripening (0 to 45 days) (Mozzarella, Crescenza, and Gorgonzola).
Cheeses were supplied in triplicate (different batches from the same factory) by local cheese markets and were stored at 4°C for a few hours before analyses. All the analyses were carried out at least in duplicate for each batch of cheese (total of six analyses for each cheese variety).
Compositional analysis.
Moisture and pH were determined as reported by the International Dairy Federation (22, 23). Soluble and total nitrogen (N) were determined by the micro-Kjeldahl method (21).
Concentrations of GABA in cheeses.
Thirty grams of cheese was suspended in 90 ml of 50 mM phosphate buffer, pH 7.0, and treated for 10 min with a stomacher (PBI International, Milan, Italy). The suspension was kept at 40°C for 1 h under gentle stirring (150 rpm) and centrifuged at 3,000 × g for 30 min at 4°C. The supernatant was filtered through Whatman no. 2 paper, and the pH of the extract was adjusted to 4.6 using 1 N HCl. The suspension was centrifuged at 10,000 × g for 10 min. Finally, the supernatant was filtered through a Millex-HA 0.22-μm-pore-size filter (Millipore Co., Bedford, MA). Total and individual free amino acids (FAAs) contained in the pH 4.6-soluble nitrogen fraction were analyzed by a Biochrom 30 series amino acid analyzer (Biochrom Ltd., Cambridge Science Park, England) with a sodium cation-exchange column (20 by 0.46 cm [inner diameter]). A mixture of amino acids at known concentrations (Sigma Chemical Co., Milan, Italy) was added with cysteic acid, methionine sulfoxide, methionine sulfone, tryptophan, ornithine, glutamic acid, and GABA and used as standard. Proteins and peptides in the samples were precipitated by addition of 5% (vol/vol) cold solid sulfosalicylic acid, holding the samples at 4°C for 1 h, and centrifuging them at 15,000 × g for 15 min. The supernatant was filtered through a 0.22-μm-pore-size filter and diluted, when necessary, with sodium citrate (0.2 M, pH 2.2) loading buffer. Amino acids were postcolumn derivatized with ninhydrin reagent and detected by absorbance at 440 (proline and hydroxyproline) or 570 (all the other amino acids) nm.
Enumeration and isolation of lactic acid bacteria.
Samples (20 g) of cheeses were diluted in 180 ml of sodium citrate (2%, wt/vol) solution and homogenized with a Lab-Blender 400 stomacher (PBI International Milan, Italy). Serial dilutions were made in one-quarter-strength Ringer's solution and plated on DeMan, Rogosa, Sharpe (MRS) (lactobacillus) or M17 (coccus) agar (Oxoid Ltd., Basingstoke, Hampshire, England) for viable counts. Mesophilic or thermophilic lactic acid bacteria were enumerated after incubation at 30 or 42°C for 48 to 72 h. At least 10 colonies for each medium and cheese variety, possibly with different morphologies, were isolated from the highest plate dilution. Gram-positive, catalase-negative, nonmotile rod and coccus isolates were cultivated in MRS or M17 broth (Oxoid Ltd.) at 30 or 42°C for 24 h and restreaked into MRS or M17 agar. All the isolates considered for further analyses showed the capacity of acidifying the culture medium. Microbial cultures were stored at −20°C in 10% (vol/vol) glycerol.
Synthesis of GABA by lactic acid bacteria isolated from cheeses.
Twenty-four-hour-old cells of 440 presumptive lactic acid bacterium strains were harvested by centrifugation (9,000 × g for 15 min at 4°C), washed twice with sterile 0.05 M potassium phosphate buffer (pH 7.0), and resuspended in sterile physiological solution (0.85% NaCl) at an A620 of 2.5, which corresponded to a cell density of ca. 8.5 to 9.0 log CFU ml−1. GAD activity was measured as described previously (33). The reaction mixture consisted of 900 μl of 50 mM sodium acetate buffer (pH 4.7) containing 2 mM l-glutamate, 0.1 mM pyridoxal phosphate, 100 μl of cell suspension, and 0.05% (wt/vol) NaN3 (final concentration). After 24 h at 30°C, the concentration of GABA was determined using a Biochrom 30 series amino acid analyzer (Biochrom Ltd.).
Genotypic identification by 16S rRNA gene sequence analysis.
Genomic DNA from each strain was extracted as reported by De Los Reyes-Gavilán et al. (10) from 2-ml samples of overnight cultures grown in MRS or M17 broth. Two primer pairs (Invitrogen Life Technologies, Carlsbad, CA), LacbF/LacbR and LpCoF/LpCoR (7), were used to amplify a 16S rRNA gene fragment of presumptive lactic acid bacteria. Fifty microliters of each PCR mixture contained 200 μM of each 2′-deoxynucleoside 5′-triphosphate (dNTP), 1 μM of both forward and reverse primers, 2 mM MgCl2, 2 U of Taq DNA polymerase (Invitrogen) in the supplied buffer, and approximately 50 ng of DNA. The expected amplicons of about 1,400 and 1,000 bp (after amplification with primer pairs LacbF/LacbR and LpCoF/LpCoR, respectively) were eluted from the gel and purified by the GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences, Piscataway, NJ). DNA sequencing reactions were performed by PRIMM srl (San Raffaele Biomedical Science Park, Milan, Italy). Taxonomic strain identification was performed by comparing the sequences of each isolate with those reported in the Basic BLAST database (2).
Random amplified polymorphic DNA-PCR (RAPD-PCR) analysis.
Genomic DNA was extracted as reported above. Two primers (Invitrogen) with arbitrarily chosen sequences (M13, 5′-GAGGGTGGCGGTTCT-3′; P4, 5′-CCGCAGCGTT-3′; and P7, 5′-AGCAGCGTGG-3′) (6, 55) were used singly in two series of amplifications. The reaction mixture contained 200 μM of each dNTP, 1 to 2 μM primer, 1.5 to 3 μM MgCl2, 1.25 U Taq DNA polymerase (Invitrogen), 2.5 μl PCR buffer, 25 ng DNA, and sterile double-distilled water to 25 μl. For amplifications with primer P4, the PCR program comprised 45 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 35°C, and elongation for 2 min at 72°C; the cycles were preceded by denaturation at 94°C for 4 min and followed by elongation at 72°C for 5 min. For primer M13, amplification reactions were performed using one cycle at 94°C for 60 s (denaturing), 42°C for 20 s (annealing), and 72°C for 2 min (elongation). PCR products were separated by electrophoresis (2 h at 130 V) on a 1.5% (wt/vol) agarose gel (Invitrogen), and the DNA was detected by UV transillumination after staining with ethidium bromide (0.5 μg/ml). The molecular weight of the amplified DNA fragments was estimated by comparison with a 1 Kb Plus DNA Ladder (Invitrogen). Gels were acquired using a UNIsave gel documentation system camera, model GAS9200/1/2/3, version 11 (UVItec Limited, Cambridge, United Kingdom). Electrophoretic profiles were compared using Quantity One software (Bio-Rad, Milan, Italy).
Acidification kinetics and synthesis of GABA during milk fermentation.
Harvested cells of lactic acid bacteria were washed in 50 mM phosphate buffer (pH 7.0), centrifuged at 9,000 × g for 15 min at 4°C, and resuspended in reconstituted skimmed milk (RSM) at a cell density of ca. 6.8 log CFU ml−1. RSM was supplemented with l-monosodium glutamate (20 mM) and incubated at 30°C for 24 h, and the pH was recorded online. Acidification data were modeled according to the Gompertz equation as modified by Zwietering et al. (57), where y is log (ΔpH Δt−1, units of pH h−1), k is the initial level of the dependent variable to be modeled, A (ΔpH) is the difference in pH (units) between the initial value (pH0) and the value reached after 18 h, μmax is the maximum acidification rate (ΔpH h−1), λ is the length of the latency phase of acidification expressed in hours, and t is time. After incubation, the pH 4.6-soluble nitrogen fraction was prepared as described elsewhere. Total and individual FAAs contained in the pH 4.6-soluble nitrogen fraction were analyzed using a Biochrom 30 series amino acid analyzer (Biochrom Ltd.).
Molecular characterization of the GAD gene.
Total DNAs were obtained as described above. Primers designed from highly conserved regions of GAD, CoreF/CoreR (5′-CCTCGAGAAGCCGATCGCTTAGTTCG-3′ and 5′-TCATATTGACCGGTATAAGTGATGCCC-3′, respectively) (PRIMM), were used to amplify genes for GABA-synthesizing enzymes of selected lactic acid bacteria (34, 40). Fifty microliters of each PCR mixture contained 200 μM of each dNTP, 1 μM of both primers, 2 μM MgCl2, 2 U of Taq DNA polymerase (Invitrogen) in the supplied buffer, and ca. 50 ng of DNA. PCR amplification was performed using the GeneAmp PCR System 9700 thermal cycler (Applied Biosystems). Amplification conditions were changed according to the primer used. PCR products were separated by electrophoresis on a 1.5% (wt/vol) agarose gel (Gibco BRL, France) and stained with ethidium bromide (0.5 μg ml−1). The amplicons obtained were eluted from the gel and purified by the GFX PCR DNA and Gel Band Purification Kit (Amersham Biosciences). DNA sequencing reactions were performed by PRIMM srl. Sequence comparison was performed by using the Basic BLAST database. Translation of nucleotide sequences analyses was performed by using OMIGA software (Oxford Molecular, Madison, WI) or the ExPASy translation routine at the ExPASy Moleculary Biology Server of the Swiss Institute of Bioinformatics (http://ca.expasy.org/). Similarity researches were performed with the advanced BLAST algorithm available at the National Center for Biotechnology Information site (htpp://www.ncbi.nlm.nih.gov/). Sequence alignments were conducted with the ClustalW algorithm at the ClustalW server at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw/index.html).
Resistance to simulated gastric and intestinal fluids.
Simulated gastric and intestinal fluids were created (13, 43). Stationary-phase-grown cells of lactic acid bacteria were harvested at 9,000 × g for 15 min at 4°C, washed with physiologic solution (0.85% NaCl), and resuspended in 50 ml of simulated gastric juice (ca. 10 log CFU ml−1) which contained NaCl (125 mM liter−1), KCl (7 mM liter−1), NaHCO3 (45 mM liter−1), and pepsin (3 g liter−1) (Sigma) (56). The final pH was adjusted to 2.0, 3.0, and 8.0. The value pH 8.0 was used to investigate the influence of the components of the simulated gastric juice apart from the effect of low pH (13). The effect of nitrite on lactic acid bacteria was also investigated by adding N (1 mg liter−1), l-ascorbic acid (5 mg liter−1), iodide (1 mg liter−1), or thiocyanate (30 mg liter−1) (54). The suspension was incubated at 37°C with gentle agitation at 100 revolutions min−1 (29, 54). Aliquots of this suspension were taken at 0, 90, and 180 min, and viable counts were determined. The effect of gastric digestion was also determined by suspending cells in RSM (25 mg ml−1) before inoculation of simulated gastric juice at pH 2.0. The final pH after the addition of RSM was ca. 3.0. This condition was assayed to simulate the effect of the food matrix during gastric transit (56). After 180 min of gastric digestion, cells were harvested and resuspended in simulated intestinal fluid which contained 0.1% (wt/vol) pancreatin and 0.15% (wt/vol) oxgall bile salt (Sigma) at pH 8.0. The suspension was incubated at 37°C under gentle agitation at 100 revolutions min−1, and aliquots were taken at 0, 90, and 180 min (13).
Synthesis of GABA under simulated GI conditions.
RSM (25 mg/ml), containing ca. 9 log CFU ml−1 of lactic acid bacteria, was subjected to pepsin and pancreatin digestion as described above (43). After digestion, the suspension was further incubated for 24 h at 37°C, at 100 revolutions min−1, to mimic the synthesis of GABA by bacteria colonizing the GI tract. Digested samples were recovered after 2, 4, 16, and 24 h of incubation (8) and subjected to free fatty acid analysis. All treatments were carried out also using RSM without bacterial inoculum as the control.
Statistical analysis.
Experimental data were subjected to analysis of variance, and pairwise comparison of treatment means was achieved using Tukey's procedure at P < 0.05 using a statistical software program, Statistica for Windows (Statistica 6.0 per Windows 1998). Multivariate statistical analysis and principal component analysis were performed using Statistica 6.0 and Statistical software for MS Excel per Windows 1998.
Nucleotide sequence accession numbers.
The sequences in this study were deposited in GenBank, National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov). The accession numbers were EF174472, EF174473, EF174474, and EF174475 for the GAD gene fragments of Lactobacillus paracasei PF6, Lactobacillus delbrueckii subsp. bulgaricus PR1, Lactobacillus plantarum FC48, and Lactococcus lactis PU1, respectively.
RESULTS
Concentrations of GABA in cheeses.
Twenty-two Italian cheese varieties having features that may influence directly or indirectly the synthesis of GABA were considered in this study. The values of pH, moisture, and pH 4.6-soluble N were in the range of 4.68 to 6.70, 30 to 60%, and 7.0 to 34%, respectively. The concentrations of total FAAs and glutamate, contained in the pH 4.6-soluble N extracts of the 22 Italian cheese varieties, markedly varied from 19.17 to 231,800.0 mg kg−1 and from 0.0 to 7,190.0 mg kg−1, respectively. The concentration of GABA varied from 0.260 to 391 mg kg−1. The lowest concentrations were found for Vento d'Estate (0.260 mg kg−1), Mozzarella (0.32 mg kg−1), and Crescenza (0.77 mg kg−1) cheeses. The majority of the cheeses (Parmigiano Reggiano, Barricato San Martino, Ubriaco di Raboso, Caciocavallo, Gorgonzola, Canestrato Pugliese, Casciotta di Urbino, Pecorino del Tarantino, Pecorino Piemontese, Flor di Capra, Caprino di Cavalese, Caprino di Valsassina, and Capritilla) had concentrations of GABA which ranged from 4 to 100 mg kg−1. The highest values of GABA were found in Pecorino Marchigiano (289 mg kg−1), Pecorino del Reatino (290 mg kg−1), Pecorino Leccese (290 mg kg−1), Pecorino Umbro (330 mg kg−1), and, especially, Pecorino di Filiano (391 mg kg−1). The values of pH for the cheeses containing the highest concentrations of GABA were 4.68 to 5.70. Overall, no statistical correlation was found between the concentration of GABA and the levels of l-glutamate or other free fatty acids. As determined by multivariate statistical analysis, the use of sheep milk and cheese ripening for 1.5 to 5 months seemed to be related to the highest concentration of GABA. The other technological traits or cheese characteristics did not show a statistical correlation.
Synthesis of GABA by lactic acid bacteria isolated from cheeses.
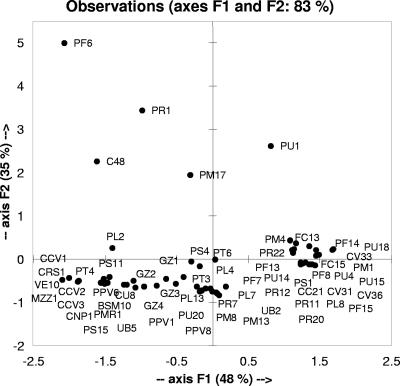
The number of presumptive mesophilic and thermophilic lactobacilli in cheeses varied from 6.90 to 8.51 log CFU g−1 and from ≤3.0 to 6.28 log CFU g−1, respectively. Lactococci and streptococci were ≤3.0 to 7.8 log CFU g−1 and ≤3.0 to 5.43 log CFU g−1, respectively. As expected, the highest numbers of thermophilic lactobacilli and streptococci were found in cheeses where primary starters were used. For each cheese, 20 gram-positive, catalase-negative, nonmotile, and acidifying isolates (total of 440) were randomly taken from the plates containing the highest dilutions and screened for GAD activity. Only 61 isolates synthesized GABA under in vitro conditions at 30°C for 24 h, pH 4.7. All these isolates were further identified by partial sequencing of the 16S rRNA gene (Table 1). Twelve species were found: Lactobacillus plantarum (17 isolates), Leuconostoc mesenteroides (two), Weissella cibaria (one), Lactobacillus paracasei (16), Lactobacillus brevis (three), Lactobacillus casei (five), Streptococcus thermophilus (six), Leuconostoc pseudomesenteroides (two), Lactococcus lactis (two), Lactobacillus delbrueckii subsp. bulgaricus (two), Enterococcus durans (one), and Lactobacillus rhamnosus (two). Two isolates of unidentified Lactobacillus species were also found. Except for Gorgonzola, Pecorino cheeses especially contained the highest numbers of GABA-producing strains (four to six). Apart from the cheese source, 17 and 16 isolates corresponded to the species L. plantarum and L. paracasei, respectively. Overall, 53 isolates were mesophilic lactic acid bacteria (45 mesophilic lactobacilli) and only eight were thermophilic lactic acid bacteria (six S. thermophilus and two L. delbrueckii subsp. bulgaricus strains). To exclude clonal relatedness, three primers, M13, P4, and P7, with arbitrarily chosen sequences, were used to characterize the 61 GABA-producing isolates by RAPD-PCR analysis. The reproducibility of RAPD fingerprints was assessed by comparing the PCR products obtained from three separate cultures of the same strain. The size of the amplified fragments ranged from 100 to 1,000 bp, and the number of fragments varied from one to seven per primer per isolate. The RAPD profiles generated by the above primers were highly discriminative and reproducible with consistent fragment patterns. The polymorphic bands in total distinguished the isolates from each other with at least a 2.5% dissimilarity level (data not shown). The capacity for synthesizing GABA of the 61 isolates was further assayed in RSM. After 24 h of fermentation at 30°C, all isolates reached the cell density of 8.5 to 9.0 log CFU g−1. The kinetics of acidification was characterized by a median value of ΔpH of 2.28. The majority of the strains caused ΔpHs which ranged from 2.00 to 2.71. The median value for μmax was 0.03 ΔpH min−1. The median value for the concentration of GABA was 1.71 mg kg−1, and the 25th and 75th percentiles of the data ranged from 1.0 to 3.67 mg kg−1, respectively. L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, L. plantarum C48, and L. brevis PM17, which showed the highest synthesis of GABA (99.9, 63.0, 36.0, 16.0, and 15.0 mg kg−1, respectively), were located out of the error bars of the box plot (data not shown). Overall, the concentration of total FAAs in the fermented RSM showed increases which ranged from 120 to 390.6 mg kg−1. The concentrations of GABA, glutamic acid, and total FAAs of each fermented RSM were subjected to principal component analysis using a covariance matrix (Fig. 1). The first two principal components explained ca. 83.26% of the total variance. PC1 showed the distribution of the samples according to the concentration of FAAs, while PC2 differentiated samples based on the concentration of GABA. The fermented RSMs started with L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, L. plantarum C48, or L. brevis PM17 were characterized by the highest synthesis of GABA and were located in different zones of the plane delimited by the two principal components.
TABLE 1.
Species of lactic acid bacteria producing GABA identified in 22 Italian cheese varieties
| Cheese | Species |
|---|---|
| Parmigiano Reggiano | L. plantarum PMR1 |
| Barricato San Martino | L. mesenteroides BSM10 |
| Vento d'Estate | W. cibaria VE10 |
| Ubriaco di Raboso | L. paracasei UB2, L. brevis UB5 |
| Caciocavallo | L. plantarum CCV1 and CCV2, L. casei CCV3 |
| Gorgonzola | S. thermophilus GZ1, GZ2, GZ3, and GZ4 |
| Crescenza | S. thermophilus CRS1 |
| Mozzarella | S. thermophilus MZZ1 |
| Canestrato Pugliese | L. plantarum CNP1 |
| Casciotta di Urbino | L. lactis CU8 |
| Pecorino Piemontese | L. plantarum PPV1 and PPV8, L. pseudomesenteroides PPV6 |
| Pecorino Marchigiano | L. plantarum PM8 and PM13, L. paracasei PM1 and PM4, L. brevis PM17 |
| Pecorino Umbro | L. lactis PU1; L. paracasei PU4, PU18, PU15, and PU20; Lactobacillus sp. strain PU14 |
| Pecorino del Reatino | L. delbrueckii subsp. bulgaricus PR1 and PR7; L. plantarum PR11, PR12, and PR20; L. pseudomesenteroides PR22 |
| Pecorino Sardo | L. paracasei PS1 and PS11, L. plantarum PS15, L. mesenteroides PS4 |
| Pecorino di Filiano | L. paracasei PF6, PF8, and PF13; L. plantarum PF14; Lactobacillus sp. strain PF7; E. durans PF15 |
| Pecorino del Tarantino | L. plantarum PT3, L. paracasei PT4, L. brevis PT6 |
| Pecorino Leccese | L. paracasei PL2, PL4, and PL13; L. plantarum PL7 and PL8 |
| Caprino di Valsassina | L. rhamnosus CV33 and CV36, L. casei CV31 |
| Caprino di Cavalese | L. casei CC21 |
| Flor di Capra | L. casei FC13 and FC15 |
| Capritilla | L. plantarum C48 |
FIG. 1.
Score plot of the first and second principal components after principal component analysis based on the concentrations of GABA, glutamic acid, and total FAAs of RSM started with lactic acid bacteria. Each fermented milk is indicated by the code of the lactic acid bacterium used for fermentation. The values are the averages of three batches of each milk fermentation.
Molecular characterization of the GAD genes.
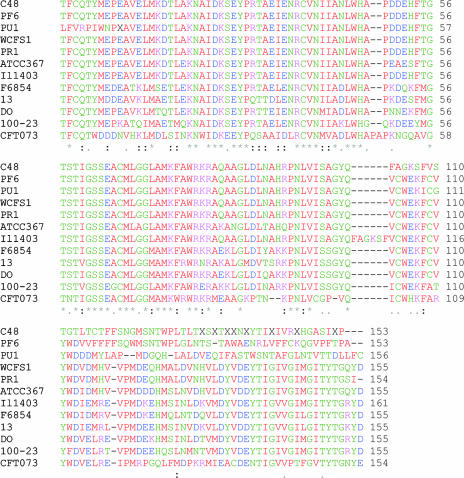
L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48, corresponding to the highest GABA-producing strains, were screened for GAD genes. Primers designed from highly conserved region of GAD genes gave a PCR product of approximately 540 bp for all the strains. The predicted amino acid sequences of L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48 showed 98, 100, 86, and 95% identity to gadB of L. plantarum WCFS1 (accession number NP_786643.1), respectively. Alignments of the internal GAD gene fragment of L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48 with the eight most similar sequences of the GAD gene are shown in Fig. 2.
FIG. 2.
Alignment of the internal deduced amino acid sequences of GAD of Lactobacillus paracasei PF6, Lactobacillus delbrueckii subsp. bulgaricus PR1, Lactococcus lactis PU1, and Lactobacillus plantarum C48 with other similar GAD sequences from Lactobacillus plantarum WCFS1 (accession number NP_786643.1), Lactobacillus brevis ATCC 367 (accession number YP_795941.1), Lactococcus lactis subsp. lactis Il1403 (accession number NP_267446.1), Listeria monocytogenes strain F6854 (accession number ZP_00234896.1), Clostridium perfringens strain 13 (accession number NP_562974.1), Enterococcus faecium DO (accession number ZP_00603789.1), Lactobacillus reuteri 100-23 (accession number ZP_01274543.1), and Escherichia coli CFT073 (accession number NP_753818.1). The deduced amino acid sequence was analyzed using ClustalW (1.81).
Resistance to simulated gastric and intestinal fluids of GABA-producing strains.
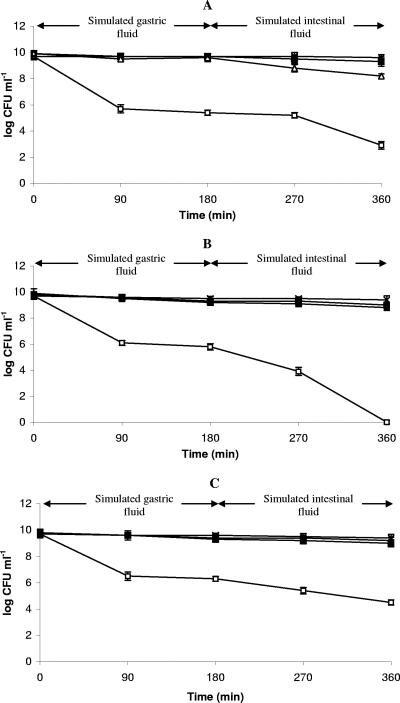
L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. plantarum C48 were incubated at 37°C in simulated gastric fluid at pH 2.0, 3.0, and 8.0. L. lactis PU1 was the only strain which had a very poor survival in all the conditions assayed (data not shown). Therefore, it was excluded from further characterization. After 180 min of incubation in simulated gastric juice at pH 3.0, all the other strains showed decreases lower than 1 log cycle with respect to their initial cell density (10 to 9.6 log CFU ml−1). At pH 8.0 no decrease of survival was found for all strains (Fig. 3A to C). After 180 min at pH 2.0, all strains decreased to 6.0 to 5.0 log CFU ml−1. When RSM (25 mg/ml) was added to the juice at pH 2.0, lactobacilli were resistant to the simulated gastric juice. Probably, this was due to the increased value of pH (3.0) caused by addition of RSM or to the direct protective effect on microbial cells by the food matrix. After 180 min of gastric digestion, cells were exposed to simulated intestinal fluid for a subsequent 180 min at pH 8.0 (Fig. 3A to C). Cell survival depended on the pH of gastric digestion. The decrease for cells previously treated at pH 8.0, 3.0, or 2.0 with the addition of RSM was always lower than 1 log cycle. When the gastric digestion was at an initial pH of 2.0, the survival of simulated intestinal fluid was approximately 3 and 4 log CFU ml−1 for L. paracasei PF6 and L. plantarum C48, respectively. Nonculturable cells of L. delbrueckii subsp. bulgaricus PR1 were found in 1 ml of sample.
FIG. 3.
Survival of selected Lactobacillus paracasei PF6 (A), Lactobacillus delbrueckii subsp. bulgaricus PR1 (B), and Lactobacillus plantarum C48 (C) under gastric conditions (0 to 180 min) at pH 8.0 (▾), 3.0 (▪), and 2.0 (□) and at 2.0 with RSM added (11%, wt/vol) (▿) and with further intestinal digestion (180 to 360 min) at pH 8.0. The values were the averages of three replicates, and standard deviations are indicated by vertical bars.
Synthesis of GABA under GI conditions.
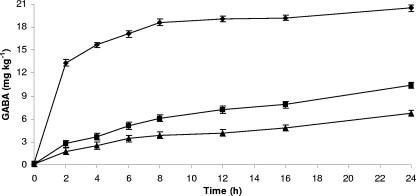
The hypothesis to be investigated concerned the capacity of selected strains to synthesize GABA during gut transit and without the addition of l-glutamate. RSM containing 9 log CFU ml−1 of L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, or L. plantarum C48 was subjected to sequential hydrolysis by pepsin (pH 2.0) and pancreatin (pH 8.0) as described above. After digestion, the suspensions were further incubated for 24 h at 37°C under stirring conditions, to mimic the bacterial GI transit. As previously shown (Fig. 3A to C), RSM protected the cells and the numbers of the three strains did not vary with respect to the initial cell density. Nevertheless, all strains synthesized GABA at a lower concentration than that found in fermented RSM (30°C for 24 h, initial pH 6.6) (Fig. 4). Among the three strains, L. paracasei PF6 showed the highest synthesis of GABA, reaching ca. 20 mg kg−1 after 24 h of incubation.
FIG. 4.
Synthesis of GABA of Lactobacillus paracasei PF6 (⧫), Lactobacillus delbrueckii subsp. bulgaricus PR1 (▪), and Lactobacillus plantarum C48 (▴) in RSM after incubation under GI conditions and further incubation at 37°C for 24 h. The values were the averages of three replicates, and standard deviations are indicated by vertical bars.
DISCUSSION
Milk and dairy products provide a rich source of valuable proteins, minerals, and vitamins. The nutritional significance of proteins includes macronutrient as well as physiological and functional aspects. Besides bioactive proteins, dairy products may also provide bioactive peptides (15, 45). First, this study reports the presence of GABA in 22 Italian cheese varieties. Overall, cheeses characterized by the shortest ripening (Mozzarella and Crescenza), nonconventional ripening (Barricato San Martino and Vento d'Estate) (11), or the longest ripening (Canestrato Pugliese and Parmigiano Reggiano) (14) contained the lowest concentrations of GABA. Among the technological traits differentiating the cheeses, only the type of milk and duration of ripening were statistically correlated with the concentration of GABA. Indeed, sheep cheeses ripened for 5 months such as Pecorino Marchigiano, Pecorino del Reatino, Pecorino Leccese, Pecorino Umbro, and Pecorino di Filiano contained the highest levels of GABA (289 to 391 mg kg−1). Nomura et al. (32) also analyzed seven commercial cheeses (Camembert, Gouda, blue, cream, Cheddar, Edam, and Emmental) and reported concentrations of GABA of 177.0, 48.0, 7.1, and 4.2 mg kg−1 for Gouda, Cheddar, blue, and Edam, respectively. All of the 22 Italian cheese varieties contained relevant numbers of mesophilic nonstarter lactic acid bacteria, as members of the autochthonous microbiota, and thermophilic lactic acid bacteria, when used as primary starters. Nevertheless, only a small part (ca. 14%) of the total 440 isolates (20 for each cheese variety) showed the capacity for synthesizing GABA under in vitro conditions and during RSM fermentation. Preliminarily, RAPD-PCR analyses showed genetic variability between the GABA-producing strains used in this study. RAPD-PCR based on at least three primers has been shown to be an effective tool in resolving intraspecific differences also for lactic acid bacteria (6, 55). Interestingly, the best GABA-producing strains, L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, and L. brevis PM17, were isolated from Pecorino di Filiano, Pecorino del Reatino, Pecorino Umbro, and Pecorino Marchigiano cheeses, respectively, which had the highest concentrations of GABA. The only exception was L. plantarum C48 isolated from Capritilla goat cheese. Other reports also showed the synthesis of GABA by primary starters such as L. lactis, S. thermophilus, and L. delbrueckii subsp. bulgaricus (17, 32-34) and nonstarter lactic acid bacteria such as L. plantarum, L. paracasei, and L. brevis (17, 27, 42). Strains of L. plantarum and L. paracasei corresponded to the major part of the GABA-producing isolates from the 22 Italian cheese varieties. Nevertheless, the capacity of synthesizing the highest levels of GABA was markedly strain dependent. Apart from other technological conditions, the presence of strains with particular aptitudes for synthesizing GABA during cheese ripening seemed to be the major prerequisite for the manufacture of GABA-enriched cheeses. To the best of our knowledge, this report is the first to show the synthesis of GABA also by L. casei, L. rhamnosus, W. cibaria, L. mesenteroides, L. pseudomesenteroides, and E. durans.
The genetics of GABA have been elucidated in Escherichia coli (50), Lactococcus lactis subsp. lactis (34), and L. brevis (42). Sanders et al. (47) sequenced the L. lactis subsp. lactis gadCB gene and suggested that it encoded a glutamate-dependent acid resistance mechanism comprised of glutamate-GABA antiporter and GAD. Nomura et al. (34) indicated that L. lactis subsp. lactis contains a single GAD gene (gadB), while the gram-negative E. coli (50) and Shigella sp. (49) contain two GAD genes. The functional properties of the two E. coli isozymes were identical (9). The partial GAD sequences found in this study showed high identity with gadB sequences from L. plantarum WCFS1 (accession number NP_786643.1), L. brevis ATCC 367 (accession number YP_795941.1), L. lactis subsp. lactis Il1403 (accession number NP_267446.1), Listeria monocytogenes strain F6854 (accession number ZP_00234896.1), Enterococcus faecium DO (accession number ZP_00603789.1), Lactobacillus reuteri 100-23 (accession number ZP_01274543.1), Clostridium perfringens strain 13 (accession number NP_562974.1,) and E. coli CFT073 (accession number NP_753818.1). Physiologically, the expression of GAD genes is assumed to control the acidification of the cytosolic environment by decarboxylating an acid substrate (glutamate) into a neutral compound (GABA) via incorporation of H+. GABA would then be exported into the extracellular environment, thereby contributing to alkalinization (for a review see reference 5). The partial GAD sequences found in this study could facilitate further studies of gene expression (42).
During RSM fermentation at 30°C for 24 h, L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, L. lactis PU1, L. plantarum C48, and L. brevis PM17 synthesized concentrations of GABA (99.9 to 15 mg kg−1) higher than those found for other cheese starters in skim milk (32, 33) and Bifidobacterium longum (41). A new type of GABA-enriched fermented milk was manufactured by using two starters: L. casei and L. lactis. L. casei hydrolyzed milk proteins into l-glutamate, and L. lactis subsp. lactis converted it into GABA (20). Potentially, the lactic acid bacteria selected in this study could be used in mixture for the manufacture of a fermented milk containing elevated levels of GABA. Foods enriched with GABA were defined as “foods for specified health use” by the Japanese government (48). Recently, some studies reported that dietary materials or products containing GABA caused a decrease of blood pressure in spontaneously hypertensive rats and hypertensive humans (20, 25, 31). A daily intake of fermented milk (10 mg of GABA) for 12 weeks decreased blood pressure by 17.4 Hg in hypertensive patients (20, 25). The amount of GABA in 100 g fermented milk synthesized by L. paracasei PF6 still represents the minimum effective daily dose to get positive effects. Some of the selected strains also showed tolerance and synthesis of GABA under simulated GI conditions. Acid and bile salt treatments were combined in this study, since they have both individual and combined effects (4). The time chosen for treatments in simulated gastric (180 min) and intestinal (further 180 min) fluids mimicked the in vivo times between entrance to and release from the stomach and intestine during digestive processes (4). L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, and L. plantarum C48 showed high resistance at pH 2.0 when RSM was added to acid and bile salt fluids. The synthesis of GABA by L. paracasei PF6, L. delbrueckii subsp. bulgaricus PR1, and L. plantarum C48 was also found in RSM under simulated GI conditions and without addition of l-glutamate as the precursor. First, this study showed the synthesis of GABA by lactic acid bacteria under GI conditions. Overall, the screening of lactic acid bacteria based on the capacity to synthesize GABA under GI conditions may be considered another biotechnological trait to select probiotics. Fermented milk enriched in GABA by lactobacilli selected in this study would be of interest for the food industry as it could be considered a health-oriented dairy product with a potential antihypertensive effect. Further studies are in progress to show the decrease of blood pressure in spontaneously hypertensive rats and hypertensive humans.
Footnotes
Published ahead of print on 21 September 2007.
REFERENCES
- 1.Adeghate, E., and A. S. Ponery. 2002. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell 34:1-6. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Grapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki, H., Y. Furuya, Y. Endo, and K. Fujimoto. 2003. Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA- tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 67:1806-1808. [DOI] [PubMed] [Google Scholar]
- 4.Chou, L., and B. Weimer. 1999. Isolation and characterization of acid- and bile-tolerant isolates from strains of Lactobacillus acidophilus. J. Dairy Sci. 82:23-31. [DOI] [PubMed] [Google Scholar]
- 5.Cotter, P. D., and C. Hill. 2003. Surviving the acid text: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 67:429-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Angelis, M., A. Corsetti, N. Tosti, J. Rossi, M. R. Corbo, and M. Gobbetti. 2001. Characterization of non-starter lactic acid bacteria from Italian ewe cheeses based on phenotypic, genotypic, and cell wall protein analyses. Appl. Environ. Microbiol. 67:2011-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Angelis, M., S. Siragusa, M. Berloco, L. Caputo, L. Settanni, G. Alfonsi, M. Amerio, A. Grandi, A. Ragni, and M. Gobbetti. 2006. Isolation, identification and selection of potential probiotic lactobacilli from pig faeces to be used as additives in pelleted feeling. Res. Microbiol. 157:792-801. [DOI] [PubMed] [Google Scholar]
- 8.De Angelis, M., C. G. Rizzello, E. Scala, C. De Simone, G. A. Farris, F. Turrini, and M. Gobbetti. 2007. Probiotic preparation has the capacity to hydrolyze proteins responsible for wheat allergy. J. Food Prot. 70:135-144. [DOI] [PubMed] [Google Scholar]
- 9.De Biase, D., A. Tramonti, R. A. John, and F. Bossa. 1996. Isolation, overexpression, and biochemical characterization of the two isoforms of glutamic acid decarboxylase from Escherichia coli. Protein Expr. Purif. 8:430-438. [DOI] [PubMed] [Google Scholar]
- 10.De Los Reyes-Gavilán, C. G., G. K. Y. Limsowtin, P. Tailliez, L. Séchaud, and J. P. Accolas. 1992. A Lactobacillus helveticus-specific DNA probe detects restriction fragment length polymorphisms in this species. Appl. Environ. Microbiol. 58:3429-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Cagno, R., S. Buchin, S. de Candia, M. De Angelis, P. F. Fox, and M. Gobbetti. 2007. Characterization of Italian cheeses ripened under non-conventional conditions. J. Dairy Sci. 90:2689-2704. [DOI] [PubMed] [Google Scholar]
- 12.Diplock, A. T., P. J. Aggett, M. Ashwell, F. Bornet, E. B. Fern, and M. B. Roberfroid. 1999. Scientific concepts of functional foods in Europe—consensus document. Br. J. Nutr. 81:1-27. [PubMed] [Google Scholar]
- 13.Fernández, M. F., S. Boris, and C. Barbés. 2003. Probiotic properties of human lactobacilli strains to be used in the gastrointestinal tract. J. Appl. Microbiol. 94:449-455. [DOI] [PubMed] [Google Scholar]
- 14.Gobbetti, M., M. De Angelis, R. Di Cagno, and C. G. Rizzello. 2007. The relative contributions of starter cultures and non-starter bacteria to the flavour of cheese, p. 121-156. In B. Weimer (ed.), Improving the flavour of cheese. Woodhead Publishing, Cambridge, England.
- 15.Gobbetti, M., L. Stepaniak, M. De Angelis, A. Corsetti, and R. Di Cagno. 2002. Latent bioactive peptides in milk proteins: proteolytic activation and significance in dairy processing. Crit. Rev. Food Sci. Nutr. 42:223-239. [DOI] [PubMed] [Google Scholar]
- 16.Hagiwara, H., T. Seki, and T. Ariga. 2004. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 68:444-447. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa, K., Y. Ueno, S. Kawamura, R. Taniguchi, and K. Oda. 1997. Production of γ-aminobutyric acid by lactic acid bacteria. Seibutsu-kogaku Kaishi 75:239-244. [Google Scholar]
- 18.Hayakawa, K., M. Kimura, K. Kasaha, K. Matsumoto, H. Sansawa, and Y. Yamori. 2004. Effect of a gamma-aminobutyric acid-enriched dairy product on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br. J. Nutr. 92:411-417. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi, T., H. Hayashi, and K. Abe. 1997. Exchange of glutamate and γ-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 179:3362-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue, K., T. Shirai, H. Ochiai, M. Kasao, K. Hayakawa, M. Kimura, and H. Sansawa. 2003. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 57:490-495. [DOI] [PubMed] [Google Scholar]
- 21.International Dairy Federation. 1964. Determination of the protein content of processed cheeses products. Standard 25. International Dairy Federation, Brussels, Belgium.
- 22.International Dairy Federation. 1970. Determination of dry matter content in whey cheese. Standard 58. International Dairy Federation, Brussels, Belgium.
- 23.International Dairy Federation. 1989. Determination of pH. Standard 115A. International Dairy Federation, Brussels, Belgium.
- 24.Jakobs, C., J. Jaeken, and K. M. Gibson. 1993. Inherited disorders of GABA metabolism. J. Inherit. Metab. Dis. 16:704-715. [DOI] [PubMed] [Google Scholar]
- 25.Kajimoto, O., H. Hirata, S. Nakagawa, Y. Kajimoto, K. Hayakawa, and M. Kimura. 2004. Hypotensive effect of fermented milk containing γ-aminobutyric acid (GABA) in subjects with high normal blood pressure. Nippon Shokuhin Kagaku Kogaku Kaishi 51:79-86. [Google Scholar]
- 26.Kinefuchi, M., M. Sekiya, A. Yamazaiki, and K. Yamamoto. 1999. Accumulation of GABA in brown rice by high pressure treatment. Nippon Shokuhin Kagaku Kaishi 46:323-328. [Google Scholar]
- 27.Komatsuzaki, N., J. Shima, S. Kawamoto, H. Monose, and T. Kimura. 2005. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 22:497-504. [Google Scholar]
- 28.Leroy, F., and L. de Vuyst. 2004. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 15:67-78. [Google Scholar]
- 29.Mao, Y., M. P. Doyle, and J. Chen. 2006. Role of colonic acid exopolysaccharide in the survival of enterohaemorrhagic Escherichia coli O157:H7 in simulated gastrointestinal fluids. Lett. Appl. Microbiol. 42:642-647. [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka, H. 2005. Treatment of germinated wheat to increase levels of GABA and IP6 catalyzed by endogenous enzymes. Biotechnol. Prog. 21:405-410. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura, Y., N. Yamamoto, K. Skai, and T. Takano. 1995. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J. Dairy Sci. 78:1253-1257. [DOI] [PubMed] [Google Scholar]
- 32.Nomura, M., H. Kimoto, Y. Someya, S. Furukawa, and I. Suzuki. 1998. Production of γ-aminobutyric acid by cheese starters during cheese ripening. J. Dairy Sci. 81:1486-1491. [DOI] [PubMed] [Google Scholar]
- 33.Nomura, M., H. Kimoto, Y. Someya, S. Furukawa, and I. Suzuki. 1999. Novel characterization for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int. J. Syst. Bacteriol. 49:163-166. [DOI] [PubMed] [Google Scholar]
- 34.Nomura, M., I. Nakajima, Y. Fujita, M. Kobayashi, H. Kimono, I. Suzuki, and H. Aso. 1999b. Lactococcus lactis contains only glutamate decarboxylase gene. Microbiology 154:1375-1380. [DOI] [PubMed] [Google Scholar]
- 35.Oh, S. H. 2003. Stimulation of gamma-aminobutyric acid synthesis activity in brown rice by a chitosan/Glu germination solution and calcium/calmodulin. J. Biochem. Mol. Biol. 36:319-325. [DOI] [PubMed] [Google Scholar]
- 36.Oh, S. H., and C. H. Oh. 2003. Brown rice extracts with enhanced levels of GABA stimulate immune cells. Food Sci. Biotechnol. 12:248-252. [Google Scholar]
- 37.Oh, S. H., J. R. Soh, and Y. S. Cha. 2003. Germinated brown rice extract shows a nutraceutical effect in the recovery of chronic alcohol-related symptoms. J. Med. Food 6:115-121. [DOI] [PubMed] [Google Scholar]
- 38.Ohmori, M., T. Yano, J. Okamoto, T. Tsushida, T. Murai, and M. Higuchi. 1987. Effect of anaerobically treated tea on blood pressure of spontaneous hypertensive rats. Nippon Nogeikagaku Kaishi 61:1449-1451. [Google Scholar]
- 39.Okada, T., T. Sugishita, T. Murakami, H. Murai, T. Saikusa, T. Hotorino, A. Onoda, O. Kajimoto, R. Takahashi, and T. Takahashi. 2000. Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. Nippon Shokuhin Kagaku Kaishi 47:596-603. [Google Scholar]
- 40.Park, K. B., and S. H. Oh. 2004. Cloning and expression of a full-length glutamate decarboxylase gene from Lactobacillus plantarum. J. Food Sci. Nutr. 9:324-329. [Google Scholar]
- 41.Park, K. B., and S. H. Oh. 2005. Production and characterization of GABA rice yogurt. J. Food Sci. Biotechnol. 14:518-522. [Google Scholar]
- 42.Park, K. B., and S. H. Oh. 2007. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour. Technol. 98:312-319. [DOI] [PubMed] [Google Scholar]
- 43.Pasini, G., M. Simonato, M. Giannattasio, A. D. B. Peruffo, and A. Curioni. 2001. Modification of wheat flour proteins during in vivo digestion of bread dough, crumb and crust: an electrophoretic and immunological study. J. Agric. Food Chem. 49:2254-2259. [DOI] [PubMed] [Google Scholar]
- 44.Rhyu, M. R., E. Y. Kim, H. Y. Kim, B. H. Ahn, and C. B. Yang. 2000. Characteristics of the red rice fermented with fungus Monascus. Food Sci. Biotechnol. 9:21-26. [Google Scholar]
- 45.Rizzello, C. G., I. Losito, M. Gobbetti, T. Carbonara, M. D. De Bari, and P. G. Zambonin. 2005. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 88:2348-2360. [DOI] [PubMed] [Google Scholar]
- 46.Saikusa, T., T. Horino, and Y. Mori. 1994. Accumulation of γ-aminobutyric acid (GABA) in the rice germ during water soaking. Biosci. Biotechnol. Biochem. 58:2291-2292. [Google Scholar]
- 47.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 48.Sanders, M. E. 1998. Overview of functional foods: emphasis on probiotic bacteria. Int. Dairy J. 8:341-347. [Google Scholar]
- 49.Small, P. L. C., and S. R. Waterman. 1998. Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 6:214-216. [DOI] [PubMed] [Google Scholar]
- 50.Smith, D. K., T. Kassam, B. Singh, and J. F. Elliott. 1992. Escherichia coli has two homologous glutamate decarboxylase genes that map to distinct loci. J. Bacteriol. 174:5820-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueno, H. 2000. Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. 10:67-79. [Google Scholar]
- 52.Ueno, Y., K. Hayakawa, S. Takahashi, and K. Oda. 1997. Purification and characterization of glutamate decarboxylase from Lactobacillus brevis IFO 12005. Biosci. Biotechnol. Biochem. 61:1168-1171. [DOI] [PubMed] [Google Scholar]
- 53.Wong, C. G., T. Bottiglieri, and O. C. Snead III. 2003. GABA, γ-hydroxybutyric acid, and neurological disease. Ann. Neurol. 54(Suppl. 6):S3-S12. [DOI] [PubMed] [Google Scholar]
- 54.Xu, J., X. Xu, and W. Verstraete. 2001. The bactericidal effect and chemical reactions of acidified nitrite under conditions simulating the stomach. J. Appl. Microbiol. 90:523-529. [DOI] [PubMed] [Google Scholar]
- 55.Zapparoli, G., S. Torriani, and F. Dellaglio. 1998. Differentiation of Lactobacillus sanfranciscensis strains by randomly amplified polymorphic DNA and pulsed-field gel electrophoresis. FEMS Microbiol. Lett. 166:324-332. [Google Scholar]
- 56.Zárate, G. A., S. P. Chaia, and G. O. González. 2000. Viability and β-galactosidase activity of dairy propionibacteria subjected to digestion by artificial gastric and intestinal fluids. J. Food Prot. 63:1214-1221. [DOI] [PubMed] [Google Scholar]
- 57.Zwietering, M. H., I. Jongeberger, F. M. Roumbouts, and K. van 't Riet. 1990. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 56:1875-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]