Abstract
Escherichia coli O157:H7 causes life-threatening outbreaks of diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome in humans and significant economic loss in agriculture and could be a potential agent of bioterrorism. Although the prevalence of E. coli O157:H7 in cattle and other species with which humans have frequent contact is high, human infections are relatively uncommon, despite a low infectious dose. A plausible explanation for the low disease incidence is the possibility that not all strains are virulent in humans. If there are substantial differences in virulence among strains in nature, then human disease may select for high virulence. We used a gnotobiotic piglet model to investigate the virulence of isolates from healthy cattle and from humans in disease outbreaks and to determine the correlation between production of Shiga toxin 1 (Stx1) and Stx2 and virulence. Overall, E. coli O157:H7 strains isolated from healthy cattle were less virulent in gnotobiotic piglets than strains isolated from humans during disease outbreaks. The amount of Stx2 produced by E. coli O157:H7 strains correlated with strain virulence as measured by a reduction in piglet survival and signs of central nervous system disease due to brain infarction. The amount of Stx1 produced in culture was not correlated with the length of time of piglet survival or with signs of central nervous system disease. We suggest that disease outbreaks select for producers of high levels of Stx2 among E. coli O157:H7 strains shed by animals and further suggest that Stx1 expression is unlikely to be significant in human outbreaks.
Escherichia coli O157:H7 causes both outbreaks and sporadic cases of diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (HUS). E. coli O157:H7 isolates produce several factors that are believed to contribute to virulence, including Shiga toxin 1 (Stx1), Stx2, and several proteins encoded in the locus of enterocyte effacement pathogenicity island (18, 31, 44). Stxs are A-B-type toxins that inhibit protein synthesis in affected cells, particularly endothelial cells in small blood vessels (8, 9, 37, 38). Following bacterial colonization of the intestine, the toxins are thought to enter the systemic circulation by crossing the intestinal mucosa and to be carried systemically on the surfaces of polymorphonuclear leukocytes (1, 2, 61). Stxs are then transferred to endothelial cells and cause damage with fibrin deposition in and thrombosis of arterioles and other small- to medium-sized blood vessels in certain target organs, including the kidney, colon, and brain, which result in infarcts in these organs (14, 43, 44, 51, 60, 66).
Based on studies in various animal models, Stxs also appear to be capable of causing direct damage to tissues other than the endothelium, including the villous epithelium in the small intestine (26), the renal tubular epithelium (42, 67), neurons (19), and myelinated nerves in the central nervous system (CNS) (21). Most patients fully recover from bloody diarrhea, but some develop life-threatening diseases of the kidneys and CNS; approximately 15% of the cases in children result in HUS (60). With early implementation of aggressive supportive therapy, including dialysis, the risk of death due to renal failure has been reduced substantially. However, about one-third of HUS cases involve significant encephalopathy, and brain injury is the leading cause of death associated with acute HUS (47, 54).
E. coli O157:H7 infections are typically contracted through consumption of contaminated food or contact with contaminated water, animal feces, or infected animals. Several disease outbreaks have been associated with contaminated beef, suggesting that cattle are an important reservoir for human infection (5, 15). However, despite the high prevalence of E. coli O157:H7 in cattle herds (16, 29, 55) and the low infectious dose in humans (57, 63, 64), the incidence in humans is quite low.
The discrepancy between the present and historic incidence in humans and the prevalence of E. coli O157:H7 in cattle today and in beef products in the past suggest that there is significant variability among the typical strains harbored by animals and the strains isolated from severe human infections. Kim et al. reported that E. coli O157:H7 strains isolated from diseased humans were members of a different lineage than strains typically isolated from healthy cattle (27). Gally et al. compared 30 E. coli serotype O157 strains from human disease cases in Scotland to a similar number of strains isolated from asymptomatic Scottish cattle for production of hemolysin, EspP, Tir, and EspD (32, 33, 49, 50). While few genotypic differences were observed among the strains from the two sources, strains isolated from infected humans produced significantly more EspD and Tir than strains isolated from cattle. Ritchie et al. compared E. coli O157:H7 strains isolated from sporadic cases of HUS with isolates obtained from cattle pastures and found that both basal and mitomycin C-induced Stx2 production by HUS-associated E. coli O157:H7 was significantly greater than production by bovine isolates (46).
We have reported that oral challenge of 1-day-old gnotobiotic pigs with E. coli O157:H7 strains expressing both Stx1 and Stx2 or only Stx2 and not Stx1 results in signs of CNS disease, vascular lesions, hemorrhage, and infarcts in the brain (18). Other workers reported similar observations (13, 65). Administration of anti-Stx2 serum protects gnotobiotic piglets inoculated with Stx2+ E. coli O157:H7 against the development of brain vascular lesions, associated brain infarcts, and CNS clinical signs, providing evidence that the lesions are directly or at least indirectly caused by Stx2 (10, 12).
More recent studies have further shown that myelin degeneration occurs in the brain and that glomerular, arteriolar, and tubular lesions occur in the kidneys of gnotobiotic piglets inoculated with Stx2+ E. coli O157:H7 (21, 42). In the kidneys, thrombotic microangiopathy (TMA), which is the morphological hallmark of HUS in humans, was consistently seen, and small preglomerular arterioles were demonstrated to express Gb3/CD77 (21, 42). Larger arteries and glomerular capillary endothelial cells were not found to express Gb3/CD77 (21). In these two studies, despite developing renal lesions, the piglets did not develop clinical signs of HUS (21, 42). In the one study in which they were investigated, clinical pathology laboratory parameters of HUS (uremia, hemolytic anemia, and thrombocytopenia) also were not detected (21); however, all of the piglets developed signs of CNS dysfunction, and five of six animals developed cerebellar microhemorrhages, lesions which were attributable to Stx-induced damage (21). In addition, the arachnoidea and endothelial cells of small arteries in the CNS expressed Gb3/CD77 (21). In the study of Pohlenz et al. (42), a retrospective histological examination of formalin-fixed kidneys from gnotobiotic piglets inoculated with different serotypes of Stx-producing E. coli was conducted. In addition to finding TMA and other vascular system-based lesions, as previously reported by Gunzer et al. (21), Pohlenz et al. (42) found that piglets frequently developed renal tubular cell apoptosis and necrosis. The sites of the renal tubular lesions matched the sites where Stx2 binding and Gb3/CD77 expression were identified immunohistochemically (21). Gb3/CD77 and Stx2 binding were also demonstrated in cells of the glomeruli and arteriolar endothelium (21). Piglets inoculated with other serotypes of E. coli that expressed variants of Stx2 (Stx2c, Stx2d, Stx2d1, and Stx2d2) had renal lesions similar to those caused by Stx2+ E. coli O157:H7 (42).
In a previous study, we found that parenteral injection of Stx1 into gnotobiotic piglets results in the same vascular and ischemic lesions that are caused by Stx2+ E. coli O157:H7. Thus, the differences between the two toxins in infectious disease appear to be due to relative toxicity (14). The vascular lesions in gnotobiotic pigs are essentially identical to those reported in human cases (65), suggesting that this model is relevant to the study of human E. coli O157:H7 disease (17). Furthermore, in humans, as in the gnotobiotic pig model, Stx2 is more frequently associated with extraintestinal disease, including HUS and CNS disease, than Stx1 is (40, 68). Purified Stx2 is 1,000 times more toxic than Stx1 to human renal endothelial cells (30) and causes severe renal damage in mice at a lower dose than Stx1 (62). Administration of Stx2 to baboons also causes HUS, while comparable concentrations of Stx1 do not (53). Thus, substantial evidence has been presented that Stx2 is a major contributor to sequellae associated with E. coli O157:H7 infection.
Based on findings from previous studies, we hypothesized that E. coli O157:H7 isolates from cattle feces are, on average, less virulent than isolates from human patients with HUS. The main objective of the present study was to test this hypothesis using gnotobiotic piglets. A second objective was to determine the extent to which expression levels of Stx1 and/or Stx2 correlate with differences in virulence. A third objective was to test the hypothesis that CNS lesions but not renal lesions are correlated with the clinical demise of piglets.
MATERIALS AND METHODS
Bacterial strains.
Ten E. coli O157:H7 strains originally isolated from human disease outbreaks were obtained from Timothy J. Barrett of the Centers for Diseases Control and Prevention in Atlanta, GA. Ten other E. coli O157:H7 strains originally isolated from healthy dairy cattle in the National Dairy Heifer Survey conducted by the National Animal Health Monitoring System of the U.S. Department of Agriculture were obtained from William Cray, Jr., of the National Animal Disease Center in Ames, IA (Table 1) (4). Strain EDL933, which is highly virulent in 1-day-old gnotobiotic piglets, was used as a positive control (18). All strains were shown by PCR to possess the stx1, stx2, and eae genes. Strains were stored in liquid nitrogen and cultured on blood agar plates (5% sheep blood in heart infusion agar [Difco Laboratories, Detroit, MI]). For piglet challenge, colony sweeps of bacteria were inoculated into 3 ml tryptic soy broth (Difco) and incubated for 18 h at 37°C to obtain a concentration of approximately 1 × 109 CFU/ml. To assess the relative amount of Stx produced by each strain in vitro, the bacterial concentration of each strain was normalized to an optical density at 280 nm of 1.00.
TABLE 1.
Relationship between Stx2 toxin titers for E. coli O157:H7 strains (in vitro) and the symptoms and survival of piglets infected with E. coli
| Group | Strain | Reference or source | Stx2 titera | Piglets
|
|||
|---|---|---|---|---|---|---|---|
| Mean survival time (days)b | % with CNS disease | % with brain infarctions | n | ||||
| Control | EDL933 | 45 | 161 | 4.3 | 100 | 90 | 10 |
| Human | 3234-86 | 39 | 32 | 6.4 | 80 | 100 | 5 |
| B8763 | 41 | 25 | 5.2 | 100 | 80 | 5 | |
| A7785 | 45 | 20 | 8.0 | 20 | 20 | 5 | |
| C9490 | 3 | 32 | 5.2 | 60 | 20 | 5 | |
| B1189 | 51 | 102 | 3.8 | 100 | 80 | 5 | |
| C509 | 48 | 102 | 3.4 | 100 | 100 | 5 | |
| C6183 | T. J. Barrett | 25 | 5.2 | 80 | 60 | 5 | |
| C8779 | 3 | 32 | 4.0 | 100 | 80 | 5 | |
| C7927 | 7 | 20 | 6.0 | 80 | 60 | 5 | |
| C4193 | 58 | 10 | 7.2 | 40 | 40 | 5 | |
| Bovine | 2890 | 4 | 64 | 5.2 | 80 | 80 | 5 |
| 2893 | 4 | <2 | 8.0 | 0 | 20 | 5 | |
| 2903 | 4 | 25 | 8.0 | 0 | 0 | 5 | |
| 2909 | 4 | 16 | 6.6 | 40 | 20 | 5 | |
| 3032 | 4 | 6 | 8.0 | 0 | 20 | 5 | |
| 2922 | 4 | <2 | 8.0 | 0 | 0 | 5 | |
| 2918 | 4 | <2 | 8.0 | 0 | 0 | 5 | |
| 2939 | 4 | 40 | 5.2 | 100 | 100 | 5 | |
| 2891 | 4 | 10 | 6.4 | 40 | 20 | 5 | |
| 2977 | 4 | 51 | 5.4 | 80 | 80 | 5 | |
Relative concentration of Stx2 (in arbitrary units) as measured with a verotoxigenic E. coli reverse passive latex agglutination assay.
Up to 8 days postinoculation.
Gnotobiotic challenge studies.
Gnotobiotic piglets were derived by closed hysterotomy and reared in sterile isolators using standard procedures (34). One pig from each litter used in the study was inoculated with positive control strain EDL933 to limit strain-by-litter bias and to ensure the susceptibility of pigs in the litter to E. coli O157:H7. Litters containing control pigs that did not succumb to EDL933 within 8 days of challenge were removed from the study. Each of the 20 E. coli O157:H7 strains tested for virulence was inoculated into five gnotobiotic piglets. Litters of piglets were arbitrarily assigned to specific E. coli strains, and typically the piglets were divided for testing with three or four strains. To further reduce strain-by-litter bias, piglets from at least two different litters were used to evaluate each strain, and piglets within litters were randomly assigned to individual strains. All piglets were challenged per os with ∼3 × 109 CFU at 24 to 30 h after birth, when it was demonstrated that the piglets were independently feeding. Following challenge, piglets were observed for clinical signs of illness, including diarrhea and CNS disease, at least three times daily for 8 days or until they exhibited signs of CNS disease or were unable to eat. Signs ascribed to CNS disease included head tilt, circling, lethargy, an inability to stand, lateral recumbency, and/or paddling (tonic-clonic convulsions). At 8 days postinoculation, or when signs of CNS disease were exhibited, piglets were euthanized and subjected to necropsy. At necropsy, pigs were examined for gross lesions, including intestinal hemorrhage and mesocolonic edema. Specimens of the jejunum, ileum, cecum, spiral colon, rectum, liver, lungs, heart, stomach, urinary bladder, kidneys, spleen, mesenteric lymph nodes, thymus, and spinal cord and the entire brain were collected for histological analysis. Brains were sectioned at the level of the medulla oblongata (olivary nucleus), cerebellum with medulla oblongata (cerebellar peduncles), midbrain (corpora quadrigemina), and two levels of cerebrum (interthalamic adhesion and genu of corpus callosum [18]). A section of the colon was also collected for aerobic and anaerobic bacterial culture to confirm the presence of the inoculated strain and to assess contamination.
Histological examinations.
Specimens collected for histologic analysis were fixed in 10% neutral buffered formalin and processed for histopathology by standard procedures, and tissues were stained with hematoxylin and eosin. Tissue sections were subjected to routine microscopic examination for lesions; however, in kidney tissue sections, the numbers of glomeruli and arterioles with lesions compatible with Stx-induced damage (16, 37) were counted, and the resulting data were included in the statistical analyses as separate counts. The pathologist conducting microscopic examinations of the tissues (R.A.M.) was blinded to the treatment groups of the piglets during the study.
Vero cell cytotoxicity assay.
Bacterial strains were tested for Stx expression using a Vero cell cytotoxicity assay (20). Bacteria were inoculated into 3 ml of tryptic soy broth and incubated for 4 h at 37°C. Secreted toxins were extracted with polymyxin B (25). Vero cells (CCL-91; American Type Culture Collection) were plated with Dulbecco's minimum Eagle's medium (HyClone) containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in 96-well U-bottom microtiter plates to obtain a concentration of 2 × 104 cells/well and incubated until the cells were confluent (18 h at 37°C in 5% CO2). The concentration of toxin from each strain was determined in triplicate in each of five separate trials. Extracts containing toxin from each E. coli O157:H7 strain were added to Dulbecco's minimum Eagle's medium in the 96-well plates at an initial dilution of 1:6 and diluted further in twofold increments. The Vero cells in test wells were incubated with the diluted toxin extracts for 2 days. After incubation, detached cells, medium, and toxin were removed by vigorous shaking. The remaining cells were fixed with 2% formalin in 67 mM phosphate-buffered saline (pH 7.4) for 1 min, after which the fixative was removed. Attached cells were stained with 0.13% crystal violet in 5% ethanol-2% formalin-phosphate-buffered saline for 20 min. Excess stain was removed by rinsing the wells gently with distilled water. The plates were air dried, and the optical density at 620 nm of the attached cells was determined spectrophotometrically. The toxin titer (50% cytolethal dose) was calculated by determining the reciprocal of the dilution required to kill 50% of the Vero cells in a well of a 96-well U-bottom microtiter plate.
Stx1 and Stx2 quantification.
Relative concentrations of Stx1 or Stx2 produced by E. coli O157:H7 strains during culture in tryptic soy broth at 37°C for 4 h were determined after polymyxin B extraction by measurement with a verotoxigenic E. coli reverse passive latex agglutination assay (Denka Seiken Co. Ltd., Tokyo, Japan) (24).
Immunohistochemistry.
Immunohistochemical detection of E. coli O157 antigen in sections of urinary bladders from infected gnotobiotic piglets was conducted by a modification of methods described previously (6). Rabbit anti-O157 antiserum (1:1,000 dilution; E. coli Reference Laboratory, Pennsylvania State University) was used as the primary antiserum. Commercial goat anti-rabbit immunoglobulin G and a streptavidin-alkaline phosphatase kit (DakoCytomation LSAB2 System-AP) were used for subsequent antigen detection steps.
Statistical analyses.
The statistical significance of data (P < 0.05) was analyzed with Student's t test, by analysis of variance (ANOVA) for mean days for the “human” and “bovine” sources, and by analysis of covariance (ANCOVA) for mean days for the “human” and “bovine” sources, as well as the Stx1/Stx2 ratio. Logistic regression was used for data for “CNS,” “diarrhea,” “edema,” “attaching and effacing (A/E) lesions,” and “infarcts” since these data were binomially distributed. For both ANCOVA and logistic regression, the interaction between the source (“human” and “bovine”) and the Stx1/Stx2 ratio was not statistically significant.
RESULTS
E. coli EDL933 causes A/E lesions and CNS disease in gnotobiotic piglets.
Eleven litters of piglets were utilized in this study. A control piglet in one litter did not become clinically ill. This litter was removed from the study to limit the effect of piglet disease resistance on the assessment of strain virulence. For the remaining 10 litters, the positive controls inoculated with strain EDL933 exhibited signs of CNS disease or died 2 to 5 days postinoculation (mean ± standard deviation, 3.7 ± 0.9 days). Nine control piglets were euthanized, and one died spontaneously. Eight of 10 control piglets exhibited diarrhea. One of the two controls that did not develop diarrhea died 3 days postinoculation. The other control exhibited signs of CNS disease on day 3 and was euthanized. The early demise of these two control pigs may have precluded the development of diarrhea in them. Nine of the 10 controls had microscopic evidence of brain infarction. Histological sections from the intestines of various control pigs exhibited attached bacteria with lesions consistent with microvillus effacement. Mesocolonic edema was observed at necropsy in euthanized control pigs. The results for the 10 litters support the proposition that the study measured differences in E. coli O157:H7 strain virulence rather than variation in piglet susceptibility among litters. Nine of 10 litters contained both piglets that developed CNS disease and piglets that failed to develop CNS disease (as a consequence of challenge with different E. coli strains). For only 1 of 20 strains (C9490) were the challenge outcomes different for pigs from different litters.
On average, E. coli O157:H7 strains isolated from human outbreaks are more virulent in gnotobiotic piglets than strains isolated from healthy cattle.
Piglets inoculated with E. coli O157:H7 strains isolated from cattle and from patients involved in disease outbreaks varied in their manifestations of clinical signs and in the presence and extent of lesions (Table 1). Several strains were as virulent as or more virulent than EDL933, while other strains were relatively attenuated. Based on the development of CNS disease or death of inoculated piglets, all of the human isolates were virulent, while only one-half of the bovine strains were virulent. Despite great variability within the groups, the human isolates were significantly more likely to cause CNS disease and/or death (Table 2) (76% versus 34%; P = 0.023, logistic regression). In addition, the numbers of days that piglets survived after challenge were significantly less for pigs that received outbreak strains than for pigs that received bovine isolates (5.4 days versus 6.9 days; P = 0.032, ANOVA). Pigs inoculated with human disease strains showed a greater, but not a statistically significant greater, incidence of brain infarcts (64% versus 34%; P = 0.07, logistic regression). There was no significant difference between treatment groups in terms of mesocolonic edema, diarrhea, or A/E lesions.
TABLE 2.
Disease indicators for piglets infected with bovine or human strains of E. coli O157:H7a
| Symptom | No. of piglets with symptom
|
|
|---|---|---|
| Bovine strains (n = 50) | Human strains (n = 50) | |
| Death or CNS disease | 17b | 38 |
| Brain infarcts | 17 | 32 |
| Diarrhea | 45 | 49 |
| A/E lesions | 34 | 38 |
| Mesocolonic edema | 41 | 47 |
Five piglets were infected with 10 strains of bovine origin and 10 strains of human origin. The potential postchallenge survival time, 8.0 days (termination of the study), was reduced to 6.9 and 5.4 days by bovine and human isolates, respectively (P < 0.03, ANOVA).
Significantly different (P < 0.05, ANOVA).
CNS disease symptoms are correlated with postmortem observation of brain necrosis (infarcts).
We previously observed that piglets typically die within several hours of manifestation of clinical signs consistent with disease of the CNS (18). To minimize piglet suffering and to preclude postmortem autolysis of tissues, piglets were euthanized immediately after CNS disease symptoms were observed. Appropriate recognition of signs of CNS disease was established by the high correlation between signs of CNS disease and the postmortem observation of infarcts in sections of brain tissue (R2 = 88.4% and P < 0.001, ANOVA). Brain necrosis was not correlated with the presence of mesocolonic edema, diarrhea, or A/E lesions, nor was diarrhea correlated with the presence of mesocolonic edema or A/E lesions.
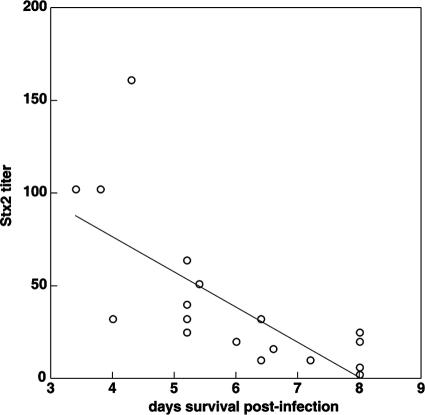
Stx2 production is correlated with the virulence of E. coli O157:H7 strains.
The amount of Stx2 produced by E. coli O157:H7 strains was correlated with a reduction in survival to the end of the study (P < 0.001, ANCOVA) (Table 3 and Fig. 1). Stx2 production was also correlated with manifestation of signs of CNS disease (P = 0.003) (Table 3) and brain necrosis (infarcts) (P = 0.003) (Table 3), but not with mesocolonic edema, diarrhea, or A/E lesions. The correlation coefficients and significance were similar whether Stx2 data obtained from Vero cell cytotoxicity assays or enzyme-linked immunosorbent assays were used. The amount of Stx1 produced in culture was not correlated with the length of time that a piglet survived, with manifestation of CNS disease or brain infarcts, or with the presence of mesocolonic edema, diarrhea, or A/E lesions (Table 3).
TABLE 3.
Correlation between outcome variables and Stx1 and Stx2 productiona
| Stx | Outcome variable | P value |
|---|---|---|
| Stx1 | Mean no. of days of survival | 0.093 |
| CNS disease | 0.623 | |
| Diarrhea | 0.832 | |
| Mesocolonic edema | 0.061 | |
| A/E lesions | 0.963 | |
| Brain infarcts | 0.955 | |
| Stx2 | Mean no. of days of survival | <0.001 |
| CNS disease | 0.003 | |
| Diarrhea | 0.078 | |
| Mesocolonic edema | 0.099 | |
| A/E lesions | 0.155 | |
| Brain infarcts | 0.003 |
ANCOVA (mean number of days of survival) and logistic regression (all other variables) were used for assessment.
FIG. 1.
Relationship between Stx2 titer and number of days of survival postinfection.
Kidney lesions.
Lesions consistent with the lesions previously described (21, 42) were observed in the kidneys of most pigs in the study, including those challenged with positive control strain EDL933. Of 109 piglets whose renal tissue was examined histologically, 85% had lesions in the glomeruli, 59% had lesions involving arterioles, 52% had lesions in both glomeruli and arterioles, 61% had lesions involving tubules, 91% had lesions in either or both glomeruli and arterioles, and 95% had lesions involving one or more of the three areas. The lesions in the glomeruli included capillary endothelial swelling, capillary congestion, fibrin-platelet thrombi, thickening and fibroplasia of Bowman's capsule, and proliferation of mesangial cells. Each individual glomerulus that was counted as positive exhibited two or more of these lesions. Arterioles with lesions exhibited TMA with endothelial swelling and proliferation, concentric intimal thickening, fibrinoid change of the tunica intima and media, and fibrin thrombi. The tubular lesions included necrosis and/or apoptosis, dilatation, and luminal accumulation of sloughed epithelial cells or neutrophils. The number of tubular lesions per tissue section was not quantified since some of the changes (e.g., dilatation) were too numerous to count and not necessarily specific for toxin-induced damage. Statistical analysis did not establish a significant correlation between kidney glomerular or arteriolar lesions and CNS disease (P = 0.57 and P = 0.098, respectively, Student's t test). Nor was a significant correlation observed between kidney lesions and Stx2 production by challenge strains (for Stx2 versus glomerular lesions, R2 = 0.2% and P = 0.28, as determined by regression analysis; for Stx2 versus arteriolar lesions, R2 = 0.2% and P = 0.28, as determined by regression analysis).
Atypical lesions.
Lesions that were either uncommon or not previously described in this model were observed in some animals, but they did not appear to be due to a unique virulence attribute of any particular strain. Purulent cystitis that was mild to moderate and subacute to chronic was seen in 14 of 105 (13.3%) piglets whose urinary bladder was microscopically examined. Lesions in the urinary bladder were characterized by infiltration of the mucosal epithelium with neutrophils, by infiltration of the propria submucosa with lymphocytes, by smaller numbers of macrophages, and by granulation tissue formation. In 8 of 14 piglets with cystitis (57.1%), inflammatory lesions were associated with coccobacillary bacterial attachment to the surfaces of mucosal transitional epithelial cells. Immunohistochemical analysis to detect the O157 antigen (6) was conducted using urinary bladder sections from all piglets in which bacterial colonization was observed. Adherent bacteria, when present, stained positive for the O157 antigen in all cases, confirming that E. coli O157:H7 had infected the urinary bladder. In addition to the aforementioned renal lesions, two piglets had mild interstitial nephritis and two piglets had mild pyelitis (inflammation of the pelvic epithelium of the kidney); both of the latter piglets also had cystitis. Eight of 126 piglets (6.3%) developed bronchopneumonia, and there were significant lesions in two animals. Lung lesions appeared to have been caused by aspiration of small amounts of milk containing bacterial inoculum. Five piglets had mild erosive gastritis.
In addition to A/E lesions, other clinically significant lesions were seen in the lower small and large intestines of some piglets. Necrosuppurative inflammation of the ileum, cecum, and colon was seen in five piglets (4.0%), and lesions were ulcerative in two of these animals. Three piglets (2.4%) had lesions suggestive of ischemic bowel necrosis. In one of these animals, lesions were associated with widespread thrombosis of small blood vessels in multiple organs, including the ileum, cecum, and colon. In the second piglet, coagulative necrosis of villi in the ileum was seen without widespread thrombosis in other organs. In the third piglet, lesions were extensive, with full-thickness necrosis of the ileal mucosa (Fig. 2) and necrohemorrhagic colitis (Fig. 3). Mucosal necrosis in the colon spared the deep portions of the crypts and was characterized by loss of the superficial portions and replacement by suffusive hemorrhage that filled the lamina propria.
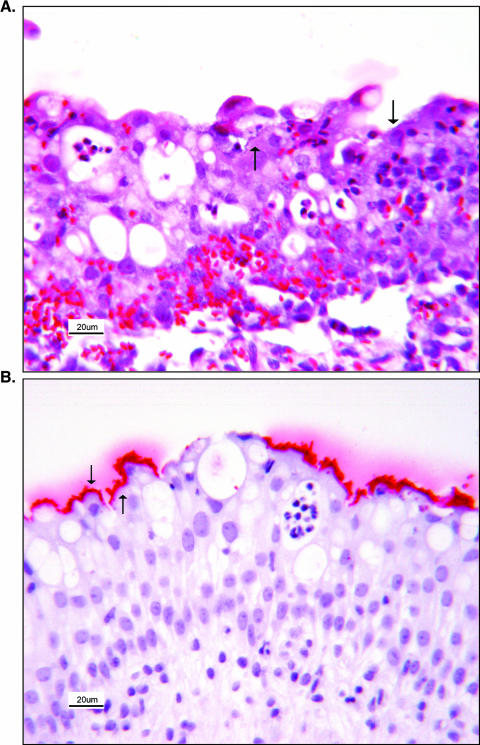
FIG. 2.
Urinary bladder of a gnotobiotic piglet (animal 15627) infected with E. coli O157:H7. (A) Neutrophils have multifocally infiltrated the mucosa. The mucosa and propria submucosa are hemorrhagic. Foci of bacterial colonization (arrows) are present on the mucosal surface, with bacteria attached to transitional epithelial cells. (B) Additional sections were cut from the same block of paraffin-embedded tissue and stained immunohistochemically for E. coli O157 antigen. The bacteria attached to transitional epithelial cells and colonizing the mucosa were identified as E. coli O157; the red reaction product indicated a positive result (arrows). Tissue sections were counterstained with hematoxylin.
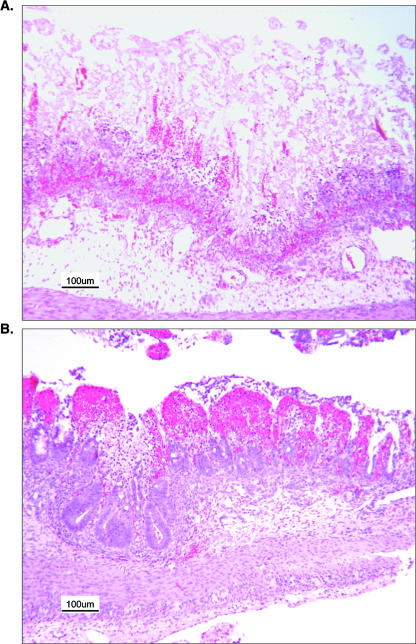
FIG. 3.
Intestines of a gnotobiotic piglet (animal 12573) infected with E. coli O157:H7. (A) Ileum. There is coagulative necrosis involving the full thickness of the mucosa and remnants of surviving epithelium at the bases of some crypts in the process of regeneration. Hyperemia and hemorrhage are evident at the junction of the mucosa with the submucosa, but hemolysis obscures evidence of hemorrhage superficial to this region. Blood vessels in the mucosa are hyperemic, and some of them (not seen at this magnification) are necrotic and thrombosed. There is edema of the submucosa with associated lymphatic blood vessel dilatation. (B) The superficial mucosa has undergone necrosis and replacement with suffusive hemorrhage that fills the lamina propria. Necrosis has spared the deeper portions of the crypts in the mucosa. Blood vessels in the mucosa are hyperemic, and some of them (not seen at this magnification) are necrotic and thrombosed. The submucosa is edematous. Tissue sections were stained with hematoxylin and eosin.
DISCUSSION
We showed that E. coli O157:H7 strains isolated from healthy cattle are, on average, less virulent in gnotobiotic piglets than E. coli O157:H7 strains isolated from human disease outbreaks. Virulence was highly correlated with in vitro production of Stx2 but not Stx1. We suggest that disease outbreaks may select for producers of high levels of Stx2 among E. coli O157:H7 strains shed by animals. This hypothesis is consistent with the findings of Ritchie et al., who reported that both the basal and mitomycin-enhanced Stx2 production by isolates from patients with HUS was greater than that by isolates from cattle (46). Gally et al. showed that strains isolated from sporadic or outbreak cases of disease had enhanced expression of EspD and Tir compared to bovine isolates, despite essentially identical strain genotypes (Stx production was not tested) (32, 33, 49, 50). Together, these observations suggest that there may be a general regulatory difference in virulence genes between strains with high virulence and strains with low virulence and that there is selection for greater virulence factor expression in more virulent strains.
A potential mechanism governing Stx2 expression differences is suggested by the observations of Muniesa et al. regarding Stx2 variability among outbreak strains (36). Isolated strains were grouped based on the amount of phage released after mitomycin C induction. Strains that released fewer phage were found to harbor two phage types, φLC159 and φSC370, although only φSC370 was detected in supernatants of induced cultures. When φSC370 was absent, large amounts of φLC159 were released, and the higher level of phage production was accompanied by an increased amount of Stx2 in the cultures. Some relationship was detected between phage production and the severity of the disease observed in patients from which the E. coli O157:H7 strains were isolated (36). These observations suggest that interaction between different phages may affect toxin regulation. Kim et al. revealed the existence of two distinctly different lineages of E. coli O157:H7 through octamer-based genome scanning (27). Human and bovine isolates were not randomly distributed between lineages. Restriction fragment length polymorphism analysis with lambdoid phage genomes indicated that phage-associated polymorphisms segregate precisely along lineages predicted by octamer-based genome scanning. Thus, highly virulent strains (which also produce high levels of Stx2) may represent a particular lineage of E. coli O157:H7 strains.
A few pigs developed lesions not previously described for gnotobiotic piglets infected with E. coli O157:H7. Most notable of these were cystitis and hemorrhagic colitis lesions. Infection by E. coli O157:H7 or other Stx-producing E. coli strains of the urinary tract has not been reported in animal models but can be a prodrome to HUS in humans (11, 23, 52, 56, 59). Hemorrhagic colitis is a common feature in clinically recognized human infections, but this condition has not been previously reported to be an outcome of experimental infectious challenge in nonprimate animal models. The several instances of necrotic and/or hemorrhagic lesions in the intestines of pigs in this study indicate that these animals are subject to these lesions, although they may be uncommon. Necrohemorrhagic intestinal lesions in the gnotobiotic piglet model were presumably secondary to ischemic necrosis and were essentially identical to the lesions which we saw previously in gnotobiotic piglets inoculated with Stx1 (14). They also closely resembled lesions seen in human patients with E. coli O157:H7 infections (22, 28, 35).
Recently, previously unrecognized lesions in the kidneys of gnotobiotic piglets infected with E. coli O157:H7 were reported (21, 42). Collectively in the two studies, renal lesions involved changes in the glomeruli, the afferent arterioles and small arteries, and the tubules. Glomeruli were affected with endothelial swelling, narrowing of the capillary lumina, congestion, and thrombosis. TMA preferentially affected afferent arterioles and, to a lesser extent, small arteries, whereas larger arteries were unaffected. Tubular epithelial apoptosis, degeneration, and necrosis were also seen (42). The locations of the vascular and tubular lesions matched the locations where Stx2 binding and the Stx2 receptor (Gb3) were identified immunohistochemically (42). In the present study, based on histological examination, 95% of the piglets had lesions consistent with those previously described which involved one or more of the three target sites (i.e., arterioles, glomeruli, and tubules). However, the presence of the lesions correlated with neither parameters of life-threatening clinical illness nor the relative amount of Stx2 produced by the challenge strains. Since previous studies have provided strong evidence that renal lesions in piglets are due to Stx2 and that these lesions resemble lesions seen in human patients with HUS, has been proposed that the gnotobiotic piglet is a relevant model for HUS (21, 42). However, a consistent finding in different studies, including the present study, is that the piglets die from brain damage and not from the clinical features of HUS (i.e., hemolytic anemia, thrombocytopenia, and renal failure). In the present study the hypothesis was that CNS lesions and not renal lesions are correlated with the clinical demise of the piglets; this was found to be the case. It may be that a piglet has greater susceptibility to Stx2-mediated damage in the brain and that the life-threatening nature of the lesions that result cause death before renal lesions have a chance to progress to clinical HUS. Alternatively, another possible explanation is that other putative factors necessary for the development of clinical HUS (60) are not present in the neonatal gnotobiotic piglet model.
Acknowledgments
This study was funded in part by USDA-CSREES NC-1007 Multi-state Research, Enteric Diseases of Swine and Cattle: Prevention, Control and Food Safety.
Footnotes
Published ahead of print on 21 September 2007.
REFERENCES
- 1.Acheson, D. W., R. Moore, S. De Breucker, L. Lincicome, M. Jacewicz, E. Skutelsky, and G. T. Keusch. 1996. Translocation of Shiga toxin across polarized intestinal cells in tissue culture. Infect. Immun. 64:3294-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson, D. W. K., L. L. Lincicome, M. S. Jacewicz, and G. T. Keusch. 1998. Shiga toxin interaction with intestinal epithelial cells, p. 140-147. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, DC.
- 3.Anonymous. 1993. Update: multistate outbreak of Escherichia coli O157:H7 infections from hamburgers—western United States, 1992-1993. Morb. Mortal. Wkly. Rep. 42:258-263. [PubMed] [Google Scholar]
- 4.Anonymous. 1994. USDA: APHIS: VS National Dairy Heifer Evaluation Project Report: Escherichia coli O157:H7 in U.S. dairy calves. National Animal Health Monitoring System, Center for Epidemiology and Animal Health, Ft. Collins, CO.
- 5.Armstrong, G. L., J. Hollingsworth, and J. G. Morris, Jr. 1996. Emerging foodborne pathogens: Escherichia coli O157:H7 as a model of entry of a new pathogen into the food supply of the developed world. Epidemiol. Rev. 18:29-51. [DOI] [PubMed] [Google Scholar]
- 6.Baehler, A. A., and R. A. Moxley. 2000. Escherichia coli O157:H7 induces attaching-effacing lesions in large intestinal mucosal explants from adult cattle. FEMS Microbiol. Lett. 185:239-242. [DOI] [PubMed] [Google Scholar]
- 7.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 8.Brown, J. E., S. W. Rothman, and B. P. Doctor. 1980. Inhibition of protein synthesis in intact HeLa cells by Shigella dysenteriae 1 toxin. Infect. Immun. 29:98-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, J. E., M. A. Ussery, S. H. Leppla, and S. W. Rothman. 1980. Inhibition of protein synthesis by Shiga toxin: activation of the toxin and inhibition of peptide elongation. FEBS Lett. 117:84-88. [DOI] [PubMed] [Google Scholar]
- 10.Chae, C., R. A. M. Moxley, J. Christopher-Hennings, D. H. Francis, and M. J. Wannemuehler. 1994. Shiga-like toxin-II-producing Escherichia coli O157:H7 infection in gnotobiotic piglets: protection against brain vascular lesions with SLT-II antiserum, p. 241-244. In M. A. Karmali and A. G. Goglio (ed.), Recent advances in verocytotoxin-producing Escherichia coli infections. Elsevier Science B.V., Amsterdam, The Netherlands.
- 11.Chiurchiu, C., A. Firrincieli, M. Santostefano, M. Fusaroli, G. Remuzzi, and P. Ruggenenti. 2003. Adult nondiarrhea hemolytic uremic syndrome associated with Shiga toxin Escherichia coli O157:H7 bacteremia and urinary tract infection. Am. J. Kidney Dis. 41:E4. [DOI] [PubMed] [Google Scholar]
- 12.Donohue-Rolfe, A., I. Kondova, J. Mukherjee, K. Chios, D. Hutto, and S. Tzipori. 1999. Antibody-based protection of gnotobiotic piglets infected with Escherichia coli O157:H7 against systemic complications associated with Shiga toxin 2. Infect. Immun. 67:3645-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donohue-Rolfe, A., I. Kondova, S. Oswald, D. Hutto, and S. Tzipori. 2000. Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J. Infect. Dis. 181:1825-1829. [DOI] [PubMed] [Google Scholar]
- 14.Dykstra, S. A., R. A. Moxley, B. H. Janke, E. A. Nelson, and D. H. Francis. 1993. Clinical signs and lesions in gnotobiotic pigs inoculated with Shiga-like toxin I from Escherichia coli. Vet. Pathol. 30:410-417. [DOI] [PubMed] [Google Scholar]
- 15.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faith, N. G., J. A. Shere, R. Brosch, K. W. Arnold, S. E. Ansay, M. S. Lee, J. B. Luchansky, and C. W. Kaspar. 1996. Prevalence and clonal nature of Escherichia coli O157:H7 on dairy farms in Wisconsin. Appl. Environ. Microbiol. 62:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis, D. H., J. E. Collins, and J. R. Duimstra. 1986. Infection of gnotobiotic pigs with an Escherichia coli O157:H7 strain associated with an outbreak of hemorrhagic colitis. Infect. Immun. 51:953-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis, D. H., R. A. Moxley, and C. Y. Andraos. 1989. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect. Immun. 57:1339-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii, J., T. Kita, S. Yoshida, T. Takeda, H. Kobayashi, N. Tanaka, K. Ohsato, and Y. Mizuguchi. 1994. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H- in mitomycin-treated mice. Infect. Immun. 62:3447-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentry, M. K., and J. M. Dalrymple. 1980. Quantitative microtiter cytotoxicity assay for Shigella toxin. J. Clin. Microbiol. 12:361-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunzer, F., I. Hennig-Pauka, K. H. Waldmann, R. Sandhoff, H. J. Grone, H. H. Kreipe, A. Matussek, and M. Mengel. 2002. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am. J. Clin. Pathol. 118:364-375. [DOI] [PubMed] [Google Scholar]
- 22.Guth, B. E., E. G. Aguiar, P. M. Griffin, S. R. Ramos, and T. A. Gomes. 1994. Prevalence of colonization factor antigens (CFAs) and adherence to HeLa cells in enterotoxigenic Escherichia coli isolated from feces of children in Sao Paulo. Microbiol. Immunol. 38:695-701. [DOI] [PubMed] [Google Scholar]
- 23.Hogan, M. C., J. M. Gloor, J. R. Uhl, F. R. Cockerill, and D. S. Milliner. 2001. Two cases of non-O157:H7 Escherichia coli hemolytic uremic syndrome caused by urinary tract infection. Am. J. Kidney Dis. 38:E22. [DOI] [PubMed] [Google Scholar]
- 24.Karmali, M. A., M. Petric, and M. Bielaszewska. 1999. Evaluation of a microplate latex agglutination method (Verotox-F assay) for detecting and characterizing verotoxins (Shiga toxins) in Escherichia coli. J. Clin. Microbiol. 37:396-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karmali, M. A., M. Petric, C. Lim, R. Cheung, and G. S. Arbus. 1985. Sensitive method for detecting low numbers of verotoxin-producing Escherichia coli in mixed cultures by use of colony sweeps and polymyxin extraction of verotoxin. J. Clin. Microbiol. 22:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keenan, K. P., D. D. Sharpnack, H. Collins, S. B. Formal, and A. D. O'Brien. 1986. Morphologic evaluation of the effects of Shiga toxin and E. coli Shiga-like toxin on the rabbit intestine. Am. J. Pathol. 125:69-80. [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, J., J. Nietfeldt, and A. K. Benson. 1999. Octamer-based genome scanning distinguishes a unique subpopulation of Escherichia coli O157:H7 strains in cattle. Proc. Natl. Acad. Sci. USA 96:13288-13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson, J. M. 2004. Update on Escherichia coli O157:H7. Curr. Gastroenterol. Rep. 6:297-301. [DOI] [PubMed] [Google Scholar]
- 29.LeJeune, J. T., T. E. Besser, D. H. Rice, J. L. Berg, R. P. Stilborn, and D. D. Hancock. 2004. Longitudinal study of fecal shedding of Escherichia coli O157:H7 in feedlot cattle: predominance and persistence of specific clonal types despite massive cattle population turnover. Appl. Environ. Microbiol. 70:377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louise, C. B., S. A. Kaye, B. Boyd, C. A. Lingwood, and T. G. Obrig. 1995. Shiga toxin-associated hemolytic uremic syndrome: effect of sodium butyrate on sensitivity of human umbilical vein endothelial cells to Shiga toxin. Infect. Immun. 63:2766-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNally, A., A. J. Roe, S. Simpson, F. M. Thomson-Carter, D. E. Hoey, C. Currie, T. Chakraborty, D. G. Smith, and D. L. Gally. 2001. Differences in levels of secreted locus of enterocyte effacement proteins between human disease-associated and bovine Escherichia coli O157. Infect. Immun. 69:5107-5114. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.McNally, A., A. J. Roe, S. Simpson, F. M. Thomson-Carter, D. E. Hoey, C. Currie, T. Chakraborty, D. G. Smith, and D. L. Gally. 2005. Differences in levels of secreted locus of enterocyte effacement proteins between human disease-associated and bovine Escherichia coli O157. Infect. Immun. 73:2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miniats, O. P., and D. Jol. 1978. Gnotobiotic pigs—derivation and rearing. Can. J. Comp. Med. 42:428-437. [PMC free article] [PubMed] [Google Scholar]
- 35.Morandi, E., C. Grassi, P. Cellerino, P. P. Massara, F. Corsi, and E. Trabucchi. 2003. Verocytotoxin-producing Escherichia coli EH O157:H7 colitis. J. Clin. Gastroenterol. 36:44-46. [DOI] [PubMed] [Google Scholar]
- 36.Muniesa, M., M. de Simon, G. Prats, D. Ferrer, H. Panella, and J. Jofre. 2003. Shiga toxin 2-converting bacteriophages associated with clonal variability in Escherichia coli O157:H7 strains of human origin isolated from a single outbreak. Infect. Immun. 71:4554-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 38.Obrig, T. G., T. P. Moran, and J. E. Brown. 1987. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem. J. 244:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostroff, S. M., P. M. Griffin, R. V. Tauxe, L. D. Shipman, K. D. Greene, J. G. Wells, J. H. Lewis, P. A. Blake, and J. M. Kobayashi. 1990. A statewide outbreak of Escherichia coli O157:H7 infections in Washington State. Am. J. Epidemiol. 132:239-247. [DOI] [PubMed] [Google Scholar]
- 40.Paton, A. W., A. J. Bourne, P. A. Manning, and J. C. Paton. 1995. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric Shiga-like toxin type II operons. Infect. Immun. 63:2450-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pavia, A. T., C. R. Nichols, D. P. Green, R. V. Tauxe, S. Mottice, K. D. Greene, J. G. Wells, R. L. Siegler, E. D. Brewer, D. Hannon, et al. 1990. Hemolytic-uremic syndrome during an outbreak of Escherichia coli O157:H7 infections in institutions for mentally retarded persons: clinical and epidemiologic observations. J. Pediatr. 116:544-551. [DOI] [PubMed] [Google Scholar]
- 42.Pohlenz, J. F., K. R. Winter, and E. A. Dean-Nystrom. 2005. Shiga-toxigenic Escherichia coli-inoculated neonatal piglets develop kidney lesions that are comparable to those in humans with hemolytic-uremic syndrome. Infect. Immun. 73:612-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson, S. E., M. A. Karmali, L. E. Becker, and C. R. Smith. 1988. The histopathology of the hemolytic uremic syndrome associated with verocytotoxin-producing Escherichia coli infections. Hum. Pathol. 19:1102-1108. [DOI] [PubMed] [Google Scholar]
- 44.Richardson, S. E., T. A. Rotman, V. Jay, C. R. Smith, L. E. Becker, M. Petric, N. F. Olivieri, and M. A. Karmali. 1992. Experimental verocytotoxemia in rabbits. Infect. Immun. 60:4154-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 46.Ritchie, J. M., P. L. Wagner, D. W. Acheson, and M. K. Waldor. 2003. Comparison of Shiga toxin production by hemolytic-uremic syndrome-associated and bovine-associated Shiga toxin-producing Escherichia coli isolates. Appl. Environ. Microbiol. 69:1059-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robson, W. L., A. K. Leung, and M. D. Montgomery. 1991. Causes of death in hemolytic uremic syndrome. Child Nephrol. Urol. 11:228-233. [PubMed] [Google Scholar]
- 48.Rodrigue, D. C., E. E. Mast, K. D. Greene, J. P. Davis, M. A. Hutchinson, J. G. Wells, T. J. Barrett, and P. M. Griffin. 1995. A university outbreak of Escherichia coli O157:H7 infections associated with roast beef and an unusually benign clinical course. J. Infect. Dis. 172:1122-1125. [DOI] [PubMed] [Google Scholar]
- 49.Roe, A. J., D. E. Hoey, and D. L. Gally. 2003. Regulation, secretion and activity of type III-secreted proteins of enterohaemorrhagic Escherichia coli O157. Biochem. Soc. Trans. 31:98-103. [DOI] [PubMed] [Google Scholar]
- 50.Roe, A. J., S. W. Naylor, K. J. Spears, H. M. Yull, T. A. Dransfield, M. Oxford, I. J. McKendrick, M. Porter, M. J. Woodward, D. G. Smith, and D. L. Gally. 2004. Co-ordinate single-cell expression of LEE4- and LEE5-encoded proteins of Escherichia coli O157:H7. Mol. Microbiol. 54:337-352. [DOI] [PubMed] [Google Scholar]
- 51.Ryan, C. A., R. V. Tauxe, G. W. Hosek, J. G. Wells, P. A. Stoesz, H. W. McFadden, Jr., P. W. Smith, G. F. Wright, and P. A. Blake. 1986. Escherichia coli O157:H7 diarrhea in a nursing home: clinical, epidemiological, and pathological findings. J. Infect. Dis. 154:631-638. [DOI] [PubMed] [Google Scholar]
- 52.Scheutz, F., B. Olesen, and A. Norgaard. 2000. Two cases of human urinary tract infection complicated by hemolytic uremic syndrome caused by verotoxin-producing Escherichia coli. Clin. Infect. Dis. 31:815-816. [DOI] [PubMed] [Google Scholar]
- 53.Siegler, R. L., T. G. Obrig, T. J. Pysher, V. L. Tesh, N. D. Denkers, and F. B. Taylor. 2003. Response to Shiga toxin 1 and 2 in a baboon model of hemolytic uremic syndrome. Pediatr. Nephrol. 18:92-96. [DOI] [PubMed] [Google Scholar]
- 54.Siegler, R. L., A. T. Pavia, R. D. Christofferson, and M. K. Milligan. 1994. A 20-year population-based study of postdiarrheal hemolytic uremic syndrome in Utah. Pediatrics 94:35-40. [PubMed] [Google Scholar]
- 55.Smith, D., M. Blackford, S. Younts, R. Moxley, J. Gray, L. Hungerford, T. Milton, and T. Klopfenstein. 2001. Ecological relationships between the prevalence of cattle shedding Escherichia coli O157:H7 and characteristics of the cattle or conditions of the feedlot pen. J. Food Prot. 64:1899-1903. [DOI] [PubMed] [Google Scholar]
- 56.Starr, M., V. Bennett-Wood, A. K. Bigham, T. F. de Koning-Ward, A. M. Bordun, D. Lightfoot, K. A. Bettelheim, C. L. Jones, and R. M. Robins-Browne. 1998. Hemolytic-uremic syndrome following urinary tract infection with enterohemorrhagic Escherichia coli: case report and review. Clin. Infect. Dis. 27:310-315. [DOI] [PubMed] [Google Scholar]
- 57.Strachan, N. J., D. R. Fenlon, and I. D. Ogden. 2001. Modelling the vector pathway and infection of humans in an environmental outbreak of Escherichia coli O157. FEMS Microbiol. Lett. 203:69-73. [DOI] [PubMed] [Google Scholar]
- 58.Swerdlow, D. L., B. A. Woodruff, R. C. Brady, P. M. Griffin, S. Tippen, H. D. Donnell, Jr., E. Geldreich, B. J. Payne, A. Meyer, Jr., J. G. Wells, et al. 1992. A waterborne outbreak in Missouri of Escherichia coli O157:H7 associated with bloody diarrhea and death. Ann. Intern. Med. 117:812-819. [DOI] [PubMed] [Google Scholar]
- 59.Tarr, P. I., L. S. Fouser, A. E. Stapleton, R. A. Wilson, H. H. Kim, J. C. Vary, Jr., and C. R. Clausen. 1996. Hemolytic-uremic syndrome in a six-year-old girl after a urinary tract infection with Shiga-toxin-producing Escherichia coli O103:H2. N. Engl. J. Med. 335:635-638. [DOI] [PubMed] [Google Scholar]
- 60.Tarr, P. I., C. A. Gordon, and W. L. Chandler. 2005. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365:1073-1086. [DOI] [PubMed] [Google Scholar]
- 61.Te Loo, D. M., V. W. van Hinsbergh, L. P. van den Heuvel, and L. A. Monnens. 2001. Detection of verocytotoxin bound to circulating polymorphonuclear leukocytes of patients with hemolytic uremic syndrome. J. Am. Soc. Nephrol. 12:800-806. [DOI] [PubMed] [Google Scholar]
- 62.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilden, J., Jr., W. Young, A. M. McNamara, C. Custer, B. Boesel, M. A. Lambert-Fair, J. Majkowski, D. Vugia, S. B. Werner, J. Hollingsworth, and J. G. Morris, Jr. 1996. A new route of transmission for Escherichia coli: infection from dry fermented salami. Am. J. Public Health 86:1142-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuttle, J., T. Gomez, M. P. Doyle, J. G. Wells, T. Zhao, R. V. Tauxe, and P. M. Griffin. 1999. Lessons from a large outbreak of Escherichia coli O157:H7 infections: insights into the infectious dose and method of widespread contamination of hamburger patties. Epidemiol. Infect. 122:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzipori, S., C. W. Chow, and H. R. Powell. 1988. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J. Clin. Pathol. 41:1099-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzipori, S., K. I. Wachsmuth, J. Smithers, and C. Jackson. 1988. Studies in gnotobiotic piglets on non-O157:H7 Escherichia coli serotypes isolated from patients with hemorrhagic colitis. Gastroenterology 94:590-597. [DOI] [PubMed] [Google Scholar]
- 67.Wadolkowski, E. A., L. M. Sung, J. A. Burris, J. E. Samuel, and A. D. O'Brien. 1990. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58:3959-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werber, D., A. Fruth, U. Buchholz, R. Prager, M. H. Kramer, A. Ammon, and H. Tschape. 2003. Strong association between Shiga toxin-producing Escherichia coli O157 and virulence genes stx2 and eae as possible explanation for predominance of serogroup O157 in patients with haemolytic uraemic syndrome. Eur. J. Clin. Microbiol. Infect. Dis. 22:726-730. [DOI] [PubMed] [Google Scholar]