Abstract
During cold storage after milk collection, psychrotrophic bacterial populations dominate the microflora, and their extracellular enzymes, mainly proteases and lipases, contribute to the spoilage of dairy products. The diversity, dynamics, and enzymatic traits of culturable psychrotrophs in raw milk from four farms were investigated over a 10-month period. About 20% of the isolates were found to be novel species, indicating that there is still much to be learned about culturable psychrotrophs in raw milk. The psychrotrophic isolates were identified and classified in seven classes. Three classes were predominant, with high species richness (18 to 21 species per class) in different seasons of the year: Gammaproteobacteria in spring and winter, Bacilli in summer, and Actinobacteria in autumn. The four minor classes were Alphaproteobacteria, Betaproteobacteria, Flavobacteria, and Sphingobacteria. The dominant classes were found in all four dairies, although every dairy had its own unique “bacterial profile.” Most but not all bacterial isolates had either lipolytic or both lipolytic and proteolytic activities. Only a few isolates showed proteolytic activity alone. The dominant genera, Pseudomonas and Acinetobacter (Gammaproteobacteria), showed mainly lipolytic activity, Microbacterium (Actinobacteria) was highly lipolytic and proteolytic, and the lactic acid bacteria (Lactococcus and Leuconostoc) displayed very minor enzymatic ability. Hence, the composition of psychrotrophic bacterial flora in raw milk has an important role in the determination of milk quality. Monitoring the dominant psychrotrophic species responsible for the production of heat-stable proteolytic and lipolytic enzymes offers a sensitive and efficient tool for maintaining better milk quality in the milk industry.
Milking procedure may be contaminated from the teat surface, the udder, milking equipment, and the milking parlor environment. Psychrotrophic bacteria are defined as those that grow at 7°C, although their optimal growth temperature is higher. During cold storage after milk collection they dominate the flora, and their extracellular enzymes, mainly proteases and lipases, contribute to the spoilage of dairy products. The extracellular enzymes can resist pasteurization (72°C for 15 s) and even ultrahigh temperature processing (UHT; 138°C for 2 s or 149°C for 10 s) (3, 19, 21, 35). The lipases, by hydrolyzing triglycerides, cause flavor defects associated with fat breakdown in cream, butter, cheese, and UHT products (4). Proteases are associated with bitterness in milk, gelation of UHT sterilized milk, and reduced yields of soft cheese. Most proteases can degrade caseins and are remarkably heat stable (3, 23). In sum, psychrotrophs play a leading role in spoilage of refrigerated milk and milk products.
The numbers of psychrotrophs that develop after milk collection depend on the storage temperature and time. Under sanitary conditions, <10% of the total microflora is psychrotrophs in contrast to >75% under unsanitary conditions (3). Psychrotrophic bacteria from numerous genera have been isolated from milk, both gram negative (Pseudomonas, Aeromonas, Serratia, Acinetobacter, Alcaligenes, Achromobacter, Enterobacter, and Flavobacterium) and gram positive (Bacillus, Clostridium, Corynebacterium, Microbacterium, Micrococcus Streptococcus, Staphylococcus, and Lactobacillus) (3, 31, 26). Of these, Pseudomonas is the most frequently reported psychrotroph in raw milk (4, 7, 9, 29, 32, 35).
In food fermentation, microbial communities are generally believed to harbor a large fraction of culturable species (24). Although conventional culture methods are still commonly used to ensure the microbiological quality of milk, very few studies have been done to identify culturable microbial communities in milk by means of molecular identification tools. Delbes et al. (6), using the 16S rRNA gene, showed that culturable bacterial communities in raw milk were highly diversified. However, these researchers analyzed only one milk sample in the winter period.
In Israel, it is estimated by the milk industries that psychrotrophs can cause about 10% lose in milk fats and proteins. The aim of the present study was to monitor the seasonal dynamics of culturable psychrotrophic communities in raw milk from four farms in Israel, to study psychrotrophic bacterial diversity with molecular tools, and to evaluate the bacterial lipolytic-proteolytic traits that may influence milk and milk products’ shelf life.
MATERIALS AND METHODS
Sampling sites.
Four farms (D, F, G, and H) in northern Israel were selected for raw milk sampling. One sample per farm was taken each month, between April 2004 and January 2005 (except for June, October, and December 2004). All of the sampled farms are equipped with modern automated milking facilities and are considered farms with very good milk quality. There is no grazing area in any of the sampled farms, and the herd's nutrition is based on a feeding mixture that is prepared in a central regional feeding center. Samples were taken under sterile conditions from a mix of several milking. The milk was stored under 5°C from the milking procedure until it was analyzed in the laboratory.
Enumeration of microorganisms.
The samples were diluted and plated on sterile standard plate count (SPC) agar, a standard medium corresponding to the American Public Health Association formulation for milk, water, food, and dairy products (Oxoid CM0463). The plates were incubated at 7°C for 10 days for psychrotrophic enumerations and at 32°C for 48 h for mesophilic enumerations. The SPC counts were performed according to Standard Methods for the Examination of Dairy Products (22).
Isolation and identification of psychrotrophic bacteria.
The diversity of the culturable psychrotrophic communities from raw milk was determined in samples that were cultured in April, May, August, and November 2004 and January 2005. Colonies with different morphologies that grew on SPC plates after 10 days at 7°C were picked and subcultured to obtain pure cultures. An average of 10 colonies per farm per month was collected. Isolated colonies were subcultured at least four times before examination of cell shape and Gram staining. Bacterial isolates were kept in SPC medium with 30% glycerol (−80°C).
Identification of isolates using 16S rRNA gene.
Universal bacterial primers, namely, 8f and 1512r, based on Escherichia coli positions, were used to amplify internal fragments of 16S rRNA gene according to the method of Felske et al. (8). A total of 8 μl of a bacterial suspension was transferred to a sterile thin-walled PCR tube. Then, 1 μl of each primer (20 pmol/μl) and 10 μl of PCR master mixture (ReddyMix; ABgene, United Kingdom) were added to the tube to make up a final reaction volume of 20 μl. Initial DNA denaturation was performed at 94°C for 4 min, followed by 33 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 50 s, and elongation at 72°C for 2 min, with a final elongation step at 72°C for 10 min. To confirm amplicon production (approximately 1,500 bp), the mixture was analyzed by electrophoresis on 1.5% agarose gel, followed by staining with ethidium bromide and visualization under UV light. The amplified PCR products were purified with a Wizard PCR product purification kit (Promega, Madison, WI). Automated sequencing with a 3100 Genetic Analyzer from Applied Biosystems was done at the sequencing center of the Technion Medical School, Haifa, Israel. Sequencing was performed for all isolates by using 8f primer (yielding a length of ca. 800 to 900 bp of the sequenced fragment), and for some of them by using 1512r primer as well (which yielded a 1,400-bp fragment). For identification of closest relatives, newly determined sequences were compared to those available in the GenBank (www.ncbi.nlm.nih.gov) databases by means of the standard nucleotide-nucleotide BLAST program (BLASTN; www.ncbi.nlm.nih.gov). A phylogenetic tree was generated by the neighbor-joining method with NJPlot (MEGA 3) based on alignments from CLUSTAL W. The bootstrap values obtained were from 1,000 iterations.
Enzyme production assays.
All isolates were tested for the production of proteolytic or lipolytic activity by agar diffusion assays at 7°C for 10 days. Proteolytic enzyme production was tested using skim milk agar (1% skim milk powder, 0.5% yeast extract, 1.5% agar). The presence of clear zones around the colonies was indicative of proteolysis. Lipolytic activity was evaluated in tributyrin agar (Himedia). Colonies surrounded by dark blue zones were deemed to evince lipolytic activity.
Statistic analysis.
The association between the mesophilic and psychrotrophic bacterial populations in raw milk samples was tested by Pearson correlation. Differences between the two kinds of populations and between species isolated on different dates were tested by one-sample t test. Repeated analysis of variance was used to determine the significance of the differences between the bacterial populations on different sampling dates. Differences between species richness and enzymatic activities of isolates belonging to different taxa were calculated by means of χ2. The entire statistical analysis was conducted with SPSS for Windows.
Nucleotide sequence accession numbers.
A total of 264 bacterial isolates were identified in the present study, and their sequences were deposited in GenBank under the accession numbers EF204205 to EF204469.
RESULTS
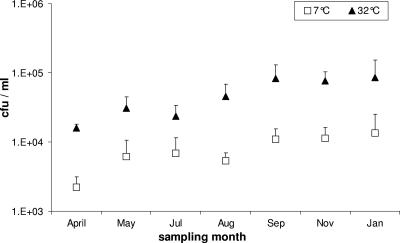
The average of culturable mesophilic bacteria on SPC agar was 4.8 × 104 ± 2.3 × 104 CFU per ml raw milk. The average percentage of the psychrotrophic bacterial population out of the total mesophilic population was 14.7% ± 6.4% (Fig. 1). Samples from the four farms showed no significant differences in psychrophilic bacterial population levels (analysis of variance repeated measures; F3,12 = 2.885, P = 0.08). Significant differences were found in levels of the psychrotrophic and mesophilic populations on the different dates of sampling (one-sample t test: t7 = 4.142, P = 0.004). Significant correlation was found between the psychrotrophic and mesophilic population dynamics during the sampling period (Pearson correlation test; r = 0.860, P = 0.013, n = 7), indicating similar dynamics in the two populations (Fig. 1). The psychrotrophic and mesophilic communities increased or decreased by the same pattern in the different seasons (Fig. 1).
FIG. 1.
Psychrotrophic (□) and mesophilic (▴) bacterial population dynamics in raw milk sampled between April 2004 and January 2005. Each sampling date represents an average of samples from four farms (mean + the standard error).
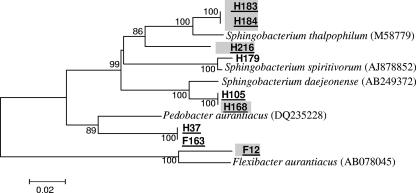
The diversity of the culturable psychrotrophic communities from raw milk sampled from the four farms (D, F, G, and H) in April, May, August, and November 2004 and January 2005 was investigated. All isolates were able to grow at both 7 and 32°C. The 264 psychrotrophic bacterial isolates identified in the present study (Table 1) and their enzymatic traits are listed in full in Tables S1 to S7 in the supplemental material. A total of 20% of the identified isolates were new unidentified species with similarities of <97.5% to the closest known relative in the GenBank (Table 1 and see Tables S2 to S7 in the supplemental material). Of these, six were most probably new genera with similarities of less than 93.8% to the closest known relative in the GenBank (Table 1 and Tables S4 and S7 in the supplemental material). The isolates were found to belong to seven classes: Alphaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Bacilli, Actinobacteria, Flavobacteria, and Sphingobacteria. The dominant classes were Gammaproteobacteria, Bacilli, and Actinobacteria, and the dominant genera were Pseudomonas, Acinetobacter (Gammaproteobacteria) Leuconostoc, Lactococcus (Bacilli), and Microbacterium (Actinobacteria). Isolates from Sphingobacteria class were found infrequently, and only from two farms (H and F: Table 1 and Table S7 in the supplemental material). Of nine isolates identified in this class, five were new species, and three were new genera (Fig. 2).
TABLE 1.
Bacterial isolates from raw milk sampled from four farms (D, F, G, and H) from April 2004 to January 2005a
| Isolate(s) | Classb | Accession no. | Closest relative in GenBank database |
|---|---|---|---|
| F8, H162, H167 | α | EF204205 to -7 | Brevundimonas bullata |
| H172 | α | EF204208 | Brevundimonas diminuta |
| G124 | α | EF204209 | Brevundimonas nasdae |
| G125 | α | EF204210 | Brevundimonas vesicularis |
| H196 | β | EF204211 | Janthinobacterium lividum |
| H208, H209, H212, H213, H214 | β | EF204212 to -16 | Delftia tsuruhatensis |
| H103, H177, H211 | γ | EF204217 to -19 | Stenotrophomonas maltophilia |
| D13, D14, D15, D16, F9, G9, G10, G11, G25, G94, G96, G101 | γ | EF204220 to -31 | Pseudomonas brennerii |
| D18 | γ | EF204232 | Pseudomonas fluorescens |
| D21, D26, D27, F1, F14, F15, F16, F17, F18, F19, F20, F21, F29, F121, D19 | γ | EF204233 to -47 | Pseudomonas putida |
| F112, F117, F127 | γ | EF204248 to -50 | Pseudomonas synxantha |
| F165 | γ | EF204251 | Pseudomonas veronii |
| F118, H202 | γ | EF204252 and -3 | Pseudomonas sp. |
| H36 | γ | EF204255 | Moraxella osloensis |
| D10, F5, G30, G13 | γ | EF204256 to -9 | Acinetobacter baumannii |
| D12, F166 | γ | EF204260 and -1 | Acinetobacter calcoaceticus |
| D25 | γ | EF204262 | Acinetobacter haemolyticus |
| F22, F24, F25, F26, F27, F146, F147, H13, H104 | γ | EF204263 to -71 | Acinetobacter johnsonii |
| D5, D6, D119, F32, G8, G14, G15, G16, G26, G29, G34, G38, H12 | γ | EF204272 to -84 | Acinetobacter lwoffii |
| H22 | γ | EF204285 | Psychrobacter arenosus |
| G28, G40, G132, H97 | γ | EF204286 to -9 | Psychrobacter faecalis |
| F162 | γ | EF204254 | Psychrobacter phenylpyruvica |
| H210 | γ | EF204290 | Aeromonas media |
| H201 | γ | EF204291 | Enterobacter amnigenus |
| F4, H200 | γ | EF204292 and -3 | Klebsiella oxytoca |
| G115, G116 | γ | EF204294 and -5 | Serratia fonticola |
| F10, F11 | γ | EF204296 and -7 | Serratia marcescens |
| F125 | Bacilli | EF204298 | Exiguobacterium undae |
| D105 | Bacilli | EF204299 | Kurthia gibsonii |
| H119 | Bacilli | EF204300 | Facklamia ignava |
| H96 | Bacilli | EF204301 | Staphylococcus equorum |
| G36 | Bacilli | EF204302 | Staphylococcus hominis |
| G138a | Bacilli | EF204303 | Staphylococcus saprophyticus |
| G127, H92, H93 | Bacilli | EF204304 to -6 | Staphylococcus sciuri |
| H16 | Bacilli | EF204307 | Macrococcus caseolyticus |
| H20 | Bacilli | EF204308 | Aerococcus viridans |
| H181a | Bacilli | EF204309 | Trichococcus pasteurii |
| G117 G97 | Bacilli | EF204310 and -1 | Carnobacterium maltaromaticum |
| H126a, H127 | Bacilli | EF204312 and -3 | Carnobacterium viridans |
| F122, G129, G130, G141 | Bacilli | EF204314 to -17 | Enterococcus faecium |
| F154, F155, F157, G139, H166, H180, H206 | Bacilli | EF204318 to -24 | Enterococcus aquimarinus |
| G6 | Bacilli | EF204325 | Leuconostoc citreum |
| D28, F170, G1, G2, G3, G4, G17, G18, G19, G20, G31, G41, G98, G119, G120 | Bacilli | EF204326 to -35, EF204336 to -40 | Leuconostoc mesenteroides |
| G21 G23 | Bacilli | EF204367, EF204369 | Leuconostoc mesenteroides subsp. mesenteroides |
| D102, D104, H14, H17, H18, H19, H25, H28, H31, H32 | Bacilli | EF204341 to -50 | Streptococcus parauberis |
| F7, G35, D22, D23, D24, D29, D30, F124, F65, H207 | Bacilli | EF204351 to -60 | Lactococcus lactis |
| D8, D90, D124, F150, G22, G99, G100, G105, G118, G121 | Bacilli | EF204361 to -64, EF204368, EF204370 to -4 | Lactococcus raffinolactis |
| F116, F123 | Bacilli | EF204365 and -6 | Lactococcus raffinolactis |
| H26 | Actinobacteria | EF204375 | Arthrobacter agilis |
| D4 | Actinobacteria | EF204376 | Arthrobacter tumbae |
| F30 | Actinobacteria | EF204377 | Arthrobacter oxydans |
| F164 | Actinobacteria | EF204378 | Arthrobacter methylotrophus |
| D85, D122 | Actinobacteria | EF204379 and -80 | Cellulosimicrobium funkei |
| F144, H23 | Actinobacteria | EF204381 and -2 | Kocuria rhizophila |
| H29, H7, H21 | Actinobacteria | EF204383 to -5 | Rothia mucilaginosa |
| H126b | Actinobacteria | EF204386 | Corynebacterium casei |
| D110 | Actinobacteria | EF204387 | Brachybacterium sacelli |
| G134a | Actinobacteria | EF204388 | Brachybacterium conglomeratum |
| G122, G133, H187 | Actinobacteria | EF204389 to -91 | Pseudoclavibacter helvolus |
| D84, D103, D118, D126, H193 | Actinobacteria | EF204392 to -96 | Microbacterium lacticum |
| D2, D3, D91, D92, D93, D96, D11, 15D127, F142, F143, F145, F151, F152, F159, F173, F175, F177, F171, G123, G135, H95, H100, H101, H102, H111, H163, H204, F109, H4, H5, H6, H173, H185, H190 | Actinobacteria | EF204397 to -430 | Microbacterium oxydans or Microbacterium maritypicum |
| D114, H110b | Actinobacteria | EF204431 and -2 | Microbacterium paraoxydans |
| H34 | Actinobacteria | EF204433 | Microbacterium trichotecenolyticum |
| H39 | Actinobacteria | EF204434 | Rhodococcus corynebacterioides |
| H11, F2, G128, G138b, H99, H170, H181b, H182 | Actinobacteria | EF204435 to -42 | Rhodococcus erythropolis |
| D117 | Actinobacteria | EF204443 | Rhodococcus fascians |
| H198 | Flavobacteria | EF204444 | Flavobacterium johnsoniae |
| H205 | Flavobacteria | EF204445 | Flavobacterium frigidimaris |
| H9, H10, H15, H38 | Flavobacteria | EF204446 to -8 | Chryseobacterium hispanicum |
| H27, H197, H30, H8 | Flavobacteria | EF204454 and -5, EF204449, EF204451 | Chryseobacterium joostei |
| F108 | Flavobacteria | EF204452 | Chryseobacterium formosense |
| F13 | Flavobacteria | EF204453 | Chryseobacterium soldanellicola |
| H2 | Flavobacteria | EF204456 | Chryseobacterium piscium |
| G24, G32, G39 | Flavobacteria | EF204457 to -9 | Chryseobacterium scophthalmum |
| H1 | Flavobacteria | EF204460 | Epilithonimonas tenax |
| H105, H168 | Sphingobacteria | EF204461 and -62 | Sphingobacterium daejeonense |
| H183, H184 | Sphingobacteria | EF204463 and -4 | Sphingobacterium thalpophilum |
| H216 | Sphingobacteria | EF204465 | Sphingobacterium spiritivorum |
| H179 | Sphingobacteria | EF204466 | Sphingobacterium spiritivorum |
| F12 | Sphingobacteria | EF204467 | Flexibacter aurantiacus subsp. Excathedrus |
| H37, F163 | Sphingobacteria | EF204468 and -9 | Pedobacter aurantiacus |
The letters D, F, G, and H in the isolate names refer to the different farms from which the bacteria were isolated. Isolate names in boldface represent a probable new unidentified species (with<97.5% 16S rRNA gene similarities to any known species). Isolate names that are underlines and in boldface represent a probable new unidentified genus (with <93.8% 16S rRNA gene similarities to any known species). For more details, see Tables S1 to S7 in the supplemental material.
α, Alphaproteobacteria; β, Betaproteobacteria; γ, Gammaproteobacteria.
FIG. 2.
Phylogenetic tree of isolates belonging to the Sphingobacteria class. The tree shows the relationship based on partial sequences of the 16S rRNA gene of selected isolates. No special marks on the isolate name indicates no enzymatic activity, gray background indicates lipolytic activity; isolates with names underlined represent new species. The letters F and H in isolate names are the different farms from which the bacteria were obtained. The sequence alignment was performed by means of the CLUSTAL W program, and the tree was generated by the neighbor-joining method with Kimura two-parameter distances in MEGA 3 software. Bootstrap values (from 1,000 replicates) greater than 50% are shown at the branch points. The bar indicates 2% sequence divergence.
Species of the three dominant classes were found at all of the farms. However, the dominant classes at each farm differed significantly (χ218 = 32, P = 0.021). Gammaproteobacteria (12 species) was the predominant class at farm F, Gammaproteobacteria (7 species) and Actinobacteria (7 species) were predominant at farm D, Actinobacteria (11 species) was predominant at farm H, and Bacilli (10 species) was predominant at farm G. The species are listed in Table 1.
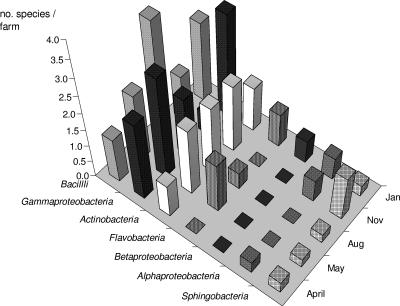
Significant differences were found (one-sample t test) between species richness per farm on the different sampling dates (t4 = 9.614, P = 0.001). The highest number of species per farm was found in January 2005 (9.3 ± 0.1); the lowest was found in April 2004 (5.3 ± 0.8). The mean of species richness per farm on the different sampling dates was calculated for every bacterial class and is presented in Fig. 3. Three bacterial classes showed dominance in different seasons of the year: Gammaproteobacteria in spring and winter (April-May and January), Bacilli in the summer (August), and Actinobacteria in autumn (November). The other four bacterial classes were present in lower species numbers than the classes listed above. Species from Sphingobacteria class were found in low numbers in all seasons. Species belonging to Alphaproteobacteria were detected only in the colder months of the year and not in summer. Representatives of Betaproteobacteria were identified only in winter (January). Flavobacteria species were found in the spring, summer, and winter, but not in autumn (Fig. 3).
FIG. 3.
The mean of species per sampling farm on different sampling dates was calculated for every bacterial class. Three bacterial classes showed dominance in different seasons of the year: Gammaproteobacteria in spring and winter (April-May and January), Bacilli in summer (August), and Actinobacteria in autumn (November).
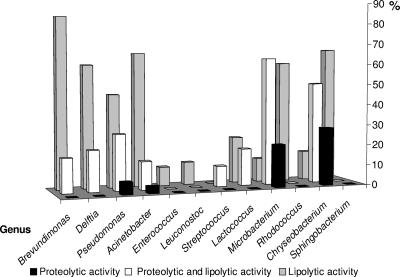
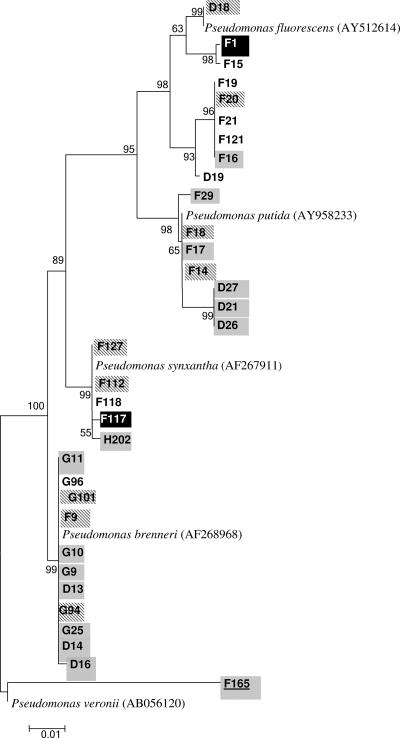
All of the isolates were screened for their ability to produce proteolytic and lipolytic enzymes. An isolate's enzymatic activity and its relatedness to a specific class proved to be linked (χ218 = 117, P < 0.001). For example, the majority of the isolates of the Bacilli class (Enterococcus, Leuconostoc Streptococcus, and Lactococcus) had no proteolytic or lipolytic activity, whereas most species from the phylum Proteobacteria had lipolytic activity (see also Tables S1 to S7 in the supplemental material). Species from the classes Alphaproteobacteria (Brevundimonas), Betaproteobacteria (Delftia), Gammaproteobacteria (Pseudomonas and Acinetobacter), Flavobacteria (Chryseobacterium), and Sphingobacteria (Sphingobacterium) showed mainly lipolytic activity, although some of the strains were both proteolytic and lipolytic (Fig. 4). Proteolytic activity as the only trait was rare and was found mainly in species that belonged to the classes Flavobacteria (Chryseobacterium) and Actinobacteria (Microbacterium) (Fig. 4). Not all of the strains of a certain species showed the same enzymatic activities. For example, 12 strains were identified as Pseudomonas brennerii (Fig. 5 and Table S3 in the supplemental material); however, four of them showed proteolytic and lipolytic activities, seven had only lipolytic activity, and one showed none of the above activities. Heterogeneity in enzymatic abilities of strains belonging to the same species was observed in other classes and species also (see Tables S1 to S7 in the supplemental material).
FIG. 4.
Percentage of isolates showing lipolytic, proteolytic, or both enzymatic activities in different genera. The number of isolates in each genera (n) is also indicated: Brevundimonas (n = 6), Delftia (n = 5), Pseudomonas (n = 33), Acinetobacter (n = 29), Enterococcus (n = 11), Leuconostoc (n = 18), Streptococcus (n = 10), Lactococcus (n = 22), Microbacterium (n = 43), Rhodococcus (n = 10), Chryseobacterium (n = 14), and Sphingobacterium (n = 6). For more details, see Fig. 2 and 5 and Tables S1 to S7 in the supplemental material.
FIG. 5.
Phylogenetic tree of isolates belonging to the genus Pseudomonas. The tree shows the relationship based on partial sequences of the 16S rRNA gene of selected isolates. No special marks on the isolate name indicates no enzymatic activity; gray background indicates lipolytic activity; black background with white letters indicates proteolytic activity; diagonal gray lines in the background of the isolate's name indicates both lipolytic and proteolytic activity; isolates with names underlined represent new species. The letters D, F, and G in isolate names are the different farms from which the bacteria were obtained. The sequence alignment was performed by means of the CLUSTAL W program, and the tree was generated by the neighbor-joining method with Kimura two-parameter distances in MEGA 3 software. Bootstrap values (from 1,000 replicates) greater than 50% are shown at the branch points. The bar indicates 1% sequence divergence.
DISCUSSION
Psychrotrophs and their extracellular enzymes play a major role in the spoilage of refrigerated milk and milk products, so it is of great importance to learn about the psychrotrophic bacteria that inhabit raw milk. Although a culturable bacterial community in raw milk has been described, most studies used classic methods for identification (11, 12, 15, 26, 28, 31). The present study used molecular tools to identify psychrotrophs in raw milk, from four farms during a period of 10 months. Bacterial mesophilic culturable counts of the milk samples were <105 CFU/ml, attesting that the milk was of good quality (2). The psychrotrophic isolates were identified and classified into seven classes (Table 1 and see Tables S1 to S7 in the supplemental material). Three classes were predominant and showed high species richness: Gammaproteobacteria, Bacilli, and Actinobacteria (with 21, 21, and 18 species, respectively). The four minor classes were Alphaproteobacteria, Betaproteobacteria, Flavobacteria, and Sphingobacteria (with 4, 2, 10, and 6 species, respectively). The dominant classes were found at all four dairies but every dairy had its own unique “bacterial profile.” All of the farms were equipped with modern facilities and used a similar feeding mixture; thus, the unique bacterial profile can be explained as a result of the different geographical location of each farm. Dairy farm G had the unique bacterial profile of Bacilli as the dominant class and relatively low levels of isolates belonging to the other classes. Different species of the Bacilli class are known to secrete antimicrobial substances (5, 17, 18, 27); hence, this may suggest that the existence of species belonging to the Bacilli class inhibited the growth of bacteria from other classes. When diversity of bacterial populations in raw milk was investigated by a direct molecular approach, the dominant clones were the phylum Firmicutes (Bacilli), followed by Proteobacteria, Actinobacteria, and Bacteroidetes (Flavobacteria) (6).
Three bacterial classes showed dominance in different seasons of the year: Gammaproteobacteria in spring and winter, Bacilli in the summer, and Actinobacteria in autumn. When heterotrophic populations in lake sediments were studied, it was shown that the bacteria maintained temperature optima above the in situ temperature, although the optimum decreased with decreasing seasonal temperature (33). The fluctuation of the psychrotrophic bacterial population during the different seasons can be explained by their species optimal temperature growth. Bacilli species (e.g., Staphylococcus, Lactococcus, Leuconostoc, and Streptococcus) with relatively high optimal temperature growth and Gamaproteobacteria species (e.g., Pseudomonas, Acinetobacter, and Psychrobacter) with relatively lower optimal temperature growth were predominant in the summer and in winter and spring, respectively.
Most but not all bacterial isolates in the present study had enzymatic activities of either lipolytic activity or both lipolytic and proteolytic activities; few showed proteolytic activity alone. Different bacterial classes exhibited different enzymatic activities (Fig. 4 and Tables S1 to S7 in the supplemental material). Five genera were predominant: Pseudomonas, Acinetobacter (gram negative), Lactococcus, Leuconostoc, and Microbacterium (gram positive). Pseudomonas and Acinetobacter were highly lipolytic, Microbacterium was highly lipolytic and proteolytic, and the lactic acid bacteria (Lactococcus and Leuconostoc) evinced very minor enzymatic ability (Fig. 4 and see Tables S1 to S7 in the supplemental material). Hence, the composition of psychrotrophic bacterial flora in raw milk has an important role in the determination of milk quality. The genus Microbacterium, which showed both lipolytic and proteolytic activity, was ubiquitous in raw milk samples in the present study. Species of Microbacterium are known to be thermoduric, namely, they can survive pasteurization processes (34). Thus, Microbacterium monitoring in raw milk can be used as an indicator of milk quality. As far as we know, Microbacterium has never been described as an important factor in determining raw milk quality. The use of a combined temporal temperature gel electrophoresis and denaturing gradient gel electrophoresis approach demonstrated that psychrotrophic populations increased within 24 h of refrigeration and that bacterial dynamics showed considerable variation between samples (20). However, the enzymatic traits of the bacterial populations were not studied. This demonstrates the importance of using a culture-dependent strategy that facilitates study of the enzymatic activities of the isolates (Fig. 4 and Tables S1 to S7 in the supplemental material). The present study also showed that studying the population species profile is not enough since species with the same identity had different enzymatic properties (Fig. 5 and Tables S1 to S7 in the supplemental material). Diversity at the strain level was also observed when the culturable microbial composition of smear-ripened cheeses was investigated (25).
About 20% of the isolates in the present study are most probably novel unidentified species (10) (Table 1 and Fig. 2). This ratio of unknown isolates is comparatively high because only few studies have used molecular tools to identify culturable bacteria in raw milk (1, 7), and none of them investigated psychrotrophic bacteria.
Chryseobacterium and Flavobacterium occur frequently in dairy products (7, 13, 16). New species of Chryseobacterium were recently isolated and characterized from raw milk (C. joostei [14]) and from a lactic acid beverage (C. shigense [30]). In the present study 14 isolates of the genus Chryseobacterium with a variety of combinations of enzymatic activities were identified (Table 1 and see Table S6 in the supplemental material). Of these, nine were unidentified species. One lipolytic and proteolytic new species from the present study has already been characterized as Chryseobacterium haifense sp. nov. (10).
We showed here that there is still a lot to learn about the composition of psychrotrophic bacterial flora from raw milk. Many novel species and genera have yet to be defined. As the new technologies reduce the initial bacterial counts of pasteurized milk to very low levels, the activity of heat-stable proteolytic and lipolytic enzymes originating from psychrotrophic bacteria will be the limiting factor in maintaining the flavor quality of fluid milk and its products. We must become acquainted with the psychrotrophic bacteria that are highly represented in the raw milk and therefore are key players in determining milk quality and develop sensitive and efficient tools to monitor their presence.
Supplementary Material
Acknowledgments
We thank Ido Izhaki for help with the statistical analysis.
This study was supported by a grant from Tnuva Research Institute (Israel).
Footnotes
Published ahead of print on 21 September 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Aquilanti, L., L. Dell Aquila, E. Zannini, A. Zocchetti, and F. Clementi. 2006. Resident lactic acid bacteria in raw milk Canestrato Pugliese cheese. Lett. Appl. Microbiol. 43:161-167. [DOI] [PubMed] [Google Scholar]
- 2.Barbano, D. M., Y. Ma, and M. V. Santos. 2006. Influence of raw milk quality on fluid milk shelf life. J. Dairy Sci. 89:E15-E19. [DOI] [PubMed] [Google Scholar]
- 3.Cousin, M. A. 1982. Presence and activity of psychrotrophic microorganisms in milk and dairy products: a review. J. Food Prot. 45:172-207. [DOI] [PubMed] [Google Scholar]
- 4.Craven, H. M., and B. J. Macauley. 1992. Microorganisms in pasteurized milk after refrigerated storage. 1. Identification of types. Aust. J. Dairy Technol. 47:38-45. [Google Scholar]
- 5.Daba, H., S. Pandian, F. Gosselin, R. E. Simard, J. Huang, and C. Lacroix. 1991. Detection and activity of a bacteriocin produced by Leuconostoc mesenteroides. Appl. Environ. Microbiol. 57:3450-3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delbes, C., L. Ali-Mandjee, and M. C. Montel. 2007. Monitoring bacterial communities in raw milk and cheese by culture-dependent and -independent 16S rRNA gene-based analyses. Appl. Environ. Microbiol. 73:1882-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dogan, B., and K. J. Boor. 2003. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 69:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felske, A., H. Rheims, A. Wolterink, E. Stackebrandt, and A. D. Akkermans. 1997. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143:2983-2989. [DOI] [PubMed] [Google Scholar]
- 9.Gunasekera, T. S., M. R. Dorsch, M. B. Slade, and D. A. Veal. 2003. Specific detection of Pseudomonas spp. in milk by fluorescence in situ hybridization using rRNA directed probes. J. Appl. Microbiol. 94:936-945. [DOI] [PubMed] [Google Scholar]
- 10.Hantsis-Zacharov, E., and M. Halpern. 2007. Chryseobacterium haifense sp. nov., a psychrotolerant bacterium isolated from raw milk. Int. J. Syst. Evol. Microbiol. 57:2344-2348. [DOI] [PubMed] [Google Scholar]
- 11.Hilton, C. D., T. Khusniati, N. Datta, and R. B. Wallace. 2002. Spoilage patterns of skim and whole milks. J. Dairy Res. 69:227-241. [DOI] [PubMed] [Google Scholar]
- 12.Holm, C., L. Jepsen, M. Larsen, and L. Jespersen. 2004. Predominant microflora of downgraded Danish bulk tank milk. J. Dairy Sci. 87:1151-1157. [DOI] [PubMed] [Google Scholar]
- 13.Hugo, C. J., P. J. Jooste, P. Segers, M. Vancanneyt, and K. Kersters. 1999. A polyphasic taxonomic study of Chryseobacterium strains isolated from dairy sources. Syst. Appl. Microbiol. 22:586-595. [DOI] [PubMed] [Google Scholar]
- 14.Hugo, C. J., P. Segers, B. Hoste, M. Vancanneyt, and K. Kersters. 2003. Chryseobacterium joostei sp. nov., isolated from the dairy environment. Int. J. Syst. Evol. Microbiol. 53:771-777. [DOI] [PubMed] [Google Scholar]
- 15.Jayarao, B. M., and D. R. Henning. 2001. Prevalence of foodborne pathogens in bulk tank milk. J. Dairy Sci. 84:2157-2162. [DOI] [PubMed] [Google Scholar]
- 16.Jooste, P. J., and C. J. Hugo. 1999. The taxonomy, ecology and cultivation of bacterial genera belonging to the family Flavobacteriaceae. Int. J. Food Microbiol. 53:81-94. [DOI] [PubMed] [Google Scholar]
- 17.Kawai, Y., T. Saito, J. Uemura, and T. Itoh. 1997. Rapid detection method for bacteriocin and distribution of bacteriocin-producing strains in Lactobacillus acidophilus group lactic acid bacteria isolated from human feces. Biosci. Biotechnol. Biochem. 61:179-182. [DOI] [PubMed] [Google Scholar]
- 18.Kawai, Y., Y. Ishii, K. Arakawa, K. Uemura, B. Saitoh, J. Nishimura, H. Kitazawa, Y. Yamazaki, Y. Tateno, T. Itoh, and T. Saito. 2004. Structural and functional differences in two cyclic bacteriocins with the same sequences produced by lactobacilli. Appl. Environ. Microbiol. 70:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koka, R., and B. C. Weimer. 2001. Influence of growth conditions on heat-stable phospholipase activity in Pseudomonas, J. Dairy Res. 68:109-116. [DOI] [PubMed] [Google Scholar]
- 20.Lafarge, V., J. C. Ogier, V. Girard, V. Maladen, J.-Y. Leveau, A. Gruss, and A. Delacroix-Buchet. 2004. Raw cow milk bacterial population shifts attributable to refrigeration. Appl. Environ. Microbiol. 70:5644-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Fandino, R., A. Olano, N. Corzo, and M. Ramos. 1993. Proteolysis during storage of UHT milk: differences between whole and skim milk. J. Dairy Res. 60:339-347. [DOI] [PubMed] [Google Scholar]
- 22.Marshall, R. T. 1992. Standard methods for the examination of dairy products, 16th ed. American Public Health Association, Washington, DC.
- 23.McPhee, J. D., and M. W. Griffiths. 2002. Psychrotrophic bacteria, Pseudomonas spp., p. 2340-2351. In H. Roginsky, J. W. Fuquay, and P. F. Fox (ed.), Encyclopedia of dairy sciences vol. 4. Academic Press, Inc., New York, NY. [Google Scholar]
- 24.Miambi, E., J. P. Guyot, and F. Ampe. 2003. Identification, isolation and quantification of representative bacteria from fermented cassava dough using an integrated approach of culture-dependent and culture-independent methods. Int. J. Food Microbiol. 82:111-120. [DOI] [PubMed] [Google Scholar]
- 25.Mounier, J., R. Gelsomino, S. Goerges, M. Vancanneyt, K. Vandemeulebroecke, B. Hoste, S. Scherer, J. Swings, G. F. Fitzgerald, and T. M. Cogan. 2005. Surface microflora of four smear-ripened cheeses. Appl. Environ. Microbiol. 71:6489-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munsch-Alatossava, P., and T. Alatossava. 2006. Phenotypic characterization of raw milk-associated psychrotrophic bacteria. Microbiol. Res. 161:334-346. [DOI] [PubMed] [Google Scholar]
- 27.Nagao, J., S. M. Asaduzzaman, Y. Aso, K. Okuda, J. Nakayama, and K. Sonomoto. 2006. Lantibiotics: insight and foresight for new paradigm. J. Biosci. Bioeng. 102:139-149. [DOI] [PubMed] [Google Scholar]
- 28.Sanjuan, S., R. Javier, and M. Garcia-Armesto. 2003. Microbial flora of technological interest in raw ovine milk during 6°C storage. I. J. Dairy Technol. 56:143-148. [Google Scholar]
- 29.Shah, N. P. 1994. Psychrotrophs in milk: a review. Milchwissenschaft 49:432-437. [Google Scholar]
- 30.Shimomura, K., S. Kaji, and A. Hiraishi. 2005. Chryseobacterium shigense sp. nov., a yellow-pigmented, aerobic bacterium isolated from a lactic acid beverage. Int. J. Syst. Evol. Microbiol. 55:1903-1906. [DOI] [PubMed] [Google Scholar]
- 31.Sorhaug, T., and L. Stepaniak. 1997. Psychrotrophs and their enzymes in milk and dairy products: quality aspects. Trends Food Sci. Technol. 8:35-41. [Google Scholar]
- 32.Ternstrom, A., A. M. Lindberg, and G. Molin. 1993. Classification of the spoilage flora of raw and pasteurized bovine milk, with special reference to Pseudomonas and Bacillus. J. Appl. Bacteriol. 75:25-34. [DOI] [PubMed] [Google Scholar]
- 33.Tison, D. L., D. H. Pope, and C. W. Boylen. 1980. Influence of seasonal temperature on the temperature optima of bacteria in sediments of Lake George, New York. Appl. Environ. Microbiol. 39:675-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washam, C. J., H. C. Olson, and E. R. Vedamathu. 1977. Heat Resistant psychrotrophic bacteria isolated from pasteurized milk. J. Food Prot. 40:101-108. [DOI] [PubMed] [Google Scholar]
- 35.Wiedmann, M., D. Weilmeier, S. S. Dineen, R. Ralyea, and K. J. Boor. 2000. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk, Appl. Environ. Microbiol. 66:2085-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.