Abstract
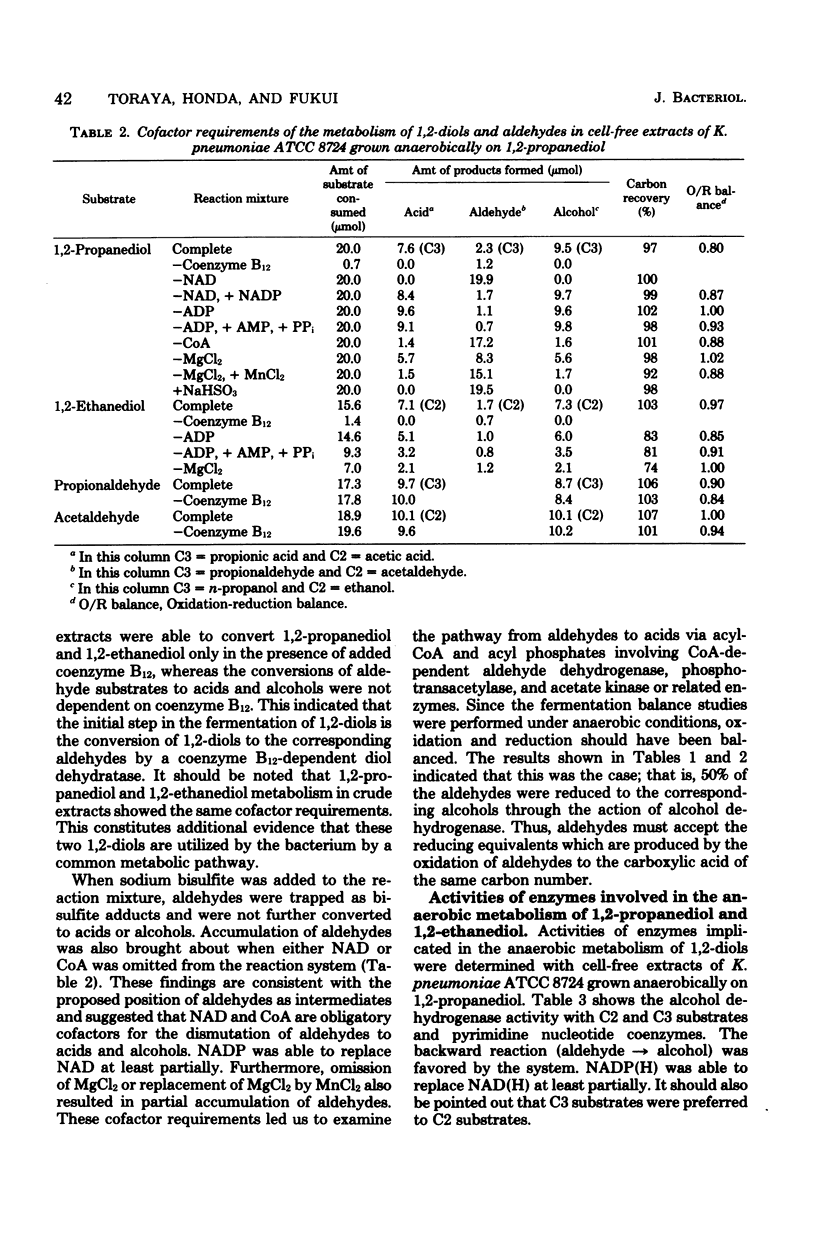
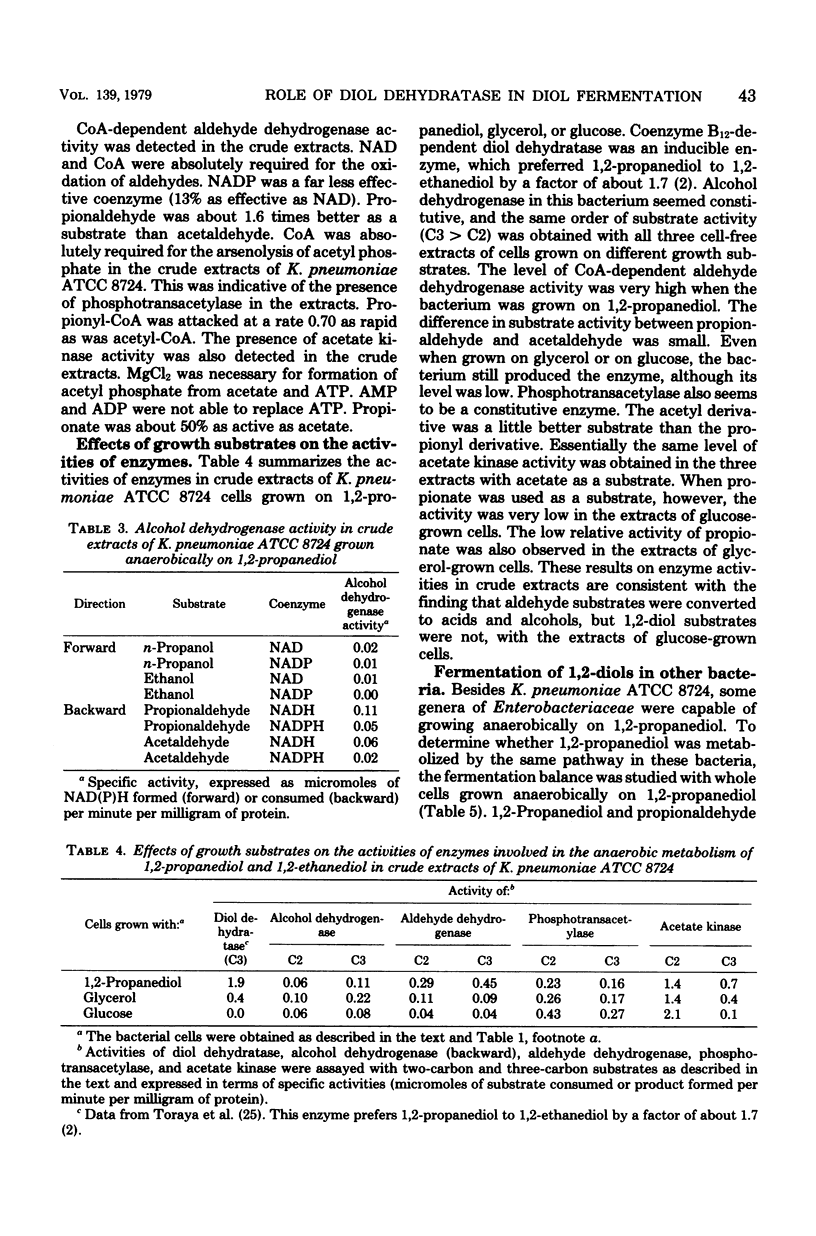
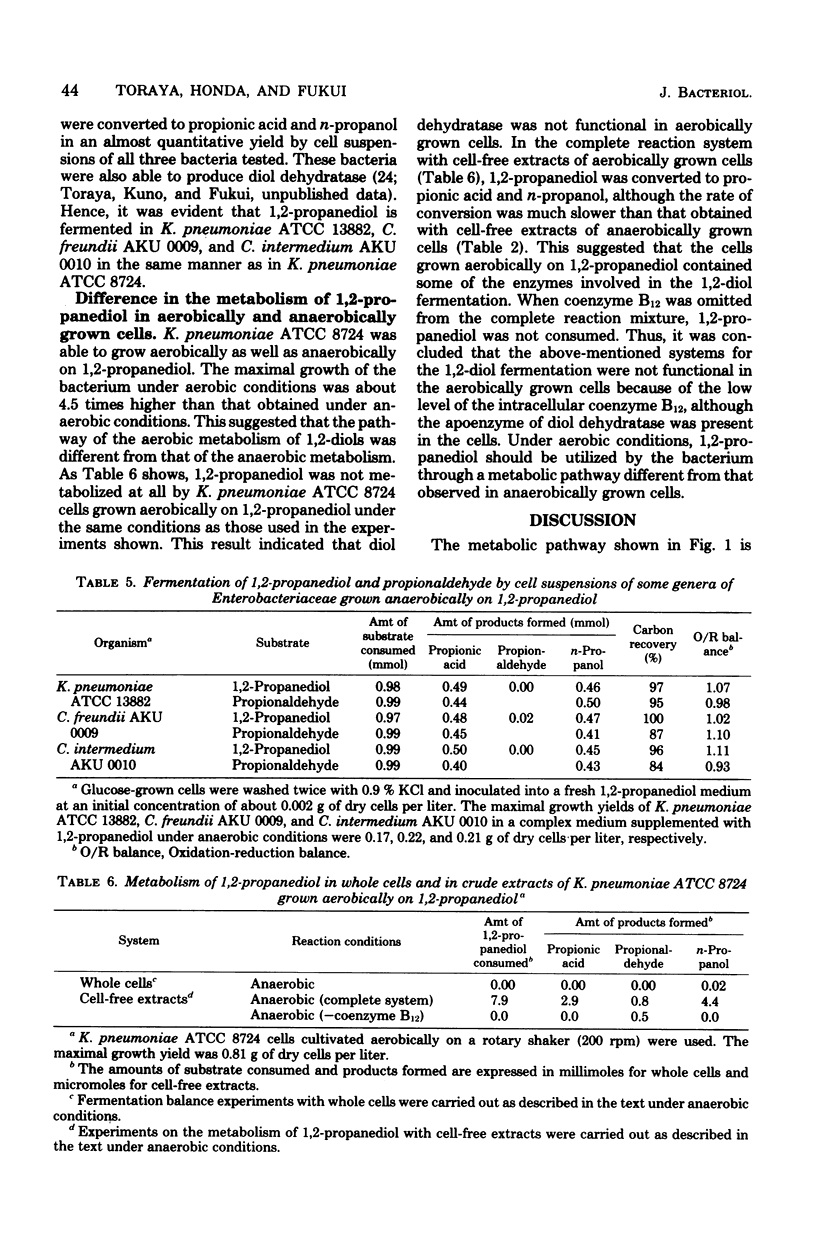
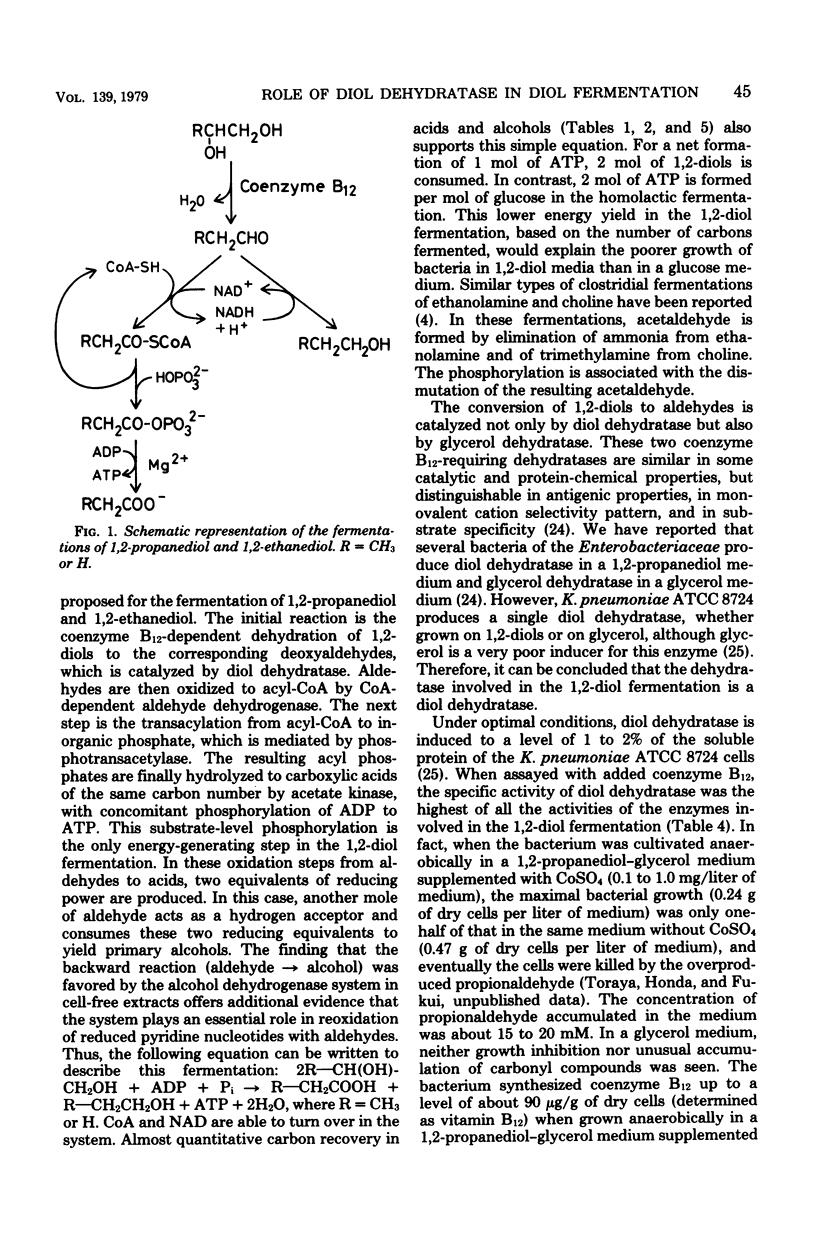
Klebsiella pneumoniae (Aerobacter aerogenes) ATCC 8724 was able to grow anaerobically on 1,2-propanediol and 1,2-ethanediol as carbon and energy sources. Whole cells of the bacterium grown anaerobically on 1,2-propanediol or on glycerol catalyzed conversion of 1,2-diols and aldehydes to the corresponding acids and alcohols. Glucose-grown cells also converted aldehydes, but not 1,2-diols, to acids and alcohols. The presence of activities of coenzyme B12-dependent diol dehydratase, alcohol dehydrogenase, coenzyme-A-dependent aldehyde dehydrogenase, phosphotransacetylase, and acetate kinase was demonstrated with crude extracts of 1,2-propanediol-grown cells. The dependence of the levels of these enzymes on growth substrates, together with cofactor requirements in in vitro conversion of these substrates, indicates that 1,2-diols are fermented to the corresponding acids and alcohols via aldehydes, acyl-coenzyme A, and acyl phosphates. This metabolic pathway for 1,2-diol fermentation was also suggested in some other genera of Enterobacteriaceae which were able to grow anaerobically on 1,2-propanediol. When the bacteria were cultivated in a 1,2-propanediol medium not supplemented with cobalt ion, the coenzyme B12-dependent conversion of 1,2-diols to aldehydes was the rate-limiting step in this fermentation. This was because the intracellular concentration of coenzyme B12 was very low in the cells grown in cobalt-deficient medium, since the apoprotein of diol dehydratase was markedly induced in the cells grown in the 1,2-propanediol medium. Better cell yields were obtained when the bacteria were grown anaerobically on 1,2-propanediol. Evidence is presented that aerobically grown cells have a different metabolic pathway for utilizing 1,2-propanediol.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELES R. H., BROWNSTEIN A. M., RANDLES C. H. beta-Hydroxypropionaldehyde, an intermediate in the formation of 1,3-propanediol by Aerobacter aerogenes. Biochim Biophys Acta. 1960 Jul 15;41:530–531. doi: 10.1016/0006-3002(60)90054-8. [DOI] [PubMed] [Google Scholar]
- ABELES R. H., LEE H. A., Jr An intramolecular oxidation-reduction requiring a cobamide coenzyme. J Biol Chem. 1961 Aug;236:2347–2350. [PubMed] [Google Scholar]
- BURTON R. M., STADTMAN E. R. The oxidation of acetaldehyde to acetyl coenzyme A. J Biol Chem. 1953 Jun;202(2):873–890. [PubMed] [Google Scholar]
- Bradbeer C. The clostridial fermentations of choline and ethanolamine. 1. Preparation and properties of cell-free extracts. J Biol Chem. 1965 Dec;240(12):4669–4674. [PubMed] [Google Scholar]
- GASTON L. W., STADTMAN E. R. Fermentation of ethylene glycol by Clostridium glycolicum, sp. n. J Bacteriol. 1963 Feb;85:356–362. doi: 10.1128/jb.85.2.356-362.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGINS C. G., MILLER O. N. Studies on the metabolism of 1, 2-propanediol phosphate in yeast. J Biol Chem. 1956 Aug;221(2):719–725. [PubMed] [Google Scholar]
- Hacking A. J., Aguilar J., Lin E. C. Evolution of propanediol utilization in Escherichia coli: mutant with improved substrate-scavenging power. J Bacteriol. 1978 Nov;136(2):522–530. doi: 10.1128/jb.136.2.522-530.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H. A., Jr, ABELES R. H. Purification and properties of dioldehydrase, and enzyme requiring a cobamide coenzyme. J Biol Chem. 1963 Jul;238:2367–2373. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER O. N., HUGGINS C. G., ARAI K. Studies on the metabolism of 1, 2-propanediol-1-phosphate. J Biol Chem. 1953 May;202(1):263–271. [PubMed] [Google Scholar]
- ROSE I. A., GRUNBERG-MANAGO M., KOREY S. R., OCHOA S. Enzymatic phosphorylation of acetate. J Biol Chem. 1954 Dec;211(2):737–756. [PubMed] [Google Scholar]
- RUDNEY H. Propanediol phosphate as a possible intermediate in the metabolism of acetone. J Biol Chem. 1954 Sep;210(1):361–371. [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Fukui S. Immunochemical evidence for the difference between coenzyme-B12-dependent diol dehydratase and glycerol dehydratase. Eur J Biochem. 1977 Jun 1;76(1):285–289. doi: 10.1111/j.1432-1033.1977.tb11594.x. [DOI] [PubMed] [Google Scholar]
- Toraya T., Honda S., Kuno S., Fukui S. Coenzyme B12-dependent diol dehydratase: regulation of apoenzyme synthesis in Klebsiella pneumoniae (Aerobacter aerogenes) ATCC 8724. J Bacteriol. 1978 Aug;135(2):726–729. doi: 10.1128/jb.135.2.726-729.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toraya T., Ushio K., Fukui S., Hogenkamp P. C. Studies on the mechanism of the adenosylcobalamin-dependent diol dehydrase reaction by the use of analogs of the coenzyme. J Biol Chem. 1977 Feb 10;252(3):963–970. [PubMed] [Google Scholar]
- Wagner O. W., Lee H. A., Jr, Frey P. A., Abeles R. H. Studies on the mechanism of action of cobamide coenzymes. Chemical properties of the enzyme-coenzyme complex. J Biol Chem. 1966 Apr 25;241(8):1751–1762. [PubMed] [Google Scholar]



