Abstract
The Flo11/Muc1 flocculin has diverse phenotypic effects. Saccharomyces cerevisiae cells of strain background Σ1278b require Flo11p to form pseudohyphae, invade agar, adhere to plastic, and develop biofilms, but they do not flocculate. We show that S. cerevisiae var. diastaticus strains, on the other hand, exhibit Flo11-dependent flocculation and biofilm formation but do not invade agar or form pseudohyphae. In order to study the nature of the Flo11p proteins produced by these two types of strains, we examined secreted Flo11p, encoded by a plasmid-borne gene, in which the glycosylphosphatidylinositol anchor sequences had been replaced by a histidine tag. A protein of approximately 196 kDa was secreted from both strains, which upon purification and concentration, aggregated into a form with a very high molecular mass. When secreted Flo11p was covalently attached to microscopic beads, it conferred the ability to specifically bind to S. cerevisiae var. diastaticus cells, which flocculate, but not to Σ1278b cells, which do not flocculate. This was true for the 196-kDa form as well as the high-molecular-weight form of Flo11p, regardless of the strain source. The coated beads bound to S. cerevisiae var. diastaticus cells expressing FLO11 and failed to bind to cells with a deletion of FLO11, demonstrating a homotypic adhesive mechanism. Flo11p was shown to be a mannoprotein. Bead-to-cell adhesion was inhibited by mannose, which also inhibits Flo11-dependent flocculation in vivo, further suggesting that this in vitro system is a useful model for the study of fungal adhesion.
The fungal adhesins are a family of cell surface proteins that mediate adherence to environmental substrates or to other cells (7, 45). Adhesins are critically important in the initial steps of fungal pathogenicity, when fungal cells must adhere to host tissue. For the common human pathogens Candida albicans and Candida glabrata, the involvement of multiple adhesins in the adherence of fungal cells to host tissue has been demonstrated (4, 5, 18, 26, 43).
Among the adhesins is the flocculin family of Saccharomyces cerevisiae cell wall proteins that mediate flocculation, which is asexual calcium-dependent cell-cell aggregation. The most recently described member of the yeast flocculin gene family, FLO11/MUC1 (24, 30), is the only flocculin expressed in the Σ1278b strain of S. cerevisiae (17), and it exhibits a wide variety of phenotypes. Some of these phenotypes are strain specific. Yeast cells of strain background Σ1278b have been shown to require FLO11 for invasive growth (23, 30), the development of pseudohyphae (24, 29), and the formation of biofilms on plastic (36), but they do not flocculate. On the other hand, the variant strain S. cerevisiae var. diastaticus, which is highly flocculent, has been shown to require FLO11 for flocculation (30). FLO11 is also required in Σ1278b strains for the formation of mats with hub and spoke structures on semisolid agar (36). The common laboratory strain background S288C does not express FLO11 due to a nonsense mutation in the transcriptional activator FLO8 (28). In some industrial strains, FLO11 mediates formation of the specialized biofilms called flors that are necessary for the production of sherry wine (19, 48). The common feature of all these phenotypes is adhesion. Commensurate with the many different pathways that regulate its expression, FLO11 has been shown to have a promoter that is among the largest described for yeast, at over 3 kb (38). Much more is known about gene regulation of FLO11 (for reviews, see references 11, 25, and 32) than about the structure and function of the protein.
We further investigated the FLO11-dependent phenotypes of S. cerevisiae var. diastaticus and found that it also differs from Σ1278b in that the haploids do not invade agar and the diploids do not form pseudohyphae. In order to investigate these strain differences in the phenotypes of FLO11 we expressed and purified the Flo11 proteins from S. cerevisiae var. diastaticus and from Σ1278b and examined their properties. An in vitro system was created for studying the adhesive characteristics of the expressed Flo11 by attaching the protein to microscopic beads and testing the adhesive properties of the beads.
Microscopic beads that can be coated covalently with proteins or ligands have been used to simplify several complex biological processes. For example, Gaur and Klotz used microscopic magnetic beads coated with extracellular matrix proteins to isolate a C. albicans adhesin gene, ALS5, by expression cloning in S. cerevisiae (13). Further work using such beads resulted in characterization of the adhesion properties of Ala1p and Als1p (12, 14, 15, 21, 22, 35). In this study, we have used this approach to study the in vitro properties of purified Flo11 proteins from two different strains.
MATERIALS AND METHODS
Flo11 phenotypic assays.
Flocculation was assayed by culturing cells to mid-log phase in yeast extract-peptone-dextrose medium (1), diluting them to equal cell densities, swirling them vigorously with a Vortex mixer, and photographing them immediately and at specified time intervals. Invasion of agar (37) and formation of biofilms on plastic (36) by haploid strains were assayed as described previously. Diploid strains were tested for pseudohyphae development on nitrogen starvation medium as described previously (16).
Construction of pFLO11-GPIΔ.
pFLO11-GPIΔ was derived from Yeplac181-PGK1p-MUC1 (abbreviated here as pFLO11), which was generously provided by the laboratory of Isak S. Pretorius (8). Yeplac181-PGK1p-MUC1 is a 2μm LEU2 mutant yeast-Escherichia coli shuttle vector containing FLO11 regulated by the constitutive PGK1 promoter (8). The ultimate source of the FLO11 gene in this plasmid is cosmid clone ATCC 70895 (American Type Culture Collection). The FLO11 sequence is identical to that found by the yeast genome sequencing project in strain background S288C (24).
A PstI-SalI fragment of 408 base pairs of the carboxyl-terminal coding region of pFLO11, including the glycosylphosphatidylinositol (GPI) anchor and PGK1 terminator, was replaced with a synthesized 44-bp insert engineered to contain a sequence of six consecutive histidine residues. The sequences of the oligonucleotides used for the six-His tag were as follows: 5′-GTCCATCACCATCACCATCACTAAGGCGCGCCTTTTTTTTTATG-3′ and 5′-TCGACATAAAAAAAAAGGCGCGCCTTAGTGATGGTGATGGTGATGGACTGCA-3′.
Growth of yeast strains.
The S. cerevisiae strains used in this study are listed in Table 1. Strains transformed with either pFLO11 or pFLO11-GPIΔ were cultured in SC−Leu selective medium (Q-Biogene, Carlsbad, CA) with twice the concentration of amino acid supplements suggested by the manufacturer for growth enhancement (2× SC−Leu). Incubation at 30°C was carried out with shaking at 250 rpm until stationary phase was achieved (A600 of approximately 4.0, corresponding to approximately 1.5 × 108 cells/ml), when supernatants were collected for analysis of the secreted protein.
TABLE 1.
Yeast strains and plasmids used in this study
| Yeast strain or plasmid | Strain background | Genotype or description | Reference or source |
|---|---|---|---|
| Strains | |||
| L5487 | Σ1278b | MATα leu2::hisG ura3-52 | 37 |
| L5487 flo11Δ | Σ1278b | MATα leu2::hisG ura3-52 flo11::URA3 | 29 |
| L5489 | Σ1278b | MATa/α leu2::hisG/leu2::hisG ura3-52/ura3-52 | C. Styles and G. Fink |
| YIY345 | S. cerevisiae var. diastaticus | MATaura3 leu2-3,112 his4 sta0 | 47 |
| YIY345 flo-1 | S. cerevisiae var. diastaticus | MATaura3 leu2-3,112 his4 sta0flo11::URA3 | 30 |
| YeAD5 | S. cerevisiae var. diastaticus | MATα leu2-3,112 ura3-Δ227 arg4 STA1 | 30 |
| M1800D × YIY319 | S. cerevisiae var. diastaticus | MATa/α leu2-3,112/LEU2 arg4/ARG4 ura3/URA3 | 6 |
| FY86 | S288C | MATα his3Δ200 ura3-52 leu2Δ1 | 46 |
| Plasmids | |||
| pFLO11 | YEplac181-PGK1p-MUC1 | 8 | |
| pFLO11-GPIΔ | YEplac181-PGK1p-MUC1-GPIΔ | This work; derived from YEplac181-PGK1p-MUC1 (8) |
Column purification of proteins.
Cultures were centrifuged at 5,000 rpm for 10 min, supernatants were collected, and pellets were washed with deflocculation buffer (20 mM sodium citrate-5 mM EDTA) to remove any Flo11p possibly bound to cell surfaces (30). The pH of pooled supernatants and washes was adjusted from a value of ∼3.3 to 7.0 with 1 M Tris base. Prepared supernatant was then applied to a Ni-nitrilotriacetic acid-agarose column (Qiagen, Inc., Valencia, CA) of approximately 2.5 cm by 15 cm at 4°C. Wash buffer (phosphate-buffered saline [PBS], pH 7.0, containing 20 mM imidazole) was subsequently applied to the column. Absorbance at 280 nm was monitored until washing was judged to be complete, i.e., A280 readings approached zero. Flo11p was then eluted with 250 mM imidazole in PBS, pH 7.0. Two-milliliter fractions were collected, and the purified Flo11p was stored at −80°C.
Purified Flo11p fractions were concentrated using centrifugal filter devices (Microcon YM-30 and Centricon YM-30; Millipore Corp., Billerica, MA). Total protein in the concentrated samples was quantified following a modification of the Bradford method (Coomassie Plus protein assay reagent; Pierce Biotechnology, Perbio, Rockford, IL).
Western blot analyses.
Protein samples were boiled in sodium dodecyl sulfate (SDS) sample buffer that contained 10% SDS and 100 mM dithiothreitol before being separated in discontinuous SDS-polyacrylamide minigels (3.9% separating gel-5% resolving gel; 7.3 × 8.3 cm). High-molecular-weight protein markers were used (Rainbow; Amersham Life Science, Amersham International, Buchinghamshire, England). Before being blotted, the Coomassie-stained gels were soaked in deionized water for 15 min, equilibrated in 25 mM Tris-192 mM glycine-1% SDS for 1 hour at room temperature, and transferred to 25 mM Tris-192 mM glycine-0.1% SDS for another hour of incubation. Proteins were blotted onto polyvinylidene difluoride (PVDF) membranes (Immobilon Millipore P; Millipore Corp., Billerica, MA), and the membranes were washed as described previously (10). Membranes were incubated with a mouse monoclonal immunoglobulin G primary antibody specific for a consecutive sequence of five histidine residues (penta-His; Qiagen, Inc., Valencia, CA) diluted 1:2,000 in 3% bovine serum albumin in TBS (10 mM Tris-HCl, pH 7.5, 150 mM NaCl), followed by incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G secondary antibody diluted 1:2,000 in 10% nonfat dried milk powder in TBS at room temperature.
Antibody binding to His-tagged protein was assessed using a chemiluminescence assay according to the manufacturer's protocol (ECL Western detection reagents; Amersham Pharmacia Biotech, Inc., Piscataway, NJ). Treated blots were exposed to autoradiographic film (HyperfilmECL; Amersham Pharmacia Biotech, Inc., Piscataway, NJ) for intervals of 2 to 5 min.
Assay for terminal mannose.
To assess the binding of Flo11p to Galanthus nivalis agglutinin (GNA), a plant lectin that specifically interacts with α(1-3)-, α(1-6)-, or α(1-2)-linked terminal mannose residues, protein samples were blotted directly onto a PVDF membrane in a slot blot manifold (Hoefer Scientific Instruments, San Francisco, CA). The membrane was incubated for 30 min in 20 ml of blocking reagent (Roche Diagnostics, Mannheim, Germany), washed two times for 10 min each in 50 ml buffer 1 (TBS-1 mM MgCl2-1 mM MnCl2, pH 7.5), and washed one time for 10 min in TBS (0.05 M Tris-HCl-0.15 M NaCl, pH 7.5). Ten microliters of digoxigenin-labeled GNA (Roche Diagnostics, Mannheim, Germany) was added to 10 ml buffer 1, and the blot was incubated in the solution for 1 hour at room temperature. Three 10-min washes with 50 ml of TBS were performed, followed by a 1-h incubation in a solution of 10 μl anti-digoxigenin antibody linked to alkaline phosphatase (Roche Diagnostics, Mannheim, Germany) added to 10 ml TBS. Washing was again performed three times with 50 ml TBS for 10 min. The blot was immersed in a substrate solution containing 200 μl 4-nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate (Roche Diagnostics, Mannheim, Germany) and 10 ml buffer 2 (0.1 M Tris-HCl-0.05 M MgCl2-0.1 M NaCl, pH 9.5) until color development was complete.
Preparation of Flo11-coated beads.
Dynal M-450 tosyl-activated beads (Dynal Biotech, Inc., Lake Success, NY) are polystyrene beads that are approximately the size of yeast cells (4.5 μm in diameter). The surface of each bead is activated with p-toluenesulfonyl chloride to facilitate the covalent binding of proteins through their amino or sulfhydryl groups. Beads at a concentration of 4 × 108 beads/ml were pipetted in 25-μl aliquots into 0.5-ml tubes. Beads were collected using a strong magnet (Magnetight separation stand; Novagen, Inc., Madison, WI) for 1 to 2 min, and supernatants were discarded. The beads were gently resuspended in 100 μl of buffer A (0.1 M sodium phosphate, pH 7.4) for 2 min and collected on the magnetic stand for 1 minute. Buffer was pipetted off and discarded. Five micrograms of Flo11p was added to each tube, i.e., to 1 × 107 washed beads, along with buffer A to maintain the initial total volume of 25 μl. Each tube was vortexed for 2 min and incubated at 30°C for 16 to 24 h with slow, tilt rotation. Beads were also incubated without added Flo11p as a control. Upon completion of incubation, beads were collected for 2 to 3 min in the magnetic stand, and supernatants were removed. Beads were washed twice for 5 min each at 4°C in buffer D, which is PBS, pH 7.4, containing 0.1% fetuin, a glycoprotein which we have shown not to inhibit Flo11-dependent adhesion (data not shown) and which is therefore suitable as a blocking agent. Washing was then performed in buffer E (0.2 M Tris, pH 8.5, with 0.1% fetuin), with incubation at 20°C with slow, tilt rotation for 24 h. Washing was performed in buffer D once for 5 min at 4°C. Flo11p-coated beads and control beads without added Flo11p were stored at a concentration of 4 × 108 beads/ml at 4°C.
Bead adhesion assay.
Adhesion of protein-coated beads to cells was assayed by a modification of the method of Gaur et al. (14). Overnight cultures of yeast strains to be combined with the coated beads were diluted to ∼2 × 108 cells/ml, and 500-μl aliquots were pelleted and washed twice with an equivalent volume of deflocculation buffer. Washed cells were then resuspended in deflocculation buffer containing 20 mM calcium, to promote adhesion (2), combined with 2.5 μl of coated beads, or 1 × 106 beads, in a total volume of 1 ml. Reaction mixes were vortexed vigorously, and wet mounts were prepared on glass slides for immediate microscopic viewing. Adhesion of yeast cells to beads was assessed by counting beads, utilizing a light microscope with a 40× objective (Leica Microsystems, Inc., Allendale, NJ), and scoring them as belonging to one of two categories, i.e., beads bound to yeast cells and beads not bound to yeast cells. Values for each category were calculated as percentages of the total number of beads counted with respect to that category. To assay adhesion of Σ1278b cells, the cells were first incubated in SC medium plus 0.1% glucose because this strain requires glucose starvation for maximum Flo11-dependent adhesion (36).
To assess the ability of d-mannose to affect the binding of Flo11p-coated beads to yeast cells, the adhesion assay was modified so that 1 × 108 S. cerevisiae var. diastaticus YIY345 cells in 1 ml were placed in a microcentrifuge tube with 2.5 μl beads coated with column-purified Flo11p derived from Saccharomyces cerevisiae var. diastaticus. Beads and cells were pelleted and resuspended in 1 ml deflocculation buffer, vortexed, and centrifuged for 5 min at 3,000 × g. Buffer was pipetted off, and the procedure was repeated once. d-Mannose at a concentration of 1 M in deflocculation buffer was then added to produce a total volume of 1 ml, and the washed beads and cells were incubated in the sugar for 5 min at 30°C with shaking. Calcium was then added to 20 mM, the tube was vortexed vigorously, and a wet mount was prepared.
RESULTS
Yeast cells from strain backgrounds Σ1278b and S. cerevisiae var. diastaticus differ in expression of FLO11-dependent phenotypes.
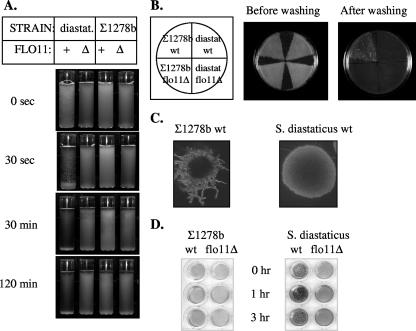
It was previously shown that Σ1278b strains express FLO11 (29), as do strains of S. cerevisiae var. diastaticus (30). However, the phenotypic expression of FLO11 differs in these two strains. S. cerevisiae var. diastaticus strains flocculate very strongly in a FLO11-dependent way, as they begin to settle out of solution immediately after being mixed (Fig. 1A). Σ1278b strains are virtually nonflocculent in comparison, remaining entirely suspended even 2 h after being mixed (Fig. 1A). Σ1278b diploid strains, on the other hand, invade agar and form pseudohyphae (16, 17), while S. cerevisiae var. diastaticus strains do not (Fig. 1B and C). Both S. cerevisiae var. diastaticus strains and Σ1278b (36) form biofilms on polystyrene microtiter wells (Fig. 1D). To explore the basis for these strain-specific differences in phenotypic expression, we purified the Flo11 protein from each strain and tested its properties.
FIG. 1.
FLO11-dependent phenotypes of yeast cells of S. cerevisiae var. diastaticus and those of the Σ1278b strain background. (A) Flocculation assay of S. cerevisiae var. diastaticus wild-type (+) (strain YIY345) and FLO11 deletion (Δ) (strain YIY345 flo-1) strains and of Σ1278b wild-type (strain L5487) and FLO11 deletion (strain L5487 flo11Δ) strains. Log-phase cultures were adjusted to contain equal cell numbers, vortexed vigorously, and photographed at the indicated time intervals. (B) Agar invasion assay. The strains described above were patched onto yeast extract-peptone-dextrose agar in the arrangement shown on the diagram. They were cultured for 3 days at 30°C, followed by 2 days at room temperature, and photographed before and after being washed with a gentle stream of water. (C) Pseudohyphae development by diploid strains. Cells of each background (Σ1278b strain L5489 and S. cerevisiae var. diastaticus strain M1800D × YIY319) were cultured on nitrogen starvation medium as described previously (16). A representative colony of each strain was photographed. (D) Adherence to polystyrene. Log-phase cultures were suspended in 0.1% glucose, transferred to a 96-well polystyrene plate, incubated at 30°C for the indicated time periods, and stained with crystal violet as previously described (36). The wells were washed with water repeatedly and photographed.
A GPI anchor is required for cell surface localization of Flo11.
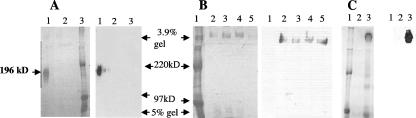
Many fungal adhesins localize to the cell surface through either a cell membrane-anchored or a cell wall-anchored GPI moiety (31). Flo11p has been localized to the yeast cell wall (17, 30). We replaced the GPI anchor with a histidine tag to facilitate secretion of the protein into the medium, from which it could then be purified using nickel columns. When the GPI anchor of FLO11 was replaced by a six-histidine tag, yeast cells of strain background Σ1278b transformed with GPI anchorless FLO11 (pFLO11-GPIΔ) secreted Flo11p (Fig. 2A, lane 1), whereas isogenic yeast strains transformed with wild-type FLO11 (pFLO11) did not secrete Flo11p (Fig. 2A, lane 2). Using the same plasmids in S. cerevisiae var. diastaticus also resulted in secretion of the protein (Fig. 2C). This protein was by far the most abundant protein in the concentrated medium, as shown by Coomassie blue staining of the gels in Fig. 2. Therefore, the GPI anchor is critically required for cell surface localization of Flo11p, and its removal results in the accumulation of significant amounts of the protein in the medium.
FIG. 2.
SDS-polyacrylamide gel electrophoresis of Flo11 proteins secreted from yeast cells of two strain backgrounds. In each case, the Coomassie-stained polyacrylamide discontinuous gel (3.9% stacking gel and 5% resolving gel) is shown on the left, while the corresponding Western blot using anti-six-His antibody is shown on the right. (A) Lane 1, culture supernatant of strain L5487 flo11Δ (Σ1278b background) transformed with pFLO11-GPIΔ; lane 2, culture supernatant of L5487 flo11Δ transformed with pFLO11 (GPI anchor sequences included); lane 3, protein molecular size marker. (B) Purified secreted Flo11p from the Σ1278b strain aggregated in a form that remained in the stacking gel. Secreted Flo11p from strain L5487 flo11Δ transformed with pFLO11-GPIΔ was purified on a nickel column. Lane 1, protein size marker; lane 2, fraction 7; lane 3, fraction 8; lane 4, fractions 9 and 10; lane 5, fractions 13 and 14. (C) Secreted Flo11p from S. cerevisiae var. diastaticus strain YIY345 flo-1 transformed with pFLO11-GPIΔ produced a large form that remained in the stacking gel. Lane 1, protein size marker; lane 2, culture supernatant of YIY345 transformed with pFLO11; lane 3, culture supernatant of strain YIY345 transformed with pFLO11-GPIΔ.
Secreted Flo11p forms aggregates.
The culture supernatant of Σ1278b strain L5487 flo11Δ transformed with pFLO11-GPIΔ contained a protein of approximately 196 kDa (Fig. 2A, lane 1). The identity of the protein as Flo11p was verified by immunodetection with anti-His antibody. However, a much higher-molecular-mass form of Flo11p (>220 kDa) was detected after the same supernatant was purified on a nickel column (Fig. 2B). Fractions collected from the nickel column are shown in Fig. 2B. The higher-molecular-weight form remained in the stacking gel. Total protein quantification showed that purification over Ni-nitrilotriacetic acid resin concentrated Flo11p approximately three times (up to 35 μg/ml in peak fractions) (data not shown). The culture supernatant of S. cerevisiae var. diastaticus strain YIY345 transformed with pFLO11-GPIΔ most often exhibited the high-molecular-weight form of Flo11p, even before column purification (Fig. 2C, lane 3), although it was also observed in the 196-kDa form (data not shown). Once formed, this high-molecular-weight form of the protein could not be disaggregated by any of the methods tried, which included varying the temperature of incubation of the protein samples, the addition of EDTA at up to 500 mM, and the addition of 50% formamide. Formation of the high-molecular-weight form of Flo11p was also not dependent on the stage of culture growth or the cell density (data not shown). Cell wall-adhesive glycoproteins are known to form aggregates such as this (3, 39, 42).
Flo11p is a mannoprotein.
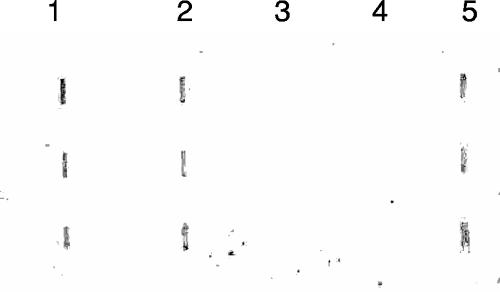
One common characteristic of fungal adhesins is that they contain a large number of residues that are susceptible to N- or O-linked glycosylation (31). Flo11p contains a central domain in which about 50% of the residues are serine and threonine, which are potential O-linked glycosylation sites. Since the predicted molecular mass of Flo11p derived from its amino acid sequence is 137,000 Da, the larger mass observed on the stained gels and immunoblots suggests that the FLO11 protein product is modified. To test for mannose modification, purified Flo11p samples from YeAD5 (S. cerevisiae var. diastaticus background) and L5487 flo11Δ (Σ1278b background) transformed with pFLO11-GPIΔ were blotted onto PVDF membranes and treated with digoxigenin-labeled GNA, a plant lectin that specifically binds to α(1-3)-, α(1-6)-, and α(1-2)-linked terminal mannose residues. Figure 3 shows that Flo11p proteins from both yeast strains are mannoproteins possessing terminal mannose residues (Fig. 3, lanes 1 and 2). The positive control for this assay was carboxypeptidase Y (Fig. 3, lane 5), a known mannoprotein. The glycoproteins fetuin and transferrin, which do not contain mannose, served as negative controls.
FIG. 3.
Flo11 is a mannoprotein. A slot blot is presented, showing purified Flo11 protein applied to a PVDF membrane and treated with a digoxigenin-labeled plant lectin that specifically interacts with terminal mannose residues (GNA). Each lane contains three replicates of a protein sample. Lane 1, nickel column-purified Flo11p from S. cerevisiae var. diastaticus strain YeAD5 transformed with pFLO11-GPIΔ; lane 2, column-purified Flo11p from Σ1278b strain L5487 flo11Δ transformed with pFLO11-GPIΔ; lane 3, fetuin (negative control); lane 4, transferrin (negative control); lane 5, carboxypeptidase Y (positive control).
Beads coated with Flo11p derived from either Σ1278b or S. cerevisiae var. diastaticus bind S. cerevisiae var. diastaticus cells.
Yeast strain Σ1278b and S. cerevisiae var. diastaticus both express the FLO11 gene (unlike the standard laboratory strain S288C), but only S. cerevisiae var. diastaticus exhibits Flo11-dependent flocculation. One hypothesis is that the Flo11 proteins produced in these two strains are modified or localized differently, enabling only one form to mediate flocculation. Another possibility is that additional factors in the yeast cell wall are responsible for the different activities of Flo11p in these strains.
In order to examine these hypotheses, an in vitro model system was created for the study of Flo11-dependent adhesion by coating plastic beads with Flo11p secreted from the two strains of yeast. The Flo11 protein secreted into the medium by pFLO11-GPIΔ-bearing cells was covalently bound to polystyrene beads of approximately the size of a yeast cell. Dynal M450 tosyl-activated magnetic beads (4.5 μm in diameter) (Dynal Biotech, Inc., Lake Success, NY) are derivatized with p-toluenesulfonyl chloride to facilitate the covalent binding of proteins to the surfaces of the beads. The Flo11 protein produced by both Σ1278b and S. cerevisiae var. diastaticus plasmid-bearing cells was used to coat these beads. Since the secreted Flo11p was encoded by the plasmid, it would have the same primary sequence in both strains, which is identical to the sequence from the S288C strain background, as determined by the yeast genome sequencing project (24). The coated beads were then tested for the ability to adhere to cells of various types.
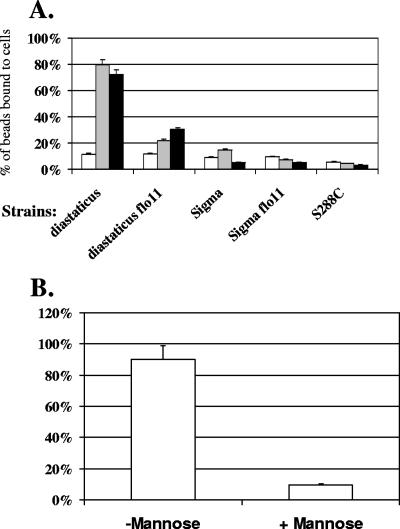
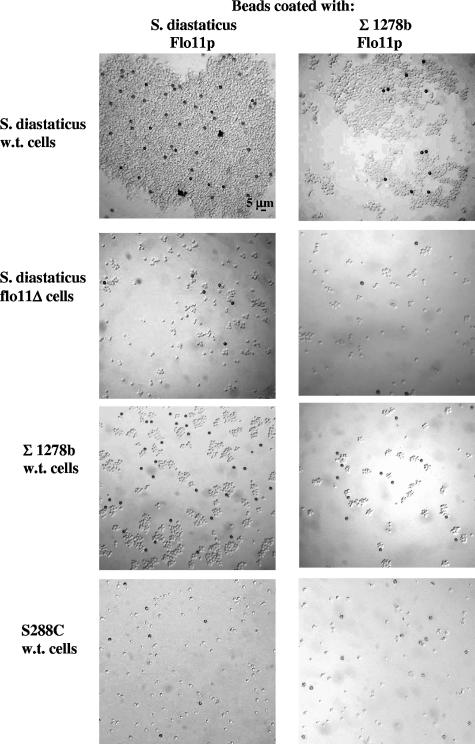
Uncoated beads did not bind to cells of either strain (see Fig. 5A). However, when they were coated with Flo11p from either yeast strain, the beads adhered to S. cerevisiae var. diastaticus cells (Fig. 4, top row, and 5A). Therefore, purified Flo11p is sufficient to bind to the cell wall of S. cerevisiae var. diastaticus. Flo11p produced by the nonflocculent stain Σ1278b was fully capable of mediating bead-to-cell adhesion. Furthermore, the same result was achieved whether the 196-kDa form of Flo11 (from Σ1278b) or the large form (>220 kDa; from purified Σ1278b Flo11 or from S. cerevisiae var. diastaticus) (data not shown) was used. These data establish Flo11p as an adhesion molecule.
FIG. 5.
(A) Quantitation of bead adhesion assay. Each column was based on the mean ± standard error for three independent experiments, with at least 200 beads counted in each. Uncoated beads (white bars) showed no specific binding to cells; beads coated with purified Flo11p secreted from either S. cerevisiae var. diastaticus strain YIY345 (gray bars) or Σ1278b strain L5487 flo11Δ (black bars) showed specific binding to S. cerevisiae var. diastaticus wild-type cells. (B) Mannose inhibits adhesion of coated beads to yeast cells, just as it inhibits flocculation of these cells. Beads coated with Flo11p derived from S. cerevisiae var. diastaticus strain YIY345 were added to cells of strain YIY345, with and without mannose treatment, and the numbers of beads bound and not bound to cells were counted for each case.
FIG. 4.
Bead adhesion assay. Flo11p-coated beads bound specifically to S. cerevisiae var. diastaticus cells in a FLO11-dependent manner. Dynal beads coated with purified secreted Flo11p from either the S. cerevisiae var. diastaticus or Σ1278b strain background were added to different strains of yeast cells, mixed, and photographed. All photos were taken with a 40× objective in the differential interference contrast setting. Beads (approximately 4.5 μm) are visible as dark particles. Cells used were from the following strains: S. diastaticus w.t., strain YIY345; S. diastaticus flo11Δ, strain YIY345 flo-1; Σ1278b w.t., strain L5487; S288C w.t., strain FY86. A quantitative analysis of this assay is shown in Fig. 5A.
A target of Flo11p adhesion is other Flo11p molecules.
We previously established that mannose inhibits Flo11-dependent flocculation (2), leading us to hypothesize that the target of Flo11p adhesion is the mannoprotein layer of the yeast outer cell wall. Since Flo11p itself is a mannoprotein (Fig. 3), we tested the ability of the Flo11-coated beads to adhere to S. cerevisiae var. diastaticus cells deleted for the FLO11 gene. Figures 4 (second row) and 5A show that the coated beads failed to adhere to cells lacking Flo11p. Likewise, the coated beads did not bind to cells of strain background S288C (Fig. 4, row 4, and Fig. 5A), which does not express FLO11. Therefore, Flo11p itself is an important part of the adhesive target on the cell wall, if not the only target.
Flo11-coated beads did not adhere to yeast cells of strain background Σ1278b, regardless of the source of Flo11p.
This result (Fig. 4, row 3) supports the hypothesis that it is differences in the cell wall that are responsible for the different abilities of Σ1278b and S. cerevisiae var. diastaticus to flocculate in a Flo11-dependent manner. These cell wall differences could be in auxiliary factors required for binding, or they could be differences in the Flo11p receptor molecules anchored in the cell wall.
The adhesion assays were quantitated by counting adherent beads and nonadherent beads in a mixture of cells and beads, using a microscope. The average of three independent assays for each mixture is shown in Fig. 5A. Flo11p from the two strains, i.e., Σ1278b and S. cerevisiae var. diastaticus, was shown to bind similarly to cells. Among the in vivo properties of Flo11-expressing cells is the ability to adhere to agar (24, 29) and to polystyrene (36). These Flo11-coated beads, however, were not observed to bind to agar or plastic (data not shown). It may be that the proper experimental conditions for such binding have not been found, that the specific gravity of the magnetic beads is too high to permit adhesion, or that other factors are involved in binding to these substrates.
Mannose inhibits Flo11-dependent adhesion.
Since mannose inhibits Flo11-dependent flocculation in vivo (2), an in vitro adhesion assay was performed to directly assess the ability of d-mannose to affect the binding of Flo11-coated beads to yeast cells. When beads coated with Flo11p derived from S. cerevisiae var. diastaticus strain YeAD5 were combined with S. cerevisiae var. diastaticus YIY345 cells and then incubated in 1 M d-mannose, no binding of beads to cells was detected (Fig. 5B). Glucose at the same concentration did not inhibit binding (data not shown). Flo11p is a member of the class of Flo1-type flocculins, whose defining characteristic is inhibition by mannose but not by glucose, maltose, or sucrose (2). Therefore, this in vitro system utilizing coated beads to test Flo11p adhesion to cells reflects the in vivo properties of mannose inhibition. This finding provides further evidence that mannose is a component to which Flo11p binds on the cell and that Flo11p functions in a lectin-like manner in S. cerevisiae var. diastaticus.
DISCUSSION
This study directly demonstrates that Flo11p is an adhesion molecule. Yeast cells of three different strain backgrounds were used in these studies. S288C has been used as a standard laboratory strain of yeast for many years; it does not express FLO11 and consequently does not form pseudohyphae or exhibit other FLO11-dependent properties (28, 38). The first yeast strain shown to form pseudohyphae and to invade a substrate was Σ1278b (16), which exhibits constitutive suppression of the stress response due to high cyclic AMP levels (40). Cells with the Σ1278b background exhibit many FLO11-dependent characteristics besides pseudohyphae, including agar invasion (29, 37), biofilm formation, and adhesion to plastic (36), but they do not flocculate. Saccharomyces cerevisiae var. diastaticus, on the other hand, flocculates very strongly, but when FLO11 is deleted flocculation is abolished (30). However, this work shows that in spite of its expression of Flo11p, S. cerevisiae var. diastaticus does not invade agar or form pseudohyphae. These two strains thus represent a naturally occurring experiment to determine the factors that govern Flo11-dependent adhesion. In order to investigate these factors, Flo11 proteins were purified from these two strains and their properties were tested.
When the GPI anchor sequences were removed from Flo11p, the protein accumulated in the extracellular medium. Two forms of Flo11p were observed, with one form of 196 kDa and another, very large form, which did not enter the separating gel. Purification of secreted Flo11p on nickel columns always resulted in conversion of the 196-kDa form to the larger form. We suggest that the large form represents an aggregate of Flo11 protein. Such aggregates have frequently been seen in SDS gels containing membrane and cell wall proteins (3, 9, 33, 39, 42). At least three groups have purified mannose-specific lectins from yeast surfaces, and similar SDS-resistant aggregation properties were displayed by all of them (3, 39, 42). Bony et al. (3) extracted Flo1p from cell walls by using hot SDS-β-mercaptoethanol and observed that the protein migrated in SDS gels as a very high-molecular-mass protein in a highly heterogeneous fashion. The fastest-migrating forms of Flo1p exhibited molecular masses of about 200 kDa, while the largest forms remained at the top and did not enter the resolving gel (3), just as we found for Flo11p. When the GPI anchor sequences of Flo1p were removed, the protein was secreted into the medium in a form that produced a fuzzy, heterogeneous, high-molecular-mass band in gels (3). Treatment with endo-β-N-acetylglucosaminidase H resulted in more protein entering the gel, suggesting that Flo1p is N glycosylated. However, the enzyme-treated Flo1p remained larger than predicted from the amino acid sequence, suggesting O glycosylation as well (3).
Consistent with the model of flocculins as glycoproteins, a homolog of Flo1p has been demonstrated to have a sugar content of 63% (42). This protein also exhibited properties of aggregation; gel filtration studies revealed an active aggregate with an apparent molecular mass of >700 kDa. The present study establishes Flo11p as a mannoprotein.
Flo11p functioned as an adhesin in vitro when it was attached to beads. The coated beads bound only to cells of the strain background that exhibits Flo11-dependent flocculation, namely, S. cerevisiae var. diastaticus, not to Σ1278b strains, which do not flocculate. This binding was not due to nonspecific trapping of the beads in the large flocs of S. cerevisiae var. diastaticus, since uncoated beads did not bind. Both strains produced Flo11p that is mannosylated, and both produced Flo11p that adheres to S. cerevisiae var. diastaticus cells equally well. In vitro model systems such as this one should prove to be very useful for further understanding fungal adhesion.
The work presented here is the first to identify an adhesive target of Flo11p. Since the outer layer of the yeast cell wall consists largely of mannoproteins (20, 27), it seemed likely that Flo11p would bind to the side chains of the cell wall mannoproteins. It has been thought that the specificity of fungal lectins may be quite broad (41). Somewhat surprisingly, we found that an essential component (and perhaps the only component) of the adhesive target of Flo11p is, in fact, other Flo11p molecules. Flo11p receptors on the cell wall are required for adhesion of Flo11-coated beads; it remains to be seen whether they are sufficient. Strain-specific differences in these Flo11p receptors in the cell wall could possibly explain the differential adhesion of S. cerevisiae var. diastaticus versus Σ1278b cells to the coated beads. Such receptor differences could be quantitative or qualitative.
Homotypic adhesion has been demonstrated for the ALS adhesins of Candida albicans (21, 22). Yeast cell aggregation mediated by cloned ALS proteins has properties characteristic of amyloid protein aggregation (35), and alterations in these properties could, in theory, produce phenotypic variation. Another possible mechanism of fungal adhesion is suggested by the observation that in Als5p, the threonine-rich repeat domain can mediate homotypic adhesion to repeats on the surfaces of apposing cells (34). Such tandem repeats are a hallmark of yeast cell wall proteins and are also found in Flo11p. In the yeast flocculin Flo1p, variation in the length and number of tandem repeats has been shown to occur between strains and to correlate with functional variation in adhesive phenotypes (44). It is possible that mechanisms such as these underlie the strain-specific variation we see in FLO11-dependent phenotypes.
Acknowledgments
We thank Isak S. Pretorius, Florian Bauer, and Marco Gagiano for the plasmid Yeplac181-PGK1p-MUC1 and for helpful advice.
This work was financially supported by NIH grant R15AI43927 and NSF grant MCB-9973776 to A.M.D.
This paper is dedicated to the memory of Robert T. Simpson, scientist, physician, and mentor.
Footnotes
Published ahead of print on 5 October 2007.
REFERENCES
- 1.Amberg, D. C., D. J. Burke, and J. N. Strathern. 2005. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 2.Bayly, J. C., L. M. Douglas, I. S. Pretorius, F. F. Bauer, and A. M. Dranginis. 2005. Characteristics of Flo11-dependent flocculation in Saccharomyces cerevisiae. FEMS Yeast Res. 5:1151-1156. [DOI] [PubMed] [Google Scholar]
- 3.Bony, M., D. Thines-Sempoux, P. Barre, and B. Blondin. 1997. Localization and cell surface anchoring of the Saccharomyces cerevisiae flocculation protein Flo1p. J. Bacteriol. 179:4929-4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cormack, B. P., N. Ghori, and S. Falkow. 1999. An adhesin of the yeast pathogen Candida glabrata mediating adherence to human epithelial cells. Science 285:578-582. [DOI] [PubMed] [Google Scholar]
- 5.De Las Penas, A., S. J. Pan, I. Castano, J. Alder, R. Cregg, and B. P. Cormack. 2003. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 17:2245-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dranginis, A. M. 1989. Regulation of STA1 gene expression by MAT during the life cycle of Saccharomyces cerevisiae. Mol. Cell. Biol. 9:3992-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dranginis, A. M., J. M. Rauceo, J. E. Coronado, and P. N. Lipke. 2007. A biochemical guide to the yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. Rev. 71:282-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagiano, M., D. van Dyk, F. F. Bauer, M. G. Lambrechts, and I. S. Pretorius. 1999. Msn1p/Mss10p, Mss11p and Muc1p/Flo11p are part of a signal transduction pathway downstream of Mep2p regulating invasive growth and pseudohyphal differentiation in Saccharomyces cerevisiae. Mol. Microbiol. 31:103-116. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher, S. R., and R. T. Leonard. 1987. Electrophoretic characterization of a detergent-treated plasma membrane fraction from corn roots. Plant Physiol. 83:265-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher, S. R., S. E. Winston, S. A. Fuller, and J. G. R. Hurrell. 1998. Immunoblotting and immunodetection, p. 6.2.1-6.2.20. In J. S. Bonafacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. M. Yamada (ed.), Current protocols in cell biology. John Wiley and Sons, Inc., New York, NY.
- 11.Gancedo, J. M. 2001. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25:107-123. [DOI] [PubMed] [Google Scholar]
- 12.Gaur, N. K., and S. A. Klotz. 2004. Accessibility of the peptide backbone of protein ligands is a key specificity determinant in Candida albicans SRS adherence. Microbiology 150:277-284. [DOI] [PubMed] [Google Scholar]
- 13.Gaur, N. K., and S. A. Klotz. 1997. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect. Immun. 65:5289-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaur, N. K., S. A. Klotz, and R. L. Henderson. 1999. Overexpression of the Candida albicans ALA1 gene in Saccharomyces cerevisiae results in aggregation following attachment of yeast cells to extracellular matrix proteins, adherence properties similar to those of Candida albicans. Infect. Immun. 67:6040-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaur, N. K., R. L. Smith, and S. A. Klotz. 2002. Candida albicans and Saccharomyces cerevisiae expressing ALA1/ALS5 adhere to accessible threonine, serine, or alanine patches. Cell. Commun. Adhes. 9:45-57. [DOI] [PubMed] [Google Scholar]
- 16.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 17.Guo, B., C. A. Styles, Q. Feng, and G. R. Fink. 2000. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97:12158-12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyer, L. L. 2001. The ALS gene family of Candida albicans. Trends Microbiol. 9:176-180. [DOI] [PubMed] [Google Scholar]
- 19.Ishigami, M., Y. Nakagawa, M. Hayakawa, and Y. Iimura. 2004. FLO11 is essential for flor formation caused by the C-terminal deletion of NRG1 in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 237:425-430. [DOI] [PubMed] [Google Scholar]
- 20.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 21.Klotz, S. A., N. K. Gaur, D. F. Lake, V. Chan, J. Rauceo, and P. N. Lipke. 2004. Degenerate peptide recognition by Candida albicans adhesins Als5p and Als1p. Infect. Immun. 72:2029-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klotz, S. A., N. K. Gaur, J. Rauceo, D. F. Lake, Y. Park, K. S. Hahm, and P. N. Lipke. 2004. Inhibition of adherence and killing of Candida albicans with a 23-mer peptide (Fn/23) with dual antifungal properties. Antimicrob. Agents Chemother. 48:4337-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchin, S., V. K. Vyas, and M. Carlson. 2002. Snf1 protein kinase and the repressors Nrg1 and Nrg2 regulate FLO11, haploid invasive growth, and diploid pseudohyphal differentiation. Mol. Cell. Biol. 22:3994-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambrechts, M. G., F. F. Bauer, J. Marmur, and I. S. Pretorius. 1996. Muc1, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA 93:8419-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen, P. Wang, X. Pan, M. Waugh, and J. Heitman. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64:746-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, F., and S. P. Palecek. 2003. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot. Cell 2:1266-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipke, P. N., and R. Ovalle. 1998. Cell wall architecture in yeast: new structure and new challenges. J. Bacteriol. 180:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, H., C. A. Styles, and G. R. Fink. 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo, W. S., and A. M. Dranginis. 1998. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by S. cerevisiae. Mol. Biol. Cell 9:161-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo, W. S., and A. M. Dranginis. 1996. FLO11, a yeast gene related to the STA genes, encodes a novel cell surface flocculin. J. Bacteriol. 178:7144-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlean, P. 1997. Biogenesis of yeast wall and surface components, p. 229-362. In J. R. P. Broach, Jr., and E. W. Jones (ed.), The molecular biology of the yeast Saccharomyces, vol. III. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 32.Palecek, S. P., A. S. Parikh, and S. J. Kron. 2002. Sensing, signalling and integrating physical processes during Saccharomyces cerevisiae invasive and filamentous growth. Microbiology 148:893-907. [DOI] [PubMed] [Google Scholar]
- 33.Podlisny, M. B., B. L. Ostaszewski, S. L. Squazzo, E. H. Koo, R. E. Rydell, D. B. Teplow, and D. J. Selkoe. 1995. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J. Biol. Chem. 270:9564-9570. [DOI] [PubMed] [Google Scholar]
- 34.Rauceo, J. M., R. De Armond, H. Otoo, P. C. Kahn, S. A. Klotz, N. K. Gaur, and P. N. Lipke. 2006. Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryot. Cell 5:1664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rauceo, J. M., N. K. Gaur, K. G. Lee, J. E. Edwards, S. A. Klotz, and P. N. Lipke. 2004. Global cell surface conformational shift mediated by a Candida albicans adhesin. Infect. Immun. 72:4948-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds, T. B., and G. R. Fink. 2001. Baker's yeast, a model for fungal biofilm formation. Science 291:878-881. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, R. L., and G. R. Fink. 1994. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 8:2974-2985. [DOI] [PubMed] [Google Scholar]
- 38.Rupp, S., E. Summers, H. J. Lo, H. Madhani, and G. Fink. 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18:1257-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shankar, C. S., and S. Umesh-Kumar. 1994. A surface lectin associated with flocculation in brewing strains of Saccharomyces cerevisiae. Microbiology 140:1097-1101. [DOI] [PubMed] [Google Scholar]
- 40.Stanhill, A., N. Schick, and D. Engelberg. 1999. The yeast Ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol. Cell. Biol. 19:7529-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratford, M. 1992. Yeast flocculation: receptor definition by mnn mutants and concanavalin A. Yeast 8:635-645. [DOI] [PubMed] [Google Scholar]
- 42.Straver, M. H., V. M. Traas, G. Smit, and J. W. Kijne. 1994. Isolation and partial purification of mannose-specific agglutinin from brewer's yeast involved in flocculation. Yeast 10:1183-1193. [DOI] [PubMed] [Google Scholar]
- 43.Sundstrom, P. 2002. Adhesion in Candida spp. Cell. Microbiol. 4:461-469. [DOI] [PubMed] [Google Scholar]
- 44.Verstrepen, K. J., A. Jansen, F. Lewitter, and G. R. Fink. 2005. Intragenic tandem repeats generate functional variability. Nat. Genet. 37:986-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verstrepen, K. J., and F. M. Klis. 2006. Flocculation, adhesion and biofilm formation in yeasts. Mol. Microbiol. 60:5-15. [DOI] [PubMed] [Google Scholar]
- 46.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 47.Yamashita, I., T. Maemura, Y. Hatano, and S. Fukui. 1985. Polymorphic extracellular glucoamylase genes and their evolutionary origin in the yeast Saccharomyces diastaticus. J. Bacteriol. 161:574-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zara, S., A. T. Bakalinsky, G. Zara, G. Pirino, M. A. Demontis, and M. Budroni. 2005. FLO11-based model for air-liquid interfacial biofilm formation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]