Abstract
Morphogenesis control by chemical signaling molecules is beginning to be highlighted in Candida biology. The present study focuses on morphogenic compounds produced in situ by Candida albicans and Candida dubliniensis during planktonic and biofilm growth that may at least partially substantiate the effect promoted by supernatants in morphogenesis. For both species, planktonic versus biofilm supernatants were analyzed by headspace-solid-phase microextraction and gas chromatography-mass spectrometry. Both planktonic cells and biofilm supernatants of C. albicans and C. dubliniensis contained isoamyl alcohol, 2-phenylethanol, 1-dodecanol, E-nerolidol, and E,E-farnesol. Alcohol secretion profiles were species, culture mode, and growth time specific. The addition of exogenous alcohols to the cultures of both species inhibited the morphological transition from the yeast to the filamentous form by up to 50%. The physiological role of these alcohols was put to evidence by comparing the effects of a 96-h cultured supernatant with synthetic mixtures containing isoamyl alcohol, 2-phenylethanol, E-nerolidol, and E,E-farnesol at concentrations determined herein. All synthetic mixtures elicited a morphological effect similar to that observed for the corresponding supernatants when used to treat C. albicans and C. dubliniensis cultures, except for the effect of the 96-h C. dubliniensis planktonic supernatant culture on C. albicans. Overall, these results reveal a group of alcohol extracellular signaling molecules that are biologically active with C. albicans and C. dubliniensis morphogenesis.
Candida albicans is a major human pathogen causing life-threatening disseminated infections. Candidiasis epidemiology was reviewed recently (25) and emphasized the increase in cases of invasive Candida infections due mainly to an augmentation ascribed to non-Candida albicans Candida species. This increase is supported by the increased use of immunosuppressive therapy and broad-spectrum antimycotic prophylaxis (25). In particular, Candida dubliniensis, a non-Candida albicans Candida species, colonizes mostly the adult population infected with immunodeficiency virus, causing oral lesions and bloodstream infections (32).
Some mechanisms have been implicated in C. albicans and C. dubliniensis pathogenicity, namely, the morphological switch from the yeast to the filamentous form. In this sense, one of the key issues in C. albicans and C. dubliniensis is the elucidation of the mechanisms involved in morphogenesis control.
Some environmental factors have been reported as determinants of morphological regulation, particularly in C. albicans. For instance, hypha-inducing conditions include stimuli such as an increase in temperature, in pH level, in serum, nutrient starvation, and in cell density (11). In particular, at high cell densities, C. albicans grows in the yeast form, while at low densities, filamentation is observed (15). Cell density regulation is a key process not only in the planktonic mode of growth but also in biofilms, which are found in many medical devices and constitute a clinically relevant issue (9). In biofilms, this process of cell density regulation may allow overpopulation and nutrient load control, as well as filamentation regulation, which are important players in sessile development (27). The morphological switch mediated by autoregulatory metabolites produced by C. albicans cells has been investigated. Namely, 2-phenylethanol, tryptophol (3, 20), and farnesoic acid (23) were reported to control cellular germination. Moreover, Hornby et al. (15) showed that C. albicans produces E,E-farnesol that, when accumulated at a threshold level, inhibits the mycelial growth in a cell density-dependent trend. Subsequent studies demonstrated that this molecule, produced in situ by planktonic C. albicans cultures, also prevents biofilm formation (28). Increasing knowledge of cell-cell communication molecules highlighted tyrosol as a quorum-sensing signal in C. albicans (4). Regarding morphological control, tyrosol accelerates C. albicans germ tube formation under inducing conditions but does not elicit this effect under noninducing conditions. Although it is produced by planktonic and biofilm cells (1), the effect of tyrosol on biofilm formation is not clear yet (1, 3).
Nowadays, it is known that among Candida species, E,E-farnesol effects are not restricted to C. albicans. Specifically, E,E-farnesol prevents the yeast-to-pseudohyphae transition in C. dubliniensis (13) but has no effect on Candida parapsilosis morphology (30), although it reduces biofilm formation in both of these Candida species (17, 19).
In this study, the main goal was to gain insight into C. albicans and C. dubliniensis morphogenesis control through signaling molecules released into the environment.
MATERIALS AND METHODS
Chemicals.
All standard alcohols were obtained from Sigma. Stock solutions were prepared in methanol (except for isoamyl alcohol) and then used at the following concentrations: isoamyl alcohol at 46 mM, 23 mM, and 46 μM; 2-phenylethanol at 500 μM and 5 μM; 1-dodecanol at 200 μM, 2 μM, and 2 nM; and E-nerolidol and E,E-farnesol at 1.5 μM and 1.5 nM, respectively. Mixtures mimicking supernatant fractions from 96-h cultures contained isoamyl alcohol, 2-phenylethanol, E-nerolidol, and E,E-farnesol at the following concentrations: 94 μM, 70 μM, 3.2 nM, 18 nM and 48 μM, 87 μM, 9.5 nM, 5.3 nM (from C. albicans planktonic and biofilm forms, respectively); and 77 μM, 61 μM, 1.5 nM, 2.0 nM and 53 μM, 93 μM, 5.3 nM, 1.5 nM (from C. dubliniensis planktonic and biofilm forms, respectively).
Strains and culture conditions.
Two Candida species strains were used in this study, C. albicans CECT 1472 (Colección Española de Cultivos Tipo, Spain) and C. dubliniensis CBS 7987 (Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands).
Prior to each experiment, both Candida species were maintained at 37°C in Sabouraud's dextrose agar. Afterward, cells were grown in Sabouraud's dextrose broth at 37°C for 24 h on a mechanical shaker at 130 rpm. Subsequently, cells were washed twice with ultrapure water and counted using a hematocytometer. Standardized cell suspensions were prepared at a cell density of 1 × 10 6 cells/ml, unless otherwise stated, in 1× RPMI 1640 medium (Sigma) buffered with morpholinepropanesulfonic acid (Sigma) (at a final concentration of 0.165 M for a pH of 7.0).
Preparation of culture supernatants.
For planktonic supernatant preparation, 120 ml of a standardized cell suspension was inoculated in 300-ml flasks and incubated at 37°C with shaking at 130 rpm. After every 24 h of incubation, the culture medium was partially renewed (30 ml). At the selected times, supernatant fractions were filter sterilized (0.22-μm pore size) and stored at 4°C.
For biofilm supernatant, standardized cell suspensions (4 ml) were inoculated into six-well plates and incubated at 37°C with shaking at 130 rpm. After 3 h, nonadherent microorganisms were removed, and fresh medium was added to allow biofilm formation from 24 to 96 h at 37°C with shaking at 130 rpm. After every 24 h of incubation, broth was partially renewed (1 ml). The supernatant fractions were prepared as described above and stored.
Biomass dry weight measurements.
At the end of the incubation periods, 1 ml of either planktonic or resuspended biofilm cells was filtered through preweighed filters (0.45 μm) and washed three times with ultrapure sterilized water. Filters were dried at 60°C until constant weight and cell dry weight were determined. This step was repeated at least four times.
Planktonic and biofilm cells characterization.
Planktonic cultures were prepared as described above, and at selected times, cellular morphology was observed directly by light microscopy.
Biofilms were formed by seeding 2 ml of the standardized cell suspension into 24-well plates and incubating the plates for 3 h at 37°C with shaking at 130 rpm. Then, nonadherent cells were aspirated, and fresh medium was added. Plates were incubated at 37°C with shaking at 130 rpm. At the selected time points, biofilms were washed three times with ultrapure sterile water. Samples were dehydrated with alcohol (using 70% ethanol for 10 min, 95% ethanol for 10 min, and 100% ethanol for 20 min) and air dried for 20 min. Samples were kept in a desiccator until the bottoms of the wells were cut and coated with gold. Biofilm examination was performed with a Leo scanning electron microscope (Cambridge). Biofilm analyses were repeated three times.
Supernatant alcohol analyses.
Supernatant alcohols were extracted using headspace-solid-phase microextraction (HS-SPME) and analyzed by gas chromatography-mass spectrometry (GC-MS). The SPME holder for manual sampling and the fiber used were purchased from Supelco (Aldrich, Bellefonte, PA). The SPME device included a fused silica fiber, partially cross-linked Carbowax/divinylbenzene (Sigma) with 65-μm film thickness, containing a liquid polymer, and solid particles (26). The Carbowax/divinylbenzene-coated fiber is recommended for small and polar molecules (with a molecular weight between 40 and 275), such as those under study. The SPME fiber was conditioned at 250°C for 30 min in the GC injector port, according to the manufacturer's recommendations. For headspace sampling, 20 ml of each supernatant fraction was introduced into a 60-ml glass vial. The vial was capped with a poly-tetrafluoroethylene septum and an aluminum cap (Chromacol Ltd., Herts, United Kingdom) after 4 g of NaCl and a 20-mm by 5-mm stirring bar (at 200 rpm) were added and placed in a thermostatically controlled bath adjusted to 40°C ± 0.1°C for 15 min to transfer the compounds from the sample to the headspace. Following this step, the SPME fiber was manually inserted into the sample vial headspace for 45 min and then introduced into the GC injection port at 250°C and kept for 5 min for alcohol thermal desorption. The GC-MS parameters were established according to the report by Coelho et al. (5). The injection port was lined with a 0.75-mm-inside-diameter splitless glass liner. The desorbed volatile compounds were separated in a GC-MS Agilent Technologies 6890 N Network gas chromatography unit equipped with a fused silica capillary column (30-m by 0.32-mm inside diameter; 0.25-μm film thickness; DB-FFAP model; J&W Scientific Inc., Folsom, CA) connected to an Agilent 5973 quadrupole mass selective detector. The splitless injection mode was used (5 min). The oven temperature was programmed from 35°C to 220°C at 2°C/min, and the transfer line was heated at 250°C. Helium carrier gas had a flow of 1.7 ml/min. The mass spectrometer was operated in the electron impact mode at 70 eV, scanning the range of 33 to 300 m/z in a 1-s cycle, in a full scan acquisition mode. The identification of Candida metabolites was achieved by comparing the GC retention times and mass spectra with those of the pure standard compounds. All mass spectra were also compared with the data system library (Wiley 275 database). A comparable analysis was done with growth medium, and no interfering substances were found in or near the retention times of those compounds. The quantification was performed using a preparation of RPMI medium solutions containing pure standards under the same conditions as those of the samples. For each compound, appropriate concentration ranges were chosen in order to include sample concentrations. Standard curves were generated for GC-MS peak areas versus the concentration of each compound (R2 > 0.98), with quantification performed above the quantification limit. All measurements were made with at least two replicates, each replicate representing the analysis of one different aliquot (20 ml) of each supernatant fractions. This approach allows the GC peak area data to be used as an indirect approach to estimate the relative content of each volatile compound. Blanks, corresponding to the analysis of the coating fiber that was not subjected to any extraction procedure, were run between sets of three analyses. Alcohol analyses in planktonic and biofilm supernatant fractions were carried out in duplicate for each sample with an agreement of results within ≤15%.
Filamentation and growth assays.
Standardized cell suspensions were prepared in 2× RPMI medium and diluted (1:1) with either C. albicans or C. dubliniensis supernatant prepared as described above. Cultures were incubated for 12 h at 37°C with shaking at 130 rpm. A control consisting of RPMI medium without culture supernatant was used.
Standardized cell suspensions were prepared in RPMI medium with the specific compounds at specified concentrations or in synthetic mixtures mimicking supernatant fractions from 96-h cultures. All the suspensions were incubated for 12 h at 37°C with shaking at 130 rpm. For each experiment, an equal volume of solvent was used as an appropriate control, as well as culture medium without the specific compound. Cell morphology was evaluated according to the method described by Henriques et al. (13). All experiments were repeated at least three times with duplicate samples, and 10 fields per slide with at least 150 cells were examined. Morphological analyses of methanol-treated and untreated controls had agreement of more than 97%. Assays regarding supernatant effects were standardized against cell dry weight.
To investigate the effect of isoamyl alcohol, 2-phenylethanol, 1-dodecanol, E-nerolidol, and E,E-farnesol on Candida cell growth, batches of cultures were grown for 12 h at 37°C with shaking at 130 rpm in the presence and absence of these alcohols as well as appropriate methanol controls, and cell optical density was measured at 620 nm. Optical densities of these cells were compared to those of the RPMI controls. Cell growth monitoring was performed in three independent assays. No growth inhibition was detected in the methanol-treated controls.
Statistical analysis.
Statistical analysis was performed using GraphPad Prism, version 5.00 software for Windows.
Normality of data distribution was tested by the Kolmogorov-Smirnov method.
A two-tailed paired t test method was used to determine differences between (i) the means of optical densities of C. albicans and C. dubliniensis cultures grown in RPMI medium and those grown in RPMI medium plus the tested alcohols, (ii) the means of the effects of 96-h supernatant versus those of the corresponding synthetic mixture on both Candida species' filamentation inhibition, and (iii) the means of RPMI medium-grown cells versus that of methanol controls in growth and filamentation assays.
Statistical significance values of the groups' means of (i) the cultures' cell dry weights, (ii) the effects of culture supernatants, and (iii) the effects of synthetic alcohols versus supernatants on Candida species morphology were evaluated using a one-way analysis of variance. Subsequent comparisons were performed using Tukey's post-hoc test.
A two-way analysis of variance was used to test for eventual interactions between (i) the cultures' cell dry weights and (ii) the effects of culture supernatants on cell morphology and Candida species.
RESULTS
Characterization of planktonic and biofilm cells.
The morphology of C. albicans and C. dubliniensis planktonic cells, which produced the supernatant, was monitored by light microscopy with 24-, 48-, 72-, and 96-h cultures (results not shown). Most of the C. albicans cultures at 24 h consisted of cells in the hyphal form, while cultures from 48 and 72 h showed an increase in the yeast form, and pseudohyphae were found. Twenty-four-hour C. dubliniensis cultures presented a prevalence of pseudohyphae, but cultures from 48 to 96 h showed an increase in yeast forms.
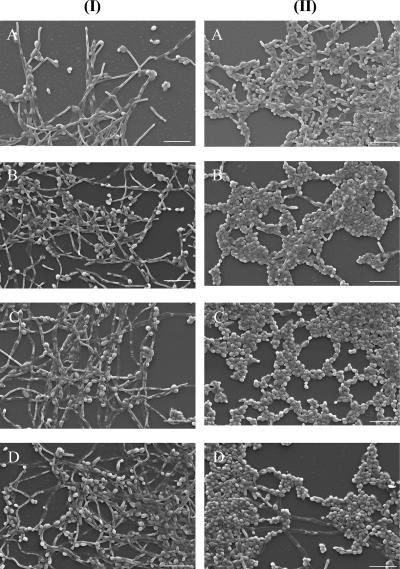
Scanning electron microscopy observation of C. albicans biofilms showed cells in the filamentous form and some in the yeast form, despite the biofilm's age (Fig. 1, column I). C. dubliniensis 24- and 48-h biofilms comprised pseudohyphae and yeast forms (Fig. 1, column II, panels A and B). However, 72-h biofilms were composed mostly of yeast cells, and pseudohyphae were rarely observed (Fig. 1, column I, panel C). Additionally, 96-h C. dubliniensis biofilms (Fig. 1, column II, panel D) contained, again, a heterogeneous mixture of yeast and pseudohypha forms.
FIG. 1.
Scanning electron microscopy of C. albicans (I) and C. dubliniensis (II) biofilms grown over 24 h (A), 48 h (B), 72 h (C), and 96 h (D) (magnification, ×1,000). Bar, 20.0 μm.
Effect of planktonic and biofilm culture supernatants on C. albicans and C. dubliniensis filamentation.
One of the aims of this work was to assess the effect of C. albicans and C. dubliniensis planktonic and biofilm supernatants on inter- and intraspecies morphological transition. It should be noted that for each assay, the percentage of inhibition reveals the relationship between the percentage of hypha inhibition in C. albicans and the percentage of pseudohypha inhibition in C. dubliniensis. Also, the averages of biomass dry weight from C. albicans planktonic cells were significantly higher than those of the biofilms, except at 24 h. An opposite relationship was observed for C. dubliniensis cultures. Comparing both species' cell dry weights, no statistical differences were detected, except for the 24-h biofilms.
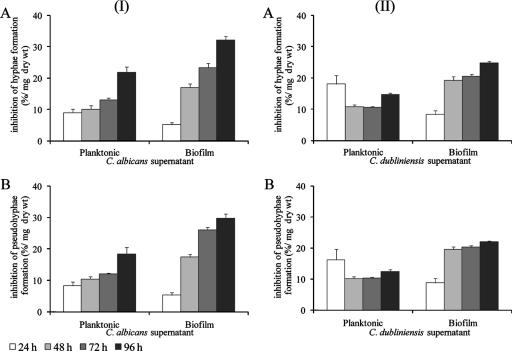
C. albicans supernatants displayed an inhibitory activity in the morphological transition from the yeast to the filamentous form for both species (Fig. 2, column I). For C. albicans cells treated with their own planktonic supernatant (Fig. 2, column I, panel A), the percentages of inhibition of hypha formation significantly increased from 48 to 96 h. The evaluation of the effect of the same supernatants on C. dubliniensis (Fig. 2, column I, panel B) showed the highest inhibition of pseudohypha formation for the 96-h fraction (P < 0.001). The C. albicans biofilm supernatant markedly increased the percentage of inhibition with increasing culture time in both of the Candida species (Fig. 2, column I, panels A and B).
FIG. 2.
Effect of time on C. albicans supernatants (I) and C. dubliniensis supernatants (II) from planktonic and biofilm cultures on the morphology of 12-h planktonic growth of C. albicans (A) and C. dubliniensis (B). Filamentation was assessed as the number of hyphae in C. albicans and number of pseudohyphae in C. dubliniensis per 10 slides counted in duplicate samples. Control cells were incubated in RPMI medium, representing >90% of hyphae plus 2% of pseudohyphae for C. albicans and >60% of pseudohyphae for C. dubliniensis. The percentages of inhibition in the three independent assays were determined as a mean percentage of reduction of that for controls, as a function of mg of dry weight ± standard deviation. For both planktonic and biofilm cultures, at every 24 h of incubation, the medium was partially renewed, as described in Materials and Methods.
Furthermore, the use of the C. dubliniensis supernatant, from either planktonic or biofilm cells, also prevented the transition from yeast to filamentous form not only in C. albicans but in C. dubliniensis as well (Fig. 2, column II). Regarding the planktonic supernatant effect on the morphological transition of C. albicans (Fig. 2, column II, panel A) and C. dubliniensis (Fig. 2, column II, panel B) cells, the highest effect was observed for the 24-h fraction (P < 0.001). Additionally, the 24-h biofilm supernatant of C. dubliniensis had a significantly lower effect in both Candida species (Fig. 2, column II).
Biofilm spent media from both C. albicans (Fig. 2, column I) and C. dubliniensis (Fig. 2, column II) cultured for longer periods (72 and 96 h) were significantly more effective in the reduction of filamentation than their planktonic equivalents in both species.
Overall, the present results show that supernatant composition may be dependent on the Candida species, differing not only due to the mode of growth but also to the growth time.
Characterization of planktonic and biofilm supernatant composition.
Supernatant samples were analyzed by HS-SPME GC-MS. Comparison between the retention times and mass spectra of the library and those of the chemically synthesized standards identified five of the peaks as isoamyl alcohol, 2-phenylethanol, 1-dodecanol, E-nerolidol, and E,E-farnesol (results not shown). The results, expressed as a function of cell dry weight (Table 1), reveal that these compounds are continuously excreted by planktonic and biofilm cells.
TABLE 1.
Concentration of secreted alcohols in C. albicans and C. dubliniensis planktonic and biofilm supernatant fractionsa
| Culture | Culture time (h) | Concn of secreted alcohol per g (dry wt) of cells for:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
C. albicans
|
C. dubliniensis
|
||||||||
| Isoamyl alcohol (μmol/g [dry wt] of cells) | 2-Phenylethanol (μmol/g [dry wt] of cells) | E-Nerolidol (nmol/g [dry wt] of cells) | E,E-Farnesol (nmol/g [dry wt] of cells) | Isoamyl alcohol (μmol/g [dry wt] of cells) | 2-Phenylethanol (μmol/g [dry wt] of cells) | E-Nerolidol (nmol/g [dry wt] of cells) | E,E-Farnesol (nmol/g [dry wt] of cells) | ||
| Planktonic | 24 | 64.09 | 7.37 | 0.7 | 0.02 | 68.79 | 15.4 | 0.74 | 0.07 |
| 48 | 58.23 | 18.44 | 2.42 | 0.59 | 42.15 | 14.35 | 2.29 | 2.13 | |
| 72 | 59.36 | 24.62 | 2.03 | 0.25 | 20 | 19.57 | 0.79 | 0.2 | |
| 96 | 57.91 | 43.22 | 1.96 | 11.08 | 32.9 | 25.8 | 0.64 | 0.84 | |
| Biofilm | 24 | 12.94 | 5.29 | 0.46 | 0.02 | 15.32 | 7.26 | 1.3 | 0.15 |
| 48 | 45.17 | 29.39 | 2.94 | 0.16 | 25.9 | 41.87 | 3.07 | 1.59 | |
| 72 | 72.49 | 37.12 | 7.53 | 0.5 | 22.7 | 31.46 | 4.09 | 0.65 | |
| 96 | 46.12 | 88.77 | 8.8 | 5.08 | 37.99 | 67.13 | 3.8 | 1.05 | |
Alcohol secretion analyses of planktonic and biofilm supernatant fractions per cell dry weight were carried out in duplicate for each sample, with an agreement of results within ≤15%. For both planktonic and biofilm cultures, at every 24 h of incubation, the medium was partially renewed, as described in Materials and Methods.
The amounts of isoamyl alcohol in C. albicans planktonic supernatants were very similar (Table 1). However, the 24-h biofilm cultures released less isoamyl alcohol than those biofilms cultured for longer periods. For C. dubliniensis planktonic cells (Table 1), the highest amount of isoamyl alcohol released was observed for 24-h cultures, while for biofilm supernatants, isoamyl alcohol concentration were slightly increased with maturation.
Considering the release of 2-phenylethanol by C. albicans planktonic and biofilm cells (Table 1), the level of this aromatic alcohol in the supernatant medium increased during the transition from 24 to 96 h of growth time. In contrast, C. dubliniensis biofilm and planktonic cells exhibited different alcohol secretion patterns (Table 1). Indeed, culture media from 96-h biofilms contained higher 2-phenylethanol levels than the ones obtained from 24-h biofilms, in contrast to an approximately constant level of 2-phenylethanol production exhibited by planktonic culture supernatant.
Amounts of E-nerolidol in C. albicans planktonic supernatants (Table 1) were approximately constant from 48 to 96 h. However, in C. albicans biofilm supernatants, the concentration of this sesquiterpenol rose continuously along with biofilm development, leveling off at 72 h. C. dubliniensis displayed a different E-nerolidol excretion profile, according to the growth mode (Table 1). In fact, planktonic cells that excreted higher levels of this alcohol belong to the 48-h population. In biofilms, the highest amounts of E-nerolidol were detected in samples recovered at more than 48 h of growth. For E,E-farnesol excretion by C. albicans, a similar profile was observed for both planktonic and sessile cells (Table 1), attaining the highest level of excretion at 96 h of growth. In the case of C. dubliniensis, E,E-farnesol reached the maximum experimental concentration in 48-h supernatant fractions.
1-Dodecanol was detected in all supernatant samples from both Candida species at concentrations lower than 2.79 nM (results not shown).
Effect of secreted alcohols on C. albicans and C. dubliniensis filamentation and growth.
The addition of 46 mM isoamyl alcohol to both Candida species growth media inhibited cell growth (Table 2). However, at 46 μM and 23 mM concentrations of this compound, filamentation was inhibited. Reduction of the isoamyl alcohol concentration significantly repressed filamentation in both Candida species (Table 2).
TABLE 2.
Effect of secreted alcohols on the growth and filamentation inhibition of planktonic C. albicans and C. dubliniensisa
| Compound | Conc |
C. albicans
|
C. dubliniensis
|
||
|---|---|---|---|---|---|
| % of growth inhibition ± SD | % of hypha inhibition ± SD | % of growth inhibition ± SD | % of pseudohypha inhibition ± SD | ||
| Isoamyl alcohol | 46 mM | 77.7 ± 1.4 | NA | 89.2 ± 0.5 | NA |
| 23 mM | 95.8 ± 3.0 | 92.4 ± 2.1 | |||
| 46 μM | 68.4 ± 12.3 | 70.7 ± 15.6 | |||
| 2-Phenylethanol | 500 μM | 95.3 ± 1.23 | 91.5 ± 10.7 | ||
| 5 μM | 71.4 ± 15.7 | 72.9 ± 17.0 | |||
| 1-Dodecanol | 200 μM | 98.7 ± 7.9 | 93.3 ± 0.8 | NA | |
| 2 μM | 69.9 ± 16.8 | 74.5 ± 9.6 | |||
| 2 nM | 57.8 ± 14.3 | 54.1 ± 12.1 | |||
| E-Nerolidol | 1.5 μM | 64.8 ± 4.7 | 64.6 ± 7.8 | ||
| 1.5 nM | 51.1 ± 9.3 | 68.3 ± 5.0 | |||
| E,E-Farnesol | 1.5 μM | 67.5 ± 10.1 | 74.1 ± 17.8 | ||
| 1.5 nM | 63.5 ± 10.8 | 57.8 ± 4.8 | |||
Percentage of growth inhibition was determined as a reduction of optical density at 620 nm of three independent assays compared to that of controls. Mean optical densities for the controls were 0.51 for C. albicans and 0.57 for C. dubliniensis. Filamentation was assessed as the number of hyphae in C. albicans and the number of pseudohyphae in C. dubliniensis per 10 slides, counted in duplicate samples. Control cells were incubated in RPMI medium, presenting >90% of hyphae plus 2% of pseudohyphae for C. albicans and >60% of pseudohyphae for C. dubliniensis. Percentages of inhibition in the three independent assays were determined as mean percentages of reduction of that for controls ± standard deviation (SD). NA, not applicable.
In the present study, two 2-phenylethanol concentrations were assayed, 5 and 500 μM, and no arrest of cell growth was observed for both concentrations. The assay at 5 μM concentration showed a 71.4% ± 15.8% reduction in hyphal formation for C. albicans, with a 72.9% ± 17.0% inhibition of pseudohyphal formation for C. dubliniensis.
Additionally, effects of 1-dodecanol were assayed at concentrations of 2 nM, 2 μM, and 200 μM. C. albicans revealed no growth inhibition at any of the concentrations assayed. In contrast, 200 μM 1-dodecanol reduced C. dubliniensis cell growth in more than 90% (Table 2). However, 1-dodecanol elicits morphogenic inhibition in both Candida species, even at a concentration of 2 nM.
Under the present conditions, 1.5 nM or 1.5 μM of E-nerolidol promoted a reduction in both Candida species' filamentation (Table 2), without growth inhibition. It may be stressed that for both Candida species, no significant differences were observed concerning the effects triggered by E-nerolidol at 1.5 nM and 1.5 μM.
E,E-farnesol control (1.5 μM) was included, and the percentages of inhibition obtained were similar to those described previously (13). Additionally, 1.5 nM E,E-farnesol was observed to inhibit cellular germination in both Candida species (Table 2). Similar to the effects of E-nerolidol, a decrease in E,E-farnesol concentration from 1.5 μM to 1.5 nM did not result in a significant decrease in filamentation inhibition for C. dubliniensis.
On the whole, the results show that these compounds, also present in supernatant fractions (Table 1), regulate yeast cell morphology but do not result in significant growth constraints.
Effect of planktonic and biofilm culture simulated supernatant on C. albicans and C. dubliniensis filamentation.
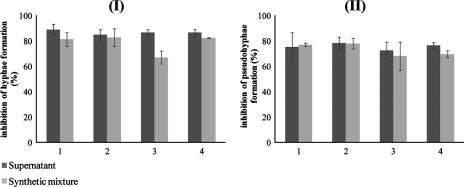
Considering all the conditions assayed (Fig. 3), no significant differences between the means of the effects of supernatants and those of synthetic mixtures on Candida filamentation inhibition were encountered, except for the effect of C. dubliniensis planktonic supernatant on C. albicans (Fig. 3, chart I, condition 3) (P = 0.02).
FIG. 3.
Effect of 96-h supernatants and corresponding synthetic mixtures on the morphology of 12-h planktonic cells of C. albicans (I) and C. dubliniensis (II). Condition 1, C. albicans planktonic supernatant; 2, C. albicans biofilm supernatant; 3, C. dubliniensis planktonic supernatant, and 4, C. dubliniensis biofilm supernatant. Filamentation was assessed as the number of hyphae in C. albicans and number of pseudohyphae in C. dubliniensis per 10 slides counted in duplicate samples. Control cells were incubated in RPMI medium, representing >90% of hyphae plus 2% of pseudohyphae for C. albicans and >60% of pseudohyphae for C. dubliniensis. The percentages of inhibition in the three independent assays were determined as the means of percentage of reduction of that for controls ± standard deviations.
DISCUSSION
Within the predominantly human pathogenic fungi are the well-recognized species C. albicans and the emerging species C. dubliniensis. Both Candida species are phenotypically very similar, and a unifying feature is that both are polymorphic, undergoing interconversion into filamentous and yeast forms. However, C. dubliniensis appears to have less ability to cause disease, and to ascertain this issue “will enhance our understanding of candidal pathogenesis in general” (32).
Results show (Fig. 2, column I, panel A, and column II, panel B) that the addition of supernatant medium from planktonic and biofilm cultures prevents the filamentation of planktonic cells of the same species. Similar results were observed by crossing supernatant fractions between species (Fig. 2, column I, panel B, and column II, panel A). These data are in accordance with those of previous reports (17) showing inter- and intraspecies effects of C. albicans and C. dubliniensis supernatant, although different strains were used.
Isoamyl alcohol, 2-phenylethanol, 1-dodecanol, E-nerolidol, and E,E-farnesol were detected in supernatants of planktonic and biofilm forms of both Candida species by HS-SPME GC-MS. Different studies have reported the effects of these alcohols on the filamentation of Saccharomyces cerevisiae and C. albicans (3, 7, 8, 14, 15). Previous reports also showed that C. albicans yeast cells secret tyrosol and tryptophol at concentrations that are within micromolar levels (1, 3). However, those compounds were not detected in the present study. Failure to detect tyrosol and tryptophol may have been due to the SPME experimental conditions used and/or to the high solubility of these compounds in water.
The E,E-farnesol concentrations determined in the supernatants (Table 1) were lower than 11.08 nM. Hornby and Nickerson (16) reported that six out of seven C. albicans reference strains and clinical isolates produce E,E-farnesol at concentrations in hundreds of nanomolar. The exception was the reference strain C. albicans ATCC 10231, for which E,E-farnesol, if present, was below the limit of detection for the method used (22.5 nmol/g [dry weight] of cells). However, using HS-SPME/GC-MS, the limit of detection obtained for E,E-farnesol was 0.01 nM (results not shown). Therefore, it seems likely that C. albicans ATCC 10231 could produce E,E-farnesol within the levels observed for C. albicans CECT 1472 and C. dubliniensis CBS 7987, used herein.
A time-based comparison of supernatant composition (Table 1) highlights the differential excretion patterns of these alcohols, as previously inferred from supernatant effects with Candida species planktonic morphology assays shown in Fig. 2.
Concerning C. dubliniensis, neither of the quantified compounds (Table 1) supports the high effect triggered by its 24-h planktonic supernatant in the inhibition of self-filamentation (Fig. 2, column II, panel B). Probably, other molecules not identified in this analysis may contribute to the result observed.
Moreover, from 48 h onward, the alcohol concentrations in C. albicans supernatants (planktonic and biofilm) are higher than those in C. dubliniensis (Table 1). These results suggest that during its ongoing growth, C. dubliniensis can be overcome by C. albicans, which corroborates one of the hypotheses postulated by Sullivan et al. (32), whereby C. albicans would be more “robust” than C. dubliniensis. In fact, previous reports concerning C. albicans and C. dubliniensis fit these results. Reports of in vitro studies showed that C. albicans has growth-competitive advantages over C. dubliniensis, more evident at 96 h for planktonic than for biofilm growth conditions (18). Results presented in Fig. 3 may support this behavior, since it can be inferred that C. dubliniensis secretes substances into the environment that specifically regulate C. albicans morphogenesis. Conversely, in vivo studies (31) showed that similar CFU counts were obtained for both species at day 2, although the C. dubliniensis yeast number decreased to nondetectable values at days 8 and 10.
Other competitive interactions described in the literature show that 2-phenylethanol selectively inhibits certain gram-positive and gram-negative bacteria (12), which could provide growth advantages to C. albicans and C. dubliniensis.
Another important aspect is that a distinct biofilm alcohol secretion pattern was found, which is consistent with the microbiological regulation of a multicellular behavior that includes protection from the environment, nutrient availability, and metabolic cooperation. Results from Table 1 show that supernatants from biofilm cells at the latest growth states contain more 2-phenylethanol and E-nerolidol than the planktonic supernatants. Interestingly, a similar relationship has been described for prostaglandins (2) and tyrosol secretion by C. albicans (1). However, this behavior was not observed for isoamyl alcohol and E,E-farnesol. This may explain, at least in part, the higher percentage of inhibition of filamentation induced by biofilm supernatant, in contrast to that of the planktonic supernatants (Fig. 2).
An earlier study with C. albicans reported that a mixture of nerolidol isomers was two times less active than E,E-farnesol (15). However, the results obtained in this work show that both E-nerolidol and E,E-farnesol have an equivalent level of cell morphology activity (Table 2).
Herein, 5 μM of 2-phenylethanol and 2 nM of 1-dodecanol inhibited (>50%) the morphological transition from yeast to filamentous form in both Candida species (Table 2). Other authors described morphological changes only at higher concentrations, e.g., 500 μM for 2-phenylethanol (3) and 10 μM for 1-dodecanol (14). These discrepancies may be due to the distinct design of filamentation assays, although different strains' sensitivities to these alcohols may not be excluded.
Furthermore, isoamyl alcohol is equivalent to 2-phenylethanol and to tryptophol as an inhibitor of C. albicans and C. dubliniensis filamentous growth but is a stimulator of S. cerevisiae filamentation (3, 6). This differential response may be supported by the fact that the S. cerevisiae response to isoamyl alcohol involves the phosphorylation of Cdc28 at tyrosine 19 by Swe1 (21), a protein not involved in C. albicans filamentation (33).
Data in Table 2 show that these alcohols elicit a morphogenic inhibition at physiological concentrations in both Candida species. When 96-h supernatants from both species were mimicked with isoamyl alcohol, 2-phenylethanol, E-nerolidol, and E,E-farnesol compositions, similar percentages of inhibition were achieved (Fig. 3). These results suggest that the major morphogenic compounds produced in situ by C. albicans and C. dubliniensis in the extracellular medium have been identified. The exception was the mixture that mimicked the C. dubliniensis planktonic supernatant, which elicited a lower morphological response with C. albicans than the supernatant (Fig. 3, chart I, condition 3), suggesting that C. dubliniensis may release a nonidentified compound(s) that specifically controls C. albicans morphogenesis.
Chen and Fink (3) suggested that in S. cerevisiae, 2-phenylethanol and tryptophol might have some synergic effects regarding invasive growth. However, the effect triggered by the specific mixtures of alcohols (Fig. 3) is slightly lower than that expected from the percentages of inhibition obtained for each single compound (Table 2). These observations suggest two hypotheses: a competition phenomenon and a saturation process.
Furthermore, it should be stressed that these studies were performed in vitro. Environmental conditions might influence the properties of microorganisms, and in the laboratory, it is difficult to mimic natural conditions and their heterogeneous parameters (24, 29). In an attempt to approximate the natural environment under experimental conditions, RPMI medium was used. This may in part simulate human host conditions; however, the amino acid composition of this growth medium might determine the molecules detected (Table 1), namely, isoamyl alcohol and 2-phenylethanol. In addition, cell culture supernatant media were partially replaced every 24 h, and in fact, in the environment, partial nutrient replacement may occur. Nevertheless, metabolites detected at each time point assayed (Table 1) may also account for additional metabolism, but this experimental parameter was maintained along all culture times. However, in vivo, the effects and excretion patterns may certainly differ. Some authors noted that the E,E-farnesol concentrations needed to block germination in C. albicans (22) and C. dubliniensis (13) in serum are different from the concentrations observed in RPMI. So, in vivo, the effectiveness of these alcohols under the control of morphogenesis may be distinct. Moreover, E,E-farnesol is not produced under anoxia (10), which is important for some tissue invasion. Thus, it is reasonable to ask if the regulation of synthesis and/or secretion of the other alcohols identified within this study could be different in vivo.
In summary, several lines of evidence provide new insights into C. albicans and C. dubliniensis metabolites, as well as their roles in morphogenesis control. Specifically, isoamyl alcohol, 2-phenylethanol, E-nerolidol, and E,E-farnesol were identified in the extracellular medium of planktonic and biofilm cells. Besides, these alcohols were shown to inhibit the morphological transition in these Candida species, to similar degrees, at physiological concentrations. Despite cell morphology, signaling molecules were secreted at different profiles depending on the Candida species' stage and mode of growth (planktonic and biofilm). Overall, this represents a breakthrough in understanding morphogenesis control in both Candida species, and inter- and intraspecies interactions may now be better understood.
Acknowledgments
We thank Fundação para a Ciência e Tecnologia (FCT), Portugal, for support through grant SFRH/BD/28222/2006 and project POCI/BIO/61112/2004.
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Alem, M. A., M. D. Oteef, T. H. Flowers, and L. J. Douglas. 2006. Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development. Eukaryot. Cell 5:1770-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alem, M. A. S., and L. J. Douglas. 2005. Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 54:1001-1005. [DOI] [PubMed] [Google Scholar]
- 3.Chen, H., and G. R. Fink. 2006. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 20:1150-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, H., M. Fujita, Q. Feng, J. Clardy, and G. R. Fink. 2004. Tyrosol is a quorum-sensing molecule in Candida albicans. Proc. Natl. Acad. Sci. USA 101:5048-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho, E., S. M. Rocha, I. Delgadillo, and M. A. Coimbra. 2006. Headspace-SPME applied to varietal volatile components evolution during Vitis vinifera L. cv. “Baga” ripening. Anal. Chim. Acta 563:204-214. [Google Scholar]
- 6.Dickinson, J. R. 1996. “Fusel” alcohols induce hyphal-like extensions and pseudohyphal formation in yeasts. Microbiology 142:1391-1397. [DOI] [PubMed] [Google Scholar]
- 7.Dickinson, J. R., M. M. Lanterman, D. J. Danner, B. M. Pearson, P. Sanz, S. J. Harrison, and M. J. Hewlins. 1997. A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 272:26871-26878. [DOI] [PubMed] [Google Scholar]
- 8.Dickinson, J. R., L. E. Salgado, and M. J. E. Hewlins. 2003. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J. Biol. Chem. 278:8028-8034. [DOI] [PubMed] [Google Scholar]
- 9.Donlan, R. M. 2001. Biofilm formation: a clinically relevant microbiological process. Clin. Infect. Dis. 33:1387-1392. [DOI] [PubMed] [Google Scholar]
- 10.Dumitru, R., J. M. Hornby, and K. W. Nickerson. 2004. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 48:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, J. F. 2000. Transcription factors in Candida albicans: environmental control of morphogenesis. Microbiology 146:1763-1774. [DOI] [PubMed] [Google Scholar]
- 12.Fraud, S., E. L. Rees, E. Mahenthiralingam, A. D. Russell, and J. Y. Maillard. 2003. Aromatic alcohols and their effect on Gram-negative bacteria, cocci and mycobacteria. J. Antimicrob. Chemother. 51:1435-1436. [DOI] [PubMed] [Google Scholar]
- 13.Henriques, M., M. Martins, J. Azeredo, and R. Oliveira. 2007. Effect of farnesol on Candida dubliniensis morphogenesis. Lett. Appl. Microbiol. 44:199-205. [DOI] [PubMed] [Google Scholar]
- 14.Hogan, D. A., A. Vik, and R. Kolter. 2004. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol. Microbiol. 54:1212-1223. [DOI] [PubMed] [Google Scholar]
- 15.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hornby, J. M., and K. W. Nickerson. 2004. Enhanced production of farnesol by Candida albicans treated with four azoles. Antimicrob. Agents Chemother. 48:2305-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabra-Rizk, M. A., M. Shirtliff, C. James, and T. Meiller. 2006. Effect of farnesol on Candida dubliniensis biofilm formation and fluconazole resistance. FEMS Yeast Res. 6:1063-1073. [DOI] [PubMed] [Google Scholar]
- 18.Kirkpatrick, W. R., J. L. Lopez-Ribot, R. K. Mcatee, and T. F. Patterson. 2000. Growth competition between Candida dubliniensis and Candida albicans under broth and biofilm growing conditions. J. Clin. Microbiol. 38:902-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laffey, S. F., and G. Butler. 2005. Phenotype switching affects biofilm formation by Candida parapsilosis. Microbiology 151:1073-1081. [DOI] [PubMed] [Google Scholar]
- 20.Lingappa, B. T., M. Prasad, Y. Lingappa, D. F. Hunt, and K. Biemann. 1969. Phenethyl alcohol and tryptophol: autoantibiotics produced by the fungus Candida albicans. Science 163:192-194. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Anaya, C., J. R. Dickinson, and P. E. Sudbery. 2003. In yeast, the pseudohyphal phenotype induced by isoamyl alcohol results from the operation of the morphogenesis checkpoint. J. Cell Sci. 116:3423-3431. [DOI] [PubMed] [Google Scholar]
- 22.Mosel, D. D., R. Dumitru, J. M. Hornby, A. L. Atkin, and K. W. Nickerson. 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl. Environ. Microbiol. 71:4938-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh, K. B., H. Miyazawa, T. Naito, and H. Matsuoka. 2001. Purification and characterization of an autoregulatory substance capable of regulating the morphological transition in Candida albicans. Proc. Natl. Acad. Sci. USA 98:4664-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palková, Z. 2004. Multicellular microorganisms: laboratory versus nature. EMBO Rep. 5:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pillonel, L., J. O. Bosset, and R. Tabacchi. 2002. Rapid preconcentration and enrichment techniques for the analysis of food volatile. A review. Lebensm. Wiss. Technol. 35:1-14. [Google Scholar]
- 27.Ramage, G., S. P. Saville, D. P. Thomas, and J. L. Lopez-Ribot. 2005. Candida biofilms: an update. Eukaryot. Cell 4:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramage, G., S. P. Saville, B. L. Wickes, and J. L. Lopez-Ribot. 2002. Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl. Environ. Microbiol. 68:5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ritz, K., and J. W. Crawford. 1999. Colony development in nutritionally heterogeneous environments, p. 49-74. In N. A. R. Gow, G. D. Robson, and G. M. Gadd (ed.), The fungal colony, 1st ed. British Mycological Society, Cambridge University Press, Cambridge, United Kingdom.
- 30.Rossignol, T., M. E. Logue, K. Reynolds, M. Grenon, N. F. Lowndes, and G. Butler. 2007. Transcriptional response of Candida parapsilosis following exposure to farnesol. Antimicrob. Agents Chemother. 51:2304-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stokes, C., G. P. Moran, M. J. Spiering, G. T. Cole, D. C. Coleman, and D. J. Sullivan. 2007. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet. Biol. 44:920-931. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan, D. J., G. P. Moran, and D. C. Coleman. 2005. Candida dubliniensis: ten years on. FEMS Microbiol. Lett. 253:9-17. [DOI] [PubMed] [Google Scholar]
- 33.Wightman, R., S. Bates, P. Amornrrattanapan, and P. Sudbery. 2004. In Candida albicans, the Nim1 kinases Gin4 and Hsl1 negatively regulate pseudohypha formation and Gin4 also controls septin organization. J. Cell Biol. 164:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]