Abstract
Endocytosis is the process by which substrates enter a cell without passing through the plasma membrane but rather invaginate the cell membrane and form intracellular vesicles. Rab7 regulates endocytic trafficking between early and late endosomes and between late endosomes and lysosomes. LeRab7 in Lentinula edodes is strongly homologous to Rab7 in Homo sapiens. Receptors for activated C kinase-1 (LeRACK1) and Rab5 GTPase (LeRAB5) were isolated as interacting partners of LeRab7, and the interactions were confirmed by in vivo and in vitro protein interaction assays. The three genes showed differential expression in the various developmental stages of the mushroom. In situ hybridization showed that the three transcripts were localized in regions of active growth, such as the outer region of trama cells, and the subhymenium of the hymenophore of mature fruiting bodies and the prehymenophore of young fruiting bodies. The existence of endocytosis in the mycelium and hymenophores was confirmed by the internalization of FM4-64. LeRAB7 was partially colocalized with the AM4-64 and was located in the late endocytic pathway. This is the first report of the presence of endocytosis in homobasidiomycetes. LeRAB7, LeRAB5, and LeRACK1 may contribute to the growth of L. edodes and cell differentiation in hymenophores.
Homobasidiomycetes differentiate dramatically during their development from mycelium stage to fruiting body, in which basidiospores are produced for reproduction. The homobasidiomycete Lentinula edodes (Shiitake) is one of the most popular edible mushrooms. An understanding of the molecular mechanism that controls the initiation and maturation of the fruiting body will thus be invaluable for the cultivation of L. edodes.
Endocytosis is a biological process by which substrates enter a cell through the invagination of the cell membrane and the formation of intracellular vesicles, all of which takes place without passage through the plasma membrane. In eukaryotic cells, it is an essential process in the recycling or removal of membrane proteins and lipids, the transport of proteins and lipids into cells for degradation and the uptake of signal molecules (34). Other cellular activities are also mediated by endocytic mechanisms, including the uptake of extracellular nutrients, the regulation of cell surface receptor expression, the maintenance of cell polarity, and the presentation of antigens (34). The process of endocytosis involves complex and tightly regulated steps in membrane trafficking and fusion. Fusion specificity is controlled by several protein factors, including Rabs, SNAP receptors, and tethering proteins (47). The mechanism of endocytosis in plants and animals has been extensively studied (34), and evidence for endocytosis has been found in budding yeasts (18) and filamentous fungi, such as Aspergillus nidulans (45), Neurospora crassa (16), and Ustilago maydis (14). The cellular slime mold Dictyostelium also feeds through an endocytic pathway (31).
The family of 60 Rab GTPases constitutes the largest and most diverse group of Ras-like small G proteins (68). They control a variety of important cellular processes, such as endocytosis, exocytosis, and vesicular trafficking, probably by assembling the general tethering, docking, and fusion machinery (63). Rab GTPases are anchored to the cytoplasmic face of intracellular compartments via a geranyl-geranyl group in the lipid bilayer, and such interactions are important for their proper functioning (49). Rabs are also involved in the actin- and microtubule-based transportation of cellular cargo (40, 46). The early stage of endocytosis involves the budding of vesicles from the plasma membrane and their fusion with early endosomes. A number of proteins that function in early endosomes have been isolated (59, 62), but relatively little is known about the process of lysosomal fusion. Rab7, which is a gene identified in Homo sapiens, controls the late stages of endocytosis, including early to late endosomal fusion and transport between late endosomes and lysosomes (6). It also plays a role in vacuolar biogenesis and the targeting of proteins to the vacuole (42). Rab7 appears to be conserved across species, and many of its homologues have been found in both plants (22) and fungi (42, 66). Recently, several interacting partners of Rab7 have been isolated, such as RILP (for Rab-interacting lysosomal protein) (7), Rabring 7 (36), and ORP-1L [for oxysterol-binding protein-related protein-1L] (25). These interacting proteins have different binding domains from Rab7, and their overexpression has different effects on membrane trafficking. The effectors of Rab7 indicate that it should have multiple interacting partners.
In the present study, an endocytosis-related gene, LeRab7, and its interacting partners, LeRab5 and LeRACK1, were isolated in L. edodes. The temporal and spatial expressions of these genes during fruiting body formation were studied. LeRab7 and LeRACK1 were expressed most strongly in the primordium, whereas LeRab5 was transcribed at similar levels throughout the various developmental stages. All three transcripts were localized in the active growth regions of fruiting bodies, where morphological differentiation and the divergence of specific cell types occur. Through the use of the endocytic dye FM4-64, the present study also shows endocytosis to occur in the mycelium and gill tissue of mature fruiting bodies. Treatment with different drugs revealed the internalization of the dye to be effected through endocytosis, and immunolabeling showed LeRAB7 to be colocalized with the internalized dye, which indicates that the protein is part of the endocytic pathway in mushrooms. As in yeasts, plants, and animals (8, 26, 32, 53), the mycelium of L. edodes is sensitive to brefeldin A (BFA) and wortmannin. Treatment with the two drugs followed by immunolabeling showed that LeRAB7 is probably located in the late endosomes and fungal vacuoles.
MATERIALS AND METHODS
Strain and culture conditions.
The dikaryotic L. edodes strain L54 was used in the present study. The media and the culture conditions used were as described previously (30).
EST study of the primordium stage of L. edodes.
LeRab7 was isolated in expressed sequence tag (EST) studies. The procedures used in the construction of the primordial cDNA library of L. edodes and the sequencing of the cDNA clones to obtain the ESTs were as described previously (39).
Yeast two-hybrid assay.
Yeast two-hybrid assay was performed using the Matchmaker library construction and screening kit (Clontech), which contains the yeast strains, expression vectors and media. The full-length cDNA of LeRab7 was cloned into the bait vector pGBKT7 and transformed into yeast strain Y187 through the polyethylene glycol-lithium acetate method by using the Yeastmaker yeast transformation system (Clontech). The subsequent procedures used were as described previously (56).
RACE.
Since the cDNA clone of LeRab5 that was isolated from the yeast two-hybrid system was not full length, 5′ rapid amplification of cDNA ends (RACE) was carried out by using a FirstChoice RLM-RACE kit (Ambion) according to the manufacturer's protocols. A 3′ gene-specific outer primer (5′-ACCTTGATGAATAACCAGTCTA-3′), inner primer (5′-TCAGTCCTTCTTCCTCAGCATA-3′), and 5′ gene-specific primer (5′-TCAAGCCGACCCCTCAATA-3′) were used for the amplification of the RACE product, which was then cloned into a TA TOPO cloning vector (Invitrogen) and sequenced.
In vitro Co-IP assay.
To confirm the interaction found in the yeast two-hybrid system, in vitro translation was performed, followed by coimmunoprecipitation (Co-IP) assay. Full-length LeRab5 was cloned from the total cDNA, which was made from primordial RNA by ImProm-II reverse transcriptase (RT; Promega), and was ligated to the pGADT7 vector. Together with LeRab7 and LeRACK1, LeRab5 was translated in vitro. The translations were performed by using a TNT T7 polymerase-coupled reticulocyte lysate system and a transcend tRNA chemiluminescent nonradioactive detection system (Promega) as described by the manufacturer. The amount of tRNA added to the reactions was twice that recommended to increase the intensity of the signals. The Co-IP assay was performed by using a Matchmaker Co-IP kit (Clontech) according to the procedures in the user manual. The Rab proteins LeRAB5 and LeRAB7 were incubated with 100 μM GTP at 30°C for 1 h before binding. LeRAB5 and LeRACK1 were added in excess, and the binding time for the two proteins and the incubation of the antibody was lengthened to 2 h. Protein A-beads were incubated at 4°C overnight. The bound proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by using a chemiluminescent detection system.
RNA extraction.
Total RNA was isolated from the mycelium, primordium, young fruiting body, mature fruiting body, and gill tissue of L. edodes. The cells were homogenized under liquid nitrogen, and RNA was extracted by using TRI Reagent (Molecular Research Center).
Northern blotting.
Five micrograms of total RNA from various life cycle stages of L. edodes was denatured and fractionated on a 1.2% formaldehyde gel and transferred to a nylon membrane (Hybond-N+; Amersham). Purified full-length LeRab7, LeRab5, and LeRACK1 cDNA samples were labeled as DNA probes using a PCR DIG probe synthesis kit (Roche). The blotted membranes were hybridized with denatured probes in 50% formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 2% blocking solution, 50 mM sodium phosphate, 0.1% N-lauroylsarcosine, and 7% (wt/vol) SDS at 42°C. The sizes of the transcripts were estimated from the migration distance of the bands compared to an RNA molecular weight marker.
Quantitative real-time RT-PCR.
One microgram of total RNA form the mycelium, primordium, young fruiting body, mature fruiting body, and gill tissue before and after sporulation (a total of six stages) was used for the real-time PCR. The RNA samples were first treated with DNase I and EDTA (Invitrogen) and then transcribed into cDNA by using TaqMan reverse transcription reagents (Applied Biosystems) according to the manufacturer's protocol. The primers used for the real-time PCR were determined by using Primer Express software (Applied Biosystems) and checked to ensure the avoidance of primer-dimer formation by using OLIGO software (version 4.0; National Biosciences). Real-time PCR was performed with a ABI Prism 7700 sequence detection system (Applied Biosystems). Two microliters of 10-fold-diluted cDNA product and 0.3 μl of each of the 10-μM gene-specific primers were used in a 20-μl PCR with a PCR SYBR green master mix (Applied Biosystems) according to the manufacturer's protocol. Each reaction was performed in duplicate, and a no-temple-control was prepared for each pair of primers. LePma was used as the housekeeping gene for normalization. The primer sequences were as follows: LePma forward primer (5′-TGTGTACCCCAGCTCATTTCCT-3′) and reverse primer (5′-CGATGTTCCAAACCCAGACAAT-3′), LeRab7 forward primer (5′-AGCGGTGTCGGAAAGACATCT-3′) and reverse primer (5′-CCCAAAGCTGCATCGTAACAA-3′), LeRab5 forward primer (5′-GCCGACCCCTCAATAGTGATT-3′) and reverse primer (5′-CATCAGTCCTTCTTCCTCAGCA-3′), and LeRACK1 forward primer (5′-TCCATTGCATGGTCTGCTGA-3′) and reverse primer (5′-GAACCTGCCTACACACGTCAAA-3′). Melting-curve analysis was performed after each run by using SDS1.91 software to ensure that the specific reactions occurred. The experiments were carried out for at least three independent biological samples. The results from the different RNA lots were subjected to an analysis of variance to verify the statistical significance between the samples.
In situ RNA-RNA hybridization.
Young and mature fruiting bodies of L. edodes were fixed with Bouin's solution at 4°C overnight and then immersed in 70% diethyl pyrocarbonate-ethanol at 4°C for 2 days. The fruiting bodies were dehydrated with diethyl pyrocarbonate-ethanol series and embedded in paraffin. Longitudinal 8-μm ultrathin cryosections were mounted onto slides that were precoated with poly-l-lysine. The full-length cDNA sequence of LeRab7, LeRab5, and LeRACK1 were cloned into the TA TOPO cloning vector (Invitrogen) for the synthesis of the RNA probes. Sense (control) and antisense RNA probes were prepared by in vitro transcription with digoxigenin-UTP by T7 and T3 RNA polymerase, respectively (Roche Diagnostics). The procedures for the in situ hybridization were those described by So et al. (54). The color-developed sections were mounted in distilled water and viewed with a Nikon microscope Microphot-FX. The images were captured with a Nikon DXM 1200 digital camera and analyzed with Nikon ACT-1 software version 2.12.
Tracing the endocytic pathway with FM4-64 dye.
Mycelial cells and gill tissue of L. edodes were used for the FM dye internalization. The mycelium of the dikaryotic L. edodes strain L54 was grown in potato dextrose broth in 25°C as a liquid culture. The broth was used as the incubating and washing medium in the subsequent steps. Five hundred microliters of mycelium culture was placed in ice for 10 min to lower its metabolic activity, and gill tissue from a mature fruiting body was thinned with a razor blade before similarly being incubated in ice. The samples were put into an ice-cold medium that contained 12 μM FM4-64 (Molecular Probes) for 8 min, washed four times with the medium, and transferred to a fresh medium at 25°C. Samples were collected at various time points (0, 20, 40, 60, and 120 min) for microscopic observation, which was carried out immediately after harvesting. All of the confocal fluorescence images were collected with a Bio-Rad Radiance 2100 system with a ×60 or ×100 objective oil lens. The filter set for the FM4-64 had an excitation wavelength of 543 nm and used an HQ660LP emission filter. Images were processed by using Adobe Photoshop software as described previously.
Effects of drug treatment on the internalization of FM4-64 dye.
To illustrate the nature of the internalization of the FM4-64 dye, drugs with different metabolic inhibitions of 30 mM sodium azide plus 30 mM sodium fluoride (Sigma) were used. In two other experiments, cytochalasin D at 20 μM (from a dimethyl sulfoxide stock) and benomyl at 3 or 6 μg/ml were applied. The drugs were added at 25°C 1 h before loading with the FM4-64 dye. The same concentration of drugs was added to all culture media and washing solutions. The procedures that followed were the same as those already described.
Characterization of the LeRAB7 antiserum.
A synthetic peptide that corresponded to N-terminal 15 amino acids (5′-YVNKRFSNQYKATIG-3′) of LeRab7 was used as an antigen to immunize rabbits for the production of LeRAB7 polyclonal antiserum (Genemed Synthesis). Two microliters of total protein extracts from a mature fruiting body and in vitro-translated LeRAB7 were subjected to SDS-PAGE analysis and Western blotting to characterize the LeRAB7 antibody. Preimmune serum was used as a negative control to show the specificity of the antibody.
Immunofluorescence staining.
Mycelium cultured in potato dextrose broth was fixed in 4.5% paraformaldehyde in sodium phosphate-EGTA buffer (50 mM sodium phosphate buffer [pH 7.0], 5 mM EGTA, 0.02% sodium azide) at 25°C for 30 min. The fixed cells were incubated in 1% cellulase Onozuka R-10 (Yakult Honsha) in sodium phosphate buffer at 25°C for 30 min and washed twice with sodium phosphate-EGTA buffer. After incubation with 0.05% Triton X-100 in sodium phosphate-EGTA buffer at 25°C for 3 min, the cells were washed twice with 1% bovine serum albumin in 1× phosphate-buffered saline and incubated for 30 min to block nonspecific binding. The primary antibody (anti-LeRAB7 antiserum) was incubated in 0.25% bovine serum albumin, 0.25% gelatin, 0.05% NP-40, and 0.02% sodium azide in 1× phosphate-buffered saline buffer (blocking buffer 2) with the permeabilized cells at 4°C overnight. After being washed with the same buffer three times, the cells were further incubated with Alexa Fluor 488-conjugated anti-rabbit immunoglobulin G secondary antibody (Molecular Probes) in blocking buffer 2 at 25°C for 1 h. After three more washes with the same buffer, the cells were mounted on the slides and observed with a confocal laser-scanning microscope. The filter set for the Alexa Fluor 488 had an excitation wavelength of 488 nm and comprised a 560DCLPXR dichroic mirror and an HQ545/40 emission filter, and the set for the AM4-64 had an excitation wavelength of 543 nm and used an HQ660LP emission filter.
Effects of BFA and wortmannin on FM dye internalization and labeling of LeRAB7.
In this test, 17.5 μM BFA and 16.5 μM wortmannin were used as the drug concentrations. The two chemicals were added at 25°C 2 h before loading with FM4-64 dye. The same concentration of chemicals was added to all culture media and washing solutions. The procedures that followed were the same as those used to trace the endocytic pathway with FM4-64 dye.
In other experiments, mycelial cells were added to 17.5 μM BFA and 16.5 μM wortmannin 2 h before incubation with AM4-64. The cells were then labeled with LeRAB7. The subsequent steps were the same as those used in the immunofluorescence staining.
RESULTS
Isolation of LeRab7.
Fruiting in L. edodes is the process of development from mycelium to fruiting body. The molecular mechanism that underlies this process is still poorly understood. To isolate genes for more extensive studies, a collection of ESTs from a cDNA library of primordia of L. edodes strain L54 was established. Random clones were partially sequenced from their 3′ ends, and homologous sequences were searched for in GenBank. Among these ESTs, the cDNA clone PEL0339 had a strong homology with Rab7 in Homo sapiens (78% identity, P = 3e-88). A complete mRNA sequence of the cDNA clone was obtained by primer walking. The complete coding sequence was 817 bp long and encoded a protein of 226 amino acid residues with a molecular mass of 25.6 kDa (39). The gene was named LeRab7 (accession no. AF407337).
In vivo protein interaction assay: yeast two-hybrid analysis.
Yeast two-hybrid analysis was used to isolate the interacting proteins of LeRAB7. A translational fusion of c-myc tag and full-length LeRab7 was cloned into the yeast expression vector pGBKT7 (Promega) and used as the bait protein to screen the mating library of L. edodes primordial cDNA for potential interacting partners. The expression of c-myc-LeRAB7 fusion protein was confirmed by Western blot analysis of the total protein extracts of the yeast using anti-c-myc monoclonal antibody (data not shown). There were about 4 × 104 transformants growing on the SD/−Trp−Leu−His (TDO) and SD/−Trp−Leu−His−Ade (QDO) media. A total of 420 clones on the QDO plates had inserts larger than 800 bp in the PCR screening, and 143 clones generated a positive result in the β-galactosidase filter assay. The primordial cDNA clones were sequenced and searched with BLASTX. Ten clones were selected based on their strong sequence similarities with known genes with low P values (P ≤ 1e-18) and correct reading frames of translated sequences. To eliminate false positives, the plasmids carrying the potential interacting primordial cDNA were isolated from their yeast hosts and cotransformed into yeast strain AH109 together with the bait plasmid (LeRab7- pGBKT7). The yeast cells were grown on QDO plates, and the interactions were again confirmed by β-galactosidase filter assay (data not shown).
Two clones were isolated as interacting partners of LeRAB7. One clone had a sequence identity of 1,056 bp to L. edodes guanine nucleotide-binding protein beta subunit 1 (accession no. AF407335) and encoded a protein of 312 amino acid residues with a molecular mass of 34 kDa. It also had a 74% identity with the amino acid sequence of RACK1 (a receptor for activated C kinase-1) of the fungus Paracoccidiodes brasiliensis. RACK1 belongs to a family of proteins that contain different numbers of structural Trp-Asp (WD) repeats, and itself contains the seven consecutive amino acids 41 to 48 with long WD repeats (51). The isolated gene was named LeRACK1. Another clone encoded part of the Ypt5 of Schizosaccharomyces pombe, which is a homologue of Rab5 in Homo sapiens. This clone was 730 bp long and contained only the 3′ portion of the coding sequence, the 3′ untranslated region, and the poly (A) tail. About 150-bp of the 5′ portion of the gene and the ATG start codon were obtained by 5′ RACE. The full-length 891-bp coding sequence of LeRab5 was deposited in the GenBank database (accession no. EF672053). It is predicted to encode a protein with 208 amino acid residues and an expected molecular mass of 22.8 kDa.
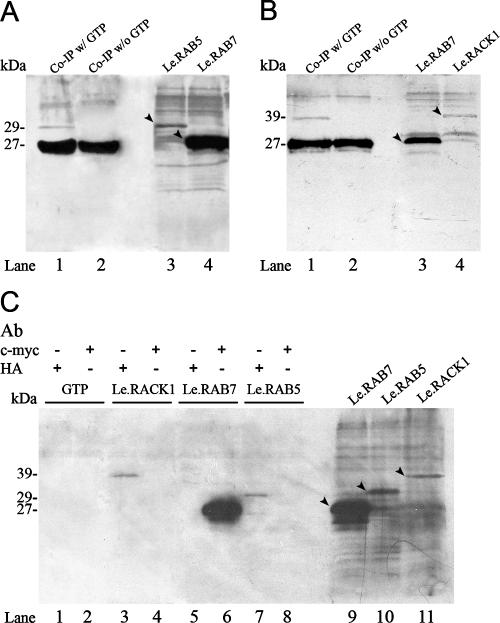
In vitro protein interaction assay: in vitro Co-IP.
Co-IP was used to confirm the protein interactions in vitro. The full-length sequence of LeRab5 was cloned from the total cDNA of the primordium stage and was ligated to the pGADT7 vector. Both pGADT7 and pGADT7-Rec were conjugated with a hemagglutinin (HA) tag, and pGBKT7 was conjugated with a c-myc tag. LeRab7-pGBKT7, LeRab5-pGADT7, and LeRack1-pGADT7-Rec were translated in vitro by using a TNT T7 polymerase-coupled reticulocyte lysate system (Promega). The in vitro-translated LeRAB7 (Fig. 1A, lane 4; Fig. 1B, lane 3; and Fig. 1C, lane 9), LeRAB5 (Fig. 1A, lane 3, and Fig. 1C, lane 10) and LeRACK1 (Fig. 1B, lane 4, and Fig. 1C, lane 11) were visualized by chemiluminiscent detection. Before the initiation of the Co-IP experiments, LeRAB7 and LeRAB5 were preincubated with 100 μM GTP to saturate the protein nucleotide binding sites with GTP. Immunoprecipitation was then performed to confirm the specificity of the c-myc and HA antibodies to different in vitro-translated proteins (Fig. 1C). In each lane in Fig. 1C, the in vitro-translated LeRAB7, LeRAB5, LeRACK1, and GTP were added with either the c-myc or HA antibody, as indicated in the figure. The three in vitro-translated proteins could only be immunoprecipitated by the antibody specific to the conjugated tag, whereas GTP was not pulled out by either antibody. The in vitro interaction of LeRAB7 and LeRAB5 is shown in Fig. 1A. LeRAB7 was simultaneously pulled out with LeRAB5 by the c-myc antibody (Fig. 1A, lane 1), but only when the LeRAB7 and LeRAB5 were preincubated with GTP (Fig. 1A, lane 2). Similarly, a GTP-dependent interaction between LeRAB7 and LeRACK1 was observed in vitro (Fig. 1B). LeRAB7 was pulled out with LeRACK1 by the c-myc antibody. Co-IP of LeRAB7 and LeRACK1 only occurred in the presence of GTP-bound LeRAB7 (Fig. 1B, lane 1) and did not take place in the absence of GTP preincubation (Fig. 1B, lane 2).
FIG. 1.
In vitro Co-IP protein interaction assay. LeRAB7 was tagged with c-Myc epitope, whereas LeRAB5 and LeRACK1 were tagged with an HA epitope. Each panel shows an autoradiogram from the SDS-PAGE analysis after in vitro transcription/translation and immunoprecipitation. The in vitro-translated proteins were biotinylated and visualized by binding with alkaline phosphatase, followed by chemiluminescent detection. The band intensities of LeRAB5 and LeRACK1 were weaker than that of LeRAB7, which may be due to different translation efficiencies on the different vectors. (A) In vitro Co-IP between LeRAB7 and LeRAB5. Lanes 3 and 4 show the in vitro-translated LeRAB5 and LeRAB7. The predicted products and their respective sizes are indicated by arrows. Lanes 1 and 2 represent the Co-IP reactions of LeRAB5 and LeRAB7 with or without GTP incubation before the mixing of the two proteins and precipitation with the c-myc antibody. LeRAB5 and LeRAB7 were coimmunoprecipitated in lane 1 (with GTP incubation), but only LeRAB7 was pulled out in lane 2 (without GTP incubation). (B) In vitro Co-IP between LeRAB7 and LeRACK1. Lanes 3 and 4 show the in vitro-translated LeRAB7 and LeRACK1. The predicted products and their respective sizes are indicated by arrows. Lanes 1 and 2 represent the Co-IP reactions of LeRAB7 with or without GTP incubation before the mixing of the two proteins. The protein mixtures were immunoprecipitated with c-myc antibody. LeRACK1 and LeRAB7 were coimmunoprecipitated in lane 1 (with GTP incubation), but only LeRAB7 was pulled out in lane 2 (without GTP incubation). (C) The immunoprecipitation of the in vitro-translated proteins and GTP was performed by using either c-myc antibody or HA antibody, as indicated (lanes 1 to 8). The predicted products and their respective sizes are indicated by arrows. The in vitro-translated LeRAB7, LeRAB5, and LeRACK1 are shown in lanes 9, 10, and 11, respectively. GTP was not pulled out by the c-myc or the HA antibody. LeRAB7 was pulled out by the c-myc antibody, whereas LeRAB5 and LeRACK1 were pulled out the by HA antibody.
Temporal expression of the genes: Northern blotting and quantitative RT-PCR.
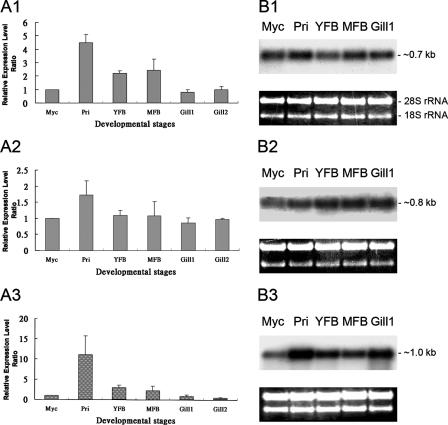
The expression of LeRab7, LeRab5, and LeRack1 during fruiting body formation was analyzed by Northern blotting and quantitative RT-PCR. The expression levels of the three transcripts at the five stages of mycelium, primordium, young fruiting body, mature fruiting body, and gill tissue of mature fruiting body before sporulation were analyzed by Northern blotting. Digoxigenin-labeled cDNA probes that corresponded to the transcripts were hybridized with the total RNA blots (Fig. 2B). Equal loadings of RNA samples from the different stages were monitored using similar intensities of 28S and 18S rRNA. The expression of LeRab7 (transcript size, ∼0.7 kb) was the strongest in the primordium (Fig. 2B1), whereas LeRACK1 (transcript size, ∼1 kb) was most strongly expressed in both the primordium and the gill tissue (hymenophore) of the mature fruiting body before sporulation (Fig. 2B3). In contrast, LeRab5 (transcript size, ∼0.8 kb) was constitutively expressed from the primordium stage to the gill tissue stage, with relatively lower expression in the mycelium stage (Fig. 2B2).
FIG. 2.
Temporal expression profiles of LeRab7, LeRab5, and LeRACK1 at different developmental stages in L. edodes. (A1 to A3) Quantitative real-time RT-PCR of the transcripts at six developmental stages. (A1) LeRab7; (A2) LeRab5; (A3) LeRACK1. The RNA amounts were normalized to the level of LePma and are shown as the expression relative to that in the mycelium. The gene expression level at the mycelium stage was taken as one. The results were calculated as the average values of three individual RNA samples, and each PCR was repeated twice. The error bars indicate standard deviations (n = 4), with P < 0.001 in the LeRab7 and LeRACK1 assay and P < 0.005 in the LeRab5 assay. (B1 to B3) Northern blotting of the transcripts at the five developmental stages. (B1) LeRab7; (B2) LeRab5; (B3) LeRACK1. Equal amounts of total RNA were loaded and confirmed by equal intensities of rRNA before the gels were blotted. Myc, mycelium; Pri, primordium; YFB, young fruiting body; MFB, mature fruiting body; Gill1, gill tissue (hymenophore) before sporulation; Gill2, gill tissue after sporulation.
The results of the Northern blot analyses were validated by quantitative RT-PCR assays. LePma (plasma membrane ATPase), which has been previously shown to express constitutively during L. edodes development (30), was used as a control transcript to normalize the expression signals of LeRab7, LeRab5, and LeRACK1. The expression levels in the six stages of mycelium, primordium, young fruiting body, mature fruiting body, and gill tissue of mature fruiting body before and after sporulation were investigated (Fig. 2A1 to A3). The expression trends of LeRab7, LeRab5, and LeRACK1 were comparable to those obtained in the Northern blot analyses (Fig. 2B1 to B3). For instance, the mRNA level of LeRab7 was highest in the primordium stage and was about fourfold that in the mycelium stage (Fig. 2A1). Similar expression levels of LeRab5 were obtained in all six developmental stages (Fig. 2A2), which is consistent with the Northern blot analysis (Fig. 2B2). The expression of LeRACK1 in the primordium stage was about 10-fold higher than in the mycelium stage (Fig. 2A3) but remained low in the gill tissue stage, which deviates from the results of the Northern blot analysis, which showed elevated expression in both the primordium and gill tissue stages (Fig. 2A3).
Spatial expression of genes: in situ RNA-RNA hybridization.
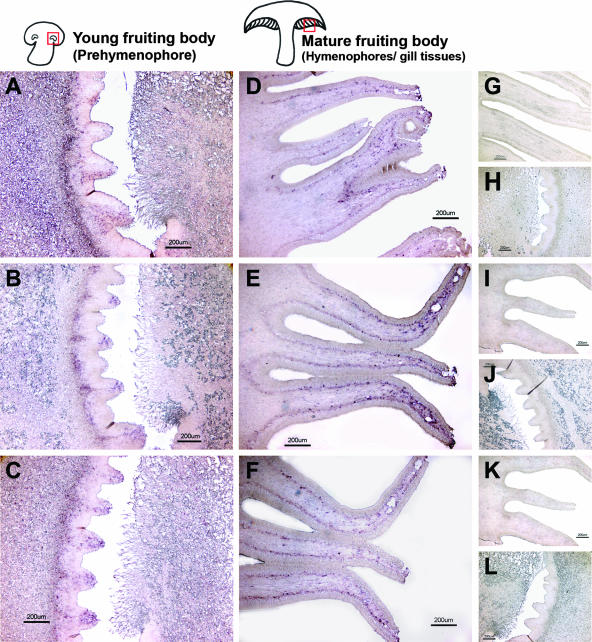
In situ RNA-RNA hybridization was used to localize the mRNA of LeRab7, LeRab5, and LeRACK1 in the fruiting body of L. edodes. These transcripts were found to be located mainly in the gill tissue and prestructure (prehymenophore) of the fruiting bodies. The hymenophore (gill tissue) is composed of the trama (the inner part of the gill tissue), the subhymenium (the middle layer), and the hymenium (the outermost layer) (27). Trama cells occupy most of the gill tissue and are able to differentiate into subhymenium, which forms the basis for hymenium formation. Basidia with basidiospores are formed in the hymenium (27). Fixed longitudinal ultrathin sections of young and mature fruiting bodies were hybridized with digoxigenin-labeled antisense RNA probes of LeRab7, LeRab5, and LeRACK1 to localize the transcripts. All three antisense probes showed specific distributions of the transcripts, whereas the sense probes gave no signals (Fig. 3). In young fruiting bodies, transcripts of LeRab7, LeRab5, and LeRACK1 were localized in tooth-like structures in the prehymenophores (Fig. 3A, B, and C). Prehymenophores, which are located in the middle of the “eye” organs of young fruiting bodies, develop into hymenophores when mushrooms mature (41). In mature fruiting bodies, transcripts of LeRab7, LeRab5, and LeRACK1 were localized in the outer region of the trama and the subhymenium of the hymenophores (Fig. 3D, E, and F). In addition, signals for the transcripts of LeRab7 and LeRab5 appeared at the tips of the hymenophores (Fig. 3D and E). Negative controls using sense probes for hybridization gave no signal in all of the samples (Fig. 3G to L).
FIG. 3.
Distributions of the LeRab7, LeRab5, LeRACK1 transcripts in young fruiting bodies and mature fruiting bodies as determined by in situ RNA-RNA hybridization. Fixed longitudinal ultrathin sections of the prehymenophores of the young fruiting bodies and the hymenophores of the mature fruiting bodies were hybridized with LeRab7 (A and B), LeRab5 (E and F), and LeRACK1 (I and J) digoxigenin-labeled RNA antisense probes, respectively. The signals were detected by alkaline phosphatase-labeled anti-digoxigenin Fab fragments and subsequent nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (BCIP) staining. Positive signals are indicated by purple Sense probes of LeRab7 (C and D), LeRab5 (G and H), and LeRACK1 (K and L) were used as negative controls to show the specificity of reactions. Magnification, ×4. Bar, 200 μm.
Endocytosis occurs in the mycelium and hymenophores of L. edodes.
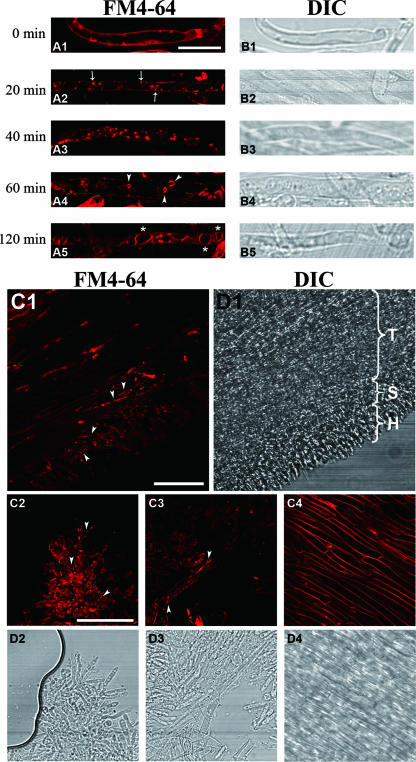
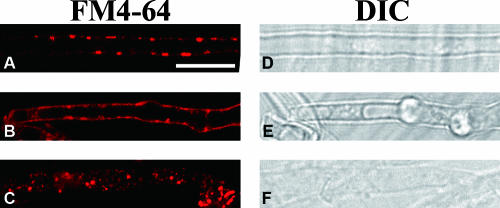
To show that endocytosis occurs in L. edodes, the membrane-selective marker FM4-64 was used to demonstrate the endocytic internalization of the plasma membrane in the mycelium and mature fruiting body gill tissue. Before loading with FM4-64, the cells were incubated at 0°C for 10 min to lower their metabolic activity. The cells were then pulse loaded with FM4-64, and the internalization of the dye was immediately traced at 0-min time points. In the mycelial hyphae, the plasma membrane was stained, and tiny bright punctuated structures were observed on the inner surface of the membrane (Fig. 4A1). The staining pattern was the same without the incubation of the cells at 0°C before the loading of the dye (data not shown). After 20 min, the bright punctuated structures developed in the mycelial cells (Fig. 4A2) and moved and vibrated along the cell length (data not shown). After 40 min, the internalized dye was found in two types of punctuated structures of different sizes. One type was similar in size to the structures observed at the 20-min time point, but most of the dye was retained in another type of large, vesicle-like structure (Fig. 4A3). At both the 60- and the 120-min time points, the dye appeared in vacuole-like hollow organelles of various sizes (0.7 to 1 μm) (Fig. 4A4). At the 120-min time point, the fluorescence intensity of the vacuolar membranes was distinctly higher than that of the plasma membranes and the vacuoles were even larger, with diameters close to the width of the mycelial cells (∼2 μm) (Fig. 4A5). No further change in dye localization was observed after this time point, which suggests that it was the final stage of dye internalization.
FIG. 4.
Time course of FM4-64 dye internalization in the mycelium and the hymenophore of a fruiting body of L. edodes. The samples in panels A1 to A5 were taken out at the time points indicated after loading with 12 μM FM4-64 at 0°C. (A1 to A3) The FM4-64 was taken up by mycelial cells and detected on the plasma membrane (A1), followed by internalization through punctuated structures (arrows) (A2), and an increase in the number and size of the circular, endosomal-like structures (A3). (A4) The dye was transported into vacuolar and hollow organelles (arrowheads) after 1 h. (A5) After 2 h, the dye was retained in large vacuolar structures (asterisks), which marked the final point of internalization. (B1 to B5) DIC images of the various time points. Bars, 10 μm. (C1) FM4-64 internalization in the hymenophore of a mature fruiting body. The images were captured after the loading of the dye at 0°C, and chasing followed after 40 min at 25°C. The appearance of punctuated structures is indicated by arrowheads. (D1) The trama (T), subhymenium (S), and hymenium (H) were observed in the DIC micrograph. (C2 to C4) Dye internalization was seen in the hymenium (C2) and subhymenium (C3) but not in the trama cells (C4). (D2 to D4) DIC images of the respective structures. Bar, 50 μm.
Endocytosis also occurred in the hymenophore of the mature fruiting body, with thin longitudinal sections of hymenophore showing FM4-64 internalization. The hymenophores had three cell types when examined with a laser-scanning confocal microscope. The hymenium at the outermost layer was small and dumbbell-shaped, the middle part was composed of long, large trama cells, and small rod-like subhymenium cells were present between these two layers (Fig. 4C1). The plasma membranes of the three cell types in the gill tissue were stained, but dye internalization was observed in the form of fluorescent spots only in the hymenium (Fig. 4C2) and subhymenium (Fig. 4C3), but not in the trama cells (Fig. 4C4). Stronger labeling was observed in the subhymenium than in the hymenium.
Validation of the active transport of FM4-64.
The effects of metabolic inhibitors, actin-depolymerizing compounds, and microtubule-depolymerizing compounds have been tested in filamentous fungi to demonstrate that intracellular membrane trafficking is energy dependent and relies on F-actin (45, 55, 67). Here, similar approaches were applied to further show that FM4-64 is internalized by endocytosis in L. edodes. A mixture of sodium azide and sodium fluoride was applied to mycelium as metabolic inhibitors, which caused the internalization of FM4-64 to halt almost completely except for a few fluorescent spots in the cells (Fig. 5A). This result suggests that the FM4-64 was internalized by active transport, which is an energy-dependent process. In the presence of cytochalasin D, an inhibitor of actin polymerization (15), the plasma membrane was uniformly labeled, and although there were small fluorescent spots attached to the inner membrane, no motile punctuated structures were found moving inside the cells. In addition, the labeling of the punctuated and vacuolar structures within the cells was fully prevented (Fig. 5B), which suggests that FM4-64 internalization depends on actin polymerization. In contrast, FM4-64 internalization was not affected by benomyl, which is a microtubule-depolymerizing agent (Fig. 5C), with the pattern of labeling remaining the same as the null control even when the concentration of the drug was doubled (data not shown). In conclusion, the internalization of FM4-64-stained membrane in L. edodes depends on energy and F-actin but not on microtubules, which are the hallmarks of active transport through endocytosis.
FIG. 5.
Effects of drugs on FM4-64 internalization. The images were collected after the loading of the dye at 0°C followed by chasing after 40 min at 25°C in the presence of a mixture of sodium azide and sodium fluoride (30 mM each) (A), cytochalasin D (20 μM) (B), and benomyl at 3 μg/ml (C). Bar, 10 μm.
Presence of LeRAB7 protein in the endosomal structures along the endocytic pathway.
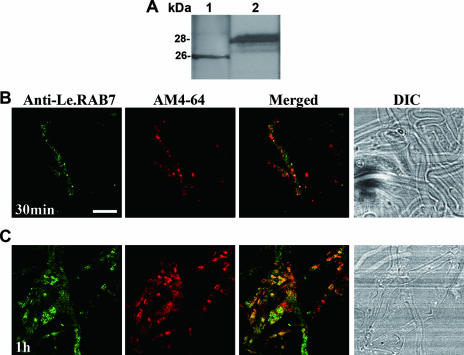
Anti-LeRAB7 immunolabeling and AM4-64 internalization were performed to show that LeRAB7 takes part in intracellular trafficking. Polyclonal anti-LeRAB7 antiserum was used as the primary antibody. A synthetic peptide that corresponded to the N-terminal amino acid sequence of LeRAB7 was used as an antigen to immunize rabbits for the production of LeRAB7 polyclonal antiserum (anti-LeRAB7). The specificity of the antiserum was established through Western blot analysis. A single main band of 26 kDa from the mycelial total protein extract of L. edodes was detected (Fig. 6A, lane 1). The molecular mass of the band agreed with that of the c-myc-tagged LeRAB7 protein produced from the in vitro translation (Fig. 6A, lane 2). The preimmune serum did not detect any protein bands from these protein extracts (data not shown).
FIG. 6.
Double labeling of mycelial cells with anti-LeRAB7 and AM4-64. (A) Characterization of the LeRAB7 antiserum. The anti-LeRAB7 detection of LeRAB7 was performed on a total protein extract of L. edodes at the mycelium stage (lane 1) and during the in vitro transcription/translation of c-myc-tagged LeRAB7 (lane 2). A single main band was observed in both lanes. (B and C) The mycelial cells were loaded with 12 μM AM4-64 at 0°C, followed by chasing at 25°C, and were fixed with 4.5% paraformaldehyde at the 30-min (B) and 60-min (C) time points. The cells were then immunostained with LeRAB7 antiserum. At the 30-min time point, the anti-LeRAB7 partly colocalized with the AM4-64. The extent of the colocalization was higher at the 60-min time point than at the 30-min time point. The yellow appearance of the merged images indicates the colocalization of the antibody and dye. Bar, 50 μm.
AM4-64 (Biotium) is spectrally identical to FM4-64 and has an additional amine group at the hydrophilic end to make it fixable for immunolabeling. The experiment with this dye was similar to that with the FM dye. The mycelial cells were incubated with 12 μM AM4-64, fixed with 4.5% paraformaldehyde at two time points (30 and 60 min), and immunolabeled with anti-LeRAB7. In the 30- and 60-min trace periods, the AM4-64-labeled structures (circular spot-like compartments) colocalized with LeRAB7. The extent of this colocalization was greater at the 60-min time point (Fig. 6C) than at the 30-min time point (Fig. 6B). In addition to the spot-like structures, both LeRAB7 and AM4-64 labeled some apparently vacuolar structures, which were possibly late endosomes or fungal vacuoles (Fig. 6C). The compartments that were labeled by anti-LeRAB7 were identified by using BFA and wortmannin in experiments that are described below.
The endocytic pathway of L. edodes is sensitive to BFA and wortmannin.
The effects of BFA and wortmannin were also tested. BFA inhibits the recycling of endosomes to the plasma membrane in plant cells (4, 21) and trafficking between the endoplasmic reticulum and Golgi bodies in animal cells (8). FM4-64 has been found to accumulate in endosomal aggregates, which are known as BFA compartments, upon treatment of cells with BFA in plants (4, 43).
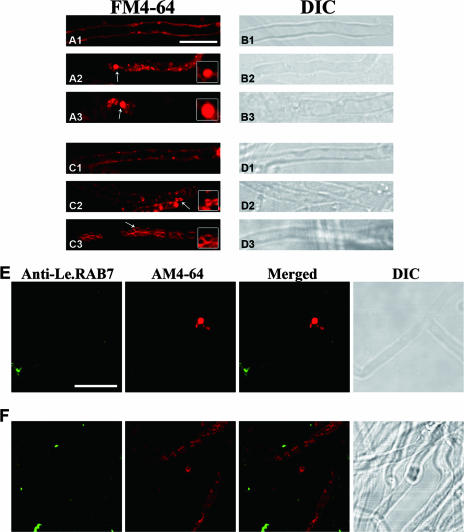
The morphology of the structures in the endocytic pathway was altered after BFA treatment in mycelial cells of L. edodes. As with the control, only the plasma membrane was labeled at the 0-min time point (Fig. 7A1). After 20 min of uptake, BFA at a concentration of 17.5 μM stimulated the FM4-64-labeled structures to form aggregates. The average diameter of the aggregates was about ∼1.5 μm, which is larger than that of the punctuated structures (∼0.5 μm) present in non-BFA-treated mycelial cells at the same time point (Fig. 7A2). The aggregates were enlarged after an hour, and there was no staining of the vacuolar structures or hollow organelles, which were presumably late endosomes and fungal vacuoles (Fig. 7A3). Similar results were obtained when 35 μM BFA was used (data not shown).
FIG. 7.
Effects of BFA and wortmannin on FM4-64 internalization and LeRAB7 labeling in mycelial cells. (A1 to A3) The cells were incubated with 17.5 μM of BFA for 2 h before loading with 12 μM FM4-64 at 0°C. Samples were taken at 0 min (A1), 20 min (A2), and 1 h (A3) after chasing at 25°C. (C1 to C3) The cells were incubated with 16.5 μM wortmannin for 2 h before loading with 12 μM FM4-64 at 0°C. (B1 to B3) Samples were taken at 0 min (B1), 20 min (B2), and 1 h (B3) after chasing at 25°C. Panels B1 to B3 and panels D1 to D3 are DIC images of the corresponding treatments and time points. The insets are enlarged images of the BFA- and wortmannin-induced structures, which are indicated by arrows. (E and F) Double labeling of mycelial cells with anti-LeRAB7 and AM4-64 after treatment with 17.5 μM BFA (E) and 16.5 μM wortmannin (F). The cells were loaded with 12 μM AM4-64 at 0°C, followed by chasing at 25°C, and were fixed after 1 h. They were then immunostained by LeRAB7 antiserum. No colocalization was observed in either treatment. Bar, 10 μm.
The mycelial cells were also treated with wortmannin, which is a phosphatidylinositol 3-kinase inhibitor of endocytosis (10) and a trafficking receptor in animal cells (53). It also inhibits protein sorting to vacuoles in plant cells (32).
Wortmannin was added to mycelium at a concentration of 16.5 μM before incubation with FM4-64. The dye stained the plasma membrane at 0 min (Fig. 7C1). The drug then induced the vacuolation of the FM4-64-labeled structure. Small vacuoles, which were normally observed after 1 h of dye internalization, appeared after only 20 min (Fig. 7C2), and the staining pattern was completely different from the punctuated pattern in the untreated cells. At the 1-h time point, the membrane of the unknown structures was uniformly labeled, and the structures were larger than the vacuoles as observed at the 30-min time point. These structures were evident throughout the mycelial cells and formed in clusters along them (Fig. 7C3).
LeRAB7 did not colocalize with the BFA- and wortmannin-induced compartments.
Previous experiments have shown LeRAB7 to colocalize with AM4-64, with the extent of the colocalization increasing between the 30- and 60-min time points. To provide more evidence for the role of LeRAB7 in endocytosis, drug treatments were administered, followed by immunolabeling. BFA at a concentration of 17.5 μM and wortmannin at a concentration of 16.5 μM were incubated with mycelial cells, and the cells were then fixed and labeled with anti-LeRAB7. Highly fluorescent aggregates formed after the BFA treatment at the 60-min time point (Fig. 7E), and LeRAB7 and AM4-64-labeled structures were observed. Little labeling of LeRAB7 was observed in the mycelial cells. In the wortmannin treatment, vacuolar compartments and their clusters appeared at the same time point, but these structures did not overlap with the LeRAB7 labels (Fig. 7F).
DISCUSSION
The presence of endocytosis is widely accepted in animal, plant, and yeast systems (18, 34). Its existence in fungi, however, is still contentious (50). Relatively little is known about the endocytic pathway of filamentous fungi, especially the basidiomycetes. Recently, more evidence has been uncovered of the existence of endocytosis in N. crassa (12), A. nidulans (45), U. maydis (14), and Magnaporthe grisea (2). For example, membrane-selective endocytic markers (e.g., FM4-64) (2, 13) and markers of fluid-phase endocytosis (e.g., Lucifer yellow) (55) have been found to be internalized in a range of filamentous fungi through active transport and are known to be actin dependent. Moreover, the genome of N. crassa encodes a complex, endocytic protein machinery (50).
Recently, a gene that encodes Rab7 was isolated in L. edodes. Rab7 plays a role in endosomal fusion and trafficking in late endocytosis (51), and provides a handle for investigating the existence of endocytosis in L. edodes. Two interacting proteins of LeRAB7 were isolated in the present study. LeRab5 is highly similar to S. pombe Ypt5, which is a homologue of Rab5 that is involved in regulating the fusion of early endosomes (1, 52). The other interacting partner was LeRACK1, which was initially identified as a guanine nucleotide-binding protein β subunit (19). RACK1 interacts with many cellular proteins and is involved in signal transduction and a multitude of other biological processes (33).
Both the temporal and the spatial expression profiles of LeRab7 and its interacting partners were analyzed. LeRab7 and LeRACK1 was expressed most strongly in the primordium (Fig. 2A1 and B1 and Fig. 2A3 and 2B3). Primordium development is characterized by a high metabolic rate and a dramatic change in growth characteristics, including extensive hyphal-hyphal interaction and cellular differentiation (35). The increased expression of LeRab7 may reflect a higher rate of late endocytosis, whereas LeRACK1 may be more involved in extensive cellular developmental processes (see below). LeRab5 was constitutively expressed in all of the developmental stages studied (Fig. 2A2 and B2). In the early stage of endocytosis Rab5 acts as a hub, and its cargo may eventually be recycled back to the membrane and transported to the Golgi compartment for protein modification or to the vacuoles for degradation (52). It is therefore reasonable that LeRab5 expresses at a similar level at all developmental stages.
All three transcripts were localized in the prehymenophores of young fruiting bodies and the outer regions of the trama and subhymenium of mature fruiting bodies, but not in the hymenium where the basidia and basidiospores are formed (Fig. 3A to F). These observations imply that these three genes may function in the development of the hymenophore from the prehymenophore and the divergence of the trama into the subhymenium (27, 28, 41). The results of the FM4-64 dye internalization in the specific cell types of the hymenophore (see below) indicate that it is likely that the two endocyte-related proteins LeRAB5 and LeRAB7 function in the cellular processes of the hymenophore in L. edodes and take part in endocytosis.
The mechanisms of the interaction between LeRAB7 and its two interacting proteins and the functions of the two protein cognates may be different. By participating in consecutive stages of the endocytic pathway, LeRAB5 and LeRAB7 may work together to control the endosomal trafficking of cargo proteins from the plasma membrane to their final destinations, such as the vacuole or Golgi. In mammalian cell lines, cargo that is destined for degradation, once it has joined a Rab5-positive early endosome, remains associated with the intermediary endosome where Rab7 accumulates. The endosome then loses its Rab5 and develops into late endosome, and the cargo is subject to lysosomal degradation (29). The transient colocalization of LeRAB5 and LeRAB7 in the putative intermediary endosome may allow the occurrence of this interaction in L. edodes.
RACK1 is a receptor that was originally identified by its ability to bind to protein kinase C (37). It is a scaffolding protein that inhibits endogenous functioning (64). It has been suggested that RACK1 facilitates cell cycle progression, cell proliferation, cell transformation, cell division, and cell spreading (9, 23, 33). It has been inferred that in L. edodes, LeRACK1 is transported to the cell through receptor-mediated endocytosis (48), and Rab proteins have been shown to interact with its cargo receptor (59). LeRACK1 may be carried by endosomes, and the interaction with LeRAB7 controls its fate inside the cell, either lysosomal degradation or the recycling of the receptor. Further investigations are needed to determine whether LeRACK1 colocalizes with the endocytic marker or the related proteins.
In the present study, FM4-64 dye, which is an endocytic marker, was shown to enter the mycelial cells and gill tissue of L. edodes through membrane internalization. During FM4-64 internalization, the sequential labeling of different structures that followed the initial labeling of the plasma membrane suggests that they are intermediates along the endocytic pathway of L. edodes (Fig. 4A1 to A5). The staining pattern was highly similar to that found in A. nidulans (45) and N. crassa (12). The punctuated structures were probably early endosomes (Fig. 4A2 and A3), as reported in A. nidulans and N. crassa hyphae, based on their similarity to the cytosolic punctuated structures found in S. cerevisiae (61). Subsequently, vacuolar hollow organelles were labeled, and their number and size increased with time (Fig. 4A4). The organelles may have been late endosomes that function during late endocytosis. The ∼2-μm vacuolar structures appeared at the final stage of FM dye internalization (Fig. 4A5), may be formed by the homotypic fusion of endosomes (45, 47), and are possibly one of the destinations on the endocytic pathway.
It is interesting that the hymenophores of the fruiting bodies of L. edodes are found to display endocytosis. Hymenophores consist of trama, subhymenium, and hymenium on which the basidia and basidiospores are formed (27). Not all of these cell types underwent dye internalization, however: the labeling of the punctuated structures only occurred in the hymenium and subhymenium (Fig. 4C1, C2, and C3). This is not surprising, because the hymenium, which mainly functions in basidiospore formation, diverges from the subhymenium. LeRab7 and LeRab5 were strongly expressed in the subhymenium and at the sides of the trama, which suggests that endocytosis takes place in specific types of cells in the hymenophore.
The dye internalization was dependent on energy and F-actin, which provides strong evidence of the existence of endocytosis in L. edodes. FM4-64 is a styryl dye that is composed of a hydrophobic tail, a dicationic head, and a nucleus. This structure becomes an energetic barrier that directs substances across the plasma membrane through simple diffusion (12). Metabolic inhibitors, sodium azide, and sodium fluoride blocked the dye internalization, leaving it to form punctuated structures on the inner side of the plasma membrane (Fig. 5A), which suggests that FM4-64 internalization is an active and energy-dependent process. F-actin is known to be involved in endocytic internalization and other membrane trafficking functions (3). Cytochalasin D depolymerizes actin and prevents actin cytoskeleton formation but does not have a general disruptive effect on cells (55); rather, it causes a similar effect to metabolic inhibitors of preventing dye translocation (Fig. 5B). Dye internalization, however, does not require a microtubule network, as was shown by the benomyl treatment (Fig. 5C), which had no observable effect on the FM4-64 localization compared to the positive control (Fig. 4A3). This result suggests that L. edodes, like other filamentous fungi, carries out endocytosis.
Rab7 plays a crucial role in controlling endosomal fusion during late endocytosis (62). To prove that LeRAB7 has a similar function, AM4-64, which is a fixable form of FM4-64, was used to determine whether LeRAB7 protein was present in the endocytic pathway. A similar technique has been used in studies of other Rab GTPases (58). As demonstrated in the double-staining experiment, the LeRAB7 protein partly colocalized with structures that were stained by the AM4-64 (Fig. 6B). LeRAB7 overlapped with the punctuated structures and the vacuolar structures at the 60-min time point (Fig. 6C), which indicates that LeRAB7 may only be present in certain compartments and probably resides in the late endosomes or vacuoles during late endocytosis. The use of other organelle markers may help to confirm this hypothesis.
Membrane traffic is displayed in yeasts and animals. In animal cells, BFA inhibits the action on GEF of ARF1, which leads to the breakdown of the Golgi. It also restricts vesicle budding events in the organelles (8). BFA disrupts budding from endosomal compartments, blocks secretion based on recycling endosomes, and thus decreases the recycling of the dye to the plasma membrane (17). The formation of aggregates in the mycelial cells after BFA treatment may be due to the accumulation of FM4-64 upon the inhibition of recycling and the inhibition of transport to the vacuoles (20) (Fig. 7A2 and A3). As a result, prevacuolar endosomal vesicles, such as early endosomes, accumulate or enlarge (43). BFA treatment followed by the labeling of anti-LeRAB7 revealed the localization of LeRAB7 in the endocytic pathway. As discussed previously, BFA affects the trafficking of early endosomes and thus inhibits the recycling endosomes (11). It also blocks the transport of proteins from early to late endosomes in yeast (24). LeRAB7 did not colocalize with the aggregates formed after BFA treatment (Fig. 7E), which shows that LeRAB7 is located in the compartments that formed after the early endosomes in the pathway, which were possibly late endosomes.
Wortmannin is an inhibitor of Vps34p (phosphatidylinositol 3-kinase) that is responsible for vacuolar protein sorting in yeasts (52). It blocks the trafficking of vacuolar proteins in plant cells (32) and affects the late endocytic pathway in animal cells (5, 26). In the mycelial cells, the membranes of unknown structures and their clusters were stained by using FM4-64 and treated with wortmannin (Fig. 7C2 and C3 and C3). This staining pattern was unique in L. edodes, and these membranes and clusters were not colocalized with LeRAB7 (Fig. 7F), which shows that these structures were not the same as the late endosomes or vacuoles present in the untreated control but may belong to other endosomal structures. The transport of substrates such as AM4-64 through these structures for the later stage of endocytosis was inhibited. These data provide evidence of the presence of wortmannin- and BFA-sensitive components in the endocytic pathway of L. edodes. However, the abnormal structures and the mechanisms by which the drugs act require characterization.
In the present study, an endocytic protein, LeRAB7, and its interacting partners, LeRAB5 and LeRACK1, were isolated. An examination of the temporal and spatial expression of these proteins shows that they may be responsible for the growth of fruiting bodies and cell differentiation in the gill tissue. FM4-64 was used as a marker to prove the existence of endocytosis in L. edodes and revealed that the process is energy dependent and relies on actin from the initiation of vesicle budding in the plasma membrane. As in animals and plants, LeRAB7 is located in the compartments at the later stages of the endocytic pathway, which are probably late endosomes and vacuoles. L. edodes has BFA- and wortmannin-sensitive components that cause the formation of different morphological structures. Thus far, this is the first report to show that endocytosis is present in the hymenophores of homobasidiomycetous mushroom. Future work will focus on the effects of LeRAB7 and its interacting proteins on the development of mushrooms. It may also be worthwhile to determine the presence of intermediary endosomes where LeRAB5 and LeRAB7 may colocalize and interact with each other.
Acknowledgments
We are grateful to Grace Sze Wan Leung for critical reading the manuscript, Winnie Wing Yan Chum for improving the manuscript, and to Liwen Jiang for valuable advice.
This study was partially supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (RGC ref. no. CUHK4147/01M).
This study was carried out in compliance with the current laws governing genetic experimentation in Hong Kong.
Footnotes
Published ahead of print on 5 October 2007.
REFERENCES
- 1.Armstrong, J., M. W. Craighead, R. Watson, S. Ponnambalam, and S. Bowden. 1993. Schizosaccharomyces pombe ypt5: a homologue of the rab5 endosome fusion regulator. Mol. Biol. Cell 4:583-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, H. A., A. Daniels, and N. D. Read. 2002. Live-cell imaging of endocytosis during conidial germination in the rice blast fungus, Magnaporthe grisea. Fungal Genet. Biol. 37:233-244. [DOI] [PubMed] [Google Scholar]
- 3.Ayscough, K. R. 2005. Defining protein modules for endocytosis. Cell 123:305-320. [DOI] [PubMed] [Google Scholar]
- 4.Baluska, F., A. Hlavacka, J. Samaj, K. Palme, D. G. Robinson, T. Matoh, D. W. McCurdy, D. Menzel, and D. Volkmann. 2002. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 130:422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brabec, M., D. Blaas, and D. Fuchs. 2006. Wortmannin delays transfer of human rhinovirus serotype 2 to late endocytic compartments. Biochem. Biophys. Res. Commun. 348:741-749. [DOI] [PubMed] [Google Scholar]
- 6.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11:467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantalupo, G., P. Alifano, V. Roberti, C. B. Bruni, and C. Brucci. 2001. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 15:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chavrier, P., and B. Goud. 1999. The role of ARF and Rab GTPases in membrane transport. Curr. Opin. Cell Biol. 11:466-475. [DOI] [PubMed] [Google Scholar]
- 9.Choi, D. S., H. Young, T. McMahon, D. Wang, and R. O. Messing. 2003. The mouse RACK1 gene is regulated by nuclear factor-κB and contributes to cell survival. Mol. Pharmacol. 64:1541-1548. [DOI] [PubMed] [Google Scholar]
- 10.Clague, M. J., C. Thorpe, and A. T. Jones. 1995. Phosphatidylinositol 3- kinase regulation of fluid phase endocytosis. FEBS Lett. 367:272-274. [DOI] [PubMed] [Google Scholar]
- 11.Emans, N., S. Zimmermann, and R. Fischer. 2002. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell 14:71-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer-Parton, S., R. M. Parton, P. C. Hickey, J. Dijksterhuis, H. A. Atkinson, and N. D. Read. 2000. Confocal microscopy of FM4-64 as a tool for analyzing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198:245-259. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, K. E., D. S. Lowry, and R. W. Roberson. 2000. Cytoplasmic cleavage in living zoosporangia of Allomyces macrogynus. J. Microsc. 199:260-269. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs, U., and G. Steinburg. 2005. Endocytosis in the plant-pathogenic fungus Ustilago maydis. Protoplasma 226:75-80. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel, M., D. Horky, A. Svoboda, and M. Kopecká. 1998. Cytochalasin D interferes with contractile actin ring and septum formation in Schizosaccharomyces japonicus var. versatilis. Microbiology 144:2331-2344. [DOI] [PubMed] [Google Scholar]
- 16.Galaghan, J., S. Calvo, K. Borkovich, E. Selker, N. D. Read, W. FitzHugh, L. J. Ma, S. Smirnov, S. Purcell, B. Rehman, T. Elkins, R. Engels, S. Wang, C. B. Nielsen, J. Butler, D. Jaffe, M. Endrizzi, D. Qui, P. PIanakiev, D. Bell-Pedersen, M. A. Nelson, M. Werner-Washburne, C. P. Selitrennikoff, J. A. Kinsey, E. L. Braun, A. Zelter, U. Schulte, G. O. Kothe, G. Jedd, W. Mewes, C. Staben, E. Marcotte, D. Greenberg, A. Roy, K. Foley, J. Naylor, N. Stange-Thomann, R. Barrett, S. Gnerre, M. Kamal, M. Kamvysselis, C. Bielke, S. Rudd, D. Frishman, S. Krystofova, C. Rasmussen, R. L. Metzenberg, D. D. Perkins, S. Kroken, D. Catcheside, W. Li, R. J. Pratt, S. A. Osmani, C. P. C. DeSouza, L. Glass, M. J. Orbach, J. A. Berglund, R. Voelker, O. Yarden, M. Plamann, S. Seiler, J. Dunlap, A. Radford, R. Aramayo, D. O. Natvig, L. A. Alex, G. Mannhaupt, D. J. Ebbole, M. Freitag, I. Paulsen, M. S. Sachs, E. S. Lander, C. Nusbaum, and B. Birren. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature 422:859-868. [DOI] [PubMed] [Google Scholar]
- 17.Geldner, N. 2004. The plant endosomal system: its structure and role in signal transduction and plant development. Planta 209:547-560. [DOI] [PubMed] [Google Scholar]
- 18.Geli, M. I., and H. Riezman. 1998. Endocytic internalization in yeast and animals: similar and different. J. Cell Sci. 111:1031-1037. [DOI] [PubMed] [Google Scholar]
- 19.Gerbasi, V. R., C. M. Weaver, S. Hill, D. B. Friedman, and A. J. Link. 2004. Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol. Biol. Cell 24:8276-8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez, L., and M. Chrispeels. 1993. Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grebe, M., J. Xu, W. Mobius, T. Ueda, A. Nakano, H. J. Geuze, M. B. Rook, and B. Scheres. 2003. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13:378-387. [DOI] [PubMed] [Google Scholar]
- 22.Haizel, T., T. Merkle, F. Turck, and F. Nagy. 1995. Characterization of membrane-bound small GTP-binding proteins from Nicotiana tabacum. Plant Physiol. 108:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermanto, U., C. S. Zong, W. Li, and L. H. Wang. 2002. RACK1, an insulin-like growth factor I (IGF-I) receptor-interacting protein, modulates IGF-I-dependent integrin signaling and promotes cell spreading and contact with extracellular matrix. Mol. Cell. Biol. 22:2345-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicke, L., B. Zanolari, M. Pypaert, J. Roher, and H. Riezman. 1997. Transport through the yeast endocytic pathway occurs through morphologically distinct compartments and requires an active secretory pathway and Sec18p/N-ethylmaleimide-sensitive fusion protein. Mol. Biol. Cell 8:13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson, M., M. Lehto, K. Tanhuanpaa, T. L. Cover, and V. M. Olkkonen. 2005. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 16:5480-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones, A. T., I. G. Mills, A. J. Scheidig, K. Alexandrov, and M. J. Clague. 1998. Inhibition of endosome fusion by wortmannin persists in the presence of activated rab5. Mol. Biol. Cell 9:323-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaneko, K., and K. Shishido. 2001. Cloning and sequence analysis of the basidiomycete Lentinus edodes ribonucleotide reductase small subunit cDNA and expression of a corresponding gene in L. edodes. Gene 262:43-50. [DOI] [PubMed] [Google Scholar]
- 28.Katsukawa, S., and K. Shishido. 2005. Analysis of recQ gene transcript in fruiting bodies of basidiomycetous mushroom Lentinula edodes. Biosci. Biotechnol. Biochem. 11:2247-2249. [DOI] [PubMed] [Google Scholar]
- 29.Lakadamyali, M., M. J. Rust, and M. Zhuang. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124:997-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung, G. S., M. Zhang, W. J. Xie, and H. S. Kwan. 2000. Identification by RNA fingerprinting of genes differentially expressed during the development of the basidiomycete Lentinula edodes. Mol. Gen. Genet. 262:977-990. [DOI] [PubMed] [Google Scholar]
- 31.Maniak, M. 2003. Fusion and fission events in the endocytic pathway of Dictyostelium. Traffic 4:1-5. [DOI] [PubMed] [Google Scholar]
- 32.Matsuoka, K., D. Bassham, N. Raikhel, and K. Nakamura. 1995. Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130:1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCahill, A., J. Warwicker, G. B. Bolger, M. D. Houslay, and S. J. Yarwood. 2002. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol. Pharmacol. 62:1261-1273. [DOI] [PubMed] [Google Scholar]
- 34.Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:572-625. [DOI] [PubMed] [Google Scholar]
- 35.Miyazaki, Y., M. Nakamura, and K. Babasaki. 2005. Molecular cloning of developmentally specific genes by representational difference analysis during the fruiting body formation in the basidiomycete Lentinula edodes. Fungal Genet. Biol. 42:493-505. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno, K., A. Kitamura, and T. Sasaki. 2003. Rabring7, a novel Rab7 target protein with a RING finger motif. Mol. Biol. Cell 14:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mochly-Rosen, D., B. L. Smith, C. H. Chen, M. H. Disatnik, and D. Ron. 1995. Interaction of protein kinase C with RACK1, a receptor for activated C-kinase: a role in beta protein kinase C mediated signal transduction. Biochem. Soc. Trans. 23:596-600. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol. 77:759-803. [DOI] [PubMed] [Google Scholar]
- 39.Ng, T. P. 2000. Expressed sequence tags and functional characterization of fruiting genes during fruit body development of edible mushroom Lerntinula edodes. M.Phil. thesis. The Chinese University of Hong Kong, Hong Kong.
- 40.Nielsen, E., F. Severin, J. Backer, A. Hyman, and M. Zerial. 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1:376-382. [DOI] [PubMed] [Google Scholar]
- 41.Nishazawa, H., Y. Miyazaki, K. Kaneko, and K. Shishido. 2002. Distribution of hydrophobin 1 gene transcript in developing fruiting bodies of Lentinula edodes. Biosci. Biotechnol. Biochem. 66:1951-1954. [DOI] [PubMed] [Google Scholar]
- 42.Ohsumi, K., M. Arioka, H. Nakajima, and K. Kitamoto. 2002. Cloning and characterization of a gene (avaA) from Aspergillus nidulans encoding a small GTPase involved in vacuolar biogenesis. Gene 291:77-84. [DOI] [PubMed] [Google Scholar]
- 43.Ovecka, M., I. Lang, F. Baluska, A. Ismail, P. Illes, and I. K. Lichtscheidl. 2005. Endocytosis and vesicle trafficking during tip growth of root hairs. Protoplasma 226:39-54. [DOI] [PubMed] [Google Scholar]
- 44.Parton, S. F., R. M. Parton, P. C. Hickey, J. Dijksterhuis, H. A. Atkinson, and N. D. Read. 2000. Confocal microscopy of FM4-64 as a tool for analysing endocytosis and vesicle trafficking in living fungal hyphae. J. Microsc. 198:246-259. [DOI] [PubMed] [Google Scholar]
- 45.Penalva, M. A. 2005. Tracing the endocytic pathway of Aspergillus nidulans with FM4-64. Fungal Genet. Biol. 42:963-975. [DOI] [PubMed] [Google Scholar]
- 46.Peranen, J., P. Auvinen, H. Virta, R. Wepf, and K. Simons. 1996. Rab8 promotes polarized membrane transport through reorganization of actin and microtubules in fibroblasts. J. Cell Biol. 135:153-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfeffer, S. R. 1999. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1:E17-E22. [DOI] [PubMed] [Google Scholar]
- 48.Qualmann, B., and M. M. Kessels. 2002. Endocytosis and the cytoskeleton. Int. Rev. Cytol. 220:96-144. [DOI] [PubMed] [Google Scholar]
- 49.Rando, R. R. 1996. Chemical biology of protein isoprenylation/ methylation. Biochim. Biophys. Acta 1300:5-16. [DOI] [PubMed] [Google Scholar]
- 50.Read, N. D., and E. R. Kalkman. 2003. Does endocytosis occur in fungi? Fungal Genet. Biol. 39:199-203. [DOI] [PubMed] [Google Scholar]
- 51.Ron, D., C. H. Chen, J. Caldwell, L. Jamieson, E. Orr, and D. Mochly-Rosen. 1994. Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of G proteins. Proc. Natl. Acad. Sci. USA 91:839-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schu, P. V., K. Takegawa, M. J. Fry, J. H. Stack, M. D. Waterfield, and S. D. Emr. 1993. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science 260:88-91. [DOI] [PubMed] [Google Scholar]
- 53.Shepherd, V. A., D. A. Orlovich, and A. E. Ashford. 1993. Cell-to-cell transport via motile tubules in growing hyphae of a fungus. J. Cell Sci. 105:1173-1178. [DOI] [PubMed] [Google Scholar]
- 54.So, W. K., H. F. Kwok, and W. Ge. 2005. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebra fish follicle-stimulating hormone and luteinizing hormone subunits: their spatial-temporal expression patterns and receptor specificity. Biol. Reprod. 72:1382-1396. [DOI] [PubMed] [Google Scholar]
- 55.Soldner, R. W., I. Schulz, A. Straube, and G. Steinberg. 2002. Dynein supports motility of endoplasmic reticulum in the fungus Ustilago maydis. Mol. Biol. Cell 13:965-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinberg, G., M. Schliwa, C. Lehmler, M. Bolker, R. Kahmann, and J. R. McIntosh. 1998. Kinesin from the plant pathogenic fungus Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J. Cell Sci. 111:2235-2246. [DOI] [PubMed] [Google Scholar]
- 57.Szeto, C. Y. Y., Leung, G. S., and H. S. Kwan. 2007. LeMAPK and its interacting partner, LeDRMIP, in fruiting body development in Lentinula edodes. Gene 393:87-93. [DOI] [PubMed] [Google Scholar]
- 58.Ueda, T., M. Yamaguchi, H. Uchimiya, and A. Nakano. 2001. Ara6, a plant-unique novel type Rab GTPase, functions in the endocytic pathway of Arabidopsis thaliana. EMBO J. 13:1378-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Sluijs, P., K. Mohrmann, M. Deneka, and M. Jongeneelen. 2001. Expression and properties of Rab4 and its effector rabaptin-4 in endocytic recycling. Methods Enzymol. 329:111-119. [DOI] [PubMed] [Google Scholar]
- 60.van IJzendoorn, S. C., M. J. Tuvim, T. Weimbs, B. F. Dickey, and K. E. Mostov. 2002. Direct interaction between Rab3b and the polymeric immunoglobulin receptor controls ligand-stimulated transcytosis in epithelial cells. Dev. Cell 2:219-228. [DOI] [PubMed] [Google Scholar]
- 61.Vida, T. A., and S. D. Emr. 1995. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol. 128:779-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vitale, G., V. Rybin, S. Christoforidis, P. Q. Thornqvist, M. McCaffrey, H. Stenmark, and M. Zerial. 1998. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 17:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitelli, R., M. Santillo, D. Lattero, M. Chiariello, M. Bifulco, C. B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272:4391-4397. [DOI] [PubMed] [Google Scholar]
- 64.Waters, M. G., and S. R. Pfeffer. 1999. Membrane tethering in intracellular transport. Curr. Opin. Cell Biol. 11:453-459. [DOI] [PubMed] [Google Scholar]
- 65.Whitmarsh, A. J., J. Cavanagh, C. Tournier, J. Yasuda, and R. J. Davis. 1998. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science 281:1671-1674. [DOI] [PubMed] [Google Scholar]
- 66.Wichmann, H., L. Hengst, and D. Gallwitz. 1992. Endocytosis in yeast: evidence for the involvement of a small GTP-binding protein (Ypt7p). Cell 71:1131-1142. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita, R. A., and G. S. May. 1998. Constitutive activation of endocytosis by mutation of myoA, the myosin I gene of Aspergillus nidulans. J. Biol. Chem. 273:14644-14648. [DOI] [PubMed] [Google Scholar]
- 68.Zerial, M., and H. McBride. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107-117. [DOI] [PubMed] [Google Scholar]