Abstract
Escherichia coli MG1655 uses several sugars for growth in the mouse intestine. To determine the roles of l-fucose and d-ribose, an E. coli MG1655 ΔfucAO mutant and an E. coli MG1655 ΔrbsK mutant were fed separately to mice along with wild-type E. coli MG1655. The E. coli MG1655 ΔfucAO mutant colonized the intestine at a level 2 orders of magnitude lower than that of the wild type, but the E. coli MG1655 ΔrbsK mutant and the wild type colonized at nearly identical levels. Surprisingly, an E. coli MG1655 ΔfucAO ΔrbsK mutant was eliminated from the intestine by either wild-type E. coli MG1655 or E. coli MG1655 ΔfucAO, suggesting that the ΔfucAO mutant switches to ribose in vivo. Indeed, in vitro growth experiments showed that l-fucose stimulated utilization of d-ribose by the E. coli MG1655 ΔfucAO mutant but not by an E. coli MG1655 ΔfucK mutant. Since the ΔfucK mutant cannot convert l-fuculose to l-fuculose-1-phosphate, whereas the ΔfucAO mutant accumulates l-fuculose-1-phosphate, the data suggest that l-fuculose-1-phosphate stimulates growth on ribose both in the intestine and in vitro. An E. coli Nissle 1917 ΔfucAO mutant, derived from a human probiotic commensal strain, acted in a manner identical to that of E. coli MG1655 ΔfucAO in vivo and in vitro. Furthermore, l-fucose at a concentration too low to support growth stimulated the utilization of ribose by the wild-type E. coli strains in vitro. Collectively, the data suggest that l-fuculose-1-phosphate plays a role in the regulation of ribose usage as a carbon source by E. coli MG1655 and E. coli Nissle 1917 in the mouse intestine.
We are interested in identifying the nutrients that Escherichia coli uses to successfully colonize the mouse large intestine in the face of competition from an extensive microflora. The large intestine of the mouse consists of the cecum and the colon, each of which contains the mucosa and the luminal contents. The two components of the mucosa are the layer of epithelial cells on the intestinal wall and the mucus layer that covers them. The relatively thick (up to 800 μm) mucus layer consists of mucin, a 2-MDa gel-forming glycoprotein and a large number of smaller glycoproteins, proteins, glycolipids, lipids, and sugars (1, 3, 15, 28, 46, 47, 51, 52). The mucus layer itself is in a dynamic state, constantly being synthesized and secreted by specialized goblet cells and degraded to a large extent by the indigenous intestinal microbes (25, 41). E. coli is present both in mucus and luminal contents; however, a large body of experimental evidence shows that growth is rapid in intestinal mucus both in vitro and in vivo but is either poor or completely inhibited in luminal contents (33, 38, 43, 52, 53, 55). It is therefore highly likely that the ability of an E. coli strain to grow in intestinal mucus plays a critical role in its ability to colonize the intestine.
The prevalent theory as to how bacteria colonize the mammalian gut has been called the nutrient-niche theory. This theory states that the 500 to 1,000 indigenous species that make up the microflora (39) can coexist in the large intestine as long as each member is able to utilize one or a few limiting nutrients better than all the others and that the rate of growth of any member of the microflora that successfully colonizes is at least equal to the washout rate from the intestine (19, 20, 21). It is the presence of a complete microflora that results in what has been termed colonization resistance, which refers to the ability of the intestinal microflora to resist colonization by an invading bacterium (2, 20, 22, 54). Due to colonization resistance, studies aimed at determining how an invading bacterium colonizes the mouse intestine are difficult, if not impossible, with conventional animals. However, intestinal colonization can be studied with the streptomycin-treated mouse, which has been used extensively to study E. coli, Salmonella enterica serovar Typhimurium, and Klebsiella pneumoniae intestinal colonization (14, 34, 42, 43).
Streptomycin treatment alters the microecology of the cecum, decreasing the populations of facultative anaerobes and some strict anaerobes and creating a niche for organisms such as E. coli (23). Nevertheless, populations of the genera Bacteroides and Eubacterium in cecal contents of streptomycin-treated mice remain largely unchanged (23). Moreover, the overall number of strict anaerobes in the cecal contents of streptomycin-treated and conventional mice is essentially identical (1 × 109 to 2 × 109 CFU/g of contents) (23). Therefore, while the streptomycin-treated mouse model is not perfect, invading microorganisms must compete for nutrients with a large number of strict anaerobes in the intestine, just as they do in conventional animals. This feature makes the streptomycin-treated mouse model the one of choice to identify the nutrients E. coli uses to colonize the mouse intestine in the face of competition from an extensive microflora.
Using the streptomycin-treated mouse model, it recently has been shown that E. coli MG1655, a human commensal strain for which the genome has been completely sequenced (5), utilizes several sugars simultaneously for growth in the mouse intestine (7). While these results support the nutrient-niche theory of colonization, the mechanisms governing the simultaneous use of sugars are largely unknown. In the present study, we report that while studying the role of l-fucose as a nutrient for E. coli MG1655 and E. coli Nissle 1917 in the mouse intestine, we found that accumulation of l-fuculose-1-phosphate, an intermediate in the degradation of l-fucose in E. coli, stimulates utilization of d-ribose. The present studies therefore raise the possibility that pool sizes of metabolic intermediates play a role in the selection of nutrients used by E. coli MG1655 and E. coli Nissle 1917 to achieve maximum colonizing ability.
MATERIALS AND METHODS
Bacterial strains.
E. coli MG1655 Strr is a spontaneous streptomycin-resistant mutant of the sequenced wild-type E. coli MG1655 strain (CGSC 7740) (37). E. coli MG1655 Strr Nalr is a spontaneous nalidixic acid-resistant mutant of E. coli MG1655 Strr (37). It is referred to in the text as E. coli MG1655. The following deletion mutants were constructed from E. coli MG1655 Strr as described below in the section on mutant construction. E. coli MG1655 Strr ΔfucAO::kan is both streptomycin resistant and kanamycin resistant and has a 1,542-bp deletion within the 1,823-bp fucA and fucO genes. It is unable to convert fuculose-1-phosphate to dihydroxyacetone phosphate and lactaldehyde and lacks both l-fuculose phosphate aldolase and propanediol oxidoreductase. It is referred to in the text as E. coli MG1655 ΔfucAO. E. coli MG1655 Strr ΔrbsK::cam has a 759-bp deletion of the 929-bp rbsK gene (7). It is unable to convert ribose to ribose-5-phosphate, lacks ribokinase, and is both streptomycin and chloramphenicol resistant. It is referred to in the text as E. coli MG1655 ΔrbsK. E. coli MG1655 Strr ΔfucAO ΔrbsK::cam has the identical 1,542-bp deletion in the fucA and fucO genes as described above but is missing the kanamycin cassette. In addition, it has a 759-bp deletion in the rbsK gene as described above. It is both streptomycin resistant and chloramphenicol resistant and is referred to in the text as E. coli MG1655 ΔfucAO ΔrbsK. E. coli MG1655 Strr ΔfucK::kan has a 993-bp deletion of the 1,448-bp fucK gene. It is unable to convert fuculose to fuculose-1-phosphate, lacks l-fuculokinase, and is both streptomycin and kanamycin resistant. Wild-type E. coli Nissle 1917 was obtained from Dean Hamer of the National Cancer Institute. E. coli Nissle 1917 Strr is a spontaneous streptomycin-resistant mutant of the wild-type strain. E. coli Nissle 1917 Strr Nalr is a spontaneous nalidixic acid-resistant mutant of E. coli Nissle 1917 Strr and is referred to in the text as E. coli Nissle 1917. The following deletion mutants were constructed from E. coli Nissle 1917 Strr as described below (see the section on mutant construction): E. coli Nissle 1917 Strr ΔfucAO::kan, E. coli Nissle 1917 Strr ΔrbsK::kan, E. coli Nissle 1917 Strr ΔfucAO ΔrbsK::cam, and E. coli Nissle 1917 Strr ΔfucK::cam. The mutants contain the same deletions as their E. coli MG1655 counterparts and are referred to in the text as E. coli Nissle 1917 ΔfucAO, E. coli Nissle 1917 ΔrbsK, E. coli Nissle 1917 ΔfucAO ΔrbsK, and E. coli Nissle 1917 ΔfucK, respectively.
Media and growth conditions.
Luria broth (LB) was made as described by Revel (48). Luria agar is LB containing 12 g of Bacto agar (Difco) per liter. MacConkey agar (Difco) was prepared according to the manufacturer's instructions. For testing the ability of E. coli strains to utilize d-ribose or l-fucose, overnight cultures grown in LB were washed twice in M9 minimal medium (no carbon source), 10 μl of the washed cultures was transferred to 10 ml of M9 minimal medium (35) containing reagent-grade glycerol (0.4%, wt/wt) as the sole carbon and energy source, and cultures were incubated at 37°C with shaking in 125-ml tissue culture bottles. Growth and the lack of growth were assessed visually. For testing the ability of l-fucose to induce the utilization of d-ribose in E. coli MG1655 and in E. coli Nissle 1917 ΔfucAO and ΔfucK mutants, the mutants were grown twice in M9 minimal medium containing glycerol (0.2%, wt/wt) as described above and then were washed and resuspended at an A600 of 0.1 in three separate 20-ml volumes of M9 minimal medium containing either 0.05% fucose, 0.15% ribose, or 0.05% fucose and 0.15% ribose. The cultures were incubated at 37°C with shaking in 125-ml tissue culture bottles. Growth was monitored spectrophotometrically (A600) using a Pharmacia Biotech Ultrospec 2000 UV/visible spectrophotometer.
Mutant construction.
Mutant E. coli strains were created by deletion mutagenesis using either a chloramphenicol cassette or a kanamycin cassette as described by Datsenko and Wanner (12). Primers used to construct deletion mutants were designed according to the E. coli MG1655 genome sequence (5). DNA procedures were as described previously (37). All constructs were verified by PCR and sequencing. The primers used to construct the ΔfucAO deletions were the following (uppercase letters, MG1655 DNA; lowercase letters, kanamycin cassette DNA): primer 1, 5′-CGCAAATGTGGCGGAATACCGACATCACGGTTGAGAGCAAACAgtgtaggctggagctgcttcg-3′; primer 2, 5′-CGTCAGTGTACGTTATCAGGATGGGATGCTGATTACGCCTACAGGCAtatgaatatcctccttag-3′. The primers used to construct the ΔrbsK deletions were the following (uppercase letters, MG1655 DNA; lowercase letters, chloramphenicol or kanamycin cassette DNA): primer 1, 5′-TGGCAGCATTAATGCTGACCACATTCTTAATCTTCAATCTTTTCCTACTCtgtaggctggagctgcttcg-3′; primer 2, 5′-GGTACGGAAGGTTGTGCGCCTTTACGTGTTACGGCAATCGCAGCGGCAGGCAtatgaatatcctccttag-3′. The primers used to construct the ΔfucK deletions were the following (uppercase letters, MG1655 DNA; lowercase letters, chloramphenicol or kanamycin cassette DNA): primer 1, 5′-CTGTGGCGCGACCAATGTCAGGGCCATCGCGGTTAATCGGCAGGGgtgtaggctggagctgcttcg-3′; primer 2, 5′-GTACGACTTAACAGCGAAGTATCAACCTGGGCGCTGCGAACCATCAtatgaatatcctccttag-3′.
Sequencing.
DNA sequencing was done at the URI Genomics and Sequencing Center, University of Rhode Island, Kingston, using the CEQ8000 genetic analysis system (Beckman Coulter, Fullerton, CA). The dye terminator cycle sequencing quick start kit (Beckman Coulter) was used in the sequencing reactions. The primers used to amplify PCR products for sequencing to determine the precise location of the deletions for E. coli MG1655 ΔfucK and E. coli Nissle 1917 ΔfucK were 5′-TATGCACAACGTTGAAGACACC-3′ and 5′-CCACAATGTGTTGCGACTTCCTC-3′. The same primers were used for sequencing. For E. coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO PCR amplification, the primers were the following: 5′-GCTTACAAACCGATTTGCATATC-3′ and 5′-GTGGGTAATTAAACGGCTAATTC-3′; for sequencing, the primers were 5′-AACAGTACTGCGATGAGTGGCAG-3′ and 5′-GCGAAGTGATCTTCCGTCACAGGT-3′. For E. coli MG1655 ΔrbsK PCR amplification, the primers were 5′-GGTTGTATGACCTGATGGTGAC-3′ and 5′-GAGAAACTG TTGAGGTAGAAACG-3′. The same primers were used for sequencing. For E. coli Nissle 1917 ΔrbsK PCR amplification, the primers were 5′-CGTTGTATGACCTGATGGTGAC-3′ and 5′-GAGAAACT GTTGAGGTAGAAACG-3′. The same primers were used for sequencing.
Mouse colonization experiments.
The method used to compare the large intestine-colonizing abilities of E. coli strains in mice has been described previously (52, 53, 55). Briefly, three male CD-1 mice (5 to 8 weeks old) were given drinking water containing streptomycin sulfate (5 g/liter) for 24 h to eliminate resident facultative bacteria (36). Following 18 h of starvation for food and water, the mice were fed 1 ml of 20% (wt/vol) sucrose containing LB-grown E. coli strains, as described in the Results section. After ingesting the bacterial suspension, both the food (Harlan Teklad Mouse and Rat Diet, Madison, WI) and streptomycin-water were returned to the mice, and 1 g of feces was collected after 5 h, 24 h, and on odd-numbered days at the indicated times. Mice were housed individually in cages without bedding and were placed in clean cages daily. Fecal samples (no older than 24 h) were homogenized in 1% Bacto tryptone, diluted in the same medium, and plated on MacConkey agar plates with appropriate antibiotics. Plates contained streptomycin sulfate (100 μg/ml) and nalidixic acid (50 μg/ml), streptomycin sulfate (100 μg/ml) and kanamycin sulfate (40 μg/ml), or streptomycin sulfate (100 μg/ml) and chloramphenicol (30 μg/ml). Antibiotics were purchased from Sigma-Aldrich (St. Louis, MO). All plates were incubated for 18 to 24 h at 37°C prior to counting. Each colonization experiment was performed at least twice, with essentially identical results. Pooled data from at least two independent experiments are presented in the figures.
RESULTS
Sequencing and growth of mutants in vitro.
All mutants used in this study were sequenced to be sure that the deletions were in the expected places in the chromosome (see Materials and Methods). In addition, each mutant was tested for growth in M9 minimal medium containing 0.4% (wt/wt) glycerol, fucose, or ribose as the sole carbon and energy source. All mutants grew normally with glycerol as the sole carbon and energy source. Furthermore, all mutants with a ΔfucAO deletion or a ΔfucK deletion failed to grow with fucose as the sole carbon and energy source, and those with a ΔrbsK deletion failed to grow with ribose as the sole carbon and energy source. Moreover, ΔfucAO ΔrbsK double mutants failed to grow on a mixture of fucose and ribose.
Mouse intestinal colonization of E. coli MG1655 ΔfucAO and ΔrbsK mutants.
Unlike its wild-type parent, E. coli MG1655 ΔfucAO cannot convert fuculose-1-phosphate to dihydroxyacetone phosphate and lactaldehyde (Fig. 1A) and therefore is unable to use fucose as a carbon and energy source. Similarly, E. coli MG1655 ΔrbsK cannot phosphorylate ribose to ribose-5-phosphate and therefore is unable to utilize ribose as a carbon and energy source (Fig. 1B). When mice were fed 105 CFU each of wild-type E. coli MG1655 and E. coli MG1655 ΔfucAO, the wild-type E. coli MG1655 grew to a level of about 108 CFU/g of feces within 1 day after feeding and then within a few days stabilized at a level of about 107 CFU/g of feces, whereas E. coli MG1655 ΔfucAO initially grew to a level of only about 107 CFU/g of feces and within a few days stabilized at a level of about 105 CFU/g of feces (Fig. 2A). When mice were fed 105 CFU each of wild-type E. coli MG1655 and E. coli MG1655 ΔrbsK, both grew at the same rate in the intestine to about 108 CFU/g of feces, and only thereafter did the E. coli MG1655 wild type, which stabilized at a level of about 107 CFU/g of feces, develop a very slight advantage, at most fourfold, over E. coli MG1655 ΔrbsK (Fig. 2B). Therefore, E. coli MG1655 appeared to use fucose during the initiation stage of the colonization process but appeared to stop using it during maintenance. In contrast, ribose did not appear to be used to any great extent during either stage of colonization.
FIG. 1.
Fucose operons and degradation pathway (A) and the ribose operon and degradation pathway (B). Arrows above genes indicate promoters and the direction of transcription. MFS, major facilitator superfamily; ABC, ATP-binding cassette.
FIG. 2.
Colonization of the mouse intestine by E. coli MG1655 ΔfucAO, E. coli MG1655 ΔrbsK, and E. coli MG1655 ΔfucAO ΔrbsK. Sets of three mice were fed 105 CFU of E. coli MG1655 (▪) and 105 CFU of MG1655 ΔfucAO (○) (A); 105 CFU of E. coli MG1655 (▪) and 105 CFU of E. coli MG1655 ΔrbsK (○) (B); 105 CFU of E. coli MG1655 (▪) and 105 CFU of E. coli MG1655 ΔfucAO ΔrbsK (○) (C); 105 CFU of E. coli MG1655 ΔfucAO (▪) and 105 CFU of E. coli MG1655 ΔfucAO ΔrbsK (○) (D); and 1010 CFU of E. coli MG1655 (▪) and 105 CFU of E. coli MG1655 ΔfucAO (○) (E). At the indicated times, fecal samples were homogenized, diluted, and plated as described in Materials and Methods. Bars representing standard errors of the log10 means of CFU per gram of feces for six mice are presented for each time point except for panels A and B, for which data from 12 mice are presented. Each colonization curve has the specific strain genotype immediately above or below it. wt, wild-type E. coli MG1655.
E. coli MG1655 ΔfucAO appears to switch to ribose in the intestine.
Since E. coli MG1655 ΔrbsK was nearly as good a colonizer as wild-type E. coli MG1655, it was expected that the colonizing ability of an E. coli MG1655 ΔfucAO ΔrbsK double mutant would closely mimic the colonizing ability of E. coli MG1655 ΔfucAO. Surprisingly, when mice were fed 105 CFU each of the wild-type E. coli MG1655 and E. coli MG1655 ΔfucAO ΔrbsK, the double mutant proved to be a far worse colonizer than expected, essentially being eliminated by day 15 after feeding (Fig. 2C). These data suggested the possibility that the E. coli MG1655 ΔfucAO mutant switched to ribose in the intestine. If true, the E. coli MG1655 ΔfucAO ΔrbsK double mutant should be a far worse colonizer than the E. coli MG1655 ΔfucAO mutant, despite differing only in the ability to utilize ribose for growth. Indeed, the E. coli MG1655 ΔfucAO ΔrbsK double mutant was rapidly eliminated in competition with E. coli MG1655 ΔfucAO (Fig. 2D), suggesting that the E. coli MG1655 ΔfucAO mutant, in contrast to the wild-type E. coli MG1655, utilizes ribose for growth in the intestine.
When mice are fed high numbers (1010 CFU/mouse) of a wild-type E. coli strain (resistant to streptomycin) and low numbers (105 CFU/mouse) of the same wild-type strain (e.g., resistant to streptomycin and nalidixic acid), they maintain the initial ratio of their input values (52), as would be expected of two strains that use all nutrients equally well. It then would be expected that if wild-type E. coli MG1655 was fed to mice in high numbers (1010 CFU/mouse) and the E. coli MG1655 ΔfucAO mutant was fed to the same mice in low numbers (105 CFU/mouse) and the only difference between the two strains was their ability to utilize fucose for growth, the ΔfucAO mutant would stabilize in numbers 5 orders of magnitude lower than those for both strains being fed to mice in equal numbers, i.e., a total of about 7 orders of magnitude. On the other hand, if the E. coli MG1655 ΔfucAO mutant was using ribose and the wild-type E. coli MG1655 was not, the E. coli MG1655 ΔfucAO mutant could conceivably grow from low numbers to higher numbers in the presence of high numbers of the wild-type strain and could colonize at the level observed when both strains were fed to the mice in low numbers, i.e., only 2 orders of magnitude lower than the level of the wild type (Fig. 1A). This is precisely what happened, i.e., when mice were fed 1010 CFU of the wild-type E. coli MG1655 and 105 CFU of the E. coli MG1655 ΔfucAO mutant, the mutant grew from a level of 5 orders of magnitude lower than that of its parent to only 2 orders of magnitude lower within a few days (Fig. 2E).
The switch to ribose appears to require fuculose-1-phosphate.
Because the E. coli MG1655 ΔfucAO mutant cannot convert fuculose-1-phosphate to dihydroxyacetone phosphate and lactaldehyde (8) and because fuculose-1-phosphate is required for induction of the fucPIKUR operon (4), E. coli MG1655 ΔfucAO most likely accumulates fuculose-1-phosphate in the presence of fucose. Since the E. coli MG1655 ΔfucAO mutant appeared to be using ribose for growth in the intestine, the question was whether the switch to ribose requires the presence of fuculose-1-phosphate. An E. coli MG1655 ΔfucK mutant, which is defective in the phosphorylation of fuculose to fuculose-1-phosphate (Fig. 1A), was employed to address this question. When mice were fed 105 CFU each of wild-type E. coli MG1655 and E. coli MG1655 ΔfucK, the mutant colonized at most fivefold below the level of its wild-type parent (Fig. 3A). When the E. coli MG1655 ΔfucK mutant was fed to mice at 105 CFU/mouse and wild-type E. coli MG1655 was simultaneously fed at 1010 CFU/mouse, the E. coli MG1655 ΔfucK mutant was eliminated (Fig. 3B). Therefore, the E. coli MG1655 ΔfucK mutant does not use ribose, and neither does it switch to any other nutrient in the intestine to allow it to grow up in the presence of high numbers of wild-type E. coli MG1655. Moreover, since the only difference between the E. coli MG1655 ΔfucK mutant and the E. coli MG1655 ΔfucAO mutant is the inability of the E. coli MG1655 ΔfucK mutant to accumulate fuculose-1-phosphate, i.e., neither mutant is able to make dihydroxyacetone phosphate and lactaldehyde (Fig. 1A), it would appear that the switch of E. coli MG1655 ΔfucAO to ribose in the intestine requires the accumulation of fuculose-1-phosphate.
FIG. 3.
Colonization of the mouse intestine by E. coli MG1655 ΔfucK. Sets of three mice were fed 105 CFU of E. coli MG1655 (▪) and 105 CFU of MG1655 ΔfucK (○) (A) or 1010 CFU of E. coli MG1655 (▪) and 105 CFU of E. coli MG1655 ΔfucK (○) (B). At the indicated times, fecal samples were homogenized, diluted, and plated as described in Materials and Methods. Bars representing standard errors of the log10 means of CFU per gram of feces for 12 mice are presented for each time point in panel A and for 6 mice in panel B. Each colonization curve has the specific strain genotype immediately above or below it. wt, wild-type E. coli MG1655.
Fucose stimulates the utilization of ribose for growth by E. coli MG1655 ΔfucAO in vitro but not for growth by E. coli MG1655 ΔfucK.
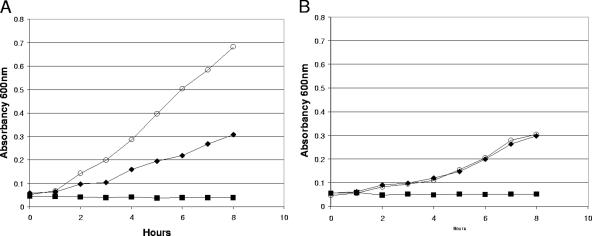
Since it appeared that fucose stimulates utilization of ribose by E. coli MG1655 ΔfucAO in the intestine, growth experiments were performed to determine whether fucose could stimulate utilization of ribose by E. coli MG1655 ΔfucAO in vitro. E. coli MG1655 ΔfucAO and E. coli MG1655 ΔfucK were grown in M9 minimal medium in the presence of 0.05% fucose, 0.15% ribose, and a mixture of 0.05% fucose and 0.15% ribose. As expected, neither strain grew with fucose as the sole carbon and energy source (Fig. 4). However, fucose stimulated more rapid growth of E. coli MG1655 ΔfucAO on ribose (Fig. 4A) but had no effect on the rate of growth of E. coli MG1655 ΔfucK on ribose (Fig. 4B). That the stimulation of ribose utilization required a functional rbsK gene was shown by the fact that the E. coli MG1655 ΔfucAO ΔrbsK mutant failed to grow on ribose in the presence or absence of fucose (data not shown). Collectively, these in vitro data support the idea that it is accumulation of fuculose-1-phosphate that stimulates growth of E. coli MG1655 ΔfucAO on ribose in the mouse intestine.
FIG. 4.
Growth of E. coli MG1655 ΔfucAO (A) and E. coli MG1655 ΔfucK (B) in M9 minimal medium in the presence of 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) ribose (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) ribose (○). E. coli MG1655 ΔfucAO and E. coli MG1655 ΔfucK were grown in M9 minimal medium containing glycerol (0.2%, wt/wt), washed, and resuspended in M9 minimal medium containing the appropriate sugars (see Materials and Methods). Incubation was at 37°C with aeration. A600 readings at the indicated times are presented. Growth experiments were performed at least three times. The results of typical experiments are shown.
Fucose has no effect on utilization of N-acetylglucosamine, but it inhibits the utilization of galactose and mannose for growth by E. coli MG1655 ΔfucAO in vitro.
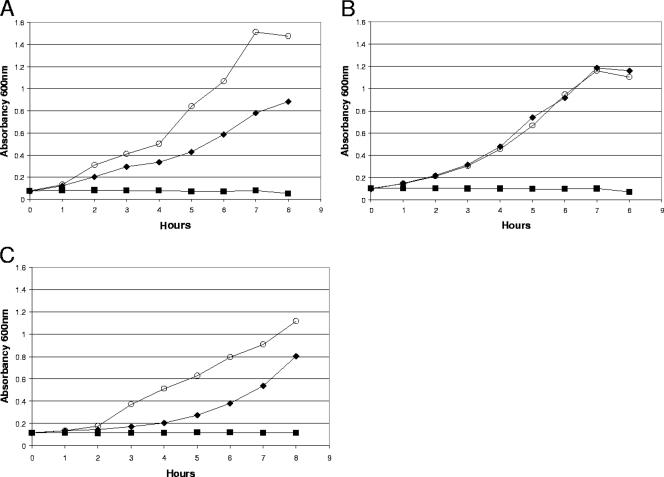
Both E. coli MG1655 ΔfucK and E. coli MG1655 ΔfucAO are unable to use fucose for growth, but E. coli MG1655 ΔfucAO switches to ribose in the intestine, whereas the switch to ribose is not made by E. coli MG1655 ΔfucK. Why then is E. coli MG1655 ΔfucK a better colonizer than E. coli MG1655 ΔfucAO when each is in competition with wild-type E. coli MG1655 (compare Fig. 2A to 3A)? One possibility was that while accumulation of fuculose-1-phosphate in E. coli MG1655 ΔfucAO stimulates utilization of ribose, it might simultaneously inhibit utilization of other sugars. Growth experiments analogous to those described for the effect of fucose on ribose utilization also were performed to determine the effect of fucose on utilization of N-acetylglucosamine, galactose, and mannose for growth by E. coli MG1655 ΔfucAO and E. coli MG1655 ΔfucK in vitro. Indeed, although 0.05% fucose had no effect on the utilization of 0.15% N-acetylglucosamine by E. coli MG1655 ΔfucAO in vitro (Fig. 5A), it greatly reduced the utilization of both galactose and mannose (Fig. 5B and C). In contrast, utilization of N-acetylglucosamine, galactose, and mannose was unaltered by fucose in the E. coli MG1655 ΔfucK mutant (data not shown). It therefore seems likely that E. coli MG1655 ΔfucAO is a worse colonizer than E. coli MG1655 ΔfucK, despite using ribose more efficiently, because accumulation of fuculose-1-phosphate inhibits its ability to compete for other sugars that are normally used for growth in the intestine.
FIG. 5.
Growth of E. coli MG1655 ΔfucAO in M9 minimal medium in the presence of 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) N-acetylglucosamine (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) N-acetylglucosamine (○) (A); 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) mannose (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) mannose (○) (B); and 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) galactose (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) galactose (○) (C). E. coli MG1655 ΔfucAO was grown in M9 minimal medium containing glycerol (0.2%, wt/wt), washed, and resuspended in M9 minimal medium containing the appropriate sugars (see Materials and Methods). Incubation was at 37°C with aeration. A600 readings at the indicated times are presented. Growth experiments were performed at least three times. The results of typical experiments are shown.
Fucose stimulates ribose utilization for growth by wild-type E. coli MG1655 in vitro.
In vitro growth experiments also were performed to determine whether fucose could stimulate utilization of ribose by wild-type E. coli MG1655 in vitro. In this case, it was necessary to use a concentration of fucose that would not allow E. coli MG1655 to grow but might still stimulate ribose utilization. Therefore, E. coli MG1655 was grown in the presence of 0.005% fucose, 0.15% ribose, or a mixture of 0.005% fucose and 0.15% ribose. Wild-type E. coli MG1655 did not grow in the presence of 0.005% fucose, but 0.005% fucose did stimulate the utilization of ribose by wild-type E. coli MG1655 (Fig. 6). It therefore appears that stimulation of ribose utilization by fucose also can occur in wild-type E. coli MG1655 at fucose concentrations too low to allow growth, suggesting the possibility that under the right conditions, fucose could signal wild-type E. coli MG1655 to utilize ribose in the intestine.
FIG. 6.
Growth of wild-type E. coli MG1655 in M9 minimal medium in the presence of 0.005% (wt/wt) fucose (▪), 0.15% (wt/wt) ribose (⧫), or a mixture of 0.005% (wt/wt) fucose and 0.15% (wt/wt) ribose (○). Wild-type E. coli MG1655 was grown in M9 minimal medium containing glycerol (0.2%, wt/wt), washed, and resuspended in M9 minimal medium containing the appropriate sugars (see Materials and Methods). Incubation was at 37°C with aeration. A600 readings at the indicated times are presented. Growth experiments were performed at least three times. The results of typical experiments are shown.
E. coli Nissle 1917 ΔfucAO also switches to ribose in the intestine.
Experiments were conducted to determine whether the switch to ribose in the intestine by E. coli MG1655 ΔfucAO was an isolated case or whether other E. coli strains carry out the switch. E. coli Nissle 1917 is a commensal strain that has been used successfully as a probiotic agent to treat gastrointestinal infections in humans since the early 1920s (50). Like E. coli MG1655 ΔfucAO, an E. coli Nissle 1917 ΔfucAO mutant colonized at a level about 2 orders of magnitude less than that of its wild-type parent when 105 CFU of each strain was fed to mice (Table 1). Also like E. coli MG1655 ΔrbsK, an E. coli Nissle 1917 ΔrbsK mutant colonized at a level of only about fourfold lower than that of its wild-type parent when 105 CFU of each strain was fed to mice (Table 1). In addition, an E. coli Nissle 1917 ΔfucAO ΔrbsK mutant colonized at a level of about 5 orders of magnitude lower than that of its wild-type parent when 105 CFU of each strain was fed to mice, i.e., greater than 2 orders of magnitude lower than expected (Table 1). Furthermore, when mice were fed 1010 CFU of wild-type E. coli Nissle 1917 and 105 CFU of the E. coli Nissle 1917 ΔfucAO mutant, the mutant grew from a level of 5 orders of magnitude lower than that of its parent to between 1.5 and 2 orders of magnitude lower within a few days (Table 1). Therefore, like E. coli MG1655 ΔfucAO, the E. coli Nissle 1917 ΔfucAO mutant appears to use ribose for growth in the intestine.
TABLE 1.
Mouse intestinal colonization of E. coli Nissle 1917 mutants relative to that of wild-type E. coli Nissle 1917
| Sugar | Defect in mutant | Inputa | Difference between log10 CFU of the wild type and log10 CFU mutant on day:
|
|
|---|---|---|---|---|
| 3 | 9 | |||
| Fucoseb | ΔfucAO | 0.13 ± 0.15 | 2.6 ± 0.5 | 2.3 ± 0.2 |
| Riboseb | ΔrbsK | 0.18 ± 0.15 | 0.03 ± 0.08 | 0.45 ± 0.23 |
| Fucose, riboseb | ΔfucAO ΔrbsK | 0.27 ± 0.33 | 2.6 ± 0.4 | 4.7 ± 0.4 |
| Fucoseb | ΔfucK | 0.22 ± 0.18 | 0.60 ± 0.15 | 1.3 ± 0.1 |
| Fucosec | ΔfucAO | 4.7 ± 0.6 | 1.3 ± 0.3 | 1.7 ± 0.2 |
| Fucosec | ΔfucK | 4.5 ± 0.5 | 5.1 ± 0.4 | 5.5 ± 0.3 |
Input values represent the mean of the log10 CFU of the E. coli Nissle 1917 mutant subtracted from the log10 CFU of the wild-type E. coli Nissle 1917 (± the log10 standard errors of the means) fed to six mice.
Mice were fed 105 CFU each of an E. coli Nissle 1917 mutant and its wild-type parent. Mice were transferred to fresh cages every day, and feces no older than 24 h were assayed every other day for 15 days. At each time point, for each mouse the log10 CFU/gram of feces for the mutant was subtracted from the log10 CFU/gram of feces for the wild type. The log10 mean of the difference ± the log10 standard error of the mean of day 3 and day 9 fecal data from at least 6 mice are shown.
The same procedure as that described in footnote b was followed, except that mice were fed 105 CFU of an E. coli Nissle 1917 mutant and 1010 CFU of its wild-type parent.
Mice also were fed 105 CFU each of wild-type E. coli Nissle 1917 and an E. coli Nissle 1917 ΔfucK mutant. The E. coli Nissle 1917 ΔfucK mutant colonized at a level of between 1 and 2 orders of magnitude lower than that of the wild-type strain (Table 1). In addition, when mice were fed 1010 CFU of wild-type E. coli Nissle 1917 and 105 CFU of E. coli Nissle 1917 ΔfucK, the E. coli Nissle 1917 ΔfucK mutant was essentially eliminated (Table 1). Collectively, these data suggest that the E. coli Nissle 1917 ΔfucK mutant does not switch to ribose in the intestine and that, like the E. coli MG1655 ΔfucAO mutant, the E. coli Nissle 1917 ΔfucAO mutant switches to ribose for growth in the intestine, mediated by the accumulation of fuculose-1-phosphate.
Fucose stimulates the utilization of ribose by wild-type E. coli Nissle 1917 and by E. coli Nissle 1917 ΔfucAO for growth in vitro.
Growth experiments were performed to determine whether fucose could stimulate utilization of ribose in the E. coli Nissle 1917 ΔfucAO mutant in vitro. The E. coli Nissle 1917 ΔfucAO mutant and the E. coli Nissle 1917 ΔfucK mutant were grown in vitro in the presence of 0.05% fucose, 0.15% ribose, and 0.05% fucose plus 0.15% ribose. As expected, neither strain grew on fucose as the sole carbon and energy source. However, fucose was able to induce more rapid growth of E. coli Nissle 1917 ΔfucAO on ribose (Fig. 7A). Fucose did not induce more rapid growth of the E. coli Nissle 1917 ΔfucK mutant on ribose (Fig. 7B). Moreover, fucose had no effect on N-acetylglucosamine utilization but inhibited both mannose and galactose utilization in the E. coli Nissle 1917 ΔfucAO mutant (data not shown). Fucose had no effect on N-acetylglucosamine, galactose, or mannose utilization by E. coli Nissle 1917 ΔfucK (data not shown). It therefore appears that as in the case of E. coli MG1655, accumulation of fuculose-1-phosphate induces more rapid growth of the commensal E. coli Nissle 1917 ΔfucAO mutant on ribose in vitro, has no effect on N-acetylglucosamine utilization, and inhibits both galactose and mannose utilization. Finally, wild-type E. coli Nissle 1917 was grown in the presence of 0.005% fucose, 0.15% ribose, or a mixture of 0.005% fucose and 0.15% ribose. Again, like E. coli MG1655, the wild-type E. coli Nissle 1917 did not grow in the presence of 0.005% fucose, but 0.005% fucose did stimulate its use of ribose (Fig. 7C).
FIG. 7.
Growth of E. coli Nissle 1917 ΔfucAO in M9 minimal medium in the presence of 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) ribose (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) ribose (○) (A); growth of E. coli Nissle 1917 ΔfucK in M9 minimal medium in the presence of 0.05% (wt/wt) fucose (▪), 0.15% (wt/wt) ribose (⧫), or a mixture of 0.05% (wt/wt) fucose and 0.15% (wt/wt) ribose (○) (B); and growth of wild-type E. coli Nissle 1917 in M9 minimal medium in the presence of 0.005% (wt/wt) fucose (▪), 0.15% (wt/wt) ribose (⧫), or a mixture of 0.005% (wt/wt) fucose and 0.15% (wt/wt) ribose (○) (C). Strains were grown in M9 minimal medium containing glycerol (0.2%, wt/wt), washed, and resuspended in M9 minimal medium containing the appropriate sugars (see Materials and Methods). Incubation was at 37°C with aeration. A600 readings at the indicated times are presented. Growth experiments were performed at least three times. The results of typical experiments are shown.
DISCUSSION
It is becoming increasingly clear that in the face of competition from a complex microflora, E. coli simultaneously uses several, presumably limiting, sugars for growth in the mouse intestine, including d-gluconate, N-acetyl-d-glucosamine, d-glucuronate, and sialic acid (7, 10, 30, 31). The simultaneous use of several sugars for growth is not unprecedented. In fact, E. coli has been shown to utilize d-glucose, d-galactose, d-maltose, d-ribose, l-arabinose, and d-fructose simultaneously in a chemostat under carbon-limiting conditions (32). It also appears that E. coli is capable of preparing itself for the appearance of alternative carbon sources when growing under glucose-limiting conditions or when utilizing acetate as the sole carbon source (18, 44). Thus, DNA microarray analysis has shown that when E. coli was grown in a chemostat under glucose-limiting conditions, genes associated with the maltose and galactose operons were up-regulated relative to batch cultures grown under conditions in which glucose was not limited (18). Similarly, with acetate as the sole carbon source, genes encoding transporters for galactose, ribose, and N-acetylglucosamine were up-regulated relative to growth with glucose as the sole carbon source (44). Moreover, using Biolog AN MicroPlate respiration analysis, it has recently been shown that when E. coli MG1655 was grown in a chemostat under glucose-limiting conditions, despite the absence of inducers a wide variety of catabolic functions were derepressed (26).
There are two divergently transcribed operons for fucose utilization in E. coli (Fig. 1A), the fucPIKUR operon, which takes fucose to fuculose-1-phosphate, and the fucAO operon, which takes fuculose-1-phosphate to dihydroxyacetone phosphate and lactaldehyde (8). Fuculose-1-phosphate is the effector of induction of both fucose operons through the FucR activator protein (4, 8, 9). In the present study, evidence is presented suggesting that accumulation of fuculose-1-phosphate through induction of the fucPIKUR operon in two commensal strains, E. coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO, stimulates growth on ribose both in the intestine and in vitro. However, in a fucAO mutant, in addition to accumulation of fuculose-1-phophate, it would be expected that the fuculose transporter (FucP) encoded by fucP, the FucR activator protein encoded by fucR, and the l-fucose mutarotase encoded by fucU also would accumulate. It recently has been reported (49) that the l-fucose mutarotase can convert β-d-pyranoribose, the form transported into the cell, to α-d-ribofuranose (49). α-d-Ribofuranose is required for ribose catabolism (40). It also has recently been reported that d-fructose possibly can enter an E. coli fucA mutant via FucP (29). If ribose were to enter ΔfucAO cells via FucP, it then could induce the ribose operon. In this case and in the case of the mutarotase, the fuculose-1-phosphate would be necessary only for inducing the fucPIKUR operon. Whether the l-fucose permease and/or the l-fucose mutarotase is involved in stimulation of ribose utilization is presently under investigation.
At the present time, it is not clear how or if fuculose-1-phosphate is involved either by itself or as an effector in combination with a regulatory protein in inducing the rbsDABCKR operon. It is possible that accumulated fuculose-1-phosphate acts as an effector of FucR to directly activate or release repression of transcription of the rbsDABCKR operon. In Bacteroides thetaiotaomicron, l-fucose has been implicated through its FucR protein, which is unrelated to the E. coli FucR protein, to both induce the fucose operon and, along with FucR, to corepress an as-yet unidentified locus named cps (control of signal production) that may be responsible for inducing the mammalian host to make hydrolysable fucosylated glycans (24). Moreover, it has been shown that transcription of the ribose operon is repressed by XylR, the xylose regulator, in the presence of xylose (27). It is therefore not farfetched to suggest the possibility that in commensal E. coli strains fuculose-1-phosphate and FucR also regulate more than one operon. It also is possible that fuculose-1-phosphate interacts with the RbsR repressor protein or an as-yet unidentified protein to relieve repression of the ribose operon. Finally, it is possible that fuculose-1-phosphate is involved in opening a gate for ribose entry into the cells via a preexisting RbsABC transporter or in allosterically activating more of the ribose gene products.
It appears that accumulation of fuculose-1-phosphate inhibits growth of both E. coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO on mannose and galactose. This finding is not unexpected; i.e., it is well known that intracellular accumulation of sugar phosphates is inhibitory to E. coli growth, although the reasons remain unclear (6, 11, 13, 16, 17, 56). However, in view of the fact that sugar phosphates normally inhibit growth, it is unexpected that accumulation of fuculose-1-phosphate would stimulate growth of E. coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO on ribose. Nevertheless, stimulation of ribose utilization by fuculose-1-phosphate appears to be a normal physiological process; i.e., fucose at a concentration too low to allow growth (0.005%) stimulated ribose utilization in both wild-type E. coli MG1655 and wild-type E. coli Nissle 1917 in vitro (Fig. 6 and 7C). In this context, although both fucose operons are activated by FucR in the presence of fuculose-1-phosphate (4, 9), it is possible that at low fucose concentrations the fucPIKUR operon is more highly expressed than the fucAO operon in wild-type E. coli MG1655, which could result in the accumulation of fuculose-1-phosphate. In fact, the two fucose operons can be expressed differentially (45, 57). The finding that low levels of fucose signal the wild-type strains to grow more rapidly on ribose raises the possibility that it also occurs in the intestine, perhaps in a minor niche. Such wild-type subpopulations presumably would be at the level of the population size of the ΔfucAO mutants in the presence of the wild-type strains, i.e., 100-fold below the wild-type population (Fig. 2E), and would go unnoticed in routine colonization experiments. Experiments designed to test this possibility are currently in progress.
The finding that E. coli MG1655 ΔfucAO and E. coli Nissle 1917 ΔfucAO mutants switch to ribose in the intestine because they accumulate fuculose-1-phosphate not only supports the nutrient-niche theory but also has far-reaching implications with respect to the stability of the commensal flora in the intestine. E. coli and presumably other members of the intestinal microflora use several limiting nutrients simultaneously for growth (7, 10, 30, 31, 37), but how any member of the microflora chooses the specific nutrients to use at any one time among those available to it is largely unknown. The data presented here suggest that the intracellular pool size of a metabolic intermediate involved in the metabolism of one nutrient plays a role in this process by signaling E. coli to rapidly switch to a second unrelated nutrient for growth. Thus, it is possible that a variety of metabolic intermediates comprise a tier of regulation designed for E. coli and perhaps other members of the microflora to more efficiently utilize the available limiting multiple nutrients to maintain growth rates sufficient to avoid washout from the intestine.
Acknowledgments
This research was supported by Public Health Service grant AI 48945 to T.C. and P.S.C.
We are grateful to Shelley Brown for excellent technical assistance.
Editor: A. Camilli
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Allan, A. 1981. Structure and function of gastrointestinal mucus, p. 637-639. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Raven Press, New York, NY.
- 2.Anderson, J. D., W. A. Gillespie, and M. H. Richmond. 1973. Chemotherapy and antibiotic-resistance transfer between enterobacteria in the human gastro-intestinal tract. J. Med. Microbiol. 6:461-473. [DOI] [PubMed] [Google Scholar]
- 3.Atuma, C., V. Strugala, A. Allen, and L. Holm. 2001. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G922-G929. [DOI] [PubMed] [Google Scholar]
- 4.Bartkus, J. M., and R. P. Mortlock. 1986. Isolation of a mutation resulting in constitutive synthesis of l-fucose catabolic enzymes. J. Bacteriol. 165:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 6.Böck, A., and F. C. Neidhardt. 1966. Properties of a mutant of Escherichia coli with a temperature-sensitive fructose-1,6-diphosphate aldolase. J. Bacteriol. 92:470-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, D. E., D. J. Smalley, D. L. Tucker, M. P. Leatham, W. E. Norris, S. J. Stevenson, A. B. Anderson, J. E. Grissom, D. C. Laux, P. S. Cohen, and T. Conway. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. USA 101:7427-7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y. M., Z. Lu, and E. C. C. Lin. 1989. Constitutive activation of the fucAO operon and silencing of the divergently transcribed fucPIK operon by an IS5 element in Escherichia coli mutants selected for growth on l-1,2-propanediol. J. Bacteriol. 171:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Y. M., Y. Zhu, and E. C. Lin. 1987. The organization of the fuc regulon specifying l-fucose dissimilation in Escherichia coli K12 as determined by gene cloning. Mol. Gen. Genet. 210:331-337. [DOI] [PubMed] [Google Scholar]
- 10.Conway, T., K. A. Krogfelt, and P. S. Cohen. December 2004, posting date. The life of commensal Escherichia coli in the mammalian intestine. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org.
- 11.Cozzarelli, N. R., J. P. Koch, S. Hayashi, and E. C. C. Lin. 1965. Growth stasis by accumulated l-glycerophosphate in Escherichia coli. J. Bacteriol. 90:1325-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englesberg, E., R. L. Anderson, R. Weinberg, N. Lee, P Hoffee, G. Huttenhauer, and H. Boyer. 1962. l-Arabinose-sensitive, l-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J. Bacteriol. 84:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre-Bonté, S., T. R. Licht, C. Forestier, and K. A. Krogfelt. 1999. Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect. Immun. 67:6152-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forstner, G. G. 1970. [1-14C]glucosamine incorporation by subcellular fractions of small intestine mucosa. J. Biol. Chem. 245:3584-3592. [PubMed] [Google Scholar]
- 16.Fradkin, J. E., and D. G. Fraenkel. 1971. 2-Keto-3-deoxygluconate-6-phosphate-aldolase mutants of Escherichia coli. J. Bacteriol. 108:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraenkel, D. G. 1968. The accumulation of glucose 6-phosphate from glucose and its effect in an Escherichia coli mutant lacking phosphoglucose isomerase and glucose 6-phosphate dehydrogenase. J. Biol. Chem. 243:6451-6457. [PubMed] [Google Scholar]
- 18.Franchini, A. G., and T. Egli. 2006. Global gene expression in Escherichia coli K-12 during short-term and long-term adaptation to glucose-limited continuous culture conditions. Microbiology 152:2111-2127. [DOI] [PubMed] [Google Scholar]
- 19.Freter, R. 1992. Factors affecting the microecology of the gut, p. 111-144. In R. Fuller (ed.), Probiotics: the scientific basis. Chapman & Hall, London, United Kingdom.
- 20.Freter, R., H. Brickner, M. Botney, D. Cleven, and A. Aranki. 1983. Mechanisms that control bacterial populations in continuous-flow culture models or mouse large intestinal flora. Infect. Immun. 39:676-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freter, R., H. Brickner, J. Fekete, M. M. Vickerman, and K. E. Carey. 1983. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 39:686-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hentges, D. J. 1983. Role of the intestinal microflora in host defense against infection, p. 311-331. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, New York, NY.
- 23.Hentges, D. J., J. U. Que, S. W. Casey, and A. J. Stein. 1984. The influence of streptomycin on colonization resistance in mice. Microecol. Ther. 14:53-62. [Google Scholar]
- 24.Hooper, L. V., J. Xu, P. G. Falk, T. Midtvedt, and J. I. Gordon. 1999. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc. Natl. Acad. Sci. USA 96:9833-9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoskins, L. 1984. Mucin degradation by enteric bacteria: ecological aspects and implications for bacterial attachment to gut mucosa, p. 51-65. In E. C. Boedecker (ed.), Attachment of organisms to the gut mucosa, vol. II. CRC Press, Inc., Boca Raton, FL. [Google Scholar]
- 26.Ihssen, J., and T. Egli. 2005. Global physiological analysis of carbon- and energy-limited growing Escherichia coli confirms a high degree of catabolic flexibility and preparedness for mixed substrate utilization. Environ. Microbiol. 7:1568-1581. [DOI] [PubMed] [Google Scholar]
- 27.Kang, H.-Y., S. Song, and C. Park. 1998. Priority of pentose utilization at the level of transcription: arabinose, xylose, and ribose operons. Mol. Cells 8:318-323. [PubMed] [Google Scholar]
- 28.Kim, Y. S., A. Morita, S. Miura, and B. Siddiqui. 1984. Structure of glycoconjugates of intestinal mucosal membranes. Role of bacterial adherence, p. 99-109. In E. C. Boedecker (ed.), Attachment of organisms to the gut mucosa, vol. II. CRC Press, Inc., Boca Raton, FL. [Google Scholar]
- 29.Kornberg, H., and C. Lourenco. 2006. A route for fructose utilization by Escherichia coli involving the fucose regulon. Proc. Natl. Acad. Sci. USA 103:19496-19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laux, D. C., P. S. Cohen, and T. Conway. 2005. Role of the mucus layer in bacterial colonization of the intestine, p. 199-212. In J. P. Nataro, P. S. Cohen, H. L. T. Mobley, and J. N. Weiser (ed.), Colonization of mucosal surfaces. American Society for Microbiology, Washington, DC.
- 31.Leatham, M. P., S. J. Stevenson, E. J. Gauger, K. A. Krogfelt, J. J. Lins, T. L. Haddock, S. M. Autieri, T. Conway, and P. S. Cohen. 2005. The mouse intestine selects non-motile flhDC mutants of Escherichia coli MG1655 with increased colonizing ability and better utilization of carbon sources. Infect. Immun. 73:8039-8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lendenmann, U., M. Snozzi, and T. Egli. 1996. Kinetics of the simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture. Appl. Environ. Microbiol. 62:1493-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Licht, T. R., T. Tolker-Nielsen, K. Holmstrøm, K. A. Krogfelt, and S. Molin. 1999. Inhibition of Escherichia coli precursor 16S rRNA processing by mouse intestinal contents. Environ. Microbiol. 1:23-32. [DOI] [PubMed] [Google Scholar]
- 34.McCormick, B. A., B. A. D. Stocker, D. C. Laux, and P. S. Cohen. 1988. The role of motility, chemotaxis, penetration through, and growth in intestinal mucus in the ability of an avirulent strain of Salmonella typhimurium to colonize the large intestines of streptomycin-treated mice. Infect. Immun. 56:2209-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 36.Miller, C. P., and M. Bohnhoff. 1963. Changes in the mouse's enteric microflora associated with enhanced susceptibility to Salmonella infection following streptomycin-treatment. J. Infect. Dis. 113:59-66. [DOI] [PubMed] [Google Scholar]
- 37.Miranda, R. L., T. Conway, T. M. P. Leatham, D. E. Chang, W. E. Norris, J. H. Allen, S. J. Stevenson, D. C. Laux, and P. S. Cohen. 2004. Glycolytic and gluconeogenic growth of Escherichia coli O157:H7 (EDL933) and E. coli K-12 (MG1655) in the mouse intestine. Infect. Immun. 72:1666-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Møller, A. K., M. P. Leatham, T. Conway, P. J. M. Nuijten, L. A. M. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 71:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore, W. E. C., and L. V. Holdeman. 1974. Human fecal flora: the normal flora of 20 Japanese. Appl. Microbiol. 27:961-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mowbray, S. L., and L. B. Cole. 1992. 1.7 Å X-ray structure of the periplasmic ribose receptor from Escherichia coli. J. Mol. Biol. 225:155-175. [DOI] [PubMed] [Google Scholar]
- 41.Neutra, M. R. 1984. The mechanism of intestinal mucous secretion, p. 33-41. In E. C. Boedecker (ed.), Attachment of organisms to the gut mucosa, vol. II. CRC Press, Inc., Boca Raton, FL. [Google Scholar]
- 42.Nevola, J. J., D. C. Laux, and P. S. Cohen. 1987. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect. Immun. 55:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newman, J. V., R. Kolter, D. C. Laux, and P. S. Cohen. 1994. The role of leuX in Escherichia coli colonization of the streptomycin-treated mouse large intestine. Microb. Pathog. 17:301-311. [DOI] [PubMed] [Google Scholar]
- 44.Oh, M. K., L. Rohlin, K. C. Kao, and J. C. Liao. 2002. Global expression profiling of acetate-grown Escherichia coli. J. Biol. Chem. 277:13175-13183. [DOI] [PubMed] [Google Scholar]
- 45.Podolny, V., E. C. C. Lin, and A. Hochschild. 1999. A cyclic AMP receptor protein mutant that constitutively activates an Escherichia coli promoter disrupted by an IS5 insertion. J. Bacteriol. 181:7457-7463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potten, C. S., and T. D. Allen. 1977. Ultrastructure of cell loss in intestinal mucosa. J. Ultrastruct. Res. 60:272-277. [DOI] [PubMed] [Google Scholar]
- 47.Quastler, H., and F. G. Sherman. 1959. Cell population in the intestinal epithelium of the mouse. Exp. Cell. Res. 17:420-438. [DOI] [PubMed] [Google Scholar]
- 48.Revel, H. R. 1967. Restriction and nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology 31:688-701. [DOI] [PubMed] [Google Scholar]
- 49.Ryu, K. S., C. Kim, I. Kim, S. Yoo, B. S. Choi, and C. Park. 2004. NMR application probes a novel and ubiquitous family of enzymes that alter monosaccharide configuration. J. Biol. Chem. 279:25544-25548. [DOI] [PubMed] [Google Scholar]
- 50.Sartor, R. B. 2005. Probiotic therapy of intestinal inflammation and infections. Curr. Opin. Gastroenterol. 21:44-50. [PubMed] [Google Scholar]
- 51.Slomiany, A., S. Yano, B. I. Slomiany, and G. B. J. Glass. 1978. Lipid composition of the gastric mucus barrier in the rat. J. Biol. Chem. 253:3785-3791. [PubMed] [Google Scholar]
- 52.Sweeney, N. J., P. Klemm, B. A. McCormick, E. Moller-Nielsen, M. Utley, M. A. Schembri, D. C. Laux, and P. S. Cohen. 1996. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect. Immun. 64:3497-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeney, N. J., D. C. Laux, and P. S. Cohen. 1996. Escherichia coli F-18 and K-12 eda mutants do not colonize the streptomycin-treated mouse large intestine. Infect. Immun. 64:3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Waaij, D., J. M. Berghuis de Vries, and J. E. C. Lekkerkerk. 1971. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 69:405-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wadolkowski, E. A., D. C. Laux, and P. S. Cohen. 1988. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infect. Immun. 56:1030-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yarmolinsky, M. B., H. Wiesmeyer, H. M. Kalckar, and E. Jordan. 1959. Hereditary defects in galactose metabolism in Escherichia coli mutants. II. Galactose-induced sensitivity. Proc. Natl. Acad. Sci. USA 45:1786-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu, Y., and E. C. Lin. 1988. A mutant crp allele that differentially activates the operons of the fuc regulon in Escherichia coli. J. Bacteriol. 170:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]