Abstract
Nucleotide-binding oligomerization domain proteins (NODs) are modular cytoplasmic proteins implicated in the recognition of peptidoglycan-derived molecules. NOD2 has recently been shown to be important for host cell cytokine responses to Mycobacterium tuberculosis, to synergize with Toll-like receptor 2 (TLR2) in mediating these responses, and thus to serve as a nonredundant recognition receptor for M. tuberculosis. Here, we demonstrate that macrophages and dendritic cells from NOD2-deficient mice were impaired in the production of proinflammatory cytokines and nitric oxide following infection with live, virulent M. tuberculosis. Mycolylarabinogalactan peptidoglycan (PGN), the cell wall core of M. tuberculosis, stimulated macrophages to release tumor necrosis factor (TNF) and interleukin-12p40 in a partially NOD2-dependent manner, and M. tuberculosis PGN required NOD2 for the optimal induction of TNF. However, NOD2-deficient mice were no more susceptible to infection with virulent M. tuberculosis than wild-type mice: they controlled the replication of M. tuberculosis in lung, spleen, and liver as well as wild-type mice, and both genotypes displayed similar lung pathologies. In addition, mice doubly deficient for NOD2 and TLR2 were similarly able to control an M. tuberculosis infection. Thus, NOD2 appears to participate in the recognition of M. tuberculosis by antigen-presenting cells in vitro yet is dispensable for the control of the pathogen during in vivo infection.
Mycobacterium tuberculosis has successfully infected one-third of the world's population and threatens to kill 10% of infected individuals during the course of their lifetime (1, 11). Few genetic loci for mycobacterial resistance or susceptibility have been identified, and it is a daunting task to uncover correlates of protection against tuberculosis (TB). Innate and adaptive immune responses are required for host defense that leads to the control of mycobacterial replication within macrophages. The infected macrophages are part of an organized granuloma consisting of multiple immune cells including macrophages, dendritic cells (DCs), and T and B lymphocytes. The interaction of M. tuberculosis with phagocytes results in the production of proinflammatory cytokines and chemokines and is crucial for coordinated innate and adaptive immune responses and thus for effective granuloma formation (10).
M. tuberculosis interacts with phagocytes via a variety of receptors (8). Although Toll-like receptors (TLRs) on macrophages and DCs are important for the recognition of M. tuberculosis (2, 31), M. tuberculosis activates these cells via both TLR-dependent and TLR-independent pathways. For example, global gene expression analysis revealed that M. tuberculosis induces gene expression in murine bone marrow-derived macrophages (BMMs) chiefly independently of MyD88, the central intracellular adaptor of TLRs (33). Expression of some proinflammatory cytokines such as interleukin-1 (IL-1) and IL-6 depends predominantly on TLR2-mediated recognition in macrophages and DCs (19, 33). However, many proinflammatory mediators including interferon-inducible protein 10, inducible nitric oxide (NO) synthase (iNOS), immune-responsive gene 1, and RANTES are induced by M. tuberculosis in BMMs in the absence of TLR2 and TLR4. IL-12p40 expression in M. tuberculosis-infected DCs is induced in a TLR2- and TLR4-independent manner; instead, its production requires TLR9 in vitro and in vivo (2, 19). Cooperation between different pattern recognition receptors also contributes to the host response to M. tuberculosis, which is demonstrated by the observation that TLR2 and TLR9 act synergistically to mediate resistance to M. tuberculosis infection (2).
TLRs are not the only receptors involved in sensing microbial infection. Nucleotide-binding oligomerization domain proteins (NODs) are members of an emerging family that have been implicated in the intracellular recognition of bacterial components (16). NOD2 recognizes muramyl dipeptide (MDP), a component of peptidoglycan (PGN) from both gram-positive and gram-negative bacteria (12-14, 17). NOD2 contains a carboxyl-terminal leucine-rich repeat domain, a central nucleotide-binding oligomerization domain, and two amino-terminal caspase recruitment domains (CARDs) (18). Following exposure to MDP, NOD2 is hypothesized to interact with the serine/threonine kinase Rip2/RICK/CARDIAK via CARD-CARD binding (21, 29, 36). RICK directly activates the NF-κB pathway through the activation of the IκB kinase complex, leading to the degradation of IκBα and the release of NF-κB (29). Data focusing on the immunological relevance of NOD2 are just starting to emerge. Mutations in human CARD15, encoding NOD2, are partially associated with susceptibility to several familial inflammatory diseases including Crohn's disease, Blau syndrome, and early-onset sarcoidosis (15, 20, 25, 28). NOD2 may therefore be important for regulating inflammatory responses (16). Further evidence that NOD2 might be involved in the control of inflammation comes from studies with NOD2-deficient (Card15−/−) mice that produced higher serum IL-12 levels than wild-type mice when systemically challenged with the TLR2 agonist PGN (40). NOD2 was also found to be required for the control of gastric infection with Listeria monocytogenes in mice yet dispensable for the control of systemic infection (22). Thus, NOD2 specifically protected against bacterial infection in the intestine, where it was required for the expression of a subgroup of intestinal antimicrobial peptides.
M. tuberculosis is an intraphagosomal pathogen; however, mycobacterial proteins and cell wall lipids access the cytosol, where they encounter intracellular molecules to modulate the host cell response (3, 4, 27). Indeed, a recent study showed that tumor necrosis factor alpha (TNF-α) production induced by sonicated M. tuberculosis in murine peritoneal macrophages was partially NOD2 dependent (9). However, the role of NOD2 in mediating immune responses that are required for the control of infection with live, virulent M. tuberculosis has not been tested. We used NOD2-deficient (Card15−/−) BMMs and DCs to show that NOD2 is required for the optimal production of proinflammatory cytokines and NO in response to M. tuberculosis infection in vitro. However, infection of Card15−/− mice and Card15−/− Tlr2−/− mice with virulent M. tuberculosis revealed that the impaired cellular responses did not result in increased susceptibility to TB. Thus, NOD2 participates in the innate recognition of M. tuberculosis, yet in vivo, redundant systems that mediate an effective immune response to M. tuberculosis even in the absence of NOD2 seem to exist.
MATERIALS AND METHODS
Mice.
Card15−/− mice were generated as previously described and used on a C57BL/6 background (n = 5 backcross generations) (30). Card15−/− Tlr2−/− mice, also on a C57BL/6 background (n = 5 backcross generations), were generated as described previously (41). Mice were housed under specific-pathogen-free conditions.
Macrophage preparation.
Bone marrow cells from 8- to 10-week-old mice were flushed from femurs and differentiated into macrophages for 7 days in Dulbecco's modified Eagle medium supplemented with 20% L-cell medium, 10% fetal bovine serum, 2 mM l-glutamine, 1 mM sodium pyruvate, and 10 mM HEPES. Cells were fed with 25% fresh medium on day 4. After 7 days in culture, BMMs were washed with phosphate-buffered saline (PBS) and seeded into tissue culture plates in Dulbecco's modified Eagle's medium containing 10% L-cell medium, 10% fetal bovine serum, 2 mM l-glutamine, and 1 mM sodium pyruvate. This results in a nearly pure macrophage population as assessed by morphology and cell surface staining of CD14, F4/80, FcγRII/III, and major histocompatibility complex class II, the latter after gamma interferon (IFN-γ) activation. Where indicated, 10 ng/ml mouse IFN-γ (R&D Systems) was added. Sixteen hours later, the cells were infected with a single-cell suspension of M. tuberculosis obtained from early-log-phase cultures (7).
DC preparation.
Bone marrow-derived DCs were prepared as previously described (23). Briefly, bone marrow was flushed out from the femur and tibia, and 2 × 106 bone marrow cells were seeded into 10-cm petri dishes in 10 ml of RPMI containing 10% fetal calf serum (HyClone Laboratories, Logan, UT) and supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), glutamine (2 mM), 2-mercaptoethanol (50 μM), and 20 ng/ml murine recombinant granulocyte-monocyte colony-stimulating factor (Peprotech Inc., Rocky Hill, NJ). On day 3, an additional 10 ml of complete medium containing granulocyte-monocyte colony-stimulating factor was added to the cultures. On days 6 and 8, the cultures were fed by changing 50% of the medium. Nonadherent cells were harvested on day 10. Resultant nonadherent cells were typically >70% CD11c+ CD11b+ as determined by fluorescence-activated cell sorter analysis.
Bacteria and microbial stimuli.
M. tuberculosis strains H37Rv, Erdman, and 1254 (ATCC 51910) were used as indicated. Bacterium-derived stimuli included 10-ng/ml lipopolysaccharide (LPS) from Salmonella enterica serovar Friedenau H909 (a kind gift by H. Brade, Research Center Borstel, Germany), 10-μg/ml MDP (Sigma), 2- to 15-μg/ml M. tuberculosis PGN, and 10-μg/ml mycoarabinogalactan PGN (mAGP).
qRT-PCR.
Four or 24 h postinfection, macrophages were lysed in TRIzol, and total RNA was isolated as described previously (33). Three hundred nanograms of RNA was transcribed into cDNA with gene-specific primers in 20 ml using 50 U of Moloney murine leukemia virus reverse transcriptase (Perkin-Elmer). To control for the presence of DNA in RNA preparations, a parallel reaction without reverse transcriptase was performed. cDNA was diluted to 100 μl and 5 μl and used for quantitative real-time PCR (qRT-PCR). PCR was performed in a volume of 15 μl on the ABI PRISM 7900HT sequence detection system (Perkin-Elmer) as previously described (33). Control reactions were subjected to the same qRT-PCR reaction. The sequences of primers and probes were previously described (33).
Quantification of cytokine and chemokine release by ELISA.
BMMs from Card15+/+ and Card15−/− mice were seeded into 48-well plates at 2 × 105 cells/well. Cells were infected with live M. tuberculosis cells at a multiplicity of infection (MOI) of 3. Supernatants were collected 24 h postinfection for TNF-α and 48 to 72 h postinfection for IL-12/IL-23p40 quantification. Concentrations of mouse TNF-α, IL-12/IL-23p40, RANTES (CCL-5), and monocyte chemoattractant protein 1 (MCP-1) (CCL-2) were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (R&D Systems and BD OptEIA, respectively). For bone marrow DC-derived supernatants, sandwich ELISA was performed using the following antibody pairs from BD Pharmingen (San Diego, CA): C15.6 and C17.8 (biotinylated) for IL-12p40, JES5-2A5 and JES5-16E3 (biotinylated) for IL-10, G281-2626 and MP6-XT3 (biotinylated) for TNF-α, and 9A5 and C17.8 (biotinylated) for IL-12p70. An OptEIA mouse IL-6 kit (BD Pharmingen, San Diego, CA) was used for IL-6 measurements.
Mouse infections.
Mice that were 8 to 10 weeks of age were infected with single-cell suspensions of logarithmic-phase cultures of M. tuberculosis by aerosol using an inhalation exposure system (Glas-Col). Animals were exposed for 40 min to an aerosol produced by nebulizing 5 ml of a bacterial suspension in PBS at a concentration of 2 × 107 bacilli/ml for low-dose challenge and 1 × 108 bacilli/ml for an intermediate-dose challenge. To obtain a high-dose challenge (2,000 CFU/lung), 5 ml of bacterial suspension (2 × 107 bacilli/ml) prepared in PBS containing 0.05% Tween 80 was nebulized.
Mice were euthanized by inhalation of CO2, and their lungs, spleen, and liver were aseptically removed and homogenized in 4 or 5 ml of PBS containing 0.05% Tween 80. To confirm the infectious dose, on day 1 postinfection, 1.6 ml of 4-ml lung homogenates was plated onto 10% oleic acid-albumin-dextrose-catalase and 0.5% glycerol-enriched 7H11 (Difco) plates. Plates were incubated at 37°C, and CFU were enumerated 14 to 21 days later. Mice were sacrificed at the indicated times postinfection; their lungs, spleens, and livers were aseptically removed and homogenized in PBS containing 0.05% Tween 80; and serial dilutions were plated onto enriched 7H11 plates for CFU enumeration. The upper left lobe of infected lungs was fixed in 10% formalin to analyze pathology in sections stained with hematoxylin and eosin. All animal studies were approved by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University.
RESULTS
Impaired NO production by NOD2-deficient macrophages in response to M. tuberculosis infection.
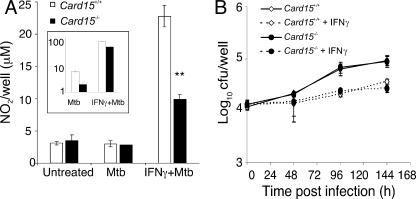
The generation of reactive nitrogen intermediates is a crucial innate immune defense mechanism against infection with M. tuberculosis. Therefore, we tested the ability of Card15−/− macrophages to generate reactive nitrogen intermediates following infection with virulent M. tuberculosis. Nitrite accumulation in supernatants of M. tuberculosis-infected BMMs was assayed 24 h postinfection. IFN-γ-primed BMMs from Card15−/− mice produced over 50% less NO than Card15+/+ macrophages following M. tuberculosis infection (Fig. 1A). Infection of cells that were not primed with IFN-γ did not result in detectable nitrite accumulation as previously described (7). Card15−/− macrophages also induced two- to threefold-lower levels of iNOS transcript than Card15+/+ macrophages in response to M. tuberculosis alone or M. tuberculosis in the presence of IFN-γ (Fig. 1A, inset). These data suggest that NOD2 is required for optimal iNOS mRNA expression and NO production in response to infection with M. tuberculosis.
FIG. 1.
Antimycobacterial activity of Card15−/− macrophages. A total of 2 × 105 BMMs from Card15+/+ (white bars and symbols) and Card15−/− (black bars and symbols) mice were seeded per well of a 48-well tissue culture plate in the presence or absence of 10 ng/ml IFN-γ. Sixteen hours after stimulation with IFN-γ, macrophages were infected with M. tuberculosis at an MOI of 3, and nitrite accumulation in cell supernatants was measured 24 h postinfection by Griess assay (A). The inset in A shows the quantification of iNOS transcript levels normalized to 1 × 104 GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcripts at the same time point. Intracellular replication of M. tuberculosis was assessed by plating lysates of infected cells onto 7H11 agar plates at the indicated time points (B). Data are representative of three independent experiments, each done with triplicate wells per group. **, P < 0.01.
NOD2 is not required for control of M. tuberculosis replication in macrophages in vitro.
To test if impaired NO production by Card15−/− macrophages resulted in an impaired control of intracellular mycobacterial replication, we infected macrophages with M. tuberculosis and measured intracellular survival by CFU (Fig. 1B). The uptake of M. tuberculosis was not affected by the lack of NOD2. Surprisingly, there was also no defect in the control of mycobacterial growth or survival in resting and IFN-γ-primed cells by Card15−/− macrophages compared to wild-type macrophages up to 6 days postinfection. Thus, despite reduced NO production, NOD2-deficient cells were still able to control the replication of intracellular M. tuberculosis.
Optimal TNF-α, IL-12p40, and RANTES production by macrophages in response to live M. tuberculosis requires NOD2.
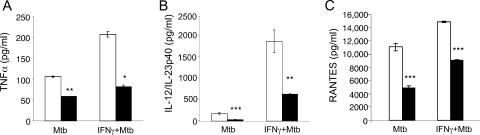
The impaired NO release of Card15−/− macrophages suggested that NOD2 is involved in macrophage activation by M. tuberculosis. We therefore tested whether the production of proinflammatory cytokines by M. tuberculosis-infected macrophages required NOD2. TNF-α levels in supernatants of M. tuberculosis-infected Card15−/− macrophages were twofold reduced compared to Card15+/+ macrophages in both the presence and absence of IFN-γ (Fig. 2A). IL-12p40 levels in culture supernatants of Card15−/− macrophages were three- and fivefold lower than those of their wild-type counterparts upon M. tuberculosis infection in the presence and absence of IFN-γ, respectively (Fig. 2B). Activation with IFN-γ enhanced cytokine production by Card15−/− macrophages although not to levels produced by IFN-γ-activated Card15+/+ macrophages. To test if the absence of NOD2 altered chemokine production in response to M. tuberculosis infection, we measured concentrations of RANTES and MCP-1 in supernatants of M. tuberculosis-infected macrophages. We found that the concentration of RANTES was twofold reduced in supernatants of Card15−/− macrophages compared to their wild-type counterparts (Fig. 2C); however, MCP-1 levels were not reproducibly altered in NOD2-deficient macrophages (data not shown). These data indicate that NOD2 is involved in macrophage activation by M. tuberculosis, but not all macrophage responses require NOD2.
FIG. 2.
Reduced cytokine production by Card15−/− macrophages in response to M. tuberculosis. A total of 2 × 105 BMMs were seeded per well of a 48-well tissue culture plate in the presence or absence of 10 ng/ml IFN-γ. Sixteen hours later, macrophages were infected with M. tuberculosis (Mtb) at an MOI of 3. TNF-α levels at 24 h (A), IL-12p40 levels at 48 h (B), and RANTES levels at 24 h (C) postinfection were determined by ELISA. Data are representative of three independent experiments, each done with triplicate wells per group. *, P < 0.05; **, P < 0.01.
NOD2 is required for cytokine production by DCs in response to live M. tuberculosis.
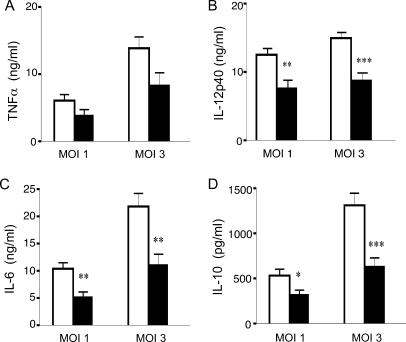
We next examined whether NOD2 also contributes to optimal cytokine production in DCs following infection with M. tuberculosis. DCs derived from wild-type and Card15−/− mice were infected with M. tuberculosis at MOIs of 1 and 3; 16 h later, the supernatants were tested for the presence of IL-12p40, IL-12p70, IL-6, IL-10, and TNF-α. As shown in Fig. 3, at both MOIs tested, there was a significant reduction in IL-12p40, IL-12p70 (data not shown), IL-6, and IL-10 in the absence of NOD2. The lack of NOD2 had a less drastic effect on TNF-α secretion by DCs. Together, the data suggest that in the absence of NOD2, DC release reduced amounts of all cytokines tested, suggesting that the NOD2 recognition system participates in the response to M. tuberculosis. However, in contrast to macrophages, the production of RANTES by M. tuberculosis-infected DCs did not require NOD2 (data not shown).
FIG. 3.
Reduced cytokine production by Card15−/− DCs in response to M. tuberculosis. A total of 2.5 × 105 DCs derived from the bone marrow of Card15+/+ and Card15−/− mice were infected with M. tuberculosis at MOIs of 1 and 3. Levels of TNF-α (A), IL-12-p40 (B), IL-6 (C), and IL-10 (D) in cell supernatants were determined by ELISA at 20 h postinfection. Data are representative of three independent experiments, each done with triplicate wells per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
mAGP-induced cytokine production is partially NOD2 dependent.
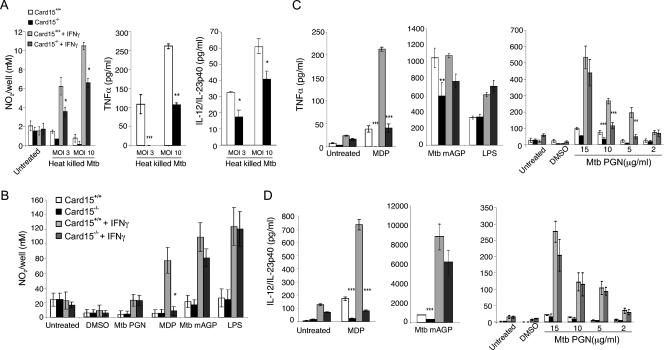
M. tuberculosis cell wall components are potent inducers of cytokines in macrophages, and this activity can be mimicked by stimulating cells with dead bacteria. We first tested if NOD2-dependent macrophage activation required live M. tuberculosis and measured NO, TNF, and IL-12p40 levels in BMMs stimulated with heat-killed M. tuberculosis. The production of the three proinflammatory mediators was reduced in Card15−/− macrophages compared to Card15+/+ macrophages (Fig. 4A), suggesting that a heat-stable component of M. tuberculosis activated BMMs in a NOD2-dependent manner. To further identify the microbial component(s) that stimulates NOD2 to mediate optimal NO and cytokine production, we treated macrophages with different cell wall components from M. tuberculosis, including purified mAGP and PGN, synthetic MDP, and LPS from S. enterica as a positive control for macrophage responsiveness because NOD2 is not required for the LPS pathway (22, 30).
FIG. 4.
Role of NOD2 in MDP-, mAGP-, and PGN-induced macrophage responses. A total of 2 × 105 BMMs were seeded per well of 48-well tissue culture plates in the presence or absence of 10 ng/ml IFN-γ. Sixteen hours later, macrophages were stimulated, and macrophage responses were measured. (A) Nitrite accumulation at 72 h, TNF-α levels at 24 h, and IL-12-p40 levels at 48 h postinfection with heat-killed M. tuberculosis (Mtb). (B) Nitrite accumulation after 72 h of stimulation with 5 μg/ml M. tuberculosis PGN, 10 μg/ml M. tuberculosis mAGP cell wall core, 10 μg/ml MDP, or 10 ng/ml LPS. (C and D) TNF-α levels at 24 h (C) and IL-12-p40 levels at 72 h (D) after stimulation with 10 μg/ml MDP, 10 μg/ml M. tuberculosis mAGP, 10 ng/ml LPS, and M. tuberculosis PGN at the indicated concentrations. Data are representative of two to three independent experiments, each done with triplicate cultures per group. Data from PGN-stimulated BMMs are from two independent experiments, each done in triplicate. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
When Card15+/+ and Card15−/− macrophages were stimulated with 10 μg/ml mAGP and 5 μg/ml PGN from M. tuberculosis, PGN induced only marginal NO release in both Card15+/+ and Card15−/− cells primed with IFN-γ (Fig. 4B). By contrast, mAGP activated IFN-γ-primed macrophages to release significant amounts of NO, but we observed no significant differences in NO production between wild-type and Card15−/− macrophages (Fig. 4B). However, stimulation with the NOD2 agonist MDP resulted in diminished NO production in Card15−/− macrophages when cells were primed with IFN-γ, consistent with previously published data (38) concerning the NOD2 dependence on MDP-induced NO production.
MDP stimulated TNF-α and IL-12p40 production in wild-type macrophages in both the absence and presence of IFN-γ in a NOD2-dependent manner (Fig. 4C and D, left), consistent with previous data from three different NOD2 loss-of-function mutant strains that are all similarly deficient in MDP responsiveness (22, 24, 30). mAGP-treated Card15−/− macrophages also produced lower TNF-α (P < 0.005) and IL-12p40 (P < 0.001) levels than wild-type macrophages. In the presence of IFN-γ, there was a similar trend of reduced TNF-α and IL-12p40 production by Card15−/− macrophages; however, this difference was not statistically significant (Fig. 4C and D, middle).
PGN is a component of mAGP that contains the NOD2 agonist MDP. TNF-α production in response to 5 μg/ml and 10 μg/ml M. tuberculosis PGN was two- to threefold reduced in the absence of NOD2 in resting and IFN-γ-primed macrophages (Fig. 4C, right). This difference subsided when the PGN concentration was increased to 15 μg/ml. PGN also induced IL-12p40 production, but this was not dependent on NOD2 at any of the concentrations tested (Fig. 4D, right).
Taken together, these results suggest that the mycobacterial cell wall fragments stimulate macrophages partially via NOD2 to induce cytokine production, but the degree and type of response depend on the structure and composition of the cell wall fragments used, highlighting the complexity of stimulation and response pathways for M. tuberculosis.
NOD2 is not required for in vivo control of M. tuberculosis.
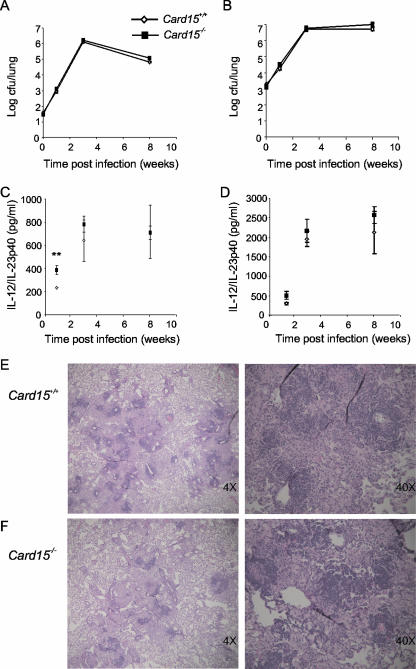
To test if impaired cytokine production by Card15−/− macrophages and DCs affects the ability of mice to control mycobacterial replication in vivo, we infected mice by aerosol with a low dose (∼35 CFU/lung), an intermediate dose (∼220 CFU/lung), and a high dose (∼1,500 CFU/lung) of M. tuberculosis and monitored growth of M. tuberculosis over time. We used two different M. tuberculosis strains, H37Rv and the clinical isolate strain 1254. None of these infections resulted in a significant difference in bacterial burdens in lungs, spleens, or livers of Card15+/+ and Card15−/− mice at weeks 1, 3, and 8 postinfection (Fig. 5A and B and data not shown). Histological analysis revealed no obvious differences in lung pathology between the two genotypes at 8 weeks postinfection (Fig. 5E and F). We measured iNOS mRNA levels by qRT-PCR in infected mouse lungs at week 1 and week 3 after low-dose infection and found no difference in iNOS mRNA expression (data not shown). Serum IL-12/IL-23p40 levels were also not different except at week 1 postinfection, when they were twofold higher in Card15−/− mice (P = 0.0089 for low-dose infection, P = 0.019 for intermediate-dose infection, and P = 0.085 for high-dose infection) (Fig. 5C and D and data not shown). Taken together, these results demonstrate that despite the impaired ability of CARD15−/− cells to produce TNF-α, IL-12/IL-23p40, IL-6, IL-10, RANTES, and NO in response to M. tuberculosis in vitro, Card15−/− mice were able to control an infection with virulent M. tuberculosis as well as wild-type mice.
FIG. 5.
Card15−/− mice control infection with M. tuberculosis. Card15+/+ and Card15−/− mice were infected by aerosol with a low dose (35 CFU) of a clinical isolate, M. tuberculosis 1254 (A), or with a high dose (1,500 CFU) of strain H37Rv (B). At the indicated time points, four to five mice of each group were sacrificed, and bacteria were enumerated in the lungs (A and B), spleens (not shown), and livers (not shown). Serum IL-12p40 levels after low-dose (C) and high-dose (D) infection were measured by ELISA. Error bars indicate standard deviations. **, P < 0.01. The upper left lobes of lungs from Card15+/+ (E) and Card15−/− (F) mice infected with a low dose of M. tuberculosis were formalin fixed and paraffin embedded at 8 weeks postinfection. Tissue sections were stained with hematoxylin and eosin. Representative sections from one mouse per genotype out of four are shown.
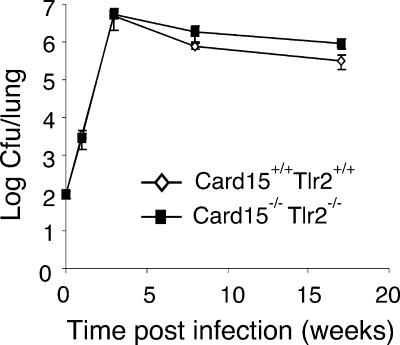
To test for a potential redundancy between NOD2- and TLR2-mediated signaling, we infected Card15−/−/Tlr2−/− mice with M. tuberculosis. As shown in Fig. 6, bacterial numbers were indistinguishable at 3 weeks postinfection in lungs from Card15+/+/Tlr2+/+ and Card15−/−/Tlr2−/− mice. At 8 and 17 weeks postinfection, CFU were twofold and threefold higher in lungs from Card15−/−/Tlr2−/− mice than in lungs from Card15+/+/Tlr2+/+ mice. However, bacterial numbers in lungs from mice of both genotypes declined from week 3 postinfection to the 17-week time point, indicating that Card15−/−/Tlr2−/− mice were able to control the infection similarly to wild-type mice.
FIG. 6.
Control of mycobacterial growth in Card15−/− Tlr2−/− mice. Card15+/+ Tlr2+/+ and Card15−/− Tlr2−/− mice were infected with a low dose of M. tuberculosis H37Rv (100 CFU). Bacterial counts in lungs were determined at weeks 1, 3, 8, and 17 postinfection (n = 4 mice per group). Error bars indicate standard deviations.
DISCUSSION
NOD2 participates in a signaling pathway that mediates recognition and responses to PGN-derived MDP (12). Because PGN from both gram-negative and gram-positive bacteria as well as that from acid-fast mycobacteria contain MDP, NOD2 is thought to function as a sensor of most bacteria, although the mechanisms involved in the direct or indirect MDP-NOD2 interaction are unresolved. Our goal was to investigate the role of NOD2 in the recognition of live, virulent M. tuberculosis by antigen-presenting cells and to test whether NOD2 is required for host resistance against M. tuberculosis infection. We demonstrated that macrophages and DCs lacking NOD2 are impaired in their ability to induce proinflammatory cytokines and NO upon infection with M. tuberculosis. NO is critical for the host defense against M. tuberculosis, and NO production by macrophages in response to mycobacterial lipoproteins or intact bacteria can occur through TLR-dependent and independent routes (5, 32, 33, 37). These data suggested that NOD2 is one of the recognition molecules required for an optimal NO response. Although NO production in NOD2-deficient cells was reduced, there was a clear distinction between different subcellular products with regard to NOD2 dependence to induce NO. NO release in response to MDP was NOD2 dependent, consistent with previous data (38), while M. tuberculosis PGN and mAGP did not require NOD2 to induce NO in BMMs. Mycobacterial lipoproteins mediate NO production via TLR2 (5, 37); however, the induction of iNOS and NO release in response to intact, live M. tuberculosis is largely TLR2 independent (32). Collectively, these data suggest that macrophages have evolved independent pathways for iNOS induction and that M. tuberculosis possesses multiple agonists that are capable of stimulating these pathways.
Human mononuclear cells from patients with Crohn's disease expressing a mutated NOD2 and peritoneal macrophages from mice lacking NOD2 responded with impaired TNF-α and IL-10 production when stimulated with sonicated M. tuberculosis (9). We found that TNF-α, IL-12p40, and RANTES production by BMMs and IL-12p40, IL-6, and IL-10 production by DCs upon infection with live, virulent M. tuberculosis were reduced in the absence of NOD2. Furthermore, MDP and mAGP, the mycobacterial cell wall core, mimicked the dependence on NOD2 for both TNF-α and IL-12p40 production. Treatment with mycobacterial PGN, a component of mAGP, also resulted in decreased levels of TNF-α in NOD2-deficient cells. IL-12p40 production was not NOD2 dependent when M. tuberculosis PGN instead of mAGP was used to stimulate macrophages. This may be the result of additional immunomodulatory features of mAGP such as mycolic acids or arabinogalactan. PGN is only one part of the highly cross-linked macromolecular structure of mAGP, and the response to such a complex agonist may not reflect the specificity of any one component. Watanabe et al. demonstrated enhanced Th1 responses, including IL-12p40 production, by PGN-treated splenocytes and splenocyte-derived antigen-presenting cells from Card15−/− mice compared to wild-type spleen macrophages (40, 41). We did not observe increased IL-12p40 production by bone marrow-derived NOD2-deficient macrophages after stimulation with PGN from M. tuberculosis in vitro. Perhaps the macrophage source is the reason for the different results. This is supported by the observation that IL-12p40 levels in the serum of M. tuberculosis-infected Card15−/− mice were increased 7 days postinfection relative to Card15+/+ mice, similar to the increased serum IL-12p40 levels in Card15−/− mice injected with PGN (40). Serum IL-12p40 is likely produced by circulating and splenic monocytes that may resemble the response of PGN-activated spleen macrophages from Card15−/− mice described previously by Watanabe et al. (40, 41).
The impaired in vitro responses of NOD2-deficient antigen-presenting cells to infection with M. tuberculosis suggested that NOD2 may be required for host defense against M. tuberculosis. However, NOD2-deficient mice were no more susceptible to M. tuberculosis infection with low and high doses than wild-type mice up to 8 weeks postinfection. The early difference in serum IL-12p40 levels between M. tuberculosis-infected Card15+/+ and Card15−/− mice subsided at later time points of infection and did not result in any detectable difference with respect to bacterial load, cellular recruitment, and lung pathology. Thus, despite impaired immune responses of NOD2-deficient cells in vitro, NOD2 was dispensable for the control of M. tuberculosis in vivo during the acute and early persistent phases of the infection. Mycobacteria possess both TLR and NOD2 agonists, and combinatory dual signaling through TLRs and NOD2 may result in synergistic host cell activation (9, 22, 39). However, mice that were doubly deficient for NOD2 and TLR2 controlled the replication of virulent M. tuberculosis as well as wild-type mice in the acute phase of the infection, and during the chronic phase, bacterial titers even declined slowly. Infection with high but not low doses of M. tuberculosis resulted in a loss of resistance in Tlr2−/− mice compared to wild-type mice (6, 31, 32, 35). We cannot rule out that Card15−/−/Tlr2−/− mice are more susceptible to infection with a high dose of M. tuberculosis than wild-type mice. It is also possible that a potential role of NOD2 in the pathogenesis of TB may become apparent in a situation of compromised immunity, for example, during the reactivation of a chronic infection. Future experiments are required to address these questions. Our current data suggest that redundant signaling systems can compensate for the loss of TLR2 and NOD2 following a low-dose aerosol challenge in mice.
Taken together, the findings of this study provide strong evidence that NOD2 is not essential for host defense in murine TB models. Recent genotyping studies of TB patients showed no significant association of six single-nucleotide polymorphisms in the leucine-rich repeat domain of NOD2 or of eight single-nucleotide polymorphisms in the Card15 promoter region with pulmonary TB in two different African populations, indicating that, at least in the populations studied, Card15 is not a major susceptibility gene for TB (26, 34).
Acknowledgments
We thank Shuangping Shi for help with initial experiments, William A. Muller for evaluation of the histopathology, and Toshiko Odaira and Elizabeth Hwang for taking care of the mice.
PGN and mAGP from M. tuberculosis were received as part of NIH NIAID contract no. HHSN266200400091C, entitled Tuberculosis Vaccine Testing and Research Materials, which was awarded to Colorado State University. This work was supported by NIH HL68525 and the I. T. Hirschl Trust to S.E., NIH AI055377 to P.S., and a Cancer Research Institute Predoctoral Fellowship Training Grant to S.G.
Editor: F. C. Fang
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Aziz, M. A., and A. Wright. 2005. The World Health Organization/International Union against Tuberculosis and Lung Disease Global Project on Surveillance for Anti-Tuberculosis Drug Resistance: a model for other infectious diseases. Clin. Infect. Dis. 41(Suppl. 4):S258-S262. [DOI] [PubMed] [Google Scholar]
- 2.Bafica, A., C. A. Scanga, C. G. Feng, C. Leifer, A. Cheever, and A. Sher. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty, W. L., and D. G. Russell. 2000. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 68:6997-7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty, W. L., H. J. Ullrich, and D. G. Russell. 2001. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur. J. Cell Biol. 80:31-40. [DOI] [PubMed] [Google Scholar]
- 5.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 6.Drennan, M. B., D. Nicolle, V. J. Quesniaux, M. Jacobs, N. Allie, J. Mpagi, C. Fremond, H. Wagner, C. Kirschning, and B. Ryffel. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrt, S., D. Schnappinger, S. Bekiranov, J. Drenkow, S. Shi, T. R. Gingeras, T. Gaasterland, G. Schoolnik, and C. Nathan. 2001. Reprogramming of the macrophage transcriptome in response to interferon-gamma and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J. Exp. Med. 194:1123-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ernst, J. D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferwerda, G., S. E. Girardin, B. J. Kullberg, L. Le Bourhis, D. J. de Jong, D. M. Langenberg, R. van Crevel, G. J. Adema, T. H. Ottenhoff, J. W. Van der Meer, and M. G. Netea. 2005. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 1:279-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, J. L., and J. Chan. 2005. What's good for the host is good for the bug. Trends Microbiol. 13:98-102. [DOI] [PubMed] [Google Scholar]
- 11.Frieden, T. R., T. R. Sterling, S. S. Munsiff, C. J. Watt, and C. Dye. 2003. Tuberculosis. Lancet 362:887-899. [DOI] [PubMed] [Google Scholar]
- 12.Girardin, S. E., I. G. Boneca, J. Viala, M. Chamaillard, A. Labigne, G. Thomas, D. J. Philpott, and P. J. Sansonetti. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869-8872. [DOI] [PubMed] [Google Scholar]
- 13.Girardin, S. E., R. Tournebize, M. Mavris, A. L. Page, X. Li, G. R. Stark, J. Bertin, P. S. DiStefano, M. Yaniv, P. J. Sansonetti, and D. J. Philpott. 2001. CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep. 2:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girardin, S. E., L. H. Travassos, M. Herve, D. Blanot, I. G. Boneca, D. J. Philpott, P. J. Sansonetti, and D. Mengin-Lecreulx. 2003. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J. Biol. Chem. 278:41702-41708. [DOI] [PubMed] [Google Scholar]
- 15.Hugot, J. P., M. Chamaillard, H. Zouali, S. Lesage, J. P. Cezard, J. Belaiche, S. Almer, C. Tysk, C. A. O'Morain, M. Gassull, V. Binder, Y. Finkel, A. Cortot, R. Modigliani, P. Laurent-Puig, C. Gower-Rousseau, J. Macry, J. F. Colombel, M. Sahbatou, and G. Thomas. 2001. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411:599-603. [DOI] [PubMed] [Google Scholar]
- 16.Inohara, N., and G. Nunez. 2003. NODs: intracellular proteins involved in inflammation and apoptosis. Nat. Rev. Immunol. 3:371-382. [DOI] [PubMed] [Google Scholar]
- 17.Inohara, N., Y. Ogura, A. Fontalba, O. Gutierrez, F. Pons, J. Crespo, K. Fukase, S. Inamura, S. Kusumoto, M. Hashimoto, S. J. Foster, A. P. Moran, J. L. Fernandez-Luna, and G. Nunez. 2003. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J. Biol. Chem. 278:5509-5512. [DOI] [PubMed] [Google Scholar]
- 18.Inohara, N., Y. Ogura, and G. Nunez. 2002. Nods: a family of cytosolic proteins that regulate the host response to pathogens. Curr. Opin. Microbiol. 5:76-80. [DOI] [PubMed] [Google Scholar]
- 19.Jang, S., S. Uematsu, S. Akira, and P. Salgame. 2004. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J. Immunol. 173:3392-3397. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa, N., I. Okafuji, N. Kambe, R. Nishikomori, M. Nakata-Hizume, S. Nagai, A. Fuji, T. Yuasa, A. Manki, Y. Sakurai, M. Nakajima, H. Kobayashi, I. Fujiwara, H. Tsutsumi, A. Utani, C. Nishigori, T. Heike, T. Nakahata, and Y. Miyachi. 2005. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105:1195-1197. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, K., N. Inohara, L. D. Hernandez, J. E. Galan, G. Nunez, C. A. Janeway, R. Medzhitov, and R. A. Flavell. 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416:194-199. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi, K. S., M. Chamaillard, Y. Ogura, O. Henegariu, N. Inohara, G. Nunez, and R. A. Flavell. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731-734. [DOI] [PubMed] [Google Scholar]
- 23.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 24.Mariathasan, S., D. S. Weiss, K. Newton, J. McBride, K. O'Rourke, M. Roose-Girma, W. P. Lee, Y. Weinrauch, D. M. Monack, and V. M. Dixit. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228-232. [DOI] [PubMed] [Google Scholar]
- 25.Miceli-Richard, C., S. Lesage, M. Rybojad, A. M. Prieur, S. Manouvrier-Hanu, R. Hafner, M. Chamaillard, H. Zouali, G. Thomas, and J. P. Hugot. 2001. CARD15 mutations in Blau syndrome. Nat. Genet. 29:19-20. [DOI] [PubMed] [Google Scholar]
- 26.Moller, M., A. Nebel, R. Kwiatkowski, P. D. van Helden, E. G. Hoal, and S. Schreiber. 2007. Host susceptibility to tuberculosis: CARD15 polymorphisms in a South African population. Mol. Cell. Probes 21:148-151. [DOI] [PubMed] [Google Scholar]
- 27.Neyrolles, O., K. Gould, M. P. Gares, S. Brett, R. Janssen, P. O'Gaora, J. L. Herrmann, M. C. Prevost, E. Perret, J. E. Thole, and D. Young. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 28.Ogura, Y., D. K. Bonen, N. Inohara, D. L. Nicolae, F. F. Chen, R. Ramos, H. Britton, T. Moran, R. Karaliuskas, R. H. Duerr, J. P. Achkar, S. R. Brant, T. M. Bayless, B. S. Kirschner, S. B. Hanauer, G. Nunez, and J. H. Cho. 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411:603-606. [DOI] [PubMed] [Google Scholar]
- 29.Ogura, Y., N. Inohara, A. Benito, F. F. Chen, S. Yamaoka, and G. Nunez. 2001. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 276:4812-4818. [DOI] [PubMed] [Google Scholar]
- 30.Pauleau, A. L., and P. J. Murray. 2003. Role of Nod2 in the response of macrophages to Toll-like receptor agonists. Mol. Cell. Biol. 23:7531-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 32.Shi, S., A. Blumenthal, C. M. Hickey, S. Gandotra, D. Levy, and S. Ehrt. 2005. Expression of many immunologically important genes in Mycobacterium tuberculosis-infected macrophages is independent of both TLR2 and TLR4 but dependent on IFN-alphabeta receptor and STAT1. J. Immunol. 175:3318-3328. [DOI] [PubMed] [Google Scholar]
- 33.Shi, S., C. Nathan, D. Schnappinger, J. Drenkow, M. Fuortes, E. Block, A. Ding, T. R. Gingeras, G. Schoolnik, S. Akira, K. Takeda, and S. Ehrt. 2003. MyD88 primes macrophages for full-scale activation by interferon-gamma yet mediates few responses to Mycobacterium tuberculosis. J. Exp. Med. 198:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockton, J. C., J. M. Howson, A. A. Awomoyi, K. P. McAdam, J. M. Blackwell, and M. J. Newport. 2004. Polymorphism in NOD2, Crohn's disease, and susceptibility to pulmonary tuberculosis. FEMS Immunol. Med. Microbiol. 41:157-160. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara, I., H. Yamada, C. Li, S. Mizuno, O. Takeuchi, and S. Akira. 2003. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47:327-336. [DOI] [PubMed] [Google Scholar]
- 36.Tanabe, T., M. Chamaillard, Y. Ogura, L. Zhu, S. Qiu, J. Masumoto, P. Ghosh, A. Moran, M. M. Predergast, G. Tromp, C. J. Williams, N. Inohara, and G. Nunez. 2004. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23:1587-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 2001. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 38.Totemeyer, S., M. Sheppard, A. Lloyd, D. Roper, C. Dowson, D. Underhill, P. Murray, D. Maskell, and C. Bryant. 2006. IFN-gamma enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J. Immunol. 176:4804-4810. [DOI] [PubMed] [Google Scholar]
- 39.Uehara, A., S. Yang, Y. Fujimoto, K. Fukase, S. Kusumoto, K. Shibata, S. Sugawara, and H. Takada. 2005. Muramyldipeptide and diaminopimelic acid-containing desmuramylpeptides in combination with chemically synthesized Toll-like receptor agonists synergistically induced production of interleukin-8 in a NOD2- and NOD1-dependent manner, respectively, in human monocytic cells in culture. Cell. Microbiol. 7:53-61. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe, T., A. Kitani, P. J. Murray, and W. Strober. 2004. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat. Immunol. 5:800-808. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe, T., A. Kitani, P. J. Murray, Y. Wakatsuki, I. J. Fuss, and W. Strober. 2006. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473-485. [DOI] [PubMed] [Google Scholar]