Abstract
Streptococcus agalactiae is frequently the cause of bacterial sepsis and meningitis in neonates. In addition, it is a commensal bacterium that colonizes the mammalian gastrointestinal tract. During its commensal and pathogenic lifestyles, S. agalactiae colonizes and invades a number of host compartments, thereby interacting with different host proteins. In the present study, the serine-rich repeat protein Srr-1 from S. agalactiae was functionally investigated. Immunofluorescence microscopy showed that Srr-1 was localized on the surface of streptococcal cells. The Srr-1 protein was shown to interact with a 62-kDa protein in human saliva, which was identified by matrix-assisted laser desorption ionization-time-of-flight analysis as human keratin 4 (K4). Immunoblot and enzyme-linked immunosorbent assay experiments allowed us to narrow down the K4 binding domain in Srr-1 to a region of 157 amino acids (aa). Furthermore, the Srr-1 binding domain of K4 was identified in the C-terminal 255 aa of human K4. Deletion of the srr-1 gene in the genome of S. agalactiae revealed that this gene plays a role in bacterial binding to human K4 and that it is involved in adherence to epithelial HEp-2 cells. Binding to immobilized K4 and adherence to HEp-2 cells were restored by introducing the srr-1 gene on a shuttle plasmid into the srr-1 mutant. Furthermore, incubation of HEp-2 cells with the K4 binding domain of Srr-1 blocked S. agalactiae adherence to epithelial cells in a dose-dependent fashion. This is the first report describing the interaction of a bacterial protein with human K4.
Keratins are the constituents of epithelial intermediate filaments (IF), which are the principal structural elements of the cytoskeleton in eukaryotic cells (14, 21). The major function of keratin IF is to provide an adaptable scaffold to epithelial cells that allows them to sustain mechanical and nonmechanical stresses. Keratins are encoded by a large multigene family with up to 54 members. They are divided in two subclasses, type I and type II, on the basis of gene structure and homology. One type I keratin and one type II keratin assemble into heterodimers, which polymerize to form IF (14). Keratin proteins have a conserved molecular structure which comprises a central rod domain with an α-helical structure flanked by non-α-helical end domains, called the head and the tail regions (25). One member of this family is keratin 4 (K4), which is produced in the stratified epithelia lining the oral mucosa, esophagus, and parts of the female genital tract (33).
A growing set of reports describe the involvement of keratins in the interaction between host and pathogens (7, 12, 42, 47, 57, 61, 66, 67). These interactions occur intracellularly as well as on the surface of epithelial cells. Thus, it is described that enteropathogenic Escherichia coli translocates two effector proteins, EspF and Tir, in the cytoplasm of epithelial cells, where they subsequently interact with K18 (7, 66). Further, convincing reports propose keratin proteins as cell surface-exposed interacting partners for ClfB of Staphylococcus aureus and for unknown bacterial receptors of Burkholderia cepacia and Porphyromonas gingivalis. Interaction of these bacteria with surface exposed keratins would mediate adherence to epithelial cells (42, 47, 57). In addition to these bacteria, Streptococcus agalactiae was shown to interact with K8 (61), even though the host cellular compartment where the interaction occurs and the implication of this interaction for the bacterium remain unknown.
Streptococcus agalactiae, also named group B Streptococcus, is the major cause of bacterial sepsis and meningitis in neonates and has emerged as an increasingly common cause of invasive diseases in immunocompromised people (5). It is also able to colonize asymptomatically multiple sites of the body, including the rectum, vagina, cervix, and throat (5). Neonates acquire S. agalactiae from colonized mothers by aspiration of infected amniotic fluid or vaginal secretions during birth (58). Furthermore, S. agalactiae colonizes the mammary glands of ruminants, where it can trigger an inflammation of the gland called mastitis (32).
Both commensal and pathogenic lifestyles require the ability of the bacterium to interact physically with host components. These interactions are crucial, notably for tissue colonization, invasion of internal compartments of the body, and evasion of immune clearance (17, 31, 43). S. agalactiae, like other gram-positive pathogens, produces proteins on its surface that interact with host factors. A subclass of these proteins are characterized by carrying a cell wall-anchoring region consisting of an LPXTG motif, a hydrophobic domain, and a short C-terminal charged tail, which allows them to be covalently attached to the cell wall by a sortase (39). In S. agalactiae, several cell wall-anchored proteins have been shown to interact with human proteins or polysaccharides (34). The FbsA protein from S. agalactiae mediates the binding of the bacterium to human fibrinogen (54), and the C5a peptidase interacts with fibronectin (8). In addition, the alpha C protein binds glycosaminoglycans (6), BibA interacts with the complement regulator C4bp (52), and the β antigen binds both the Fc part of human serum immunoglobulin A (IgA) and factor H of the complement system (3). In addition, cell wall-anchored surface proteins can also possess enzymatic activities: the C5a peptidase cleaves the C5a protein of the complement system, while the protease CspA cleaves the α-chain of fibrinogen (10, 28). Although interacting partners are known for several surface proteins, the analysis of the genome of S. agalactiae strain NEM316 predicted at least 30 surface proteins covalently anchored to the cell wall (23), indicating that the function and particularly the interacting partners of the majority of these surface proteins are still unknown.
One of these putative surface proteins of unknown function is Gbs1529 (23). The 3′ region of gbs1529 is composed of 153 imperfect direct repeats translated as SAS(T/M) (Fig. 1A) (23). This protein was recognized by Seifert et al. as a member of the streptococcal and staphylococcal serine-rich repeat protein family and was accordingly named Srr-1 for serine-rich repeat protein 1 (55). Proteins of this family are typically of high molecular weight. Their N-terminal part comprises a short serine-rich repeat region, which is followed by a nonrepeat region that typically exhibits adhesive functions. Thus, GspB and Hsa from Streptococcus gordonii are reported to bind the carbohydrate moiety of platelet membrane glycoprotein GPIbα (59), and SraP from S. aureus mediates bacterial adherence to platelets (56). In addition, Fap1 of Streptococcus parasanguinis mediates bacterial attachment to saliva-coated hydroxylapatite (68). The N-terminal adhesive domain of Srr proteins is followed by a region containing numerous serine-rich repeats and a cell wall-anchoring domain. The Srr-encoding genes are typically located within a putative operon that encodes two proteins homologous to SecA and SecY, termed SecA2 and SecY2, and several putative glycosyltransferases. The genes secA2 and secY2 are described to be necessary for the export of the Srr protein GspB, leading to the idea that this accessory Sec system is specialized to the transport of GspB (9). The four glycosyltransferases encoded by the genes clustered with gspB mediate glycosylation of the serine-rich repeat regions of GspB (60). Accordingly, Srr-1 contains both a small and a large serine-rich repeat region and is encoded by a gene clustered with homologues of secA2 and secY2 and with seven genes encoding putative glycosyltransferases (55). The adhesive function of serine-rich repeat proteins led Seifert et al. (55) and Takamatsu et al. (59) to hypothesize that Srr-1 from S. agalactiae harbors an adhesive function. Considering the ability of GspB and Hsa to bind to the sialylated carbohydrate moieties of fetuin and glycocalicin, the binding of Srr-1 to these glycoproteins was tested. However, no interaction between these glycoproteins and Srr-1 could be detected (59), leaving open the question of a possible adhesive function for Srr-1.
FIG. 1.
Schematic representation of the Srr-1 protein of S. agalactiae (A) and ability of truncated Srr-1 derivatives to bind human K4 (B and C). In the full-length Srr-1 protein, the K4 binding domain (K4-BD), the serine-rich repeat regions (vertical hatching), the membrane-spanning region (diagonal hatching), and the LPXTG cell wall-anchoring motif are indicated. The Srr-1 protein fragments produced in E. coli as hexahistidyl-tagged proteins are schematically represented in panel B. The results of immunoblot analysis presented in panel C are summarized in panel B; effective binding and undetected binding to human K4 are represented by + and −, respectively. Localization of the K4 binding domain in Srr-1 is shown in panel C. After SDS-PAGE, K4 was blotted on nitrocellulose. The membranes were incubated with anti-K4 antibodies (control) or with the protein Srr-1-N (a), Srr-1-N1 (b), Srr-1-N2N3 (c), Srr-1-N2 (d), or Srr-1-N3 (e), followed by incubation with anti-His tag antibodies.
Here we describe experiments showing the surface localization of Srr-1 in S. agalactiae and unraveling an interacting partner of this protein. We also investigated whether Srr-1 plays a role in adherence of S. agalactiae to epithelial cells.
MATERIALS AND METHODS
Bacterial strains, eukaryotic cells, and growth conditions.
S. agalactiae 6313 is a serotype III clinical isolate obtained from an infected neonate (64). All the other S. agalactiae strains tested in this study were clinical isolates of serotype Ia, Ib, or II-V (54). E. coli DH5α was used for cloning purposes, and E. coli BL21(DE3) and ER2566 (New England Biolabs) served as hosts for the production of Srr-1 and K4 fusion proteins. S. agalactiae was cultivated at 37°C in Todd-Hewitt yeast broth (THY) containing 1% yeast extract. S. agalactiae clones carrying the plasmid pG+host6 (Appligene) or pAT18 (62) were selected with erythromycin (5 μg·ml−1). E. coli was grown at 37°C in Luria-Bertani (LB) broth, and strains carrying the plasmid pG+host6, pAT18, pET28a, or pOTB7-k4 (RZPD, Berlin, Germany) were selected by supplementing the medium with ampicillin (100 μg·ml−1), erythromycin (300 μg·ml−1), kanamycin (50 μg·ml−1), or chloramphenicol (50 μg·ml−1). Growth of S. agalactiae was monitored by measuring the optical density of the culture at 600 nm.
The cell line HEp-2 (ATCC CCL-23) was obtained from the American Type Culture Collection. HEp-2 is a human epidermoid carcinoma cell line of larynx. This cell line was propagated in minimal essential medium (Gibco BRL) supplemented with 10% fetal calf serum. Tissue cultures were incubated in a humid atmosphere at 37°C with 5% CO2.
Antibodies and human proteins.
Peroxidase-labeled goat anti-mouse IgG Fab fragments, monoclonal mouse anti-His tag antibodies, monoclonal mouse anti-human-K4 antibodies, fluorescein isothiocyanate (FITC)-labeled sheep anti-mouse IgG Fab fragments, and monoclonal anti-rabbit IgG were purchased from Dianova (Hamburg, Germany), Roche Diagnostics (Penzberg, Germany), Sigma Aldrich (Steinheim, Germany), Dako Cytomation (Gostrup, Denmark) and Sigma Aldrich (Steinheim, Germany), respectively. For the generation of anti-Srr-1 antibodies, affinity-purified Srr-1-N fusion protein was size separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose. After staining with Ponceau S, the Srr-1-containing band was cut out. After the nitrocellulose membrane was dissolved in dimethyl sulfoxide, the solution was used for the immunization of mice. Immunization consisted of two subcutaneous applications of the purified protein within 2 weeks. Serum was collected 4 weeks after immunization. Animal experiments were performed according to legal provisions.
Human K4 and K6 were purified from saliva of a healthy human volunteer. For this purpose, human saliva was separated by SDS-PAGE, and the protein band of interest was excised from a Coomassie blue-stained gel. K4 and K6 were purified using an electroeluter (model 422; Bio-Rad, München, Germany) according to the manufacturer's instructions.
Preparation of crude extracts from tissues.
Murine and bovine lung, brain, and brain endothelium were homogenized using the IKA Ultra Turrax T25 dispersing instrument (Jahnke & Kunkel, Staufen, Germany). All probes were suspended in HEPES buffer (10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.05% Tween 20) and stored at −20°C with an additional 20% glycerol.
General DNA techniques.
Chromosomal DNA of S. agalactiae was isolated according to the method of Pospiech and Neumann (45). DNA manipulations were performed according to standard protocols (49). Oligonucleotides used in this study are described in Table 1 and were purchased from MWG Biotech GmbH (Ebersberg, Germany).
TABLE 1.
Oligonucleotides used in this work
| Purpose | Oligonucleotide sequencea (5′→3′) |
|---|---|
| Amplification of 5′ half of srr-1 | TTGGCAAGCAGTTAACAG |
| TCTGATAAAAGTTTAATTTCGGC | |
| Amplification of the repeat region of Srr-1 | AAGTGCTTCTACGAGTGCGTC |
| GTCTTTATCTTTTTTAGATTTTTTTCGTCC | |
| Construction of Δsrr-1 mutant | GAGCGGGGTACCCTCGCAGACGATAGACAGAAG |
| CCCATCCACTATACTTATACACTTTAACCCCTCATTTTCC | |
| TGTATAAGTATAGTGGATGGGCAATTGAGGAACGTGTTCAG | |
| CCGCGGATCCGCATTCGCATCTGAGTCAC | |
| Complementation of Δsrr-1 | GAGCAGGGTACCGGAAAATGAGGGGTTAAAG |
| CCGCGGATCCGATATGCCTTTTACCATGTC | |
| Production of: | |
| Srr-1-N and -N1 | GTGCTTTGCCATGGTTGGCAAGCAGTTAACAG |
| Srr-1-N, -N2N3, and -N2C | CCGCGGATCCTCTGATAAAAGTTTAATTTCGGC |
| Srr-1-N1 | CCGCTCGAGCTGCAACTCGCTTGATAC |
| Srr-1-N2N3 and -N2 | CATGCCATGGGTATCAAGCGAGTTGCAG |
| Srr-1-N2 | CCGCTCGAGCCGAGATCTTACATTTGTCTTAAC |
| Srr-1-N3 | CATGCCATGGTCATGAAGCTTGATGATGAAAGAC |
| K4 and K4N | CCGCGGATCCATGATTGCCAGACAGCAGTG |
| K4 and K4C | TGGCACAAGCTTCTATCGTCTCTTGTTCAG |
| K4N | TGGCACAAGCTTCTAAAGACTGTCCACCTTGGC |
| K4C | CCGCGGATCCATGAATGACGAGATCAACTTCC |
Restriction enzyme sites used for cloning are underlined. cDNA sequences between primers are in boldface.
Construction of srr-1 deletion mutant.
The srr-1 gene was deleted from the chromosome of S. agalactiae 6313 as previously described (50). In brief, two DNA fragments flanking the region to be deleted were amplified by PCR from the genome of S. agalactiae 6313. The resulting PCR products were mixed in equal amounts with each other and subjected to a crossover PCR, resulting in one PCR product. The crossover PCR product and the thermosensitive vector pG+host6 were digested with appropriate enzymes, ligated, and used to transform E. coli DH5α. The resulting pG+Δsrr-1 plasmid was introduced into S. agalactiae 6313 by electroporation, and two recombination events flanking the region of the chromosome to be deleted were subsequently selected. Successful deletion was confirmed by Southern blotting with PvuII-digested chromosomal DNAs of the S. agalactiae parental strains and of the deletion mutant.
Plasmid-mediated expression of srr-1 in S. agalactiae.
The srr-1 structural gene, including its own promoter, was cloned in the E. coli/Streptococcus shuttle vector pAT18 (62). The resulting plasmid pATsrr-1 and the vector pAT18 were introduced into S. agalactiae by electroporation.
Heterologous production and purification of Srr-1 and K4 fusion proteins.
The vector pET28a (Novagen) was used for the synthesis of hexahistidyl-tagged Srr-1 and K4 fusion proteins. The Srr-1-N-, Srr-1-N1-, Srr-1-N2N3-, Srr-1-N2-, and Srr-1-N3-encoding regions were amplified by PCR by using chromosomal DNA of S. agalactiae 6313 as the template. After digestion of the srr-1-derived PCR products and of plasmid pET28a with NcoI and XhoI or with NcoI and BamHI, srr-1 derivatives were ligated with pET28a and transformed into E. coli. The human K4 cDNA (k4) was amplified by PCR by using DNA of plasmid pOTB7-K4 (RZPD, Berlin, Germany) as the template. After digestion of the k4 PCR products and of plasmid pET28a with BamHI and HindIII, k4 was ligated into pET28a and used to transform E. coli.
Production and purification of hexahistidyl-tagged fusion proteins were performed as previously described (54).
Immunoblot analysis.
For immunoblot experiments, proteins were size separated by SDS-PAGE and electroblotted onto nitrocellulose membrane, and the membrane was blocked overnight with 1% (wt/vol) blocking reagent (Roche Diagnostics) solubilized in phosphate-buffered saline (PBS). Depending on the experiment, the membrane was incubated for 1 h with either mouse anti-His tag antibodies (1:250 in PBS), mouse anti-Srr-1 antibodies (1:300 in PBS), or mouse anti-human-K4 antibodies (1:300 in PBS). Bound primary antibodies were detected with appropriate peroxidase-labeled IgG Fab fragments as previously described (51).
MALDI-TOF mass spectrometry.
Matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) analysis was used to identify the interaction partner of Srr-1 in human saliva. The protein band of interest was excised from colloidal Coomassie blue-stained gels with a scalpel and subjected to in-gel digestion with trypsin as described previously (22).
Detection of K4 binding by enzyme-linked immunosorbent assay (ELISA).
Maxisorp 96-well plates (Nunc, Wiesbaden, Germany) were coated with human K4 (20 nM) in 300 μl of PBS at 4°C overnight, and nonspecific binding sites were blocked with 10% bovine serum albumin (BSA) in PBS for 1 h at room temperature. Different concentrations of the fusion proteins Srr-1-N, Srr-1-N1, Srr-1-N2N3, and Srr-1-N3, diluted in 200 μl of PBS, were incubated in the wells for 1 h at room temperature. Bound Srr-1 proteins were detected as previously described (27). Apparent dissociation constants (KDs) were calculated as described elsewhere (11, 35).
Binding of FITC-labeled S. agalactiae to immobilized protein.
Binding of fluorescein isothiocyanate (FITC)-labeled bacteria to immobilized proteins was quantified as described previously (26). The amount of bound bacteria was calculated as the percentage of total labeled bacteria added to each well.
Fluorescence microscopy.
The surface localization of Srr-1 was investigated by immunofluorescence microscopy. Overnight cultures of the S. agalactiae strain 6313 and the Δsrr-1 mutant were diluted 1:1,000 in PBS, and 50 μl of each suspension was placed onto a slide and subsequently heat fixed. The bacteria were blocked with 100 μl of a 10% (wt/vol) BSA solution (in PBS) for 30 min at room temperature. The BSA solution was then removed, 100 μl of an Srr-1-specific antibody solution (1:300 in PBS) was added, and the bacteria were incubated for 45 min at room temperature by shaking slowly. The cells were washed three times with 10% (wt/vol) BSA in PBS, and 100 μl of a tetramethyl rhodamine isocyanate (TRITC)-labeled anti-mouse IgG Fab fragment solution (1:10,000 in PBS) was added subsequently. All probes were incubated for 30 min at room temperature in the dark with slow shaking. After three washes with PBS, the slides were fixed with 10 μl Moviol on coverslips and were incubated overnight at 39°C in the dark. The next day, the fluorescence of the TRITC-labeled antibody was detected by fluorescence microscopy. As a negative control, the Srr-1-specific antibody solution was omitted in one series of experiment. As a positive control, the Srr-1-specific antibody solution was replaced by the FbsA-specific mouse monoclonal antibody 5H2 (1:1,000 in PBS) (44).
Adherence and internalization assays.
S. agalactiae adherence to and internalization into HEp-2 epithelial cells were assayed as described previously (26). Briefly, HEp-2 cells cultured in 24-well plates were infected with S. agalactiae at a multiplicity of infection of 10:1 and incubated at 37°C for 2 h. After washing and lysis of eukaryotic cells with distilled water, appropriate dilutions of recovered bacteria were plated on THY agar to determine the number of CFU. The data were expressed as the percentage of adherent bacteria, based on the original inoculum. To assess the number of internalized bacteria, infected cells were incubated for an additional 2 h in tissue culture medium supplemented with penicillin G (10 U) and streptomycin (0.01 mg) to kill extracellular bacteria. Intracellular bacteria were subsequently recovered and plated on THY for CFU determination. The invasion index was calculated as (number of invasive/number of adherent bacteria) × 100%. Each experiment was repeated at least three times in triplicate.
Flow cytometry.
For the detection of K4 on the surface of epithelial cells, HEp-2 cells were grown overnight in 24-well plates at a density of 3 × 105 cells/well. The next day, medium was removed, and cells were washed twice with PBS and incubated for 1 h with mouse anti-human-K4 antibodies (1:10 in PBS), mouse anti-rabbit IgG (as a negative control; 1:10 in PBS), or PBS. Afterwards, cells were washed twice with PBS and incubated for 1 h with FITC-labeled sheep anti-mouse IgG Fab fragments (1:250 in PBS). Cells were washed twice with PBS and subsequently detached from the wells by incubation for 10 min with 50 mM EDTA (pH 8.0) in PBS. The cell suspension was centrifuged for 5 min, and the cell pellet was suspended in 200 μl fluorescence-activated cell sorting buffer (PBS [pH 7.4] containing 2% fetal calf serum and 20 mM EDTA). The fluorescence intensity of each sample was measured using a FACScalibur cytometer (Becton & Dickinson, Heidelberg, Germany), and data were analyzed with the Cellquest software.
Sequence analysis.
Sequence data were analyzed using BLAST, PSI-BLAST (1, 2), and SignalP (41). GenBank and the Conserved Domain Database (37) were consulted.
Statistical analysis.
The statistical differences in adherence ability between the wild-type and mutant strains were determined by two-tailed t test analysis using Microsoft Excel 2002.
Accession numbers.
The accession number of the genomic DNA sequence of S. agalactiae NEM316 in EMBL is AL732656. In the SwissProt TREMBL database, the Srr-1 and human K4 polypeptides are available with the accession numbers Q8E473 and P19013, respectively.
RESULTS
Sequence analysis of the Srr-1-encoding region.
The open reading frame srr-1 of strain NEM316 is predicted to encode a putative surface protein of 1,310 amino acids (aa) (23). Although the SignalP program did not detect any signal peptide, the primary structure of Srr-1 bears typical features of gram-positive surface proteins. Interrogation of the Conserved Domain Database did not reveal any conserved domain in Srr-1.
Srr-1 was previously reported to have an orthologous counterpart encoded in the completely sequenced genomes of the two S. agalactiae strains 2603V/R and A909 (55). One orthologous sequence could also be found in the partially sequenced genomes of the four strains 18RS21, 515, CJB111, and H36B. No orthologue of Srr-1 was identified in the partially sequenced genome of the fifth strain, COH1. The genome of this strain encodes a paralogous protein with a C-terminal serine-rich repeat region, named Srr-2 (55). The N terminus of this paralogous protein exhibits 33% identity (expect = 2.10−56) to the N terminus of Srr-1.
PSI-BLAST analysis was performed with the amino acid sequence of Srr-1. Two iterations were performed with the low-complexity filter. This analysis revealed that the region between aa 200 and 640 of Srr-1 shares between 17% and 21% identity with the nonrepeat domain of the surface proteins belonging to the staphylococcal Clf-Sdr protein family. The expect values were between 1 × 10−30 and 4 × 10−118, suggesting a probable phylogenetic relatedness of Srr-1 with these proteins. Members of the Clf-Sdr family typically exhibit adhesive functions and are thus described to bind fibrinogen, collagen, bone sialoprotein, or K10 (4, 15, 38, 40, 42, 48, 63).
Size variation of srr-1.
Repetitive DNA sequences are likely to exhibit size variation due to a high rate of recombination between repeats (65). Consequently, size variation of the repeat region of srr-1 was investigated by PCR. For this purpose, chromosomal DNAs of 98 human clinical isolates, belonging to serotypes Ia, Ib, II, III, IV, and V, were used as templates. By using primers designed to amplify the 5′ half of the srr-1 open reading frame, a unique PCR product of approximately 2 kb was obtained for all 98 tested strains, as exemplified in Fig. 2A, which shows a selection of representative strains. The results obtained indicate a wide distribution of srr-1 in different serotypes of S. agalactiae and demonstrates size conservation of this region. In contrast, the primer pair designed to amplify the repeat region resulted in unique PCR products ranging from 0.5 to 2 kb, depending on the tested strain (Fig. 2B). This shows that the repeat region of srr-1 exhibits substantial size variation within the S. agalactiae species.
FIG. 2.
Intraspecies size variability of srr-1 in different S. agalactiae isolates. Chromosomal DNAs from 98 human S. agalactiae isolates belonging to serotypes Ia, Ib, II, III, IV, and V were used as templates for amplification by PCR of the 5′ (A) and 3′ (B) regions of srr-1. The 5′ and 3′ regions of srr-1 are bp 1 to 1928 and bp 1929 to 3930, respectively, in strain NEM316. A representative sample of the 98 tested strains is presented.
Srr-1 is a surface protein.
Several sequence features of the Srr-1 protein, i.e., the anchoring signals and homologies to other surface proteins, argue for a surface localization in S. agalactiae, although Srr-1 does not possess a typical signal peptide according to analysis performed with SignalP. We investigated by immunofluorescence microscopy whether Srr-1 is present on the surface of the cells. For this purpose, the srr-1 gene was deleted from the genome of S. agalactiae 6313, resulting in the Δsrr-1 mutant. No difference in growth rate, final optical cell density, chain length, or clumping was observed between the S. agalactiae Δsrr-1 mutant and the wild-type strain 6313 (data not shown).
To investigate whether cell wall-bound proteins can be detected in S. agalactiae 6313 and the Δsrr-1 mutant by immunofluorescence microscopy, the two strains were tested with monoclonal antibodies raised against the surface protein FbsA (44). With both strains, fluorescent signals were observed colocalizing with the bacteria (Fig. 3), demonstrating the successful detection of FbsA in the cell walls of S. agalactiae 6313 and the Δsrr-1 mutant. When S. agalactiae 6313 was probed with anti-Srr-1 serum, the bacteria revealed an intense fluorescence, whereas no fluorescence was detected when the Δsrr-1 mutant was tested with the same serum. Neither strain 6313 nor the Δsrr-1 mutant revealed a fluorescence signal when the anti-Srr-1 serum was omitted, indicating that the secondary antibody did not interact with bacterial cells. Together, these results indicate that Srr-1 is localized on the surface of S. agalactiae.
FIG. 3.
Localization of Srr-1 on the surface of S. agalactiae. S. agalactiae strain 6313 and the Δsrr-1 mutant were immobilized on slides and incubated either with anti-FbsA monoclonal antibodies (A), with anti-Srr-1 serum (B), or, as a negative control, without primary antibodies (C). Subsequently, bound antibodies were detected by measuring the fluorescence of TRITC-labeled anti-mouse IgG Fab fragments, and pictures were taken after phase-contrast and fluorescence microscopy visualization.
Srr-1 binds to human K4.
As staphylococcal serine-rich proteins interact via their N-terminal domains with host proteins (20), the N terminus of Srr-1 was functionally characterized. For this purpose, the N terminus-encoding region of srr-1 was cloned in the expression vector pET28a, placing a hexahistidyl tag at the N terminus of the gene product. The resulting Srr-1-N fragment, consisting of the N-terminal region of Srr-1 without the serine-rich repeat region (Fig. 1A), was overproduced in E. coli and purified. In order to identify a host molecule able to interact with Srr-1-N, crude extracts from different organs were tested in immunoblots for binding to Srr-1-N. Considering that S. agalactiae causes pneumonia, meningitis, and septic arthritis (5), crude protein extracts were prepared from lung of mouse and cow, from brain and brain endothelium of cow, and from joint liquid of cow and were subsequently tested for binding to Srr-1. In addition, human saliva was also tested. The protein extracts were size separated by SDS-PAGE and transferred to a membrane, which was then incubated with Srr-1-N. Bound Srr-1-N protein was detected with anti-Srr-1 antibodies. As depicted in Fig. 4A, Srr-1-N bound exclusively to a 62-kDa protein in human saliva. To unravel the identity of the interaction partner of Srr-1, human saliva was size separated on a preparative SDS-polyacrylamide gel, and proteins were subsequently stained with Coomassie blue. Two proteins, having a size close to 62 kDa, were identified as putative binding partners of Srr-1. These two proteins were isolated from the gel and subsequently subjected to MALDI-TOF mass spectrometry analysis. The MALDI-TOF spectra of these two proteins were compatible with those of K4 and K6. To discriminate between these two candidates, purified fractions of K4 and K6 (Fig. 4B) were tested by immunoblotting for binding of Srr-1-N. No interaction between Srr-1-N and purified K6 was detected, whereas a clear signal was obtained with Srr-1-N and purified K4 (Fig. 4B). This result strongly suggests that K4 is the interaction partner of Srr-1. However, since K4 was purified from saliva, Srr-1-N might bind to a different protein than K4 which would comigrate with K4. Therefore, the human K4 cDNA was cloned into pET28, thereby placing a hexahistidyl tag at the N terminus of the protein. The recombinant K4 protein was subsequently tested by immunoblotting for binding to Srr-1-N. Here again, Srr-1-N was shown to bind recombinant K4 (data not shown), demonstrating that Srr-1 interacts specifically with human K4.
FIG. 4.
Binding of Srr-1-N to human K4. (A) Binding of Srr-1-N to proteins of bovine, murine, or human origin was investigated by immunoblotting. Crude extracts from different organs (lane 1, bovine brain endothelium; lane 2, bovine lung; lane 3, bovine joint liquid; lane 4, bovine brain; lane 6, murine lung) and human saliva (lane 5) were size separated by SDS-PAGE and blotted onto nitrocellulose. The membrane was incubated with Srr-1-N fusion protein and subsequently with anti-Srr-1 antibodies. Bound primary antibodies were detected with peroxidase-labeled anti-mouse IgG Fab fragments with subsequent visualization by chemiluminescence. (B) Binding of Srr-1 to human K4 and K6 was tested by immunoblotting. Purified human K6 (lanes 1 and 3) and K4 (lanes 2 and 4) were size separated by SDS-PAGE (lanes 1 and 2), blotted onto nitrocellulose, and tested for binding to Srr-1-N (lanes 3 and 4) as described for panel A. (C) Identification of Srr-1 binding site in human K4. Full-length human K4 and the truncated proteins K4N and K4C, comprising the N-terminal and C-terminal regions of K4, respectively, were size separated by SDS-PAGE, blotted onto nitrocellulose, and tested for binding to Srr-1-N as described for panel A.
Localization of the K4 binding domain of Srr-1.
To delineate the K4 binding site in Srr-1-N, several fragments of the N-terminal region of Srr-1 were overproduced in E. coli as hexahistidyl-tagged fusion proteins (Fig. 1B), purified, and tested for binding to human K4 by immunoblotting. More precisely, two truncated fusion proteins of Srr-1-N were constructed, one termed Srr-1-N1 and consisting of the N-terminal 330 aa of Srr-1-N and the other one termed Srr-1-N2N3 and comprising the C-terminal 259 aa of Srr-1-N (Fig. 1B). Immunoblot experiments showed that Srr-1-N2N3 bound to human K4 whereas Srr-1-N1 did not (Fig. 1C).
To further narrow down the K4 binding site, two subfragments of Srr-1-N2N3 were designed, one termed Srr-1-N2 and consisting of the N-terminal 163 aa of Srr-1-N2N3 and the other termed Srr-1-N3 and comprising the C-terminal 157 aa of Srr-1-N2N3 (Fig. 1B). In immunoblot analysis, Srr-1-N3 exhibited binding to K4 whereas Srr-1-N2 did not show interaction with this protein (Fig. 1C). These findings demonstrate that a region of 157 aa in Srr-1 binds to human K4 and that the K4 binding site is localized in Srr-1 between aa 485 and 642.
Localization of the Srr-1 binding site in human K4.
To localize the region in human K4 to which Srr-1 binds, truncated hexahistidyl-tagged fusion proteins of human K4 were tested for their binding to Srr-1-N. One fusion protein, named K4N, carried the N-terminal 302 aa of K4, while the other fusion protein, K4C, comprised the C-terminal 255 aa of K4. The full-length K4 and the truncated polypeptides K4N and K4C were size separated by SDS-PAGE, blotted onto a membrane, and incubated with purified Srr-1-N protein. Bound Srr-1-N protein was detected with Srr-1-specific antibodies. As depicted in Fig. 4C, the Srr-1-N fusion protein exhibited binding to human K4C, demonstrating that the C-terminal 255 aa of human K4 are bound by Srr-1.
Srr-1 binds to human K4 in a dose-dependent and saturable manner.
The binding of full-length Srr-1-N and truncated Srr-1-N fragments to K4 was quantified by ELISA. For this purpose, K4 from saliva was immobilized in microtiter wells and incubated with increasing concentrations of the fusion proteins Srr-1-N, Srr-1-N2N3, Srr-1-N3, and Srr-1-N1. Bound fusion protein was detected with anti-His tag antibodies, followed by peroxidase-conjugated goat anti-mouse antibodies. As depicted in Fig. 5A, Srr-1-N, Srr-1-N2N3, and Srr-1-N3 bound to immobilized K4 in a dose-dependent and saturable fashion. In contrast, Srr-1-N1, which did not bind to K4 in immunoblot, revealed no interaction with K4 in ELISA. The fusion proteins Srr-1-N, Srr-1-N2N3, and Srr-1-N3 revealed for the binding to K4 apparent KDs of 9.64 × 10−9 M, 2.09 × 10−8 M, and 9.44 × 10−9 M, respectively.
FIG. 5.
Binding of Srr-1-derived proteins to immobilized human K4 in a capture ELISA (A) and attachment of S. agalactiae to immobilized human K4 (B). (A) In the ELISA, microtiter wells were coated with a fixed amount of K4, followed by the addition of increasing concentrations of the fusion proteins Srr-1-N (diamonds), Srr-1-N2N3 (squares), Srr-1-N3 (triangles), and Srr-1-N1 (circles). Bound Srr-1 fusion proteins were detected by anti-His tag antibodies, followed by peroxidase-labeled anti-mouse IgG Fab fragments. Color development was initiated by the addition of tetramethyl-benzidine substrate and stopped with H2SO4. The absorbance of the microtiter wells was measured at 450 nm. Values represent the means and standard deviations from three independent experiments, each performed in triplicate. (B) Bacterial attachment to immobilized K4 was quantified with FITC-labeled bacteria. The ordinate represents the percentage of bacteria bound to immobilized K4 in relation to the number of input bacteria. The asterisk indicates a significant (P < 0.01) difference in the binding abilities of the indicated strains. Each assay was performed at least four times in triplicate.
Contribution of Srr-1 to bacterial binding to human K4.
To analyze the importance of srr-1 for the binding of S. agalactiae to human K4, protein binding assays were performed with the S. agalactiae parental strain 6313, the Δsrr-1 mutant, and a Δsrr-1 strain with plasmid-mediated expression of srr-1. For the construction of the last strain, the srr-1 gene was cloned with its own promoter into the E. coli/Streptococcus shuttle vector pAT18, resulting in plasmid pATsrr-1. The vector pAT18 was subsequently introduced into S. agalactiae strain 6313 and the Δsrr-1 mutant, and the plasmid pATsrr-1 was introduced into the S. agalactiae Δsrr-1 mutant. The ability of the resulting S. agalactiae strains, 6313_pAT18, Δsrr-1_pAT18, and Δsrr-1_pATsrr-1, to bind to human K4 was subsequently quantified by measuring the fluorescence intensity of bound FITC-labeled bacteria. The Δsrr-1_pAT18 strain exhibited a 36% decreased binding to immobilized human K4 compared to strain 6313_pAT18 (Fig. 5B). Moreover, plasmid-mediated expression of srr-1 restored the K4 binding ability of the Δsrr-1_pATsrr-1 strain to the wild-type level. This result demonstrates that S. agalactiae requires the Srr-1 protein for full binding to human K4.
Srr-1 promotes adherence to HEp-2 cells.
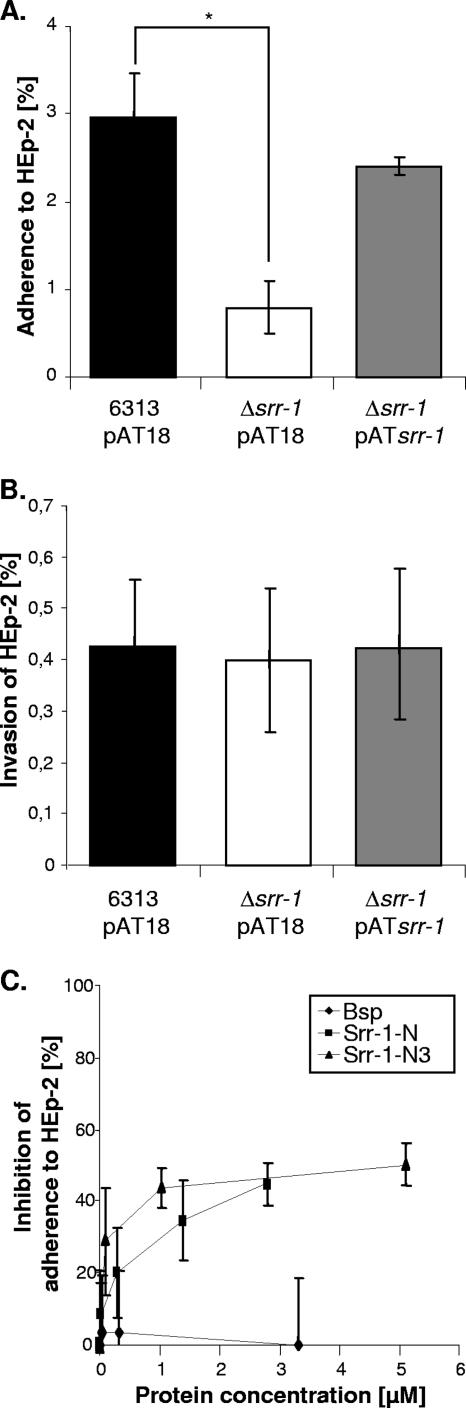
In order to investigate the importance of Srr-1 for bacterial adherence to and invasion of host cells, tissue culture experiments were performed by infecting the epithelial cell line HEp-2 with the S. agalactiae 6313_pAT18, Δsrr-1_pAT18, and Δsrr-1_pATsrr-1 strains. As depicted in Fig. 6B, the invasion of HEp-2 cells by the srr-1 mutant did not differ significantly from that by the wild type, indicating that Srr-1 does not contribute to invasion by S. agalactiae under the chosen experimental conditions. Conversely, the adherence of the srr-1 mutant to HEp-2 cells was approximately 75% lower than that of the wild type (Fig. 6A). Moreover, complementation of the srr-1 mutant with an extrachromosomal copy of the srr-1 gene completely restored its adherence to Hep-2 cells to the wild-type level (Fig. 6A).
FIG. 6.
Role of Srr-1 in bacterial adherence to and invasion of HEp-2 cells. The epithelial cell line HEp-2 was infected with equal amounts of bacteria of each strain, and the numbers of cell-adherent (A) and internalized (B) bacteria were related to the number of input bacteria. The asterisk indicates a significant difference in the adherence abilities of the indicated strains (P < 0.05). For competition experiments with soluble purified proteins (C), HEp-2 cells were incubated, prior to infection, with increasing concentrations of proteins. The adherence assay was subsequently performed with the wild-type strain S. agalactiae 6313 as for panel A. Error bars indicate standard deviations.
To further demonstrate the involvement of Srr-1 in adherence, competition experiments were performed with soluble purified Srr-1. Prior to infection with the wild-type strain S. agalactiae 6313, HEp-2 cells were incubated with increasing concentrations of soluble Srr-1-N fusion protein. The adherence assay was then performed as described above. Incubation with soluble Srr-1-N resulted in an inhibition of S. agalactiae 6313 adherence in a dose-dependent and saturable manner (Fig. 6C). The inhibition curve reached a plateau at a value corresponding to approximately 50% of inhibition, indicating that adherence was not completely abolished. As a negative control, similar experiments were performed with purified Bsp, a protein involved in the morphogenesis of S. agalactiae (46). No significant effect on adherence was observed with this protein, highlighting the specificity of inhibition by Srr-1-N. Finally, HEp-2 cells were also incubated with increasing concentrations of Srr-1-N3, which contains the K4 binding domain. As depicted in Fig. 6C, this protein inhibited the adherence of S. agalactiae to HEp-2 cells in a fashion similar to that for Srr-1-N. Together these data demonstrate that Srr-1 promotes adherence of S. agalactiae to epithelial HEp-2 cells. Moreover, since the Srr-1-N3 fragment, which bears the K4 binding domain, also interfered with the bacterial binding to HEp-2 cells, it can be speculated that the receptor recognized by Srr-1 on the surface of HEp-2 cells is K4.
K4 is accessible on the surface of HEp-2 cells.
A role of K4 as a receptor for Srr-1 would imply that K4 is accessible on the surface of HEp-2 cells. Therefore, the surface availability of K4 was investigated by flow cytometry. HEp-2 cells were incubated either with mouse monoclonal anti-K4 antibodies or, as a negative control, with mouse monoclonal anti-rabbit IgG, which is said by the manufacturer to show no cross-reaction with IgGs from other species, including human. Bound primary antibodies were detected by measuring the intensity of fluorescence of bound FITC-labeled sheep Fab fragments raised against mouse IgG. As shown in Fig. 7, an increase in fluorescence was measured with anti-K4 antibodies compared to anti-rabbit IgG. Thus, this result indicates that K4 is exposed on the surface of epithelial HEp-2 cells.
FIG. 7.
Accessibility of K4 on the surface of epithelial HEp-2 cells. Cells were incubated with either monoclonal anti-K4 antibodies (white) or monoclonal anti-rabbit IgG antibodies as a negative control (black). After washing, cells were probed with FITC-labeled Fab fragments raised against mouse IgG, and fluorescent HEp-2 cells were counted with a flow cytometer.
DISCUSSION
S. agalactiae is both a commensal and a pathogenic bacterium in humans and dairy cattle. These varying lifestyles require the bacterium to interact physically with different host components. In this report, we describe the functional characterization of Srr-1, a protein previously reported to contain serine-rich repeats (55).
Although Srr-1 did not reveal a typical signal peptide, immunofluorescence microscopy demonstrated the location of Srr-1 on the surface of S. agalactiae cells. Likewise, also for the Srr proteins GspB, Hsa, SraP, and Fap1, no signal peptide could be predicted with the program SignalP. The Srr-encoding genes, including srr-1, are located in a putative operon encoding several putative glycosyltransferases and two proteins, homologous to SecA and SecY, termed SecA2 and SecY2. The Srr protein GspB has already been demonstrated to be exported by a special Sec system, encoded by the sec genes located in its vicinity (9). It can therefore be speculated that all of the Srr proteins, including Srr-1, are recognized and exported by secretion systems, encoded by sec genes which are clustered with the srr genes. Of note, this secretion system would recognize a signal peptide with characteristics different from those of the main Sec system.
The data presented here demonstrate that the streptococcal Srr-1 protein can interact with the C terminus of human K4. S. agalactiae was reported to interact with K8 and with two other, unidentified keratins with sizes of 60 and 75 kDa (61). It is likely that the 60-kDa keratin is K4, as it has an expected size of 59 kDa.
In the present study, Srr-1 was detected on the cell surface of S. agalactiae, and the Srr-1 protein was demonstrated to contribute to attachment of S. agalactiae to human K4. This result indicates that Srr-1 is exposed on the surface of S. agalactiae cells in a fashion that allows the protein to interact efficiently with K4. S. aureus can bind keratin 10 via the surface protein ClfB (42). The apparent dissociation constant for ClfB and K10 was reported to be on the order of 10−8 molar (67), which is in the same range as the apparent KD described here for K4 and Srr-1. Both K4 and K10 are produced in suprabasal epithelial layers, including the layer constituting the apical surface of the epithelium (21, 33). O'Brien et al. proposed that binding to K10 via ClfB contributes to S. aureus colonization in the nose (42). Similarly, it could be possible that interaction of S. agalactiae with K4 via Srr-1 also contributes to colonization of host epithelia. This hypothesis is supported by data from tissue culture experiments that clearly revealed the positive role of Srr-1 in adherence of S. agalactiae to epithelial HEp-2 cells. Moreover, adherence was competitively inhibitable by the purified K4 binding domain of Srr-1. In addition, fluorescence-activated cell sorter analysis clearly demonstrated that K4 is accessible on the surface of this epithelial cell line. Of note, although keratin is a major constituent of the cytoskeleton and therefore found mainly intracellularly, several reports describe the presence of K8, K10, K18, and K19 on the surface of mammalian cells (16, 24, 29, 42). Importantly, K8 or a K8-like protein was detected on the external surface of the human hepatocellular carcinoma cell line HepG2 and on the surface of rat hepatocytes (29). Interestingly, this surface-exposed keratin is the major interacting partner of plasminogen, which is the precursor of plasmin, a protease that plays a central role in fibrinolysis (29).
The Srr-1 binding domain was localized in the C-terminal 255 aa of K4. Similarly, ClfB is also described to bind the C terminus of K10 (67). More precisely, the minimal binding of ClfB was narrowed down to the 15-aa peptide sequence YGGGSSGGGSSGGY, termed the Y-Y loop, which is imperfectly repeated in the tail region of K10 (67). The tail domain of K4 does not contain any Y-Y loop; however, it is highly enriched with glycine and serine residues, like the ClfB binding site in K10. It is therefore likely that this glycine-serine-rich region of K4 contains the Srr-1 binding site.
The K4 protein is the most abundant keratin in stratified nonepidermal epithelia. It is produced in oral, lingual, laryngeal, pharyngeal, esophageal, exocervical, and vaginal epithelia (33). In line with this, S. agalactiae is reported to colonize multiple body sites, including the vaginas and cervices of women and the throats of newborns (30). Colonization of these body sites would be crucial for the pathogenesis of the bacterium. Indeed, colonization of the female genital tract is the major source of contamination of newborn infants (58), and the colonization of the throats of newborn infants could be the route by which neonatal meningitis is initiated (13). Thus, considering the body sites colonized by S. agalactiae, it is likely that interaction between this bacterium and K4 occurs in vivo in the vagina, the exocervix, and the throat.
Although the Δsrr-1 mutant revealed a reduced binding to immobilized K4 compared to the wild type, it could still bind to human K4. This suggests that there is at least another still-unknown factor involved in the attachment of S. agalactiae to human K4. However, BLAST analysis performed with the K4 binding domain of Srr-1 did not reveal any homologous protein encoded by the genome of S. agalactiae NEM316. This suggests that the other K4 binding factor is not a protein phylogenetically closely related to Srr-1.
Similarly, the Δsrr-1 mutation did not completely abolish adherence to epithelial HEp-2 cells. This could be explained by the presence of other adhesins on the surface of S. agalactiae cells. Indeed, several surface structures of S. agalactiae are reported to be involved in adherence to host cells (8, 18, 36, 52, 53) and could be responsible for this residual adherence to HEp-2 cells.
Surface proteins often bear multiple binding activities (19). In addition, Srr-1 is homologous to serine-rich repeat proteins that interact with host components, including fibrinogen and collagen. Moreover, the K4 binding domain of Srr-1 spans a region of 157 aa, while the N-terminal region of Srr-1 is approximately 650 aa. This opens the possibility that Srr-1 is a multifunctional protein that binds other host components in addition to K4. In this regard, it would be interesting to test the ability of Srr-1 to bind to fibrinogen, collagen, or other extracellular matrix proteins. In addition, it could also be interesting to know if these other domains of Srr-1 are involved in adherence to epithelial cells. Experiments to explore this possibility are under way.
In conclusion, the present study demonstrated that Srr-1 is localized on the surface of S. agalactiae cells and that it is able to interact with human K4. The domain interacting with this mammalian protein was found to be restricted to one region of 157 aa. Finally, it was shown that Srr-1 promotes adherence of S. agalactiae to immobilized K4 and to epithelial HEp-2 cells. A growing set of reports describe the involvement of keratins in the interaction between host and pathogens (7, 12, 42, 47, 57, 61, 66, 67). However, to our knowledge, this is the first report describing a bacterial binding partner for K4.
Acknowledgments
We thank Steffen Schaffer at the Research Centre Jülich, Germany, for performing the MALDI-TOF analysis. We also thank Philipp Heinz for performing the PCR analysis to investigate the distribution of Srr-1 in S. agalactiae.
Frédéric Borges was supported by the Institut National de la Recherche Agronomique (France).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Areschoug, T., M. Stalhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 277:12642-12648. [DOI] [PubMed] [Google Scholar]
- 4.Arrecubieta, C., M. H. Lee, A. Macey, T. J. Foster, and F. D. Lowy. 2007. SdrF, a Staphylococcus epidermidis surface protein, binds type I collagen. J. Biol. Chem. 282:18767-18776. [DOI] [PubMed] [Google Scholar]
- 5.Balter, S., C. G. Whitney, and A. Schuchat. 2000. Epidemiology of group B streptococcal infections, p. 154-162. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 6.Baron, M. J., G. R. Bolduc, M. B. Goldberg, T. C. Auperin, and L. C. Madoff. 2004. Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 279:24714-24723. [DOI] [PubMed] [Google Scholar]
- 7.Batchelor, M., J. Guignot, A. Patel, N. Cummings, J. Cleary, S. Knutton, D. W. Holden, I. Connerton, and G. Frankel. 2004. Involvement of the intermediate filament protein cytokeratin-18 in actin pedestal formation during EPEC infection. EMBO Rep. 5:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. [DOI] [PubMed] [Google Scholar]
- 10.Bohnsack, J. F., X. N. Zhou, P. A. Williams, P. P. Cleary, C. J. Parker, and H. R. Hill. 1991. Purification of the proteinase from group B streptococci that inactivates human C5a. Biochim. Biophys. Acta 1079:222-228. [DOI] [PubMed] [Google Scholar]
- 11.Bozzini, S., L. Visai, P. Pignatti, T. E. Petersen, and P. Speziale. 1992. Multiple binding sites in fibronectin and the staphylococcal fibronectin receptor. Eur. J. Biochem. 207:327-333. [DOI] [PubMed] [Google Scholar]
- 12.Carlson, S. A., M. B. Omary, and B. D. Jones. 2002. Identification of cytokeratins as accessory mediators of Salmonella entry into eukaryotic cells. Life Sci. 70:1415-1426. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, Q., D. Nelson, S. Zhu, and V. A. Fischetti. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu, P. G., and L. M. Weiss. 2002. Keratin expression in human tissues and neoplasms. Histopathology 40:403-439. [DOI] [PubMed] [Google Scholar]
- 15.Davis, S. L., S. Gurusiddappa, K. W. McCrea, S. Perkins, and M. Hook. 2001. SdrG, a fibrinogen-binding bacterial adhesin of the microbial surface components recognizing adhesive matrix molecules subfamily from Staphylococcus epidermidis, targets the thrombin cleavage site in the Bbeta chain. J. Biol. Chem. 276:27799-27805. [DOI] [PubMed] [Google Scholar]
- 16.Diaz, L. A., S. A. Sampaio, C. R. Martins, E. A. Rivitti, M. L. Macca, J. T. Roscoe, Y. Takahashi, R. S. Labib, H. P. Patel, D. F. Mutasim, et al. 1987. An autoantibody in pemphigus serum, specific for the 59 kD keratin, selectively binds the surface of keratinocytes: evidence for an extracellular keratin domain. J. Investig. Dermatol. 89:287-295. [DOI] [PubMed] [Google Scholar]
- 17.Doran, K. S., and V. Nizet. 2004. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol. Microbiol. 54:23-31. [DOI] [PubMed] [Google Scholar]
- 18.Dramsi, S., E. Caliot, I. Bonne, S. Guadagnini, M. C. Prevost, M. Kojadinovic, L. Lalioui, C. Poyart, and P. Trieu-Cuot. 2006. Assembly and role of pili in group B streptococci. Mol. Microbiol. 60:1401-1413. [DOI] [PubMed] [Google Scholar]
- 19.Fischetti, V. A. 2000. Surface proteins on gram-positive bacteria, p. 11-24. In V. A. Fischetti, R. P. Novick, J. J. Ferreti, D. A. Portnoy, and J. L. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, DC.
- 20.Foster, T. J., and M. Hook. 1998. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 6:484-488. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs, E., and D. W. Cleveland. 1998. A structural scaffolding of intermediate filaments in health and disease. Science 279:514-519. [DOI] [PubMed] [Google Scholar]
- 22.Gerstmeir, R., A. Cramer, P. Dangel, S. Schaffer, and B. J. Eikmanns. 2004. RamB, a novel transcriptional regulator of genes involved in acetate metabolism of Corynebacterium glutamicum. J. Bacteriol. 186:2798-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser, P., C. Rusniok, C. Buchrieser, F. Chevalier, L. Frangeul, T. Msadek, M. Zouine, E. Couve, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499-1513. [DOI] [PubMed] [Google Scholar]
- 24.Godfroid, E., M. Geuskens, T. Dupressoir, I. Parent, and C. Szpirer. 1991. Cytokeratins are exposed on the outer surface of established human mammary carcinoma cells. J. Cell Sci. 99:595-607. [DOI] [PubMed] [Google Scholar]
- 25.Gu, L. H., and P. A. Coulombe. 2007. Keratin function in skin epithelia: a broadening palette with surprising shades. Curr. Opin. Cell Biol. 19:13-23. [DOI] [PubMed] [Google Scholar]
- 26.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2003. Analysis of RogB-controlled virulence mechanisms and gene repression in Streptococcus agalactiae. Infect. Immun. 71:5056-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 72:3495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris, T. O., D. W. Shelver, J. F. Bohnsack, and C. E. Rubens. 2003. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J. Clin. Investig. 111:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hembrough, T. A., J. Vasudevan, M. M. Allietta, W. F. Glass II, and S. L. Gonias. 1995. A cytokeratin 8-like protein with plasminogen-binding activity is present on the external surfaces of hepatocytes, HepG2 cells and breast carcinoma cell lines. J. Cell Sci. 108:1071-1082. [DOI] [PubMed] [Google Scholar]
- 30.Hickman, M. E., M. A. Rench, P. Ferrieri, and C. J. Baker. 1999. Changing epidemiology of group B streptococcal colonization. Pediatrics 104:203-209. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson, H. F., and R. J. Lamont. 1997. Streptococcal adhesion and colonization. Crit. Rev. Oral Biol. Med. 8:175-200. [DOI] [PubMed] [Google Scholar]
- 32.Keefe, G. P. 1997. Streptococcus agalactiae mastitis: a review. Can. Vet. J. 38:429-437. [PMC free article] [PubMed] [Google Scholar]
- 33.Leube, R. E., B. L. Bader, F. X. Bosch, R. Zimbelmann, T. Achtstaetter, and W. W. Franke. 1988. Molecular characterization and expression of the stratification-related cytokeratins 4 and 15. J. Cell Biol. 106:1249-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindahl, G., M. Stalhammar-Carlemalm, and T. Areschoug. 2005. Surface proteins of Streptococcus agalactiae and related proteins in other bacterial pathogens. Clin. Microbiol. Rev. 18:102-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lodish, H., A. Berk, S. L. Zipursky, P. Matsudaira, D. Baltimore, and J. E. Darnell. 1999. Molecular cell biology. W. H. Freeman and Co., New York, NY.
- 36.Maisey, H. C., M. Hensler, V. Nizet, and K. S. Doran. 2007. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 189:1464-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchler-Bauer, A., J. B. Anderson, P. F. Cherukuri, C. DeWeese-Scott, L. Y. Geer, M. Gwadz, S. He, D. I. Hurwitz, J. D. Jackson, Z. Ke, C. J. Lanczycki, C. A. Liebert, C. Liu, F. Lu, G. H. Marchler, M. Mullokandov, B. A. Shoemaker, V. Simonyan, J. S. Song, P. A. Thiessen, R. A. Yamashita, J. J. Yin, D. Zhang, and S. H. Bryant. 2005. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 33:D192-D196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDevitt, D., P. Francois, P. Vaudaux, and T. J. Foster. 1994. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol. Microbiol. 11:237-248. [DOI] [PubMed] [Google Scholar]
- 39.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni Eidhin, D., S. Perkins, P. Francois, P. Vaudaux, M. Hook, and T. J. Foster. 1998. Clumping factor B (ClfB), a new surface-located fibrinogen-binding adhesin of Staphylococcus aureus. Mol. Microbiol. 30:245-257. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien, L. M., E. J. Walsh, R. C. Massey, S. J. Peacock, and T. J. Foster. 2002. Staphylococcus aureus clumping factor B (ClfB) promotes adherence to human type I cytokeratin 10: implications for nasal colonization. Cell. Microbiol. 4:759-770. [DOI] [PubMed] [Google Scholar]
- 43.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 44.Pietrocola, G., A. Schubert, L. Visai, M. Torti, J. R. Fitzgerald, T. J. Foster, D. J. Reinscheid, and P. Speziale. 2005. FbsA, a fibrinogen-binding protein from Streptococcus agalactiae, mediates platelet aggregation. Blood 105:1052-1059. [DOI] [PubMed] [Google Scholar]
- 45.Pospiech, A., and B. Neumann. 1995. A versatile quick-prep of genomic DNA from gram-positive bacteria. Trends Genet. 11:217-218. [DOI] [PubMed] [Google Scholar]
- 46.Reinscheid, D. J., C. Stosser, K. Ehlert, R. W. Jack, K. Moller, B. J. Eikmanns, and G. S. Chhatwal. 2002. Influence of proteins Bsp and FemH on cell shape and peptidoglycan composition in group B Streptococcus. Microbiology 148:3245-3254. [DOI] [PubMed] [Google Scholar]
- 47.Sajjan, U. S., F. A. Sylvester, and J. F. Forstner. 2000. Cable-piliated Burkholderia cepacia binds to cytokeratin 13 of epithelial cells. Infect. Immun. 68:1787-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakinc, T., B. Kleine, and S. G. Gatermann. 2006. SdrI, a serine-aspartate repeat protein identified in Staphylococcus saprophyticus strain 7108, is a collagen-binding protein. Infect. Immun. 74:4615-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 50.Samen, U., B. Gottschalk, B. J. Eikmanns, and D. J. Reinscheid. 2004. Relevance of peptide uptake systems to the physiology and virulence of Streptococcus agalactiae. J. Bacteriol. 186:1398-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samen, U. M., B. J. Eikmanns, and D. J. Reinscheid. 2006. The transcriptional regulator RovS controls the attachment of Streptococcus agalactiae to human epithelial cells and the expression of virulence genes. Infect. Immun. 74:5625-5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santi, I., M. Scarselli, M. Mariani, A. Pezzicoli, V. Masignani, A. Taddei, G. Grandi, J. L. Telford, and M. Soriani. 2007. BibA: a novel immunogenic bacterial adhesin contributing to group B Streptococcus survival in human blood. Mol. Microbiol. 63:754-767. [DOI] [PubMed] [Google Scholar]
- 53.Schubert, A., K. Zakikhany, G. Pietrocola, A. Meinke, P. Speziale, B. J. Eikmanns, and D. J. Reinscheid. 2004. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect. Immun. 72:6197-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557-569. [DOI] [PubMed] [Google Scholar]
- 55.Seifert, K. N., E. E. Adderson, A. A. Whiting, J. F. Bohnsack, P. J. Crowley, and L. J. Brady. 2006. A unique serine-rich repeat protein (Srr-2) and novel surface antigen (ɛ) associated with a virulent lineage of serotype III Streptococcus agalactiae. Microbiology 152:1029-1040. [DOI] [PubMed] [Google Scholar]
- 56.Siboo, I. R., H. F. Chambers, and P. M. Sullam. 2005. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73:2273-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sojar, H. T., A. Sharma, and R. J. Genco. 2002. Porphyromonas gingivalis fimbriae bind to cytokeratin of epithelial cells. Infect. Immun. 70:96-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spellerberg, B. 2000. Pathogenesis of neonatal Streptococcus agalactiae infections. Microbes Infect. 2:1733-1742. [DOI] [PubMed] [Google Scholar]
- 59.Takamatsu, D., B. A. Bensing, H. Cheng, G. A. Jarvis, I. R. Siboo, J. A. Lopez, J. M. Griffiss, and P. M. Sullam. 2005. Binding of the Streptococcus gordonii surface glycoproteins GspB and Hsa to specific carbohydrate structures on platelet membrane glycoprotein Ibalpha. Mol. Microbiol. 58:380-392. [DOI] [PubMed] [Google Scholar]
- 60.Takamatsu, D., B. A. Bensing, and P. M. Sullam. 2004. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186:7100-7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamura, G. S., and A. Nittayajarn. 2000. Group B streptococci and other gram-positive cocci bind to cytokeratin 8. Infect. Immun. 68:2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1991. Shuttle vectors containing a multiple cloning site and a lacZ alpha gene for conjugal transfer of DNA from Escherichia coli to gram-positive bacteria. Gene 102:99-104. [DOI] [PubMed] [Google Scholar]
- 63.Tung, H., B. Guss, U. Hellman, L. Persson, K. Rubin, and C. Ryden. 2000. A bone sialoprotein-binding protein from Staphylococcus aureus: a member of the staphylococcal Sdr family. Biochem. J. 345:611-619. [PMC free article] [PubMed] [Google Scholar]
- 64.Valenti-Weigand, P., P. Benkel, M. Rohde, and G. S. Chhatwal. 1996. Entry and intracellular survival of group B streptococci in J774 macrophages. Infect. Immun. 64:2467-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Belkum, A. 1999. Short sequence repeats in microbial pathogenesis and evolution. Cell Mol. Life Sci. 56:729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viswanathan, V. K., S. Lukic, A. Koutsouris, R. Miao, M. M. Muza, and G. Hecht. 2004. Cytokeratin 18 interacts with the enteropathogenic Escherichia coli secreted protein F (EspF) and is redistributed after infection. Cell. Microbiol. 6:987-997. [DOI] [PubMed] [Google Scholar]
- 67.Walsh, E. J., L. M. O'Brien, X. Liang, M. Hook, and T. J. Foster. 2004. Clumping factor B, a fibrinogen-binding MSCRAMM (microbial surface components recognizing adhesive matrix molecules) adhesin of Staphylococcus aureus, also binds to the tail region of type I cytokeratin 10. J. Biol. Chem. 279:50691-50699. [DOI] [PubMed] [Google Scholar]
- 68.Wu, H., K. P. Mintz, M. Ladha, and P. M. Fives-Taylor. 1998. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol. Microbiol. 28:487-500. [DOI] [PubMed] [Google Scholar]