Abstract
Deletion mutants of the two sortase genes of Enterococcus faecalis OG1RF were constructed. srtC (renamed here bps for biofilm and pilus-associated sortase) was previously shown to be necessary for the production of Ebp pili and important for biofilm formation and endocarditis. Here, we report that a srtA deletion mutant showed a small (5%) yet significant (P = 0.037) reduction in biofilm relative to OG1RF, while a ΔsrtA Δbps double mutant showed a much greater reduction (74% versus OG1RF and 44% versus the Δbps mutant). In a murine urinary tract infection (UTI), the 50% infective doses of both the ΔsrtA Δbps and Δbps mutants were ∼2 log10 greater than that of OG1RF or the ΔsrtA mutant. Similarly, ∼2 log10 fewer bacteria were recovered from the kidneys after infection with the Δbps mutant (P = 0.017) and the ΔsrtA Δbps double mutant (P = 0.022) compared to wild-type strain OG1RF. In a competition UTI, the Δbps mutant was slightly, but not significantly, less attenuated than the ΔsrtA Δbps double mutant. Fluorescence-activated cell sorter analysis with Ebp-specific antibodies confirmed that a minority of OG1RF cells express Ebp pili on their surface in vitro and that Bps has a major role in Ebp pilus biogenesis but also indicated a function for SrtA in surface localization of the pilus subunit protein EbpA. In conclusion, deletion of bps had a major effect on virulence in murine UTIs, as well as biofilm; deletion of srtA from OG1RF had little effect on these phenotypes, but its deletion from a bps mutant had a pronounced effect on biofilm, suggesting that Bps and/or the proteins it anchors may compensate for the loss of some SrtA function(s).
In gram-positive bacteria, it has been known for some time that cell surface proteins can be covalently attached to peptidoglycan by a mechanism that requires sortase(s) (7, 15, 24). Sortases are extracellular transpeptidases that are found in the plasma membrane and have been shown to sort and anchor proteins that are destined for the cell surface by cleaving the conserved threonine of C-terminal LPXTG-like motifs, followed by amide bond formation between threonine and the pentaglycine cross-bridge of cell wall peptidoglycan (7, 24). Sortases can be divided into four classes, namely, A, B, C, and D, based on their structure (1). Class A sortases, which appear to be ubiquitous in many gram-positive bacteria, anchor a large number and broad range of surface proteins (7). The sortase C class of enzymes are predicted to anchor a much smaller set of substrates, and the genes coding for these are typically clustered with the substrate genes and have been shown to be involved in pilus biogenesis in addition to surface anchoring (18, 22). Genome analysis of Enterococcus faecalis V583 revealed the presence of two class A sortases (EF_2524 and SrtA [EF_3056]), one class C sortase (SrtC [EF_0194], renamed here Bps for biofilm and pilus-associated sortase) (1, 16), and 41 surface proteins bearing a cell wall sorting signal motif (19). It is believed that at least some of these surface proteins are microbial surface components recognizing adhesive matrix molecules (19) that play a role in the attachment of E. faecalis to extracellular matrix proteins and thus are likely to be important for virulence; one example is Ace, an E. faecalis adhesin to collagen and laminin (11). In addition, two other sortases, named srt-1 and srt-2, were reported in E. faecalis strain E99 containing a bee (biofilm enhancer in enterococcus) locus; however, their occurrence was rare and they were found in only 2 of 40 E. faecalis isolates tested (23).
While analyses of sortase mutants of several gram-positive pathogens have provided evidence for their wide range of roles in bacterial physiology and pathogenesis (reviewed in reference 7), only limited information is available on the role of E. faecalis sortases. A study by Kristich et al. (5) demonstrated that SrtA anchors the plasmid-encoded protein Asc10 to the enterococcal cell wall to facilitate the pheromone-induced aggregation of E. faecalis OG1RF cells. Deletions of bee locus-linked srt-1 and srt-2 of E. faecalis E99 (23) and of the ubiquitous ebp (endocarditis and biofilm-associated pilus)-linked sortase C (encoded by bps, also referred to in earlier studies as srtC) of E. faecalis OG1RF (13) have each led to a reduction in biofilm production. It was also demonstrated that bps, but not srtA, was essential for tethering of the endocarditis-associated Ebp pili by E. faecalis OG1RF. However, what other proteins may be processed by Bps or SrtA and what role these sortases may play in virulence have not been reported.
The present study further explored the role of E. faecalis sortases by deleting independently or in combination the two sortase genes (namely, srtA and bps) present in E. faecalis strain OG1RF. Here, we generated sortase (srtA with and without bps) deletion derivatives of OG1RF and evaluated these constructs for their phenotypic effects by using biofilm assays and a mouse urinary tract infection (UTI) model and for their effect on the surface localization of Ebp pilus subunit proteins.
MATERIALS AND METHODS
Bacterial strains and media.
The bacterial strains and plasmids used in this study are listed in Table 1. A total of 100 E. faecalis strains isolated over 32 years from diverse locations (the United States, Thailand, China, Argentina, Spain, Canada, Belgium, and the United Kingdom), including clinical isolates, nosocomial and community-derived fecal isolates, and animal isolates, were included. Tryptic soy broth (Difco Laboratories, Sparks, MD) containing 0.25 M glucose was used for biofilm experiments. Brain heart infusion broth (BHIB) (Difco Laboratories) and/or brain heart infusion agar (BHIA) was used for routine culturing of bacteria. BHIB containing 40% heat-inactivated horse serum (BHIS; Sigma, St. Louis, MO) was used to culture the bacteria for in vivo experiments. The antibiotics (Sigma) used in BHIA plates for the growth of OG1RF and the various mutant strains were erythromycin (10 μg/ml), fusidic acid (25 μg/ml), rifampin (100 μg/ml), and spectinomycin (1,000 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. faecalis strains | ||
| OG1RF | Laboratory strain; Rifr Fusr | 10 |
| CK111(pCF10) | Conjugative donor construct; Rifs Fuss Sper | 5 |
| TX5470 | bps deletion mutant of OG1RF; OG1RF Δbpsb | 13 |
| TX5536 | srtA deletion mutant of OG1RF; OG1RF ΔsrtA | This study |
| TX5537 | srtA and bps double-deletion mutant of OG1RF; OG1RF ΔsrtA Δbps | This study |
| E. coli strains | ||
| EC1000 | E. coli host strain for routine cloning of RepA-dependent plasmids | 6 |
| TX5497 | EC1000(pTEX5497); Chlr Eryr | This study |
| Plasmids | ||
| pCJK47 | Plasmid for markerless exchange; carries oriTpCF10, lacZ, and P-pheS*; Eryr | 4 |
| pTEX5497 | Plasmid for srtA deletion with flanking regions of srtA cloned into pCJK47; Eryr | This study |
Abbreviations: Chl, chloramphenicol; Ery, erythromycin; Fus, fusidic acid; Gen, gentamicin; Rif, rifampin; Tet, tetracycline; Spe, spectinomycin. A superscript s designates sensitivity, and a superscript r designates resistance.
Previous studies referred to bps as srtC.
General techniques.
Genomic DNA was extracted by the hexadecyltrimethylammonium bromide method as described previously (25). For the PCR primers used for amplification and sequencing, see Table S1 in the supplemental material. Pulsed-field gel electrophoresis was carried out as described earlier (9). Southern and colony lysate hybridizations were performed under high-stringency conditions (20) with probes labeled with the RadPrime DNA labeling system (Invitrogen).
Construction of sortase mutants.
Nonpolar deletion mutants of E. faecalis were constructed by allelic replacement (4, 13). By crossover PCR, fragments upstream (1 kb) and downstream (900 bp) of the complete srtA open reading frame were amplified together to create a single 1.9-kb fragment with primers listed elsewhere (see Table S1 in the supplemental material). After ligating this fragment into pCJK47 (4), the construct was then transformed into Escherichia coli EC1000 (6) cells to obtain TX5497 (Table 1). After confirming the sequence, the plasmid containing the srtA up-down fragment (pTEX5497) was electroporated into E. faecalis CK111 competent cells (5). CK111 cells containing pTEX5497 were conjugated with wild-type OG1RF and TX5470 by a standard technique (12), and single-crossover integrants (OG1RF::pTEX5497 and TX5470::pTEX5497) were selected on BHIA plates which contained rifampin (100 μg/ml) with erythromycin (10 μg/ml) or spectinomycin (1,000 μg/ml). Single colonies were purified on BHIA plates with fusidic acid (25 μg/ml) with erythromycin (10 μg/ml) and subjected to the PheS* counterselection (negative selection) system with MM9YEG agar plates supplemented with 10 mM p-Cl-Phe (4) to select for double-crossover deletions. The deletions were confirmed as correct by PCR sequencing, hybridization, and pulsed-field gel electrophoresis procedures.
Growth curve experiment.
Cultures of test bacteria grown overnight were diluted (1:20) in BHIS and grown at 37°C with gentle shaking. A reading of optical density at 600 nm (OD600) was taken every hour from 0 to 12 h and then at 24 h. At intervals of 0, 4, and 6 h, CFU counts were also determined by plating serial dilutions on BHIA.
Biofilm assay.
A biofilm density assay was carried out for wild-type E. faecalis OG1RF and its isogenic ΔsrtA, Δbps, and ΔsrtA Δbps deletion mutants according to a method previously described by Mohamed et al. (8).
UTI model.
Protocols for preparation of mice, inoculum volumes, and all others stages of the experiment were the same ones previously used in our laboratory (21). Initially groups of five mice per inoculum (102 to 106 CFU) were used for each test bacterial strain (wild-type OG1RF and the ΔsrtA, Δbps, and ΔsrtA Δbps mutants), resulting in several independent monoinfection experiments. In the second set of monoinfection experiments, groups of 10 additional mice were infected with an inoculum of 104 CFU. The urinary bladders and kidney pairs were excised, weighed, and homogenized in 1 ml and 5 ml of saline, respectively, and dilutions were plated onto BHIA for CFU counting. The 50% infective dose (ID50) of each test bacterium was determined by a previously described method (17). The detection limit of bacteria in this experiment was 10 CFU/ml of tissue homogenate. Identities of the test bacteria recovered from infected organs were confirmed by plating them on bile esculin azide agar plates (Difco Laboratories) and BHIA plates with rifampin (100 μg/ml) or by colony PCR. For the competition assay, cultures of Δbps and ΔsrtA Δbps mutants were resuspended in saline solution and mixed in approximately equal (1:1) volumes based on OD600 readings, used to infect mice, and plated for colony counts. After 24 h, CFU were recovered from organs as previously described (21). Colony lysate preparations were probed with intragenic bps, srtA (amplified with primer sets EF3056F-EF3056R for srtA and EF1094F-EF1094R for bps), and ace (2) probes, and high-stringency hybridization techniques were used to determine the ratio of Δbps mutant to ΔsrtA Δbps double mutant cells in the bacterial colonies recovered. Similar to the method previously described for E. faecalis mixed infections (13, 14), the mean virulence index of ΔsrtA Δbps mutants relative to that of Δbps mutants was calculated with the following equation: Mean virulence index = Σ[(% Δbps in inoculum)/(% ΔsrtA Δbps in inoculum)]/Σ[(% Δbps in kidneys)/(% ΔsrtA Δbps in kidneys)].
The University of Texas Health Science Center at Houston preapproved protocol and guidelines of the Animal Welfare Committee were followed throughout the course of this study.
Polyclonal antibodies and flow cytometry.
Production and purification of polyclonal antibodies against recombinant EbpA, EbpB, and EbpC proteins were described elsewhere (13). For flow cytometry analysis of surface expression of Ebp proteins, bacteria were grown in BHIS to late log phase. After being washed twice with phosphate-buffered saline (PBS), 100 μl of bacteria adjusted to an OD600 of 0.2 were suspended in 40 μl of newborn calf serum (Sigma) and incubated for 15 min at room temperature. After centrifugation at 10,000 × g for 6 min, 100 μl of 20 μg/ml preimmune or affinity-purified anti-Ebp specific antibodies in dilution buffer (PBS containing 20% newborn calf serum and 0.1% bovine serum albumin [BSA]) was added and the solution was incubated at 4°C for 2 h. The bacteria were washed twice with 400 μl of 0.1% BSA in PBS and then added to a dilution buffer containing 100 μl of 1:100-diluted goat anti-rabbit immunoglobulin G (IgG) conjugated with F(ab′)2 fragment-specific R-phycoerythrin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and incubated at 4°C for 2 h. After cells were washed three times with 400 μl of 0.1% BSA in PBS, cells were resuspended in 500 μl of 1% paraformaldehyde in PBS and analyzed with a Coulter EPICS XL AB6064 flow cytometer (Beckman Coulter, Fullerton, CA) and System II software.
Statistics.
Differences in OD570 readings in biofilm experiments were evaluated with the Kruskal-Wallis test (analysis of variance [ANOVA]) and, in some cases, also by the Mann-Whitney test. Differences in the log10 CFU of bacteria recovered from organs in monoinfections with inocula of 104 CFU were evaluated by the unpaired t test. Differences between the total numbers of infected kidney pairs/bladder (102 to 106 CFU inoculum groups combined) were evaluated by Fisher's exact test. The percentages of the Δbps mutant in the inoculum versus the percentages of the Δbps mutant in the kidneys of individual mice infected with the Δbps and ΔsrtA Δbps mutants in the competition assay were analyzed for significance by the paired t test.
RESULTS AND DISCUSSION
An estimated 8 million doctor visits in the United States are linked with UTIs each year (3). Although the significance of E. faecalis in causing infections associated with the kidney and bladder is well documented (accounting for 2 to 12% of outpatient UTIs and 7 to 16% of inpatient UTIs) (26), little is known about the factors used by E. faecalis to infect this site. To help develop therapeutic or preventive measures against E. faecalis UTIs, it is important to identify E. faecalis surface antigens which function as adhesins to urinary tract tissues. Cell wall-associated proteins of E. faecalis are the focus of our ongoing research because of their predicted central role in adhesion and, presumably, colonization of tissues (e.g., heart valves and kidneys) and infections (e.g., endocarditis and UTIs). Since these surface proteins are thought to utilize the sortase-mediated cell wall anchoring mechanism, we here constructed and analyzed different sortase mutants to indirectly assess the global effect of surface-associated proteins in biofilm formation and pathogenicity of E. faecalis in UTIs. Conceptually, we consider the sortases to be fitness factors, rather than virulence factors, since the impact of sortase deletions is presumably the result of defective localization of a number of cell wall-associated proteins, including virulence factors.
Sortase-encoding genes of E. faecalis strains.
Of the three sortase genes (srtA, EF_2524, and bps) previously identified in strain V583 (16), EF_2524 (which is part of an integrated plasmid remnant region [EF_2512 to EF_2542]) of V583 (16) was not present in seven of nine E. faecalis strains tested, including OG1RF (1, 13). Since our previous study found that bps is ubiquitous in E. faecalis (13), here we tested for the presence of srtA in 100 diverse E. faecalis strains by colony hybridization and found that this gene was also present in all of the isolates tested. The ubiquitous distribution of these two genes in diverse E. faecalis strains suggests that they are part of the core genome and have a role in E. faecalis biology. We next analyzed the almost completed genomic sequence of E. faecalis strain OG1RF (10) generated by our collaboration with the human genome sequencing center at the Baylor College of Medicine (available at http://www.hgsc.bcm.tmc.edu/projects/microbial/microbial-detail.xsp?project_id=111) and confirmed that OG1RF (which failed to hybridize with EF_2524) contains only the two ubiquitous sortase genes srtA and bps. In order to evaluate the independent roles of these sortase genes, we generated sortase (srtA and/or bps) deletion derivatives of strain OG1RF.
Deletion of E. faecalis srtA from OG1RF and from its bps deletion mutant, TX5470.
Sequencing of OG1RF ΔsrtA (TX5536) and OG1RF ΔsrtA Δbps (TX5537) confirmed the deletion of the complete srtA open reading frame, which encodes 244 amino acids (data not shown). There were no obvious differences in the growth patterns of wild-type E. faecalis OG1RF and its isogenic sortase mutants (ΔsrtA, Δbps, and ΔsrtA Δbps), suggesting that the sortases are not essential for in vitro growth of E. faecalis.
Contribution of the srtA and bps genes to biofilm formation.
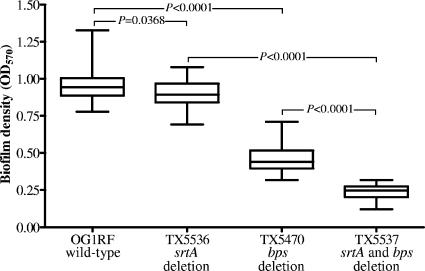
All of the sortase mutant derivatives of E. faecalis OG1RF showed decreased biofilm formation (P < 0.0001 by Kruskal-Wallis test), with reductions ranging from 5% (ΔsrtA mutant; median OD570 = 0.893; interquartile range [IQR], 0.841 to 0.968) to 74% (ΔsrtA Δbps double mutant; median OD570 = 0.247; IQR, 0.204 to 0.274) relative to wild-type E. faecalis OG1RF (median OD570 = 0.943; IQR, 0.887 to 1.004) (Fig. 1). The decrease for the ΔsrtA mutant was small and not significant (P > 0.05) by post-hoc test of Kruskal-Wallis ANOVA testing compared to OG1RF, although it was significant by the Mann Whitney test (Fig. 1) (P = 0.037), and observed in repeated experiments. The decrease seen with the ΔsrtA Δbps double mutant (see above) was much more substantial, with the median OD570 of ΔsrtA Δbps 44% less than that of Δbps (median OD570 = 0.441; IQR, 0.396 to 0.516; P < 0.001); the results for Δbps are consistent with those previously reported (13). This surprising and pronounced decrease seen with the ΔsrtA Δbps double mutant versus the Δbps mutant suggests the possibilities that (i) Bps may compensate for the loss of SrtA from OG1RF and that (ii) in the absence of Bps, SrtA performs some function that is important for biofilm formation.
FIG. 1.
Comparison of biofilm production of wild-type OG1RF and the TX5536 (ΔsrtA), TX5470 (Δbps), and TX5537 (ΔsrtA Δbps) mutants. Median values and interquartile ranges are shown. Multiple comparison of median OD570s from biofilm assays of wild-type and mutant cells by Kruskal-Wallis test (ANOVA) with Dunn's post-hoc modification showed a highly significant difference (P < 0.0001). Individual median OD570s from biofilm assays of wild-type and mutant cells were compared by Mann-Whitney test and are shown above the whiskers.
Effect of srtA and bps gene deletions in a murine UTI model.
In a recent study, we established a murine UTI model with OG1RF grown in BHIS and showed that Ebp pili are important for this strain's ability to cause infection of murine kidneys (21). Here, to test the effects of different sortase deletion mutant derivatives of OG1RF, mice were infected as previously described (21) via intraurethral catheterization with a range of CFU. The ID50s derived from monoinfection experiments showed that both the ΔsrtA Δbps (ID50 = 1.5 × 104) and Δbps (ID50 = 3.6 × 104) mutants required ∼2 log10 more cells to infect 50% of the mice than did wild-type E. faecalis OG1RF (ID50 = 1.1 × 102) or ΔsrtA (ID50 = 1.4 × 102), indicating a substantial role for Bps compared to SrtA in UTI infections. Furthermore, the comparable (∼2 log) difference between the ID50s of the bps mutant and that of strain OG1RF versus those of the previously reported ebp mutant and strain OG1RF (21) suggests that the effect seen after bps deletion is likely due to the resulting inability to form Ebp pili (13). For the total number of mouse kidneys and urinary bladders infected by the mutants compared to the number infected by wild-type E. faecalis OG1RF, see Table S2 in the supplemental material. The percentage of kidneys of mice infected summed across all inocula was 91% for wild-type OG1RF, 80% for the ΔsrtA mutant (P = 0.2650), 63% for the Δbps mutant (P = 0.0048), and 51% for the ΔsrtA Δbps double mutant (P < 0.0001). Although fewer bladders than kidneys were infected, the differences between individual strains were similar to those of kidney infections (see Table S2 in the supplemental material); the percentage of bladders of mice infected summed across all inocula was 78% for wild-type OG1RF, 76% for the ΔsrtA mutant (P = 1.0), 51% for the Δbps mutant (P = 0.0177), and 37% for the ΔsrtA Δbps double mutant (P = 0.0005).
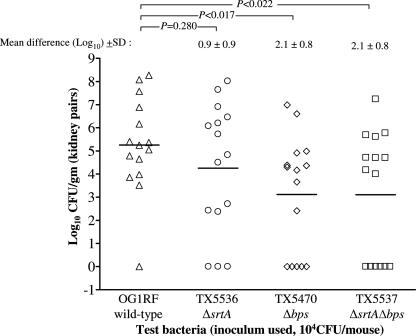
The log10 CFU recovered from the kidney pairs (n = 15) of mice infected with wild-type E. faecalis OG1RF and its isogenic mutants at a 104 CFU inoculum are shown in Fig. 2. A comparison of the mean log10 CFU of bacteria recovered from the kidneys showed that the differences between OG1RF and the Δbps mutant (2.1 ± 0.8 log10) and between OG1RF and the ΔsrtA Δbps double mutant (2.1 ± 0.8 log10) were statistically significant (P = 0.017 and P = 0.022, respectively), while the difference between OG1RF and the ΔsrtA mutant (0.9 ± 0.9, P = 0.280) was not.
FIG. 2.
Monoinfection with wild-type E. faecalis OG1RF and its isogenic mutants. Data are from 15 mice infected with 104 CFU; results are expressed as log10 CFU per gram from kidney homogenates 48 h after transurethral challenge. The log10 CFU from both kidneys were combined and averaged. A value of 1 was assigned to those kidneys with 0 CFU. Triangles represent wild-type E. faecalis OG1RF, circles represent OG1RF ΔsrtA, diamonds represent OG1RF Δbps, and squares represent OG1RF ΔsrtA Δbps. Horizontal bars represent the geometric mean. The mean difference in CFU counts of sortase mutants versus OG1RF is given as the log10 ± the standard deviation (SD). Differences in the log10 CFU of OG1RF versus the ΔsrtA, Δbps, and ΔsrtA Δbps mutants recovered from organs were evaluated by the unpaired t test.
Because the results shown above for the percentage of mice infected, although not significant by Fisher's test (see Table S2 in the supplemental material), suggested that the ΔsrtA Δbps double mutant may be more attenuated than the Δbps mutant, we next used a competition assay with an approximately equal mixture of Δbps and ΔsrtA Δbps mutant cells of two different inocula. For 17 animals, the percentage of Δbps mutant cells in the total inoculum was 42% to 43%. At autopsy, the percentages of Δbps mutant cells in the total number of CFU recovered from kidneys were in the range of 52 to 63% for five animals and 92%, 46%, and 23% for one animal each. Nine animals were not infected. The mean percentage of Δbps mutant cells in the total number of CFU of bacteria recovered from kidneys was 55.1%, demonstrating a slight outnumbering by the Δbps mutant; however, the paired t test showed no significant difference between these mutants (P = 0.1097). The mean virulence index (13, 14) of the ΔsrtA Δbps double mutant relative to the Δbps mutant in kidneys was 0.605, indicating that SrtA plays only a minor role in UTI or that Bps can compensate for the SrtA loss. Taken together, our bps mutant data corroborate the previously demonstrated role of Ebp pili (21) in murine kidney infections and the competition assay results suggest little to no additional role for SrtA in this model. This is in contrast to the role of SrtA in other gram-positive organisms, such as Staphylococcus aureus and Listeria monocytogenes, where SrtA plays a much larger role in virulence (7). However, it is possible that srtA of E. faecalis contributes more significantly to the UTI disease process in humans compared to mice or it may be more important for other infection processes such as endocarditis.
Role of sortases in surface localization and polymerization of Ebp pili.
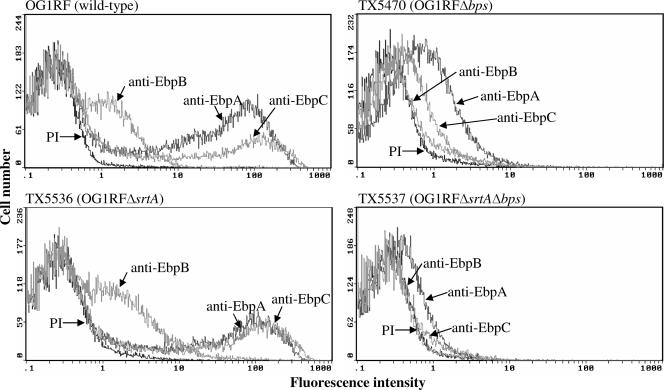
By Western blotting, we have previously shown that bps, but not srtA, is needed for polymerization of Ebp pili (13) and immunogold electron microscopy estimated that few wild-type cells (<2% of cells grown in BHIB and <20% of cells grown in tryptic soy broth-0.25 M glucose) produce Ebp pili in vitro (13). Here, we measured the surface-localized Ebp proteins of wild-type OG1RF and sortase mutants after growth in BHIS (the growth condition that enhanced levels of high-molecular-weight Ebp bands in Western blot assays) (13) by fluorescence-activated cell sorting analysis. While anti-Ace antibodies (used as a positive control) bound 98% of wild-type OG1RF grown at 46°C (11) (data not shown), the percentages of EbpA-, EbpB-, and EbpC-expressing cells in BHIS cultures of strain OG1RF were 34%, 20%, and 24%, respectively (Fig. 3) and the mean fluorescence intensities of EbpA, EbpB, and EbpC were 29.8, 2.4, and 27.1, respectively. The relatively low intensity of EbpB observed in flow cytometry analysis seemed to contradict the immunoblotting data from our earlier study (13), which showed that the EbpB protein is part of the polymeric structure. One possible explanation for these results is that EbpB may be relatively hidden beneath the surface of the Ebp pilus structure and thus not accessible to antibodies in a flow cytometry analysis, as seen with group B streptococci (22). The percentages of Δbps mutant cells with surface-localized EbpA, EbpB, and EbpC were 28%, 8%, and 10%, but with much lower mean fluorescence intensities (2.1, 3.0, and 2.0, respectively) (Fig. 3). This markedly reduced fluorescence intensity of Ebp proteins in the Δbps mutant is consistent with the role of Bps in pilus polymerization, and this observation is consistent with loss of Ebp high-molecular-weight multimers in Western blot assays of the Δbps mutant (13). Since our earlier EbpA surface localization studies by immunogold electron microscopy (13) showed staining of both the cell surface and the pili of wild-type OG1RF, the persistence of a relatively high percentage of EbpA-positive Δbps mutant cells (28%), but with a greatly reduced mean fluorescence intensity (29.8 to 2.1), suggests that the EbpA subunit protein is still located on the cell surface of the bps mutant, presumably in the monomeric form. Unlike the Δbps mutant, the ΔsrtA mutant (TX5536) showed an expression pattern similar to that of wild-type OG1RF, albeit with higher mean fluorescence intensities (49.7, 3.3, and 34.3 for EbpA, EbpB, and EbpC, respectively) and some difference in the percentage of cells, with a small decrease for EbpA (34 versus 27%) and small increases for EbpB (20 versus 26%) and EbpC (24 versus 26%) (Fig. 3). These moderately increased Ebp intensities with the srtA mutant, implying enhanced pilus production compared to that of the wild type, suggest that the presence of SrtA somehow results in interference with the action of Bps for polymerization. The mutant that lacked both sortases (TX5537) showed the least surface localization (6%, 3%, and 5% of cells with mean fluorescence intensities of 1.8, 2.4, and 2.4 for EbpA, EbpB, and EbpC, respectively) (Fig. 3). The decrease in the percentage of EbpA-stained cells of the ΔsrtA Δbps double mutant (6%) compared to the Δbps mutant (28%) indicates that SrtA may be involved in the surface anchoring of EbpA, at least in the absence of Bps; an additional decrease was also seen to a minor degree with both EbpB and EbpC. Taken together, these surface analysis studies indicate that both sortases are important for Ebp component surface localization in OG1RF, with Bps being the major one for pilus biogenesis, as well as for ascending murine UTI.
FIG. 3.
Flow cytometry analysis of surface expression of Ebp pilus proteins in wild-type E. faecalis OG1RF and its isogenic mutants. Cells were incubated with either control preimmune (PI) IgGs or anti-Ebp specific IgGs, followed by incubation with a F(ab′)2 fragment of goat anti-rabbit IgGs (heavy plus light chains) conjugated to R-phycoerythrin. Bacteria were analyzed by flow cytometry by using side scatter as the threshold of detection. Specific binding by anti-Ebp antibodies is indicated as log fluorescence intensity on the x axis, and each histogram represent 50,000 bacteria.
In summary, from this study and the previous work from our laboratory (13, 21), it appears likely that Bps, presumably via Bps-anchored surface proteins, plays an important role in both in vitro biofilm formation and in vivo murine kidney infections under the conditions tested. The results from this study also provide evidence for an additional role of SrtA or SrtA-anchored surface proteins in biofilm, particularly in the absence of Bps, but a minor role in UTI. Flow cytometry studies have shown that both sortases are important for Ebp pilus component surface localization in OG1RF, with the class C sortase Bps being the major one for pilus assembly. The ubiquitous presence of both sortase genes in E. faecalis isolates increases the likelihood that sortases in general, and Bps in particular, could be a target for disease prevention.
Supplementary Material
Acknowledgments
We thank Karen Jacques-Palaz and Charlene Thomsom for technical assistance.
This work was supported by NIH grant R37 AI 47923 from the Division of Microbiology and Infectious Diseases, NIAID, to B. E. Murray. Kelvin Kemp was supported by NIH grant T35 DK007676 from the National Institute of Diabetes and Digestive and Kidney Diseases and a Summer Scholarship Award from the Infectious Diseases Society of America.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 4 September 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 156:289-297. [DOI] [PubMed] [Google Scholar]
- 2.Duh, R. W., K. V. Singh, K. Malathum, and B. E. Murray. 2001. In vitro activity of 19 antimicrobial agents against enterococci from healthy subjects and hospitalized patients and use of an ace gene probe from Enterococcus faecalis for species identification. Microb. Drug Resist. 7:39-46. [DOI] [PubMed] [Google Scholar]
- 3.Foxman, B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A):5S-13S. [DOI] [PubMed] [Google Scholar]
- 4.Kristich, C. J., J. R. Chandler, and G. M. Dunny. 2007. Development of a host-genotype-independent counterselectable marker and a high-frequency conjugative delivery system and their use in genetic analysis of Enterococcus faecalis. Plasmid 57:131-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristich, C. J., D. A. Manias, and G. M. Dunny. 2005. Development of a method for markerless genetic exchange in Enterococcus faecalis and its use in construction of a srtA mutant. Appl. Environ. Microbiol. 71:5837-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leenhouts, K., G. Buist, A. Bolhuis, A. ten Berge, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253:217-224. [DOI] [PubMed] [Google Scholar]
- 7.Marraffini, L. A., A. C. Dedent, and O. Schneewind. 2006. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70:192-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed, J. A., W. Huang, S. R. Nallapareddy, F. Teng, and B. E. Murray. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray, B. E., K. V. Singh, J. D. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nallapareddy, S. R., X. Qin, G. M. Weinstock, M. Hook, and B. E. Murray. 2000. Enterococcus faecalis adhesin, Ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect. Immun. 68:5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nallapareddy, S. R., K. V. Singh, and B. E. Murray. 2006. Construction of improved temperature-sensitive and mobilizable vectors and their use for constructing mutations in the adhesin-encoding acm gene of poorly transformable clinical Enterococcus faecium strains. Appl. Environ. Microbiol. 72:334-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nallapareddy, S. R., K. V. Singh, J. Sillanpaa, D. A. Garsin, M. Hook, S. L. Erlandsen, and B. E. Murray. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Investig. 116:2799-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nannini, E. C., F. Teng, K. V. Singh, and B. E. Murray. 2005. Decreased virulence of a gls24 mutant of Enterococcus faecalis OG1RF in an experimental endocarditis model. Infect. Immun. 73:7772-7774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson, G. K., and T. J. Mitchell. 2004. The biology of Gram-positive sortase enzymes. Trends Microbiol. 12:89-95. [DOI] [PubMed] [Google Scholar]
- 16.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 17.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 18.Scott, J. R., and D. Zahner. 2006. Pili with strong attachments: Gram-positive bacteria do it differently. Mol. Microbiol. 62:320-330. [DOI] [PubMed] [Google Scholar]
- 19.Sillanpaa, J., Y. Xu, S. R. Nallapareddy, B. E. Murray, and M. Hook. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069-2078. [DOI] [PubMed] [Google Scholar]
- 20.Singh, K. V., T. M. Coque, G. M. Weinstock, and B. E. Murray. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323-331. [DOI] [PubMed] [Google Scholar]
- 21.Singh, K. V., S. R. Nallapareddy, and B. E. Murray. 2007. Importance of endocarditis and biofilm associated pilus (ebp) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J. Infect. Dis. 195:1671-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in gram-positive pathogens. Nat. Rev. Microbiol. 4:509-519. [DOI] [PubMed] [Google Scholar]
- 23.Tendolkar, P. M., A. S. Baghdayan, and N. Shankar. 2006. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J. Bacteriol. 188:2063-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694:269-278. [DOI] [PubMed] [Google Scholar]
- 25.Wilson, K. 1994. Preparation of genomic DNA from bacteria, p. 2.4.1-2.4.2. In F. M. Ausubel, R. Brent, R. E. Kingston, D. M. David, J. G. Scidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology. Green Publishing Associates, Brooklyn, NY. [DOI] [PubMed]
- 26.Wilson, M. L., and L. Gaido. 2004. Laboratory diagnosis of urinary tract infections in adult patients. Clin. Infect. Dis. 38:1150-1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.