Abstract
Neisseria meningitidis serogroup B is a major cause of bacterial meningitis in younger populations. The available vaccines are based on outer membrane vesicles obtained from wild-type strains. In children less than 2 years old they confer protection only against strains expressing homologous PorA, a major, variable outer membrane protein (OMP). We genetically modified a strain in order to eliminate PorA and to overproduce one or several minor and conserved OMPs. Using a mouse model mimicking children's PorA-specific bactericidal activity, it was demonstrated that overproduction of more than one minor OMP is required to elicit antibodies able to induce complement-mediated killing of strains expressing heterologous PorA. It is concluded that a critical density of bactericidal antibodies needs to be reached at the surface of meningococci to induce complement-mediated killing. With minor OMPs, this threshold is reached when more than one antigen is targeted, and this allows cross-protection.
Infectious diseases caused by Neisseria meningitidis are a significant public health concern. N. meningitidis serogroup B (MenB) caused 69% of meningococcal disease reported in Europe in 2004 (10). MenB has also caused outbreaks in several countries with annual attack rates of 5 to 50 cases per 100,000 persons, with most cases occurring in young children (5). Overall, MenB causes a substantial proportion of diseases across all ages, but the specific distribution varies by age group, with higher proportions in infants and toddlers than in older age groups (27, 33). Conjugate polysaccharide vaccines based on the capsular polysaccharide of N. meningitidis serogroups A, C, W-135, and Y have been licensed for adolescents, and pediatric development is ongoing. However, utilization of the serogroup B capsular polysaccharide as a vaccine antigen has been hampered by its poor immunogenicity and by potential concern about inducing autoantibodies that cross-react with glycosylated host antigens (11, 26). Alternative antigens are therefore being evaluated as candidates for use in a vaccine against MenB strains.
It is possible to extract the outer membrane from N. meningitidis or culture supernatant in the form of outer membrane vesicles (OMVs). Vaccines based on OMVs have been developed by using detergent extraction to reduce the lipooligosaccharide (LOS) content (13). PorA is one of the most abundant outer membrane proteins (OMPs) displaying high antigenic variability, which is used to classify meningococci (14). OMV vaccines made from single wild-type strains induce protection in children more than 4 years old in a PorA serosubtype-independent way (8). In children less than 2 years old, wild-type OMV vaccines predominantly induce PorA serosubtype-specific serum bactericidal activity (29, 41, 46). Efforts to develop cross-protective vaccines, especially in younger populations, are ongoing (32).
Ideally, a vaccine to prevent MenB disease should be safe and immunogenic in the pediatric population and elicit protection against a wide range of clinical isolates (34). In this context, we are actively pursuing the development of a multicomponent vaccine containing conserved surface antigens able to induce cross-protective immune responses. In order to limit the risk of the appearance of vaccine escape mutants, our research is oriented towards a vaccine able to interfere with several mechanisms of the meningococcal infectious process, such as iron uptake (39), toxicity (42), and adhesion (4).
To overcome limitations of recombinant expression and folding of integral OMPs, an alternative expression system in N. meningitidis was developed by taking into account the capacity of this organism to produce large amounts of OMVs in the presence of detergent. Overproduction of OMPs that might have potential as vaccine antigens was achieved by using two methodologies referred to as gene delivery and promoter replacement (35). When the overexpressed gene encodes a surface component, the resulting recombinant strain produces OMVs enriched in the desired component.
In the present study, four minor OMPs (TbpA, Hsf, NspA, and Omp85) that have already shown some potential as vaccine candidates, being surface exposed and well conserved among serogroup B neisseria strains, were overexpressed. TbpA is an integral OMP that, together with TbpB, makes up the transferrin receptor of N. meningitidis (21, 30, 36, 40). Sera from carriers and subjects with meningococcal diseases, but not sera from controls, had detectable antibodies to TbpA/B, suggesting that there is expression of TbpA/B by Neisseria in vivo (1, 18). Affinity-isolated Tbp proteins from N. meningitidis induced protection against challenge in mice after passive or active immunization (7). Omp85 is a minor antigen present in N. meningitidis and in OMVs (22, 28). Omp85 is highly conserved (12) and is an essential protein involved in the positioning and folding of other OMPs in the bacterial outer membrane (3, 17, 45). There is a correlation between the presence of antibodies against an 80-kDa protein detected by Western blotting and bactericidal activity after immunization with wild-type OMV vaccine (37). Hsf (or NhhA) is the neisserial autotransporter protein homologous to Haemophilus influenzae Hsf/Hia. Hsf is well conserved among N. meningitidis strains, its gene has been detected in all strains tested, and the protein is surface located (31). It has been suggested that Hsf acts as an adhesin (38). Recombinant Hsf is also recognized by human serum from patients and carriers (44). NspA is present on the surface of 99% of meningococcal strains tested and is well conserved (24). Immunization of mice with NspA induced protection against N. meningitidis challenge (23). Based on sequence similarity with Opa proteins, it is possible that NspA is an adhesin (43).
We developed a mouse model mimicking the PorA-specific bactericidal response observed in younger populations with wild-type OMVs. To overcome the PorA-restricted protection of wild-type OMVs, expression of PorA in the H44/76 strain was suppressed, and to avoid the presence of the polysaccharide B capsule, the cps gene complex was deleted. This deletion also removed the galE gene, resulting in the synthesis of truncated LOS. Using the porA galE, acapsulate mutant strain as the expression host, we evaluated the impact on the vaccine response of overproduction of four minor well-conserved OMPs (TbpA, Hsf, NspA, and Omp85) for which a potential role in protection has been demonstrated.
MATERIALS AND METHODS
N. meningitidis transformation.
Cells of N. meningitidis strain H44/76 (B:15:P1.7,16:L3,7) incubated overnight in the presence of 5% CO2 on chocolate base (GC) (Difco) or Mueller-Hinton (MH) (Difco) medium plates were collected in 2 ml of liquid GC or MH medium containing 10 mM MgCl2 and diluted to obtain an optical density at 550 nm (OD550) of 0.1. Two micrograms of DNA was added to the cell suspension, and this was followed by a 6-h incubation at 37°C (with shaking). After the incubation period, 100 μl of the culture, undiluted or diluted 1/10, 1/100, or 1/1,000, was spread on GC or MH medium plates containing the appropriate antibiotic (see below). Recombinant colonies appeared after 48 h of incubation at 37°C in the presence of 5% CO2.
Construction of H44/76 lacking capsular polysaccharides (cps).
Plasmid pMF121 (16) was used to construct an H44/76 derivative lacking the capsular polysaccharide. This plasmid contains the flanking regions of the gene locus coding for the biosynthesis pathway of the group B polysaccharide and an erythromycin resistance gene. Deletion of the group B polysaccharide locus resulted in loss of expression of the group B capsular polysaccharide and loss of the active copy of the galE gene, leading to galactose-deficient LOS. Erythromycin (10 μg/ml)-resistant colonies were selected, and capsule-deficient strains were identified by colony blotting using the anti-group B polysaccharide 735 monoclonal antibody (DadeBehring, Marburg, Germany). Binding of the monoclonal antibody was visualized with a biotinylated anti-mouse immunoglobulin (1/1,000; Amersham).
Construction of plasmid pCMK, targeting integration in the porA locus of H44/76.
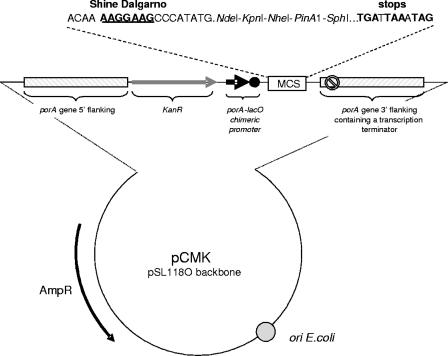
A schematic drawing of the pCMK vector is presented in Fig. 1. pCMK is a high-copy-number plasmid that replicates in Escherichia coli, was derived from a pSL1180 backbone (PharmaciaBiotech), and harbors the bla gene, thereby conferring resistance to ampicillin. In addition, pCMK contains two porA flanking regions (porA5′ and porA3′ containing a transcription terminator) necessary for homologous recombination, a selectable marker conferring resistance to kanamycin, two uptake sequences, a porA/lacO chimeric promoter repressed in the E. coli host [BL21(DE3)] expressing lacIq but transcriptionally active in N. meningitidis, and a multiple cloning site (with five sites: NdeI, KpnI, NheI, PinAI, and SphI) necessary for insertion of foreign DNA into pCMK.
FIG. 1.
Schematic representation of the pCMK vectors used to deliver genes, operons, and/or expression cassettes in the genome of N. meningitidis. MCS, multiple cloning site.
The porA5′ and porA3′ recombinogenic regions and the porA/lacO promoter were PCR amplified from genomic DNA extracted from H44/76 using oligonucleotides PorA5′Fwd, PorA5′Rev, PorA3′Fwd, PorA3′Rev, PorAlacORev, and PorAlacOFwd (Table 1) under the conditions described by the supplier of HiFi DNA polymerase (Boehringer, Mannheim, Germany) and cloned in pSL1180. The kanamycin resistance cassette was excised from pUC4K (PharmaciaBiotech) by PstI restriction and introduced between the porA5′ flanking region and the porA/lacO promoter region.
TABLE 1.
Primers used in this study
| Primer | Nucleotide sequencea | Relevant characteristic(s) |
|---|---|---|
| PorA5′Fwd | 5′-CCCAAGCTTGCCGTCTGAATACATCCCGTCATTCCTCA-3′ | HindIII, uptake sequence |
| PorA5′Rev | 5′-CGATGCTCGCGACTCCAGAGACCTCGTGCGGGCC-3′ | NruI |
| PorA3′Fwd | 5′-GGAAGATCTGATTAAATAGGCGAAAATACCAGCTACGA-3′ | BglII, stop codons |
| PorA3′Rev | 5′-GCCGAATTCTTCAGACGGCGCAGCAGGAATTTATCGG-3′ | EcoRI, uptake sequence |
| PorAlcOFwd | 5′-AAGCTCTGCAGGAGGTCTGCGCTTGAATTG-3′ | PstI |
| PorAlacORev | 5′-CTTAAGGCATATGGGCTTCCTTTTGTAA-3′ | NdeI |
| PPA1 | 5′-GCGGCCGTTGCCGATGTCAGCC-3′ | |
| PPA2 | 5′-GGCATAGCTGATGCGTGGAACTGC-3′ | |
| N01-full-NdeI | 5′-GGGAATTCCATATGAAAAAAGCACTTGCCACAC-3′ | NdeI |
| NdeI-NspA 3 | 5′-GGAATTCCATATGTCAGAATTTGACGCGCAC-3′ | NdeI |
| HSF 01-NdeI | 5′-GGAATTCCATATGATGAACAAAATATACCGC-3′ | NdeI |
| HSF 02-NheI | 5′-GTAGCTAGCTAGCTTACCACTGATAACCGAC-3′ | NdeI |
| ProD15-51X | 5′-GGGCGAATTCGCGGCCGCCGTCAACGGCACACCGTTG-3′ | EcoRI |
| ProD15-52 | 5′-GCTCTAGAGCGGAATGCGGTTTCAGACG-3′ | XbaI |
| TnRD15-KpnI/XbaI | 5′-CGCCGGTACCTCTAGAGCCGTCTGAACCACTCGTGGACAACCC-3′ | KpnI and XbaI, uptake sequence |
| TnR03Cam(KpnI) | 5′-CGCCGGTACCGCCGCTAACTATAACGGTC-3′ | KpnI |
| PorA-01 | 5′-CGCCGGTACCGAGGTCTGCGCTTGAATTGTG-3′ | KpnI |
| PorA02 | 5′-CGCCGGTACCTCTAGACATCGGGCAAACACCCG-3′ | KpnI |
| BAD16 | 5′-GGCCTAGCTAGCCGTCTGAAGCGATTAGAGTTTCAAAATTTATTC-3′ | NheI, uptake sequence |
| BAD17 | 5′-GGCCAAGCTTCAGACGGCGTTCGACCGAGTTTGAGCCTTTGC-3′ | HindIII, uptake sequence |
| BAD18 | 5′-TCCCCCGGGAAGATCTGGACGAAAAATCTCAAGAAACCG-3′ | XmaI and BglII |
| BAD19 | 5′-GGAAGATCTCCGCTCGAGCAAATTTACAAAAGGAAGCCGATATGCAACAGCAACATTTGTTCCG-3′ | BglII and XhoI |
| BAD21 | 5′-GGAAGATCTCCGCTCGAGACATCGGGCAAACACCCG-3′ | BglII and XhoI |
| BAD20 | 5′-TCCCCCGGGAGATCTCACTAGTATTACCCTGTTATCCC-3′ | XmaI, BglII, and SpeI |
Restriction sites are in bold type; uptake sequences or stop codons are underlined.
Construction of an H44/76 ΔporA strain.
The H44/76 cps strain was transformed with 2 μg of supercoiled pCMK plasmid DNA as described above and plated on kanamycin-containing plates (200 μg/ml). Kanamycin-resistant colonies were screened for deletion of the porA gene by PCR with boiled bacterial lysates using primers PPA1 and PPA2 (Table 1). The absence of PorA synthesis was further confirmed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis.
Overproduction of NspA and Hsf in H44/76 (gene delivery).
The gene coding for NspA was PCR amplified from genomic DNA extracted from H44/76 using the N01-full-NdeI and NdeI-NspA 3 oligonucleotide primers containing NdeI restriction sites (Table 1). The corresponding amplicon was digested with NdeI and inserted into the NdeI restriction site of the pCMK delivery vector.
The gene coding for Hsf was PCR amplified from genomic DNA extracted from H44/76 using the HSF 01-NdeI and HSF 02-NheI oligonucleotide primers (Table 1). Because of the sequence of the HSF 01-NdeI primer, the Hsf protein produced contained two methionine residues at the N terminus. The corresponding amplicon was subsequently cloned in the NdeI restriction site of the pCMK delivery vector. In the recombinant plasmid, designated pCMK-Hsf, we deleted the lacO gene present in the chimeric porA/lacO promoter.
Two micrograms of pCMK-NspA or pCMK-Hsf was used to transform the H44/76 Δcps strain. Kanamycin-resistant colonies were screened for deletion of the porA gene and insertion of a second copy of the nspA gene or the hsf gene by PCR using boiled bacterial lysates. The absence of PorA synthesis and overproduction of NspA or Hsf were further confirmed by SDS-PAGE analysis.
Overproduction of Omp85 in H44/76 (promoter delivery).
A promoter replacement plasmid was constructed using E. coli cloning methodologies. A DNA fragment covering nucleotides −983 to −48 with respect to the omp85 gene start codon (ATG) was PCR amplified from genomic DNA extracted from the H44/76 strain using oligonucleotides ProD15-51X and ProD15-52 containing EcoRI and XbaI restriction sites, respectively (Table 1). This fragment was subjected to restriction and inserted into the pUC18 plasmid (PharmaciaBiotech) restricted with the same enzymes. The construct that we obtained was subjected to in vitro mutagenesis using the genome priming system (with the pGPS2 donor plasmid) commercialized by New England Biolabs. Clones in which a mini-transposon (derived from Tn7 and harboring a chloramphenicol resistance gene) was inserted were selected. One clone containing a mini-transposon insertion located in the omp85 5′ flanking region, 401 bp downstream from the EcoRI site, was isolated and used for further studies. This plasmid was subjected to circle PCR mutagenesis in order to (i) delete a repeated DNA sequence (Tn7R) generated by the transposition process, (ii) insert meningococcal uptake sequences required for transformation, and (iii) insert suitable restriction sites allowing cloning of foreign DNA material, such as promoters. The circle PCR was performed using the TnRD15-KpnI/XbaI and TnR03Cam(KpnI) oligonucleotides containing uptake sequences and restriction sites (KpnI and XbaI) (Table 1). The resulting PCR fragment was gel purified, digested with Asp718 (isoschizomer of KpnI), and ligated to a 184-bp DNA fragment containing the porA promoter and generated by PCR using the PorA-01 and PorA02 oligonucleotides containing KpnI restriction sites. A recombinant plasmid (pUC OMP85) carrying a porA promoter inserted in the correct orientation was selected and used to transform H44/76 lacking capsular polysaccharide (Δcps) and PorA (ΔporA). Recombinant H44/76 clones resulting from a double-crossover event (PCR screening) were selected on GC medium containing 5 μg/ml chloramphenicol and analyzed for Omp85 synthesis.
Overproduction of TbpA in H44/76 (promoter delivery).
The tbpB gene was deleted and replaced by the Cmr/PorA promoter cassette. A 3,218-bp DNA fragment corresponding to the 509-bp 5′ flanking region of the tbpB gene, the 2,139-bp tbpB coding sequence, the 87-bp intergenic sequence, and the first 483 nucleotides of the tbpA coding sequence was PCR amplified from H44/76 genomic DNA using oligonucleotides BAD16 and BAD17 containing uptake sequences and NheI and HindIII restriction sites (Table 1). This PCR fragment was cloned in a pGEM-T vector (Promega). The plasmid was subjected to circle PCR mutagenesis in order to (i) insert suitable restriction sites allowing cloning of a Cmr/PorA promoter cassette and (ii) delete 209 bp of the 5′ flanking sequence of tbpB and the tbpB coding sequence. The circle PCR was performed using the BAD18 and the BAD19 oligonucleotides containing XmaI, BglII, and XhoI restriction sites (Table 1). The Cmr/PorA promoter cassette was amplified from the pUC OMP85 plasmid using primers BAD21 and BAD20 containing XmaI, SpeI, BglII, and XhoI restriction sites (Table 1). This PCR fragment was cloned in the circle PCR plasmid. Two micrograms of this plasmid was used to transform the H44/76 Δcps ΔporA strain. Integration by double crossover in the upstream region of tbpA directed insertion of the porA promoter directly upstream of the tbpA start codon. Recombinant H44/76 clones resulting from a double-crossover event (PCR screening) were selected on GC medium containing 5 μg/ml chloramphenicol and analyzed for TbpB down-expression and TbpA synthesis.
Culture and preparation of OMVs.
A vial of frozen N. meningitidis (recombinant or not recombinant) was thawed and streaked onto a modified Frantz agar plate, which was then incubated at 37°C for 18 h. Colonies were resuspended, added to a flask containing modified Frantz medium supplemented with the appropriate antibiotic, and incubated at 37°C for 16 h with shaking. The cells were separated from the culture broth by centrifugation at 5,000 × g at 4°C for 15 min. OMVs were isolated using deoxycholate as described previously (15).
SDS-PAGE.
OMV preparations were analyzed by SDS-PAGE. After heating for 5 min at 100°C in sample buffer, 15 μg was loaded onto the gel. After electrophoresis, gels were stained with Coomassie brilliant blue R250.
Mice and immunizations.
Outbred OF1 mice (female; 6 to 8 weeks old; also known as CF1; Charles River, Lyon, France) received three injections with OMVs via the intramuscular route on days 0, 21, and 28. With each 50-μl injection, 5 μg of antigen formulated in the GSK proprietary AS04 adjuvant (AlPO4 plus 3-O-deacyl-4′-monophosphoryl lipid A) was administered. Control mice received only adjuvant. Blood samples were collected 14 days after the third injection. The experiments complied with the relevant national guidelines of Belgium and institutional policies of GlaxoSmithKline Biologicals.
Antibody assays.
Omp85 and TbpA derived from strain H44/76 were expressed without their signal sequence in E. coli, where they accumulated in inclusion bodies. These bodies were purified and solubilized as described previously (20). The passenger domain of Hsf derived from strain H44/76 was expressed and purified from E. coli as a C-terminally His-tagged protein. NspA derived from strain B11 was expressed without its signal sequence and was purified from E. coli as an N-terminally His-tagged protein.
Enzyme-linked immunosorbent assay (ELISA) plates were coated with TbpA, Hsf, NspA, or Omp85 in phosphate-buffered saline (PBS). The assays were performed as described previously (20).
Complement-dependent bactericidal antibody assays.
Wild-type MenB strains used in this study were isolated either from cases during epidemics occurring in different regions around the world (epidemic strains) or from a case isolate in the United Kingdom (nonepidemic strain) (Table 2). The strains were grown overnight on MH agar (Difco) containing 1% (vol/vol) Polyvitex (Biomérieux) and 1% horse serum (Sigma) at 37°C in a 5% CO2 atmosphere. The bacteria were inoculated into tryptic soy broth (Becton Dickinson) with 50 μM of the iron chelator desferrioxamine mesylate (Sigma) and were grown in shaking flasks for 3 h at 37°C until an OD470 of 0.5 was reached. Each culture was then diluted in PBS containing 0.5 mM MgCl2, 0.9 mM CaCl2, and 0.1% glucose (PBS-glucose) in order to obtain an OD600 of 0.4 (bacterial suspension). The sera were heat inactivated (40 min at 56°C) and subsequently diluted 1/50 in PBS-glucose. In wells of sterile flat-bottom microtiter plates (Nunc), 25 μl of diluted test serum was mixed with 12.5 μl of baby rabbit complement (selected for the absence of bactericidal activity against the test strains; Cerdarlane Laboratories) and 12.5 μl of the bacterial suspension. Serial dilutions of test sera were treated similarly. The controls included bacteria plus complement, bacteria plus heat-inactivated complement, and test serum plus bacteria plus heat-inactivated complement. Antiserum from mice immunized three times with whole bacterial cells (5 μg of protein with AS04 per injection) was used as a positive control in the bactericidal assays and also to validate or reject each microtiter plate. The microtiter plates were then sealed and incubated with shaking (520 rpm) for 75 min at 37°C without CO2. After this incubation, 50 μl of MH medium containing 0.9% agar was added to each well. A second layer of 50 μl of PBS containing 0.9% agar was added 30 min later. After overnight incubation at 33°C in the presence of 5% CO2, the colonies were counted. The average number of CFU in the controls corresponding to bacteria plus complement was defined as 100%. The bactericidal titer was defined as the reciprocal of the serum dilution that resulted in 50% killing.
TABLE 2.
Summary of invasive N. meningitidis strains used in bactericidal assays
| Strain | Country of origin | Year isolated | PorA classification | Epidemic strain |
|---|---|---|---|---|
| H44/76 | Norway | 1976 | P1.7,16 | Yes |
| CU385 | Cuba | 1980 | P1.19,15 | Yes |
| M97-250687 | United Kingdom | 1997 | P1.19,15 | No |
| NZ124/98 | New Zealand | 1998 | P1.7,4 | Yes |
Possible additive or synergistic effects of antibodies directed against different overexpressed minor OMPs were studied by using several pooled sera. Pools were obtained from sera derived from 20 mice after immunization with the same vaccine preparation. For mixing experiments, equal volumes of pools from the same treatment group were combined and subsequently tested in the bactericidal assay.
Statistical analysis.
Differences in bactericidal antibody titers were determined by the Kruskal-Wallis nonparametric test with the one-tailed Dunn test. A P value of ≤0.05 was considered statistically significant.
RESULTS
Relevance of the mouse model to the clinical situation of infants.
OMVs prepared from wild-type strains H44/76 and Cu385 and formulated in the AS04 Adjuvant System were administered to OF1 mice (10 animals per group) via the intramuscular route at 0, 21, and 28 days. Serum samples were obtained 2 weeks after the third injection, pooled (10 sera per group), and tested for their bactericidal activity against four strains isolated from cases in different countries around the world. These strains expressed PorA which was either homologous or heterologous to the PorA of the vaccine strains.
Pooled antisera prepared from mice immunized with OMVs obtained from H44/76 producing P1.7,16 PorA were bactericidal against strain H44/76 but not against the heterologous PorA strains Cu385, M97-250687, and NZ124/98 (Table 3). Similar observations were made with pooled antisera from mice immunized with OMVs from strain Cu385 producing P1.19,15 PorA, which were bactericidal against the Cu385 and M97-250687 strains but not against heterologous PorA strains (H44/76 and NZ124/98). Control sera from mice immunized with AS04 alone exhibited no or undetectable bactericidal activity against the four strains. Positive control sera were obtained from mice immunized with the corresponding heat-inactivated whole bacteria (data not shown).
TABLE 3.
Complement-mediated bactericidal titers of sera from mice immunized with PorA+ OMV vaccinesa
| Vaccine | Bactericidal activity with strainb:
|
|||
|---|---|---|---|---|
| H44/76 (P1.7,16) | Cu385 (P1.19,15) | M97-250687 (P1.19,15) | NZ124/98 (P1.7,4) | |
| P1.7,16 OMVs | 1,568 | <100 | <100 | <100 |
| P1.19,15 OMVs | <100 | 1,082 | 870 | <100 |
The bactericidal activity is expressed as the reciprocal dilution of serum required to kill 50% of bacteria. The assay was performed with pooled sera from 10 mice per group.
The PorA serosubtypes of the strains are indicated in parentheses.
These results demonstrate that the immunogenicity of OMVs in OF1 mice reflects the immunogenicity of wild-type OMV vaccines in human infants; i.e., they predominantly induce a PorA serosubtype-specific serum bactericidal activity (41).
Overproduction of minor OMPs.
To avoid a predominant bactericidal response directed against PorA, we decided to analyze the impact of overproduction of minor OMPs in a porA knockout background. Similarly, to further increase vaccine specificity and prevent unwanted responses, such as the production of bactericidal antibodies directed against LOS, a galE mutant background was used.
Two different strategies were used to overproduce minor OMPs. TbpA and Omp85 were overproduced by promoter replacement, while Hsf and NspA were overproduced by gene delivery. In both cases, the porA promoter was selected for overexpression.
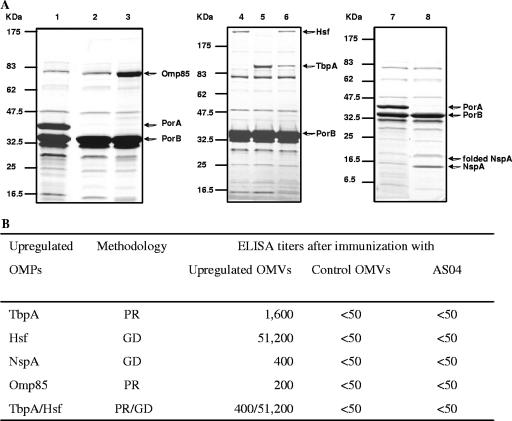
The impact of overproduction of minor OMPs on their amounts in OMVs prepared from recombinant strains was determined by SDS-PAGE. PorA was not present in OMVs purified from recombinant strains (Fig. 2A, lanes 2, 3, 4, 5, 6, and 8). Compared to wild-type OMVs (lanes 1 and 7), the levels of Omp85 (lane 3), Hsf (lanes 4 and 6), TbpA (lanes 5 and 6), and NspA (lane 8) were clearly enhanced in OMVs from the genetically modified strains. We were also able to overproduce simultaneously two different OMPs (lane 6). However, the double overproduction of TbpA and Hsf in a single strain reduced the level of TbpA compared to the level when it was overproduced alone (lanes 5 and 6), whereas the level of Hsf was not affected (lanes 4 and 6).
FIG. 2.
Overproduction of Omp85, Hsf, TbpA, and NspA. (A) Impact on the content of proteins in MenB OMVs. OMVs purified from different N. meningitidis strains were separated by SDS-PAGE and stained with Coomassie brilliant blue. The strains used are wild-type strain H44/76 (lanes 1 and 7), a galE porA mutant derivative of H44/76 (lane 2), and galE porA mutants overexpressing Omp85 (lane 3), Hsf (lane 4), TbpA (lane 5), Hsf and TbpA simultaneously (lane 6), or NspA (lane 8). In lane 8, bands corresponding to denatured and nondenatured NspA are present. (B) Impact on the induction of antibodies against minor OMPs in mice immunized with OMVs purified from either overproducing strains or control strains or in mice immunized with adjuvant alone (AS04) (20 mice per group). Overproduction was achieved by either the promoter replacement (PR) or gene delivery (GD) strategy. ELISA titers are expressed as the reciprocal dilutions of pooled sera required to obtain an OD490 of 0.5, using purified recombinant TbpA, Hsf, NspA, or Omp85.
Thus, overproduction of TbpA, Hsf, NspA, and Omp85 by gene delivery or promoter replacement resulted in significant increases in the amounts of these proteins in OMVs.
Impact of overproduction of minor OMPs on antibody responses.
Groups of 20 mice were immunized intramuscularly with different OMV preparations (5 μg protein per injection) formulated with AS04. Serum samples were obtained 2 weeks after the third dose and pooled. In ELISA, pooled sera from control mice inoculated with adjuvant alone or from mice immunized with OMVs from a nonoverproducing strain had no detectable antibodies directed against TbpA, Hsf, NspA, and Omp85 (titers, <50), whereas immunization of mice with OMVs prepared from the strain that overproduced minor OMPs elicited the production of antibodies against the respective OMPs (Fig. 2B). The most impressive increase was observed with Hsf (at least a 1,024-fold increase). The lower overproduction of TbpA in H44/76 overexpressing both tbpA and hsf affected the anti-TbpA response (titer, 400 versus 1,600).
Antibodies induced via TbpA and Hsf overproduction show additive effects in complement-mediated bactericidal activity.
The serum samples obtained from mice immunized with different OMV preparations were tested individually in serum bactericidal assays against three MenB strains (20 mice per group). These strains included parental wild-type strain H44/76 and heterologous strains Cu385 and NZ124/98. Vaccine preparations obtained from porA galE mutant strains either not overproducing OMPs (control vaccine) or overproducing Hsf alone elicited low or undetectable serum bactericidal antibody titers in mice (geometric mean titer, ≤88) with a low percentage of seroconversion (from 0 to 25% of mice developed detectable bactericidal antibodies) (Table 4). The serum bactericidal titers against H44/76 or Cu385 were slightly higher when tests were performed with sera from mice immunized with OMVs from the TbpA-overproducing strain, and the seroconversion rates were also slightly higher. However, the bactericidal responses elicited by the control vaccine and the single-OMP-overproducing-strain vaccines were not statistically different. In contrast, mice immunized with OMVs from the TbpA/Hsf-overproducing strain had significantly higher bactericidal antibody titers against H44/76 and Cu385 than mice immunized with single-overexpressed-OMP vaccines (P ≤ 0.036) or control vaccine (P ≤ 0.0003). When measured with strain NZ124/98, sera from mice immunized with TbpA/Hsf OMVs had significantly higher bactericidal antibody titers than sera from mice immunized with the control vaccine (P = 0.0007). This immunization experiment was repeated twice with similar results (data not shown).
TABLE 4.
Impact of upregulation of minor OMPs on the induction of complement-mediated killing by bactericidal antibodies in mice
| porA knockout galE OMV vaccine | MenB strain H44/76
|
MenB strain Cu385
|
MenB strain NZ124/98
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Geometric mean titer for 50% killinga | 95% Confidence interval | Seroconversion (%)b | Geometric mean titer for 50% killing | 95% Confidence interval | Seroconversion (%)b | Geometric mean titer for 50% killing | 95% Confidence interval | Seroconversion (%)b | |
| No upregulation | 71 | 46-109 | 15 | 59 | 42-82 | 5 | 50 | 50-50 | 0 |
| TbpA upregulated | 134 | 61-296 | 30 | 117 | 57-241 | 30 | 85 | 48-149 | 20 |
| Hsf upregulated | 74 | 45-122 | 15 | 73 | 44-121 | 15 | 88 | 52-149 | 25 |
| TbpA and Hsf upregulated | 727 | 342-1,546 | 90 | 244 | 117-511 | 65 | 222 | 91-542 | 45 |
The geometric mean titer for 50% killing was calculated with individual sera (n = 20)..
Percentage of responder mice (titer, >50).
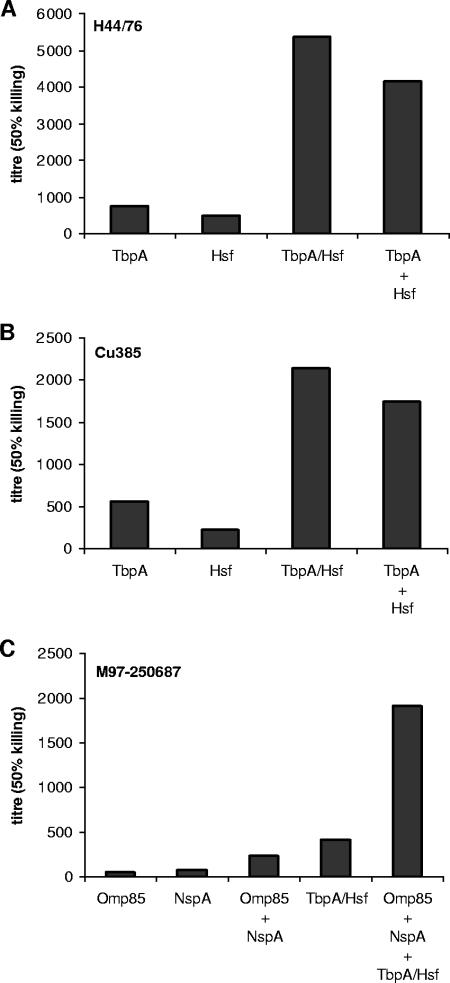
In order to confirm the additive activity of anti-TbpA and anti-Hsf antibodies in the induction of complement-mediated killing, serum-mixing experiments were performed. For this purpose, 20 sera from mice immunized with OMVs from either TbpA- or Hsf-overproducing strains were pooled. The pools were tested alone or mixed in serum bactericidal assays against strains H44/76 and Cu385. A pool of 20 sera from mice immunized with TbpA/Hsf OMVs was used as a control. The bactericidal antibody titers measured against H44/76 and Cu385 are shown in Fig. 3A and B. The sera from mice immunized with OMVs from the strain overproducing only one minor OMP had lower bactericidal titers than the sera from mice immunized with OMVs from the strain overproducing both TbpA and Hsf and than the mixed sera. The bactericidal titers of the mixed sera and TbpA/Hsf sera were similar.
FIG. 3.
Bactericidal activity against N. meningitidis strains of serum antibodies from mice immunized with OMV vaccines. Sera from 20 mice (see Table 4) were pooled, and two to four pools were combined. Bactericidal assays with (A) H44/76 and (B) Cu385 were performed using pooled sera from mice immunized with OMVs from either TbpA-, Hsf-, or Tbp/Hsf-overproducing strains or with a 1:1 (vol/vol) mixture of serum pools from mice immunized with OMVs from TbpA- and Hsf-overproducing strains. Bactericidal activity is expressed as the reciprocal antibody titer. (C) Bactericidal assays with strain M97-250687 were performed using pooled sera from mice immunized with OMVs from either Omp85-, NspA-, or TbpA/Hsf-overproducing strains or a combination thereof. The data are the means of three different mixing experiments performed using the same serum samples and are expressed as the reciprocal bactericidal titers.
Combination of minor OMPs has synergistic effects on complement-mediated bactericidal titers.
A second combination experiment was performed with OMVs from NspA- and Omp85-overproducing strains. First, pools of 20 sera from mice immunized with NspA OMVs or Omp85 OMVs were analyzed for the ability to mediate, alone or in combination, bactericidal activity against strain M97-250687 (Fig. 3C). Pooled sera from mice immunized with OMVs from strains overproducing NspA or Omp85 had low or undetectable bactericidal antibodies (titers, ≤74), but the mixture of pools displayed clear bactericidal activity against strain M97-250687 (titer, 239). Mice immunized with OMVs from the TbpA/Hsf-overproducing strain also had significant bactericidal activity against strain M97-250687 (titer determined with pooled sera, 412). To evaluate the additive effect of bactericidal antibodies directed against different minor OMPs, pooled sera from mice immunized with NspA, Omp85, and TbpA/Hsf OMVs were combined. The bactericidal activity was enhanced when the three serum pools were mixed (titer, 1,920) compared to the activity obtained with the combination of NspA plus Omp85 or the TbpA/Hsf serum pool alone (titers, 239 and 412, respectively). As observed with the other serum combinations described above, the bactericidal titer obtained with a mixture of the three sera was higher than the sum of the bactericidal titers obtained with the individual pooled sera. This indicates that there is a synergistic effect of antibodies directed against different minor OMPs, which is observed with two or more minor OMPs depending on the strain used in the bactericidal assays.
DISCUSSION
In children less than 2 years old, OMV vaccines prepared from wild-type MenB strains are able to elicit a bactericidal antibody response against homologous PorA strains but not against heterologous PorA strains (41). We developed a mouse model that mimics bactericidal antibody responses induced in infants by wild-type OMV vaccines. To avoid a PorA immunodominant response, we genetically modified strain H44/76 to knock out the porA gene. In this strain, the cps gene complex was also deleted, resulting in the absence of capsular polysaccharide and the α-chain of LOS. In our mouse model, a vaccine containing OMVs produced from this strain induced a weak or undetectable bactericidal antibody response against parental wild-type strain H44/76. The genetically modified strain was used to evaluate the impact of overproduction of minor and well-conserved OMPs on the bactericidal antibody response elicited by OMVs. We selected four minor, well-conserved OMPs with potential as vaccine antigens, which were overproduced either by promoter replacement or by gene delivery. For both overexpression strategies the strong porA promoter was used.
We demonstrated that gene delivery or promoter replacement results in overproduction of minor OMPs in OMV preparations and that overexpression is needed to elicit the production of specific antibodies in nonprimed mice immunized with OMVs from a porA knockout strain. It is noteworthy that induction of antibodies against well-conserved minor OMPs, such as NspA, could also be achieved by sequential immunizations with different wild-type OMVs produced from PorA heterologous strains (25).
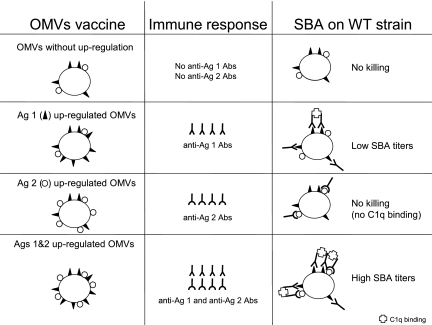
Using our mouse model, we observed that enhancement of the antibody response against one minor OMP may not be sufficient to mediate bactericidal activity against MenB strains even if very high specific antibody titers are induced (e.g., an ELISA titer of 51,200 for Hsf). Efficient complement-mediated killing was observed only when at least two minor OMPs were targeted by bactericidal antibodies. This was observed with anti-TbpA and anti-Hsf antibodies mediating bactericidal activity against heterologous MenB strains (Cu385, NZ124, and M97-250687) and also with anti-NspA and anti-Omp85 antibodies (tested only against strain M97-250687). Moreover, increasing the number of targeted minor antigens on the surface of the bacteria leads to further enhancement of bactericidal antibody titers, which are clearly higher than the simple sum of bactericidal titers obtained when only two minor OMPs are targeted. Our hypothesis is that a minimal density of bacterial surface proteins occupied by bactericidal antibodies must be reached to allow activation of the complement cascade via the classical pathway (Fig. 4). We suggest that activation of complement requires at least two adjacent antibodies, which do not necessarily have to be directed against the same antigens. However, the corresponding antigens should be close enough to each other on the bacterial surface to allow binding of a C1q molecule to at least two Fc domains, which is the first step in the initiation of the classical complement cascade (6). This situation is probably more characteristic of coccal bacteria. For example, bacillary and coccobacillary bacteria have polar localization of autotransporter proteins, but in coccal bacteria, such as N. meningitidis, expression of these proteins is observed at different loci (19). Consequently, when only one minor meningococcal OMP is targeted, the bactericidal antibodies are scattered over the surface of the bacteria and would not be able to fix the C1q factor, which is the first step of the classical pathway activation of the complement cascade leading to the formation of the membrane attack complex. To increase the density of bactericidal antibodies on the surface of the bacteria, our strategy was to target several minor, well-conserved OMPs simultaneously. This strategy could also be applied to other gram-negative bacterial species that naturally do not produce blebs by producing mutations in the tol-pal genes that result in the formation of blebs (2, 9).
FIG. 4.
Schematic representation of the impact of the antigen density on the bactericidal activity of antibodies on the surface of coccal MenB strains. A defined density is required to induce significant bactericidal antibody killing mediated by complement. This threshold density is reached when at least two minor OMPs (Ag 1 and Ag 2) are targeted by antibodies. For an efficacious OMV vaccine, at least two minor OMPs must be overproduced, resulting in vaccine-induced bactericidal antibody killing. Ag, antigen; Abs, antibodies; SBA, serum bactericidal activity. The first step of the classical pathway for activation of the complement cascade is binding of the C1q factor to antibodies, leading to the formation of the membrane attack complex.
The overproduction of several well-conserved minor OMPs induced a cross-reactive bactericidal antibody response, which has two advantages for a MenB vaccine with broad coverage. First, it allows interference with several stages of the meningococcal infection process, such as iron uptake (via TbpA), adhesion (via Hsf and possibly NspA), and vital function (via Omp85). Second, it reduces the risk of emergence of vaccine escape mutants.
In conclusion, we have demonstrated that it is possible to induce a protective bactericidal antibody response against MenB strains by immunization with OMVs from strains lacking the major, variable PorA OMP and overproducing selected well-conserved minor OMPs. Our results for a limited number of heterologous strains also suggest that the use of well-conserved minor OMPs results in a cross-protective response. This approach may be the key to development of a fully protective vaccine for meningococcal disease and may also be a strategy that is generally applicable to other host-adapted bacterial pathogens in which phase and antigenic variation of major OMPs has stifled vaccine development.
Acknowledgments
M.P.B. is supported by The Netherlands Council for Chemical Sciences (CW) with financial aid from The Netherlands Technology Foundation (STW). Work in M.P.J.'s lab is supported by program grant 284214 from the National Health and Medical Research Council of Australia. This work was supported in part by GlaxoSmithKline Biologicals, Rixensart, Belgium.
We thank U. Krause for editorial assistance.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 30 July 2007.
REFERENCES
- 1.Ala'Aldeen, D. A. A., P. Stevenson, E. Griffiths, A. R. Gorringe, L. I. Irons, A. Robinson, S. Hyde, and S. P. Borriello. 1994. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect. Immun. 62:2984-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubès. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos, M. P., and J. Tommassen. 2004. Biogenesis of the Gram-negative bacterial outer membrane. Curr. Opin. Microbiol. 7:610-616. [DOI] [PubMed] [Google Scholar]
- 4.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Aricò, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappuoli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly, M., and N. Noah on behalf of the European Meningitis Surveillance Group. 1999. Is group C meningococcal disease increasing in Europe? A report of surveillance of meningococcal infection in Europe 1993-6. Epidemiol. Infect. 122:41-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper, N. R. 1985. The classical complement pathway: activation and regulation of the first complement component. Adv. Immunol. 37:151-216. [DOI] [PubMed] [Google Scholar]
- 7.Danve, B., L. Lissolo, M. Mignon, P. Dumas, S. Colombani, A. B. Schryvers, and M. J. Quentin-Millet. 1993. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine 11:1214-1220. [DOI] [PubMed] [Google Scholar]
- 8.de Moraes, J. C., B. A. Perkins, M. C. C. Camargo, N. T. R. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. Land Gral, V. L. Gattas, H. D. G. Vasconcelos, B. D. Plikaytis, J. D. Wenger, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 9.Dubuisson, J. F., A. Vianney, N. Hugouvieux-Cotte-Pattat, and J. C. Lazzaroni. 2005. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology 151:3337-3347. [DOI] [PubMed] [Google Scholar]
- 10.EU-IBIS Network. 2006. Invasive Neisseria meningitidis in Europe 2003/2004. Health Protection Agency, London, United Kingdom.
- 11.Finne, J., M. Leinonen, and P. H. Mäkelä. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 322:355-357. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick, D. A., and J. O. McInerney. 2005. Evidence of positive Darwinian selection in Omp85, a highly conserved bacterial outer membrane protein essential for cell viability. J. Mol. Evol. 60:268-273. [DOI] [PubMed] [Google Scholar]
- 13.Frasch, C. E., L. van Alphen, J. Holst, J. T. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. J. Maiden (ed.), Meningococcal vaccines: methods and protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 14.Frasch, C. E., W. D. Zollinger, and J. T. Poolman. 1985. Serotype antigens of Neisseria meningitidis and a proposed scheme for designation of serotypes. Rev. Infect. Dis. 7:504-510. [DOI] [PubMed] [Google Scholar]
- 15.Fredriksen, J. H., E. Rosenqvist, E. Wedege, K. Bryn, G. Bjune, L. O. Froholm, A. K. Lindbak, B. Mogster, E. Namork, U. Rye, G. Stabbetorp, R. Winsnes, B. Arise, and O. Gloss. 1991. Production, characterization and control of MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease. NIPH Ann. 14:67-79. [PubMed] [Google Scholar]
- 16.Frosch, M., E. Schultz, E. Glenn-Calvo, and T. F. Meyer. 1990. Generation of capsule-deficient Neisseria meningitidis strains by homologous recombination. Mol. Microbiol. 4:1215-1218. [DOI] [PubMed] [Google Scholar]
- 17.Gentle, I., K. Gabriel, P. Beech, R. Waller, and T. Lithgow. 2004. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J. Cell Biol. 164:19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorringe, A. R., R. Borrow, A. J. Fox, and A. Robinson. 1995. Human antibody response to meningococcal transferrin binding proteins: evidence for vaccine potential. Vaccine 13:1207-1212. [DOI] [PubMed] [Google Scholar]
- 19.Jain, S., P. van Ulsen, I. Benz, M. A. Schmidt, R. Fernandez, J. Tommassen, and M. B. Goldberg. 2006. Polar localization of the autotransporter family of large bacterial virulence proteins. J. Bacteriol. 188:4841-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kortekaas, J., S. A. Müller, P. Ringler, M. Gregorini, V. E. Weynants, L. Rutten, M. P. Bos, and J. Tommassen. 2006. Immunogenicity and structural characterisation of an in vitro folded meningococcal siderophore receptor (FrpB, FetA). Microbes Infect. 8:2145-2153. [DOI] [PubMed] [Google Scholar]
- 21.Legrain, M., V. Mazarin, S. W. Irwin, B. Bouchon, M. J. Quentin-Millet, E. Jacobs, and A. B. Schryvers. 1993. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130:73-80. [DOI] [PubMed] [Google Scholar]
- 22.Manning, D. S., D. K. Reschke, and R. C. Judd. 1998. Omp85 proteins of Neisseria gonorrhoeae and Neisseria meningitidis are similar to Haemophilus influenzae D-15-Ag and Pasteurella multocida Oma87. Microb. Pathog. 25:11-21. [DOI] [PubMed] [Google Scholar]
- 23.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moe, G. R., P. Zuno-Mitchell, S. N. Hammond, and D. M. Granoff. 2002. Sequential immunization with vesicles prepared from heterologous Neisseria meningitidis strains elicits broadly protective serum antibodies to group B strains. Infect. Immun. 70:6021-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nedelec, J., J. Boucraut, J. M. Garnier, D. Bernard, and G. Rougon. 1990. Evidence for autoimmune antibodies directed against embryonic neural cell adhesion molecules (N-CAM) in patients with group B meningitis. J. Neuroimmunol. 29:49-56. [DOI] [PubMed] [Google Scholar]
- 27.Noah, N., and B. Henderson. 2002. Surveillance of bacterial meningitis in Europe 1999/2000. Communicable Disease Surveillance Centre, London, United Kingdom.
- 28.Norheim, G., A. Aase, D. A. Caugant, E. A. Høiby, E. Fritzsønn, T. Tangen, P. Kristiansen, U. Heggelund, and E. Rosenqvist. 2005. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine 23:3762-3774. [DOI] [PubMed] [Google Scholar]
- 29.Oster, P., D. Lennon, J. O'Hallahan, K. Mulholland, S. Reid, and D. Martin. 2005. MeNZB: a safe and highly immunogenic tailor-made vaccine against the New Zealand Neisseria meningitidis serogroup B disease epidemic strain. Vaccine 23:2191-2196. [DOI] [PubMed] [Google Scholar]
- 30.Pajón, R., G. Chinea, E. Marrero, D. Gonzalez, and G. Guillén. 1997. Sequence analysis of the structural tbpA gene: protein topology and variable regions within neisserial receptors for transferrin iron acquisition. Microb. Pathog. 23:71-84. [DOI] [PubMed] [Google Scholar]
- 31.Peak, I. R. A., Y. Srikhanta, M. Dieckelmann, E. R. Moxon, and M. P. Jennings. 2000. Identification and characterisation of a novel conserved outer membrane protein from Neisseria meningitidis. FEMS Immunol. Med. Microbiol. 28:329-334. [DOI] [PubMed] [Google Scholar]
- 32.Perrett, K. P., and A. J. Pollard. 2005. Towards an improved serogroup B Neisseria meningitidis vaccine. Expert Opin. Biol. Ther. 5:1611-1625. [DOI] [PubMed] [Google Scholar]
- 33.Pollard, A. J. 2004. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr. Infect. Dis. J. 23:S274-S279. [PubMed] [Google Scholar]
- 34.Poolman, J., and F. X. Berthet. 2001. Alternative vaccine strategies to prevent serogroup B meningococcal diseases. Vaccine 20:S24-S26. [DOI] [PubMed] [Google Scholar]
- 35.Poolman, J. T., C. Feron, G. Desquesne, P. A. Denoël, S. Dessoy, K. K. Goraj, D. E. Janssens, S. Kummert, Y. Lobet, E. Mertens, D. Y. Monnom, P. Momin, N. Pépin, J.-L. Ruelle, J. J. Thonnard, V. G. Verlant, P. Voet, and F. X. Berthet. 2002. Outer membrane vesicles and other options for a meningococcal B vaccine, p. 135-149. In C. Ferreirós, M. T. Criado, and J. Vázquez (ed.), Emerging strategies in the fight against meningitis: molecular and cellular aspects. Horizon Scientific Press, Wymondham, Norfolk, United Kingdom.
- 36.Rokbi, B., G. Renauld-Mongenie, M. Mignon, B. Danve, D. Poncet, C. Chabanel, D. A. Caugant, and M. J. Quentin-Millet. 2000. Allelic diversity of the two transferrin binding protein B gene isotypes among a collection of Neisseria meningitidis strains representative of serogroup B disease: implication for the composition of a recombinant TbpB-based vaccine. Infect. Immun. 68:4938-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Rønnild, G. Bjune, and H. Nøkleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarselli, M., R. Rappuoli, and V. Scarlato. 2001. A common conserved amino acid motif module shared by bacterial and intercellular adhesins: bacterial adherence mimicking cell cell recognition? Microbiology 147:250-252. [DOI] [PubMed] [Google Scholar]
- 39.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson, P., P. Williams, and E. Griffiths. 1992. Common antigenic domains in transferrin-binding protein 2 of Neisseria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae type b. Infect. Immun. 60:2391-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tappero, J. W., R. Lagos, A. M. Ballesteros, B. Plikaytis, D. Williams, J. Dykes, L. L. Gheesling, G. M. Carlone, E. A. Høiby, J. Holst, H. Nøkleby, E. Rosenqvist, G. Sierra, C. Campa, F. Sotolongo, J. Vega, J. Garcia, P. Herrera, J. T. Poolman, and B. A. Perkins. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520-1527. [DOI] [PubMed] [Google Scholar]
- 42.Thompson, S. A., and P. F. Sparling. 1993. The RTX cytotoxin-related FrpA protein of Neisseria meningitidis is secreted extracellularly by meningococci and by HlyBD+ Escherichia coli. Infect. Immun. 61:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vandeputte-Rutten, L., M. P. Bos, J. Tommassen, and P. Gros. 2003. Crystal structure of neisserial surface protein A (NspA), a conserved outer membrane protein with vaccine potential. J. Biol. Chem. 278:24825-24830. [DOI] [PubMed] [Google Scholar]
- 44.van Ulsen, P., L. van Alphen, C. T. P. Hopman, A. van der Ende, and J. Tommassen. 2001. In vivo expression of Neisseria meningitidis proteins homologous to the Haemophilus influenzae Hap and Hia autotransporters. FEMS Immunol. Med. Microbiol. 32:53-64. [DOI] [PubMed] [Google Scholar]
- 45.Voulhoux, R., M. P. Bos, J. Geurtsen, M. Mols, and J. Tommassen. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262-265. [DOI] [PubMed] [Google Scholar]
- 46.Wong, S., D. Lennon, C. Jackson, J. Stewart, S. Reid, S. Crengle, S. Tilman, I. Aaberge, J. O'Hallahan, P. Oster, K. Mulholland, and D. Martin. 2007. New Zealand epidemic strain meningococcal B outer membrane vesicle vaccine in children aged 16-24 months. Pediatr. Infect. Dis. J. 26:345-350. [DOI] [PubMed] [Google Scholar]