Abstract
The ehrlichiae express variable outer membrane proteins (OMPs) that play important roles in both pathogenesis and host defense. Previous studies revealed that OMPs are immunodominant B-cell antigens and that passive transfer of anti-OMP antibodies can protect SCID mice from fatal ehrlichial infection. In this study, we used a model of fatal monocytotropic ehrlichiosis caused by Ehrlichia bacteria from Ixodes ovatus (IOE) to determine whether OMP immunization could generate protective immunity in immunocompetent mice. Immunization of C57BL/6 mice with a purified recombinant OMP expressed by IOE omp19 generated protection from fatal IOE infection and elicited robust humoral and CD4 T-cell responses. To identify CD4 T-cell epitopes within OMPs, we performed enzyme-linked immunospot analyses for gamma interferon (IFN-γ) production using a panel of overlapping 16-mer peptides from IOE OMP-19. Five immunoreactive peptides comprising residues 30 to 45, 77 to 92, 107 to 122, 197 to 212, and 247 to 264 were identified; the strongest response was generated against OMP-19107-122. Most of the peptides are conserved between E. muris and E. chaffeensis OMP-19, and they elicited IFN-γ production in CD4 T cells from E. muris-infected mice, indicating that T-cell epitope cross-reactivity likely contributes to heterologous immunity. Accordingly, CD4 T-cell responses to both OMP-19 and OMP-19107-122 were of greater magnitude following high-dose IOE challenge of mice that had been immunized by prior infection with E. muris. Our studies cumulatively identify B- and T-cell epitopes that are associated with protective homologous and heterologous immunity during ehrlichial infection.
Ehrlichial infections are a significant cause of morbidity in both humans and animals throughout the world. Although it is likely that many immune mechanisms contribute to host defense in these infections, it is now clear that antibodies play important roles in immunity. Outer membrane proteins (OMPs) are major targets of protective antibodies, and immunodominant B-cell epitopes have been located in hypervariable regions of these antigens (11).
T cells can also play an important role(s) in ehrlichial immunity, including the secretion of gamma interferon (IFN-γ) (2). However, the identification of T-cell antigens and epitopes utilized during protective immune responses has been more challenging. Studies of a closely related rickettsia, Anaplasma marginale, have identified T-cell epitopes in major surface protein 2 (MSP2) that are associated with protective immunity in cattle (4, 6). T-cell epitopes identified in MSPs in these studies were located in both conserved and variable regions (1, 5). These data suggest that ehrlichial OMPs also express immunodominant B- and T-cell antigens and are thus potential vaccine candidates. In one study, the immunization of BALB/c mice with Erlichia chaffeensis OMP-19 prevented blood-borne E. chaffeensis infection (17), although the B- and/or T-cell epitopes involved in protection were not identified.
In this study, we addressed a possible role for OMPs in protective immunity in a model of fatal ehrlichiosis. Such an infection model has been described for Ehrlichia bacteria from Ixodes ovatus (IOE), which cause fatal monocytotropic ehrlichiosis within about 12 days of infection (9, 19). Unlike many ehrlichiae, such as E. chaffeensis, IOE causes fatal infection in immunocompetent mouse strains. Moreover, it is possible to generate immunity against fatal IOE infection following prior infection with the closely related ehrlichia E. muris (8). Protection was associated in the latter case with high OMP-19 antibody responses (8, 22). A role for T cells in protective immunity induced by heterologous infection is expected but has not yet been fully established.
Here we examined whether immunization with a recombinant OMP known to be expressed during ehrlichial infection, delivered with adjuvant, could mediate protection against fatal IOE challenge. Using this approach, mice were successfully immunized against fatal IOE infection, and the immunization was associated with robust humoral and cellular immune responses. Because T-cell epitopes recognized in the mouse have not been identified in ehrlichial infections, we have performed a characterization of OMP-19 T-cell epitope utilization following OMP immunization and in E. muris infection. These data, along with our previous studies (11), provide a complete characterization of both the T- and B-cell epitopes of a major immunodominant ehrlichial antigen of particular interest for studies of host defense and for vaccine development.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were obtained from the Jackson Laboratories, Bar Harbor, ME, and were handled in accordance with institutional guidelines for animal welfare. Institutional standards for animal welfare did not permit the use of death as an end point in the infection experiments, so mice were routinely sacrificed when deemed moribund.
Bacterial infections.
Infections were performed as described previously (2). Mice were inoculated via the peritoneum. Quantitative PCR was used to determine the bacterial copy number in the frozen aliquots, as described previously (2). We made the simplifying assumption that copy number and numbers of viable bacteria were equivalent in our experimental model. Mice were challenged with IOE at least 4 weeks following E. muris immunization. In vitro infections with E. chaffeensis were performed using bacteria obtained from infected spleen cells from SCID mice (11).
Cloning and production of IOE OMP-19.
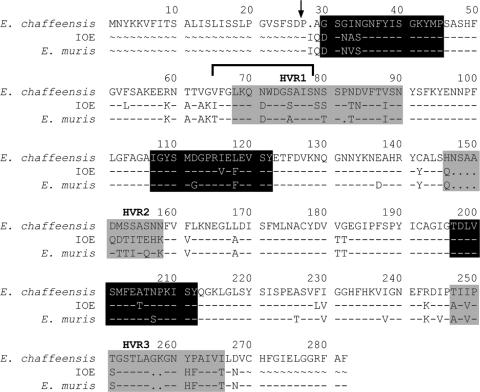
Cloning, production, and purification of IOE and E. chaffeensis OMP-19 recombinant proteins have been described previously (11, 22). The recombinant IOE OMP-19 lacked 26 amino-terminal leader residues and 13 carboxy-terminal residues relative to the E. chaffeensis and E. muris OMP-19 sequences (see Fig. 7), as the sequence of the entire IOE OMP-19 gene was not available.
FIG. 7.
Distribution of major B- and T-cell epitopes in OMP-19. B- and T-cell epitopes identified in OMP-19 are shown in a sequence alignment of the three closely related ehrlichiae used in this study. The B-cell epitope was identified previously (11) and is indicated by the horizontal bracket. The hypervariable regions are highlighted in gray, and T-cell epitopes are shown in black. The two major epitopes (OMP-1930-45 and OMP-19107-122) and a third epitope recognized by a T-cell hybridoma (OMP-19197-212) are shown. The arrow indicates the putative N-terminal signal sequence cleavage site. OMP77-92 and OMP247-264 were omitted from the schematic because they were weakly immunogenic and were not recognized by any of the hybridomas generated from immunized mice.
Enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assays were performed as described previously (11). The reciprocal antibody titers were assigned by determining the highest dilution of antibody that exhibited an A405 greater than 0.1 after subtracting the background absorbance. The background A405 values when using normal sera were typically less than 0.1.
Peptides.
Forty-six overlapping 16-mer peptides spanning the truncated amino- to carboxy-terminal residues of IOE OMP-19 were synthesized as a peptide array by New England Peptide LLC (Fitchburg, MA). The peptides overlapped by 11 amino acids. The peptides were solubilized in 50% acetonitrile to a final concentration of 2.5 mg/ml and stored at −20°C. The amino acid sequences of the peptides are shown in Table S1 in the supplemental material. The OMP-19 residue 107 to 122 (OMP-19107-122) truncation variants shown in Table 3 were also synthesized as part of the peptide array. OMP-19107-122 and OMP-19197-212 were resynthesized using conventional peptide synthesis methodologies to confirm activity.
TABLE 3.
Recognition of truncation variants of IOE OMP-19107-122 by the hybridoma BOI-6
| Peptide | Amino acid sequence | Relative responsea |
|---|---|---|
| 1 | IGYSMDGPRVEFEVSYb | 1.00 |
| 2 | GYSMDGPRVEFEVSY | 1.33 |
| 3 | YSMDGPRVEFEVSY | 1.38 |
| 4 | SMDGPRVEFEVSY | 1.36 |
| 5 | MDGPRVEFEVSY | 0.35 |
| 6 | DGPRVEFEVSY | 0.04 |
| 7 | GPRVEFEVSY | 0.17 |
| 8 | IGYSMDGPRVEFEVS | 1.47 |
| 9 | IGYSMDGPRVEFEV | 1.45 |
| 10 | IGYSMDGPRVEFE | 1.12 |
| 11 | IGYSMDGPRVEF | 0.003 |
| 12 | IGYSMDGPRVE | 0.001 |
| 13 | IGYSMDGPRV | 0.001 |
IL-2 production in response to the truncated peptides is indicated relative to the response achieved using the full-length peptide (1).
The underlined sequence indicates the minimal T-cell epitope required for T-cell activation.
OMP immunization and challenge infections.
Mice were immunized with 200 μg of purified OMP-19 in complete Freund's adjuvant (CFA) by subcutaneous injection at two or three sites on the back (100 μg per injection site). The mice received a fatal IOE challenge infection (500 bacteria; 2× 50% lethal dose [LD50]) 30 days postimmunization and were monitored thereafter for morbidity and bacterial infection.
Generation of T-cell hybridomas.
Axial and brachial lymph nodes (LNs) were harvested from IOE OMP-19-immunized mice 10 to 12 days following immunization, the tissues were dissociated, and the pooled cells were cultured at a concentration of 4 × 106 cells/ml with recombinant IOE OMP-19 (10 μg/ml) in a volume of 30 ml complete tumor medium (14). Four days later, the cells were harvested, washed with Hanks’ balanced salt solution, and cultured in complete tumor medium with interleukin-2 (IL-2) (20 units/ml). Eight days following the initiation of the culture, the cells underwent fusion with the T-cell thymoma BWα−/β−, using standard methods (14). Hybridomas were monitored for growth and were screened for responses to OMP-19-specific peptides by using an IL-2 assay as described previously (14). IL-2 was quantitated by measurement of [3H]thymidine incorporation by the HT-2 indicator cell line (14).
T-cell proliferation assay.
T cells were purified from the spleens of immunized mice by magnetic-bead cell selection as described previously (2). The purified cells were cultured with recombinant OMP-19 (10 μg/ml) in the presence of autologous spleen antigen-presenting cells (APCs) from uninfected C57BL/6 mice. After 72 h, 0.5 mCi of [3H]thymidine (New England Nuclear, Boston, MA) was added to the cultures, and 24 h later, cells were harvested and [3H]thymidine incorporation was measured using a 1205 beta plate counter (Wallac, Gaithersburg, MD).
ELISPOT.
The numbers of antigen-specific CD4 T cells in spleens or LNs were determined by using a standard enzyme-linked immunospot (ELISPOT) assay (15). Specific responses were quantitated by subtracting the number of spots, if any, detected in the absence of the specific antigen.
Statistical analyses.
Statistical analyses of the challenge studies were performed by using a log rank test. Other data were analyzed by using a one-tailed Mann-Whitney test with a confidence interval of 95%. The data were analyzed using Prism software (GraphPad Software, Inc.).
RESULTS
Recombinant OMPs mediate protection against fatal IOE infection.
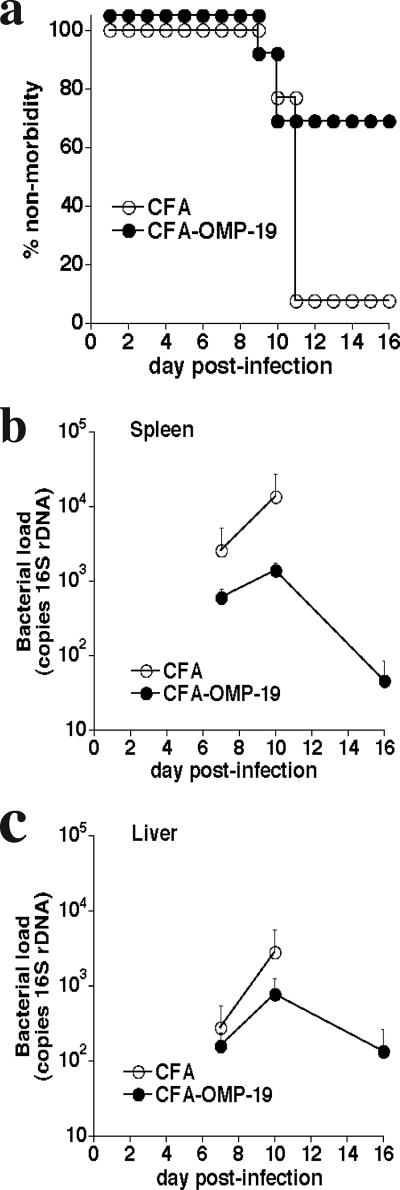
Although heterologous ehrlichial infection can generate protective immunity to fatal IOE challenge infection in immunocompetent mice, it was not known whether subunit vaccination could also be effective. In our studies, OMP-19 was chosen because this antigen is expressed and has been shown to be a target of protective antibody responses following E. chaffeensis infection (11, 20). Recombinant IOE OMP-19 was used to immunize mice subcutaneously in the presence of CFA. The mice were challenged at least 30 days later with 2× LD50 of IOE (approximately 500 bacteria [3]). Mice immunized with IOE OMP-19/CFA, but not CFA alone, were protected from IOE challenge (Fig. 1a). Protection was associated with an approximately 1-log reduction in bacterial numbers in the spleen on day 10 postinfection (Fig. 1b). Bacterial colonization was detected in the livers of both immunized and control mice but decreased to near-background levels in the surviving immunized mice by day 16 postinfection (Fig. 1c). Mice that survived initial IOE challenge exhibited significantly lower bacterial infection within 16 days postinfection and survived indefinitely (data not shown).
FIG. 1.
Immunization with IOE OMP-19 provided protection against fatal IOE challenge. (a) C57BL/6 mice were immunized with 200 μg of IOE OMP-19 in CFA or with CFA alone (control). Thirty days later, the mice were challenged with a high-dose (2× LD50) IOE inoculum, and morbidity was monitored. Data from three separate experiments of identical design were pooled and represent 13 mice per group. The differences between the two groups were significant (P = 0.0039) as determined by using a log rank test. (b, c) Level of bacterial infection in the spleens and livers of immunized and control mice was determined on the indicated days postinfection. Standard deviations of the mean are shown. Four mice were used per group. The data for the spleens were statistically significant on both day 7 (P = 0.047) and day 10 (P = 0.002) postinfection. Data from the livers were not significant on days 7 and 10 postinfection.
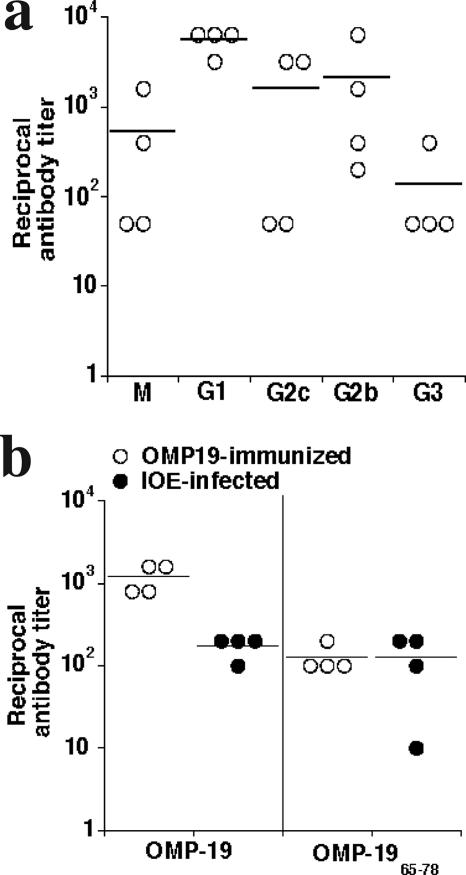
Immunity was associated with strong humoral responses against IOE OMP-19.
The ehrlichiae induce strong antibody responses in many different hosts, and we have demonstrated that antibodies can be highly effective against the ehrlichiae (21, 22). Therefore, we examined whether high antibody titers were associated with protective immunity following OMP-19 vaccination. Indeed, antibody titers to OMP-19 were robust, with reciprocal titers often exceeding 1,000 (Fig. 2a). Immunoglobulin M (IgM) and antibodies of all IgG subclasses were detected (Fig. 2a). We also examined whether the immunodominant antibody epitope previously described for E. chaffeensis OMP-19 (10) was recognized in the IOE OMP-19-immunized mice. This determination was possible because the antibody epitope (contained within residues 65 to 78) was largely conserved between IOE and E. chaffeensis OMP-19 (see Fig. 7). The E. chaffeensis peptide OMP-1965-78 was detected using serum from IOE OMP-19-immunized mice and IOE-infected mice (Fig. 2b), indicating that both immunization and infection elicit responses to this immunodominant antibody epitope.
FIG. 2.
Immunization generated high OMP-19 serum antibody titers. (a) Sera from four C57BL/6 mice were collected 30 days after immunization with OMP-19, and the serum antibody titer was measured for each of the Ig classes and subclasses. Background obtained using normal sera (typically A405 < 0.1) was subtracted, and only absorbances greater than 0.1 were considered significant. (b) An immunodominant peptide, OMP-1965-78, identified during E. chaffeensis infection was also recognized by polyclonal sera from both IOE OMP-19-immunized and IOE-infected mice. Antibody titers for individual mice are shown; horizontal lines indicate means.
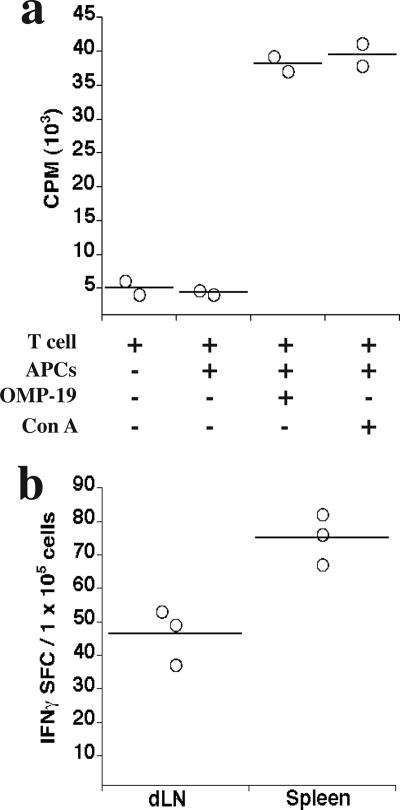
T-cell responses were elicited by OMP-19 immunization.
T-cell responses are usually associated with protective immunity against intracellular bacteria, so we next examined T-cell responses to OMP-19. T-cells purified from the spleens of IOE OMP-19-immunized mice exhibited strong proliferative responses to OMP-19 in vitro (Fig. 3a). Because IFN-γ production is required for immunity following low-dose IOE infection, we also enumerated CD4 T-cell responses by using a conventional ELISPOT analysis (Fig. 3b). IFN-γ production was identified in both spleen and draining LN (brachial) CD4 T cells 10 days following infection. This amounted to approximately 45 to 75 spot-forming cells per 1 × 105 CD4 T cells in these tissues (Fig. 3b), or 0.045 to 0.075% of the total CD4 T cells detected.
FIG. 3.
T cells from OMP-19-immunized mice proliferated and produced IFN-γ in response to specific antigens. (a) T cells were purified by negative magnetic selection from spleens of C57BL/6 mice after 10 days following IOE OMP-19 immunization. Cells were cultured with or without APCs from unimmunized mice in the presence or absence of IOE OMP-19 (10 μg/ml). Concanavalin A was used as positive control. Cells from three immunized mice were pooled and were cultured in duplicate. Proliferative responses using T cells from unimmunized mice were less than 2 × 103 cpm. (b) The frequencies of IFN-γ-producing OMP-19-specific CD4 T cells in spleens and draining brachial lymph nodes (dLN) from IOE OMP-19-immunized mice were determined by ELISPOT assay. Cells from three OMP-19-immunized mice were pooled and cultured in triplicate in the presence of OMP-19 (10 μg/ml). Data were obtained following subtraction of background from cells cultured in the absence of added antigen. Responses using CD4 T cells from unimmunized mice were at background levels. Horizontal lines indicate means. SFC, spot-forming cells.
Identification of T-cell epitopes in IOE OMP-19.
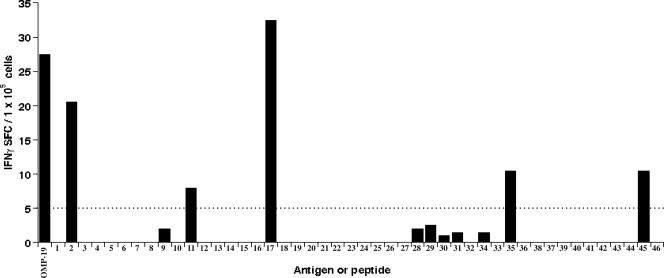
As murine ehrlichial CD4 T-cell epitopes have not been characterized, we next sought to identify the exact epitopes recognized within OMP-19. To this end, we synthesized a panel of 46 overlapping peptides spanning the entire OMP-19 sequence carboxy terminal to the signal sequence cleavage site (see Fig. 7; see also Table S1 in the supplemental material) and used these in CD4 T-cell IFN-γ ELISPOT assays. The peptides were synthesized as 16-mers and overlapped by 11 residues. Five immunogenic peptides were identified that elicited specific responses by spleen T cells (Fig. 4). These included the peptides comprised of the following amino acid residues: 30 to 45, 77 to 92, 107 to 122, 197 to 212, and 247 to 264 (Fig. 4; see also Table S1 in the supplemental material). The strongest response was generated against OMP-19107-122. These data revealed that multiple epitopes were recognized by CD4 T cells following OMP immunization. Peptide specificity was confirmed by resynthesis of OMP-19107-122 and OMP-19197-212 (data not shown).
FIG. 4.
Identification of immunoreactive peptides in IOE OMP-19. The frequencies of IFN-γ-producing OMP-19 peptide-specific T cells in spleens collected from IOE OMP-19-immunized mice were determined by ELISPOT assay. Spleen cells from two immunized mice were pooled and cultured in duplicate in the presence of either OMP-19 (10 μg/ml) or a panel of overlapping 16-amino-acid IOE OMP-19 peptides. Data shown were obtained after subtraction of background; none of the peptides elicited responses from CD4 T cells obtained from unimmunized mice. Counts above the dotted line intercepting the value of 5 SFC/1 × 105 cells were considered significant. The sequences of the peptides are available in Table S1 in the supplemental material. SFC, spot-forming cells.
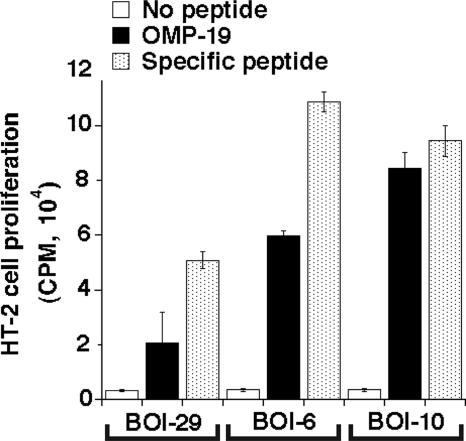
In parallel studies, six T-cell hybridomas that recognized OMP-19 were generated from similarly immunized mice (Table 1). Further analyses demonstrated that, together, the hybridomas recognized three of the peptides identified in the ELISPOT studies (comprising residues 30 to 45, 107 to 122, and 197 to 212) (Table 1). Three hybridomas were generated that recognized OMP-19107-122. Representative hybridomas generated robust production of IL-2 in response to their cognate peptides (Fig. 5). T-cell hybridomas BOI-6, -10, and -29 in most cases recognized the homologous peptides from both E. muris and E. chaffeensis and/or infected APCs, indicating that the elicited T cells were cross-reactive (Table 2). One exception was peptide OMP-19197-212. The hybridoma generated against IOE OMP-19197-212, BOI-10, recognized the variant encoded by E. chaffeensis, but not the E. muris variant. This difference is likely due to the asparagine-to-serine substitution at position 207, as serine is the only residue that is not found in the corresponding peptides encoded by the other ehrlichiae. Thus, some T cells that recognize IOE OMP-19 do not recognize the homologous epitope expressed by the related ehrlichiae (see Fig. 7).
TABLE 1.
Stimulation of IL-2 production from T-cell hybridomas by IOE OMP-19-derived peptides
| T-cell hybridoma | IL-2 production with peptides comprised of residuesa
|
||||
|---|---|---|---|---|---|
| 30-45 | 77-92 | 107-122 | 197-212 | 247-264 | |
| BOI-6 | − | − | + | − | − |
| BOI-10 | − | − | − | + | − |
| BOI-14 | − | − | + | − | − |
| BOI-29 | + | − | − | − | − |
| BOI-67 | + | − | − | − | − |
| BOI-72 | − | − | + | − | − |
+, a more than 2-fold increase in IL-2 production relative to that in cultures that lacked the specific peptide; −, response was not significant.
FIG. 5.
OMP-19 peptide specificity of T-cell hybridomas generated following IOE OMP-19 immunization. Hybridomas produced IL-2 following incubation with OMP-19 or OMP-19 peptides comprising residues 30 to 45 (for BOI-29), 107 to 122 (for BOI-6), and 197 to 212 (for BOI-10). IL-2 production was determined by measuring [3H]thymidine incorporation by the indicator cell line HT-2. Standard deviations are indicated.
TABLE 2.
Cross-recognition of related ehrlichiae by IOE OMP-19-specific T-cell hybridomas
| T-cell hybridoma | IL-2 production witha:
|
|||||
|---|---|---|---|---|---|---|
| Peptide fromb:
|
Peptide from APCs infected withc:
|
|||||
| IOE | E. muris | E. chaffeensis | IOE | E. muris | E. chaffeensis | |
| BOI-6 | + | + | + | + | + | + |
| BOI-10 | + | − | + | + | − | + |
| BOI-29 or -67 | + | +d | +e | + | + | + |
+, a greater than 2-fold increase in IL-2 production relative to cultures that lacked specific peptide; −, response was not significant.
The hybridomas were incubated with spleen APCs and the cognate peptides encoded by the indicated ehrlichiae.
IL-2 production by the hybridomas was measured following incubation with in vitro- or in vivo-infected APCs.
The IOE peptide is identical to the E. muris variant, with the exception of a conservative Val to Ala substitution at position 33.
Recombinant E. chaffeensis OMP-19 was utilized because the peptide was unavailable.
Since OMP-19107-122 was found to be a dominant peptide in both of the above analyses, we performed studies using peptide truncation variants to identify the core T-cell epitope within this sequence, which is recognized by the hybridoma BOI-6. This core epitope was found to comprise residues 110 to 119 (Table 3; Fig. 7).
In vivo CD4 T-cell responses to OMP-19 during infection.
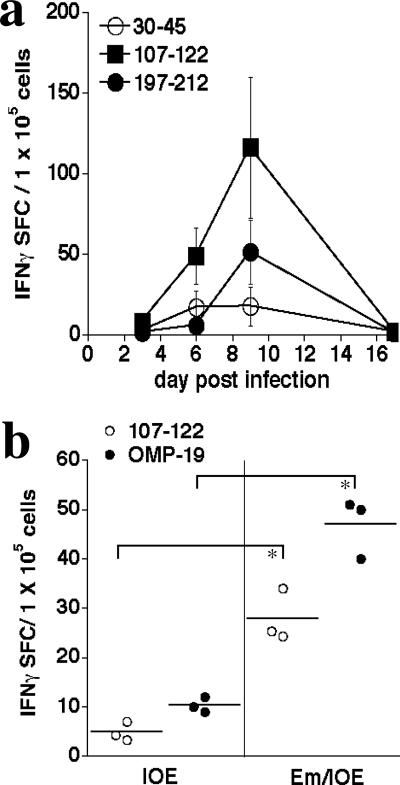
To address whether the CD4 T-cell epitopes recognized following OMP-19 vaccination were also targets for T cells following infection, we infected mice intraperitoneally with E. muris and monitored T-cell IFN-γ responses thereafter by ELISPOT assay. In these studies, the corresponding peptides from E. muris OMP-19 were utilized (except for OMP-1930-45, where the IOE peptide was used, as it is identical to the E. muris peptide with the exception of a conservative valine-to-alanine substitution at position 33 [see Fig. 7]). OMP-19-reactive T cells were first detected on day 6 postinfection and increased in number until at least day 9 postinfection (Fig. 6a). Each of the three peptides that we analyzed elicited T-cell responses, although the greatest number of responsive T cells were found to recognize OMP-19107-122, as was observed in the vaccinated mice (Fig. 4). By day 16 postinfection, the T-cell responses were no longer detectable. These data indicate that, although E. muris establishes a low-level, persistent infection (18; data not shown), persistent infection may occur in the absence of detectable spleen T-cell responses to OMP-19. Although the hybridoma BOI-10 that recognizes IOE OMP-19197-212 did not recognize E. muris OMP-19197-212, other CD4 T cells elicited during E. muris infection did recognize this peptide, indicating that some, but not all, T cells elicited by IOE OMP-19 were cross-reactive. These studies indicate that epitopes elicited by vaccination are also generated during normal infection.
FIG. 6.
Recognition of OMP-19 peptide antigens by CD4 T cells during ehrlichial infection. (a) C57BL/6 mice were infected with E. muris, and the frequencies of IFN-γ-producing OMP-19 peptide-specific CD4 T cells in spleens were determined by ELISPOT assay on the indicated days postinfection. Mean SFC (spot-forming cell) numbers and standard deviations are indicated. Three mice were used per group; data were obtained after subtraction of background obtained from cultures that did not contain added peptides. (b) ELISPOT analysis of OMP-19- and OMP-19107-122-specific CD4 T-cell responses in naive or E. muris-immunized mice 8 days after challenge with high-dose IOE (500 bacteria; 2× LD50). The differences between E. muris-immunized (Em-IOE) and nonimmunized (IOE) IOE-infected mice were statistically significant. *, P = 0.05.
As prior E. muris infection has been shown to generate protective immunity to high-dose IOE challenge (8), we next tested whether enhanced cross-reactive T-cell responses were generated following high-dose IOE challenge in the E. muris-immunized mice. ELISPOT analyses revealed elevated CD4 T-cell responses against both IOE OMP-19 and IOE OMP-19107-122 in the immunized mice (Fig. 6b). OMP-19-specific responses were largely undetectable in the nonimmunized IOE-infected mice, perhaps due to the relatively low IOE challenge inoculum (500 bacteria). These data suggest that the enhanced ELISPOT assay responses were due to the presence of effector/memory CD4 T cells in the E. muris-immunized mice.
DISCUSSION
OMPs can generate protective immunity against fatal ehrlichial infection.
This study demonstrates that protective immunity can be generated in mice following immunization with an ehrlichial OMP. A previous study indicated that OMP-19 administration could inhibit E. chaffeensis infection, although this ehrlichia is not highly virulent in immunocompetent mice (7). In contrast, protection in our studies was generated against infection with the highly pathogenic IOE, suggesting that significant immunity was generated by using our immunization strategy. In related studies, purified outer membranes from Anaplasma phagocytophila were utilized to immunize cattle, and such immunity was associated with serological responses to anaplasma MSPs (6). Although the protection described in the present paper was not achieved in all immunized mice, the data nevertheless suggest that immunization with OMPs by themselves can be an effective immunogen. It is possible that multicomponent vaccines or additional immunizations could be a useful strategy to improve immunity (12). Our vaccination strategy utilized CFA, so additional studies will be required to evaluate the efficacy of other adjuvants that are available for clinical or veterinary use.
Immunity was associated with robust humoral and cellular responses.
We reported previously that humoral immunity can be effective in both immunocompromised and immunocompetent mice (11, 22). Accordingly, OMP-19 immunization was associated with high titers of antibodies, which likely contributed to protection. Although our previous studies of OMP-19 antibodies elicited during E. chaffeensis infection identified several highly effective IgG2cs (formerly indicated as IgG2a), but not IgG1 (11, 13), all IgG isotypes (IgG1, IgG2c, IgG2b, and IgG3) were elicited in the IOE OMP-19-immunized mice. One explanation for the differences in OMP-19 isotype utilization is that ehrlichial infection, in contrast to the antigen/adjuvant combination used in the present studies, generates qualitatively distinct antibody responses. Our previous studies also identified a major B-cell epitope, OMP-1965-78, that was utilized during E. chaffeensis infection following IOE OMP-19 immunization and IOE infection. Although the OMP-1965-78 B-cell epitope was variable among several E. chaffeensis clinical isolates (16), this region was highly conserved among IOE, E. muris, and E. chaffeensis, suggesting that the latter three ehrlichiae are more closely related than has been believed (Fig. 7). We have not yet determined the role of humoral immunity in protection following IOE OMP-19 immunization, but our previous studies suggest that antibodies and/or B cells play important roles in immunity (11, 22). Not surprisingly, OMP-19 immunization also elicited robust T-cell responses. T cells from immunized mice proliferated in vitro in response to OMP-19, and ELISPOT analyses identified high numbers of antigen-specific T cells in both draining LNs and spleens. Thus, it is likely that both the B- and T-cell responses elicited by vaccination generated protective immunity.
Conserved T-cell epitopes contribute to cross-protection.
This is the first study to identify CD4 T-cell epitopes in an ehrlichia antigen. The three principal epitopes identified and studied here were conserved across IOE, E. muris, and E. chaffeensis (Fig. 7) and exhibited a high degree of similarity within the family of p28 OMPs in E. chaffeensis (data not shown). Thus, OMP-19 immunization may induce cross-protective T-cell immunity against related ehrlichial pathogens that express variable OMPs. Studies of E. muris-infected mice revealed that the epitopes utilized following immunization were also generated during normal infection. Moreover, we obtained evidence of a CD4 T-cell anamnestic response in E. muris-immunized mice following IOE challenge, suggesting that CD4 T cells play a role in protective immunological memory. Thus, our data reveal that subunit vaccination against the ehrlichiae is a viable strategy and identify T-cell epitopes that will facilitate additional studies of the naive and memory CD4 T-cell responses following ehrlichia infection.
Supplementary Material
Acknowledgments
We thank the Wadsworth Center Immunology and Peptide Synthesis Core Facilities for essential support.
The work was supported by U.S. Public Health Service grant R01 AI064678 to G.M.W.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 13 August 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Abbott, J. R., G. H. Palmer, C. J. Howard, J. C. Hope, and W. C. Brown. 2004. Anaplasma marginale major surface protein 2 CD4+-T-cell epitopes are evenly distributed in conserved and hypervariable regions (HVR), whereas linear B-cell epitopes are predominantly located in the HVR. Infect. Immun. 72:7360-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitsaktsis, C., J. Huntington, and G. M. Winslow. 2004. Production of interferon-g by CD4 T cells is essential for resolving ehrlichia infection. J. Immunol. 172:6894-6901. [DOI] [PubMed] [Google Scholar]
- 3.Bitsaktsis, C., and G. Winslow. 2006. Fatal recall responses mediated by CD8 T cells during intracellular bacteria infection. J. Immunol. 177:4644-4651. [DOI] [PubMed] [Google Scholar]
- 4.Brown, W. C., K. A. Brayton, C. M. Styer, and G. H. Palmer. 2003. The hypervariable region of Anaplasma marginale major surface protein 2 (MSP2) contains multiple immunodominant CD4(+) T lymphocyte epitopes that elicit variant-specific proliferative and IFN-gamma responses in MSP2 vaccinates. J. Immunol. 170:3790-3798. [DOI] [PubMed] [Google Scholar]
- 5.Brown, W. C., T. C. McGuire, D. Zhu, H. A. Lewin, J. Sosnow, and G. H. Palmer. 2001. Highly conserved regions of the immunodominant major surface protein 2 of the genogroup II ehrlichial pathogen Anaplasma marginale are rich in naturally derived CD4(+) T lymphocyte epitopes that elicit strong recall responses. J. Immunol. 166:1114-1124. [DOI] [PubMed] [Google Scholar]
- 6.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganta, R. R., C. Cheng, M. J. Wilkerson, and S. K. Chapes. 2004. Delayed clearance of Ehrlichia chaffeensis infection in CD4+ T-cell knockout mice. Infect. Immun. 72:159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismail, N., L. Soong, J. W. McBride, G. Valbuena, J. P. Olano, H. M. Feng, and D. H. Walker. 2004. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 172:1786-1800. [DOI] [PubMed] [Google Scholar]
- 9.Ismail, N., H. L. Stevenson, and D. H. Walker. 2006. Role of tumor necrosis factor alpha (TNF-α) and interleukin-10 in the pathogenesis of severe murine monocytotropic ehrlichiosis: increased resistance of TNF receptor p55- and p75-deficient mice to fatal ehrlichial infection. Infect. Immun. 74:1846-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, J. S., F. Chu, A. Reilly, and G. M. Winslow. 2002. Antibodies highly effective in SCID mice during infection by the intracellular bacterium Ehrlichia chaffeensis are of picomolar affinity and exhibit preferential epitope and isotype utilization. J. Immunol. 169:1419-1425. [DOI] [PubMed] [Google Scholar]
- 11.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, F. K. Chu, and G. Winslow. 2001. Outer membrane protein specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 12.Lopez, J. E., G. H. Palmer, K. A. Brayton, M. J. Dark, S. E. Leach, and W. C. Brown. 2007. Immunogenicity of Anaplasma marginale type IV secretion system proteins in a protective outer membrane vaccine. Infect. Immun. 75:2333-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 14.Mix, D., and G. M. Winslow. 1996. Proteolytic processing activates a viral superantigen. J. Exp. Med. 184:1549-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyahira, Y., K. Murata, D. Rodriguez, J. R. Rodriguez, M. Esteban, M. M. Rodrigues, and F. Zavala. 1995. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J. Immunol. Methods 181:45-54. [DOI] [PubMed] [Google Scholar]
- 16.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 69:2083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of E. chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olano, J. P., G. Wen, H. M. Feng, J. W. McBride, and D. H. Walker. 2004. Histologic, serologic, and molecular analysis of persistent ehrlichiosis in a murine model. Am. J. Pathol. 165:997-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata, S., M. Kawahara, Y. Rikihisa, H. Fujita, Y. Watanabe, C. Suto, and T. Ito. 2000. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 38:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singu, V., H. Liu, C. Cheng, and R. R. Ganta. 2005. Ehrlichia chaffeensis expresses macrophage- and tick cell-specific 28-kilodalton outer membrane proteins. Infect. Immun. 73:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winslow, G. M., E. Yager, K. Shilo, E. Volk, and F. K. Chu. 2000. Antibody-mediated elimination of the obligate intracellular bacterial pathogen Ehrlichia chaffeensis during active infection. Infect. Immun. 68:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yager, E., C. Bitsaktsis, B. Nandi, J. W. McBride, and G. Winslow. 2005. Essential role for humoral immunity during Ehrlichia infection in immunocompetent mice. Infect. Immun. 73:8009-8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.