Abstract
The control of intracellular pathogens such as Mycobacterium tuberculosis is dependent on the activation and maintenance of pathogen-reactive T cells. Dendritic cells (DCs) are the major antigen-presenting cells initiating antimycobacterial T-cell responses in vivo. To investigate if immunization strategies that aim to optimize DC function can improve protective immunity against virulent mycobacterial infection, we exploited the ability of the hematopoietic growth factor Fms-like tyrosine kinase 3 ligand (Flt3L) to expand the number of DCs in vivo. A DNA fusion of the genes encoding murine Flt3L and M. tuberculosis antigen 85B stimulated enhanced gamma interferon (IFN-γ) release by T cells and provided better protection against virulent M. tuberculosis than DNA encoding the single components. Vaccination of mice with a recombinant Mycobacterium bovis BCG strain secreting Flt3L (BCG:Flt3L) led to early expansion of DCs compared to immunization with BCG alone, and this effect was associated with increased stimulation of BCG-reactive IFN-γ-secreting T cells. BCG and BCG:Flt3L provided similar protective efficacies against low-dose aerosol M. tuberculosis; however, immunization of immunodeficient mice revealed that BCG:Flt3L was markedly less virulent than conventional BCG. These results demonstrate the potential of in vivo targeting of DCs to improve antimycobacterial vaccine efficacy.
Tuberculosis (TB) kills 2 million individuals per year and is the greatest cause of death by a single bacterial agent (26). The worldwide incidence of TB continues to rise (26), due in part to the variable efficacy displayed by the only registered vaccine for human use, Mycobacterium bovis bacillus Calmette-Guérin (BCG) (10). This has fuelled the search for more effective anti-TB vaccines, and approaches ranging from DNA vaccines to live, attenuated strains of Mycobacterium tuberculosis have been assessed; the majority of these approaches have failed to deliver improved protective efficacy compared to BCG in animal models (5). A small number of vaccines have recently entered human trials, two of which aim to improve expansion of T cells directed against a single member of the M. tuberculosis antigen 85 (Ag85) complex (15, 24). While these two approaches have shown promise in animal models and initial testing in humans, the fact that no single mycobacterial antigen is recognized by all M. tuberculosis-infected individuals (32) may limit the breadth of coverage delivered by these vaccines within the human population.
One approach to improve anti-TB vaccination is to target components of the immune response required for optimal protective efficacy. We have previously demonstrated that vaccination strategies designed to augment gamma interferon (IFN-γ) production, such as codelivery of the cytokines interleukin-12 (IL-12) and IL-23 during DNA vaccination, significantly improve protection against TB (29, 39). IL-12 is critical for the induction of Th1-like CD4+ cells, and humans and mice lacking the p40 chain of IL-12 or its receptors are highly susceptible to M. tuberculosis infection (2, 6, 8). M. tuberculosis infection of either murine or human dendritic cells (DCs), but not macrophages, polarizes naïve T cells to the Th1 phenotype due to production of IL-12 (11, 14). Infection of mice with BCG and subsequent analysis of DC and macrophage populations revealed that DCs exclusively produced IL-12 and presented antigen to T cells (17). After aerosol infection of mice with M. tuberculosis it was observed that DCs, and not macrophages, migrated specifically to the draining lymph nodes (DLNs) and initiated primary Th1 responses (4). These results indicate that DCs play a pivotal role in priming the adaptive immune response to counter infection with M. tuberculosis. It is possible that limited numbers of DCs at the site of antigen production is one factor hindering development of vaccines against diseases such as TB which are reliant on optimal priming of T-cell responses.
The Fms-like tyrosine kinase 3 ligand (Flt3L) influences the development of multiple hematopoietic lineages, in particular DCs (23). Administration of soluble Flt3L to both mice (21) and humans (22) increases the numbers of DCs in secondary lymphoid organs and blood. We therefore reasoned that codelivery of Flt3L may be a feasible strategy to improve the efficacy of anti-TB vaccines. In this report, we demonstrate that Flt3L, delivered as plasmid DNA fused to the dominant M. tuberculosis Ag85B protein, promotes increased protective immunity against aerosol M. tuberculosis infection in mice. In addition, secretion of Flt3L by a recombinant form of the BCG vaccine augments immunogenicity and improves the safety of BCG in immunodeficient animals without altering the protective efficacy.
MATERIALS AND METHODS
Bacterial strains and media.
M. tuberculosis H37Rv (= ATCC 27294) and M. bovis BCG strain Pasteur were grown in Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with oleic acid-albumin-dextrose-catalase. When required, the antibiotic kanamycin was added at a concentration of 25 μg/ml.
Construction of DNA vaccines and rBCG strains.
The pCDNA3 vector expressing the M. tuberculosis Ag85B gene has been previously described (18). The gene encoding M. tuberculosis Ag85B was amplified from M. tuberculosis genomic DNA and cloned into the BamHI/HindIII sites of pCDNA:mflt3L (16), yielding pFlt-85. Recombinant BCG (rBCG) that secretes soluble murine Flt3L (BCG:Flt3L) was constructed by amplifying the Flt3L gene from pCDNA:mflt3L and cloning it into the mycobacterium-Escherichia coli shuttle vector pJEX65, which allows detection of recombinant proteins by a C-terminal epitope tag (37). BCG containing pJEX65 alone (BCG:Ct) was used as the control strain for vaccination experiments. Vectors were transformed into BCG as previously described (31). BCG strains were grown as described previously (29), and culture supernatants were concentrated approximately 10-fold using a Nanosep 3K Omega centrifugal column (Pall Corporation, Ann Arbor, MI). The total protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Lysates and supernatants were analyzed for expression of recombinant Flt3L by immunoblotting using the anti-myc monoclonal antibody (MAb) 9E10 (Santa Cruz Biotechnology, Santa Cruz, CA) or by a murine Flt3L-specific enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Transfection of HEK 293 cells.
Six-well plates were seeded with 4 × 105 human embryonic kidney HEK 293 cells. DNA constructs were transfected into HEK 293 cells by using FuGene (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions. Supernatant was collected after 48 h, and the Flt3L concentration was determined by an ELISA (R&D Systems).
DC culture and flow cytometry.
DC precursors were derived from murine bone marrow as described previously (9). DC precursors (2 × 105 cells/well) were incubated for 6 days with supernatants from HEK 293-transfected cells or granulocyte-macrophage colony-stimulating factor (2.5 ng/ml; Peprotech, Rocky Hill, NJ) plus IL-4 (5 ng/ml; Peprotech). The expression of cell surface marker proteins was analyzed with a FACSCalibur (BD Biosciences, San Jose, CA) using the CellQuest software (BD Biosciences). Phycoerythrin-conjugated major histocompatibility complex class II (MHC-II), allophycocyanin-conjugated CD11b, biotin-conjugated CD11c, fluorescein isothiocyanate-conjugated B220, and strepavidin-PerCP were all purchased from BD Pharmingen (San Diego, CA).
Animal infections.
For immunogenicity studies, mice were inoculated intramuscularly with plasmid DNA (three inoculations of 100 μg in saline at 2-week intervals) or subcutaneously with BCG strains (5 × 105 CFU in saline), and immune responses were analyzed at the specified time points. IFN-γ release by splenocytes after DNA vaccination was determined by incubating 5 × 105 splenocytes with 3 μg/ml of M. tuberculosis Ag85B protein and 72 h later determining the level of IFN-γ released by ELISA. IFN-γ-secreting T cells were detected by incubating 5 × 105 splenocytes or DLN cells with BCG lysate (1 or 10 μg/ml) and performing an enzyme-linked immunospot assay as previously described (29). For analysis of DC populations, single-cell suspensions were prepared from spleens and DLNs of rBCG-immunized mice by digestion with collagenase and DNase. DCs were identified based on surface expression of CD11c and MHC-II and were further classified by expression of B220.
For assessment of protective efficacy, C57BL/6 mice (five mice per group) were immunized as described above for DNA vaccines and BCG strains, and 6 weeks (DNA) or 12 weeks (BCG) after vaccination mice were challenged with aerosol M. tuberculosis H37Rv using a Middlebrook airborne infection apparatus (Glas-Col, Terre Haute, IN) with an infective dose of approximately 100 viable bacilli per lung. Four weeks after the challenge, the numbers of bacteria in the lungs and spleens were determined using Bacto Middlebrook 7H11 agar.
For survival studies, RAG-1−/− mice were infected intravenously (i.v.) with 1 × 106 CFU of BCG:Flt3L or BCG:Ct. Infected mice were monitored daily and culled if they displayed signs of ill health, including reduced activity, ruffling of fur, and weight loss exceeding 15% of the weight loss of age-matched controls. Survival was calculated using a Kaplan-Meier nonparametric survival plot, and significance was assessed by the Mantel-Cox log rank test.
Statistical analysis.
Statistical analyses of the results from immunological assays and log-transformed bacterial counts were conducted using analysis of variance (ANOVA). Fisher's protected least-significant-difference ANOVA post hoc test was used for pairwise comparison of multigroup data sets. A P value of <0.05 was considered significant.
RESULTS
Ftl3L-Ag85B fusion protein is secreted by plasmid DNA in a functional form.
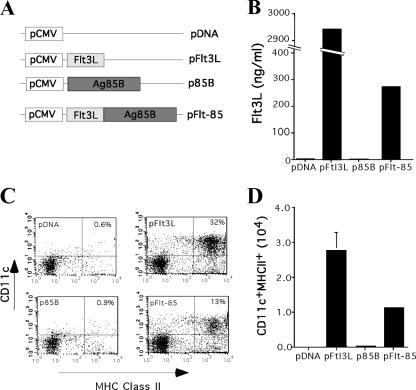
DNA vaccines were constructed which expressed either mouse soluble Flt3L (pFlt3L), the M. tuberculosis Ag85B protein (p85B), or a fusion of the two proteins (pFlt-85) (Fig. 1A). Flt3L could be detected in supernatants from HEK 293 cells transfected with pFlt3L or pFlt-85, although the fusion protein was secreted at lower levels than Flt3L alone (Fig. 1B). In order to determine if Flt3L was secreted in a functional form, supernatants from transfected HEK 293 cells were cultured with mouse bone marrow cells for 6 days. Cells displaying a DC phenotype (CD11c+ MHC-II+) were readily generated from bone marrow cultures supplemented with pFlt3L and pFlt3L-85 supernatants (Fig. 1C and D). The same population was not present in cultures supplemented with supernatants from pCDNA3- and p85B-transfected HEK 293 cells. Therefore, Flt3L can be delivered as a fusion with Ag85B in a biologically active form.
FIG. 1.
Flt3L-Ag85B is expressed by DNA vaccines in a functional form. (A) Schematic representation of pCDNA3 vectors expressing Flt3L, M. tuberculosis Ag85B (p85B), and the Flt3L-Ag85B fusion protein (pFlt-85). (B) Secretion of Flt3L by DNA-transfected HEK 293 cells. Flt3L in the culture medium of cells 3 days after transfection was detected by ELISA. (C) Generation of DCs from bone marrow progenitors using culture medium from DNA-transfected HEK 293 cells. The generation of CD11c+ MHC-II+ cells on day 6 after addition of HEK 293 supernatants to bone marrow cells was determined by flow cytometry. (D) Total number of CD11c+ MHC-II+ cells in culture.
Delivery of Flt3L-Ag85B by DNA vaccination improves protective efficacy against aerosol M. tuberculosis infection.
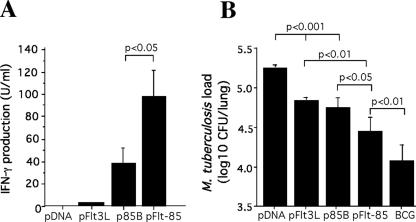
We next determined if fusion of the Flt3L molecule can augment the protective effect of the Ag85B protein. The immunopotentiating effect of Flt3L on antigen-specific immunity was investigated by vaccinating mice with DNA vectors and measuring the release of IFN-γ by Ag85B-reactive splenocytes. Vaccination with the plasmid expressing the antigen alone (p85B) resulted in the release of IFN-γ by splenocytes in response to the Ag85B protein (Fig. 2A). Vaccination with DNA encoding the fusion protein (pFlt-85) significantly enhanced antigen-specific IFN-γ release compared to vaccination with DNA encoding Ag85B alone (Fig. 2A). In order to determine if the improved immunogenicity imparted by the Flt3L-Ag85B fusion translated into enhanced protective efficacy, mice were vaccinated with DNA vectors and challenged 6 weeks after the last injection with aerosol M. tuberculosis H37Rv. Delivery of Flt3L alone conferred significant protection against M. tuberculosis challenge, and the level was equivalent to the level of protection afforded by p85B. The protective effect of Flt3L and Ag85B was significantly improved by fusion of the two proteins in the form of pFlt-85 (Fig. 2B). Therefore, Flt3L is able to augment the immunogenicity and protective efficacy of DNA vaccines encoding the M. tuberculosis Ag85B protein.
FIG. 2.
Immunogenicity and protective efficacy of DNA encoding murine Flt3L and M. tuberculosis Ag85B. (A) Splenocytes from immunized mice were cultured with 3 μg/ml Ag85B, and the level of IFN-γ released was determined by ELISA. (B) For determination of protective efficacy, 4 weeks following the final vaccination mice were infected by the aerosol route with 100 CFU of M. tuberculosis H37Rv. Four weeks after challenge, the bacterial load, expressed as the log10 CFU (mean ± standard error of the mean), was analyzed in the lung. The significance of differences between groups was determined by ANOVA. The error bars indicate standard errors of the means, and the data are representative of two separate experiments.
Secretion of Flt3L by BCG improves antimycobacterial immunity after vaccination.
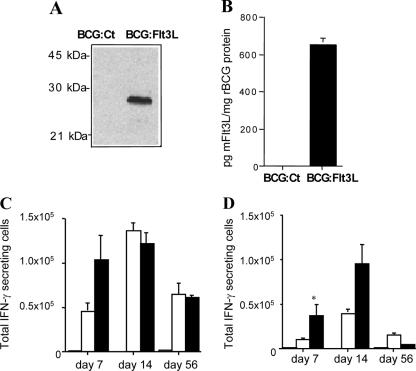
While delivery of Flt3L fused to the Ag85B protein resulted in significant protective efficacy against aerosol M. tuberculosis infection, the level did not reach that achieved by delivery of the BCG vaccine (Fig. 2B). We hypothesized that delivery of Flt3L during BCG vaccination may influence the numbers of antigen-presenting cells and increase the protective capacity of the BCG vaccine. To investigate this, an rBCG strain that secreted soluble murine Flt3L (BCG:Flt3L) was developed. Flt3L was readily detected in cell lysates of BCG:Flt3L by Western blotting with the anti-c-myc MAb 9E10 (Fig. 3A) or in culture supernatants by Flt3L-specific ELISA (Fig. 3B).
FIG. 3.
Construction and in vivo immunogenicity of BCG:Flt3L. BCG Pasteur was transformed with the control pMV261 plasmid (BCG:Ct) or plasmid pJEX73 (BCG:Flt3L), and the presence of Flt3L in cell lysates was determined by immunoblotting with the anti-c-myc MAb 9E10 (A). The presence of Flt3L in the culture supernatants of rBCG strains was determined by murine Flt3L-specific ELISA (B). To assess the immunogenicity, mice were not vaccinated (striped bar) or were vaccinated with 5 × 105 CFU of BCG:Ct (open bars) or BCG:Flt3L (solid bars), and at the indicated time points splenocytes (C) or DLN cells (D) were stimulated with 1 μg/ml BCG lysate. The number of IFN-γ-secreting cells was determined by an enzyme-linked immunospot assay. The significance of differences between BCG:Flt3L-vaccinated animals and BCG:Ct-vaccinated animals (asterisk, P < 0.05) was determined by ANOVA. The data are representative of two separate experiments.
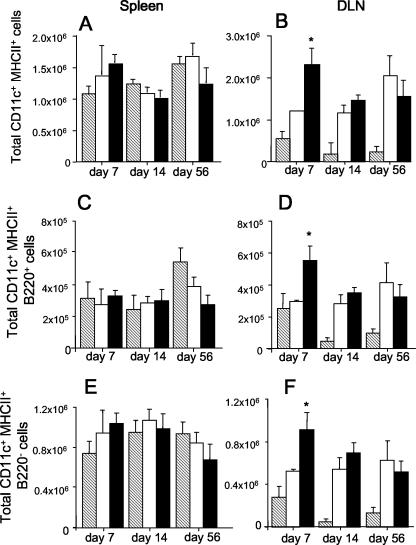
To determine if secretion of Flt3L influences the immunogenicity of BCG, mice were vaccinated with BCG:Flt3L or BCG containing the empty vector (BCG:Ct), and at various time points the numbers of IFN-γ-secreting splenocytes or DLN cells produced in response to BCG antigens were determined. At day 7 postinfection, mice vaccinated with BCG:Flt3L displayed heightened IFN-γ responses compared with those of BCG:Ct-vaccinated animals, and the difference was significant in the DLNs (Fig. 3C and 3D). The difference was less apparent at day 14, and no difference was observed at day 56 postinfection in either the spleen or the DLNs. Therefore, secretion of Flt3L had an early effect on immunogenicity. To determine if this effect was associated with expansion of DCs at these sites, cells from vaccinated mice were stained for cell surface makers and analyzed by flow cytometry. In the spleen, expression of Flt3L in BCG had no significant effect on the number of CD11c+ MHC-II+ cells at any of the time points examined (Fig. 4A). In the DLNs an increased number of CD11c+ MHC-II+ cells was observed at 7 days after infection with BCG:Flt3L compared to the number of cells after infection with BCG:Ct (Fig. 4B). The increase was apparent in both the CD11c+ MHC-II+ B220− subset (conventional DCs) and CD11c+ MHC-II+ B220− cells (plasmacytoid DCs) (Fig. 4F). No significant difference was detected at later time points (Fig. 4B, D, and F). Therefore, secretion of Flt3L augmented antimycobacterial immunity generated by rBCG early after vaccination, particularly in lymph nodes draining the site of immunization.
FIG. 4.
Influence of BCG:Flt3L on DC numbers in vaccinated mice. Mice were vaccinated subcutaneously with 5 × 105 CFU of rBCG, and at the indicated time points the numbers of CD11c+ MHC-II+ cells in the spleen (A, C, and E) and DLNs (B, D, and F) were determined by flow cytometry. The cells were further defined as cells that were B220+ (C and D) or B220− (E and F). Striped bars, unvaccinated mice; open bars, BCG:Ct-vaccinated animals; solid bars, BCG:Flt3L-vaccinated animals. The significance of differences between BCG:Flt3L-vaccinated animals and BCG:Ct-vaccinated animals (asterisk, P < 0.05) was determined by ANOVA. The data are representative of two separate experiments.
Vaccination with BCG secreting Flt3L improves vaccine safety without altering the protective efficacy.
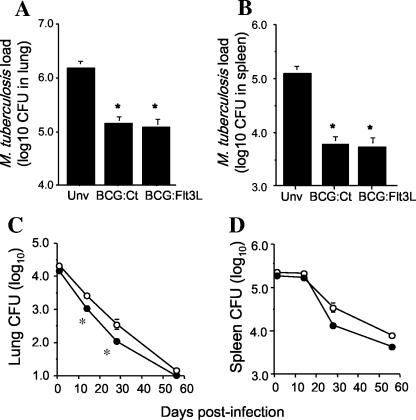
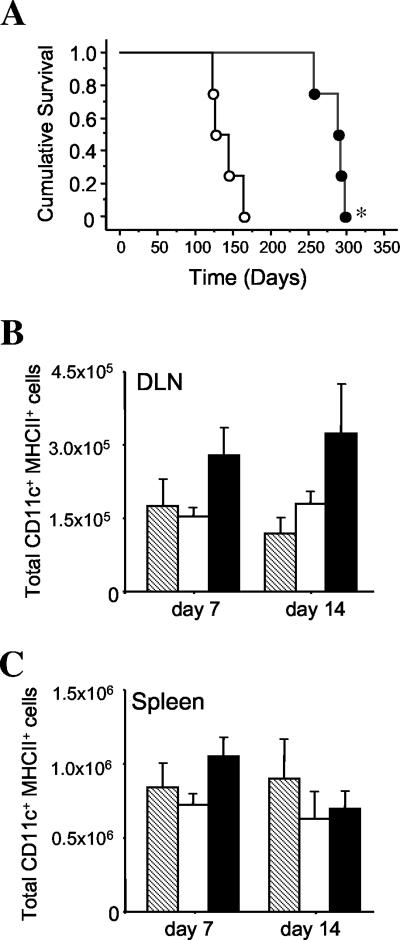
In order to determine if secretion of Flt3L by BCG influenced the protective efficacy of the vaccine, mice were vaccinated with BCG:Ct or BCG:Flt3L and 12 weeks later were challenged with aerosol M. tuberculosis. Both rBCG strains provided significant protection against M. tuberculosis compared to the results obtained for nonimmunized animals, and this effect was apparent in both the lung (Fig. 5A) and the spleen (Fig. 5B). Secretion of Flt3L did not significantly improve the protective efficacy of BCG, as BCG:Ct and BCG:Flt3L protected mice to similar extents. However, i.v. inoculation of the two strains and monitoring of the bacterial loads in organs indicated that BCG:Flt3L appeared to be more attenuated for in vivo growth than the control BCG (Fig. 5C and 5D). The difference was most apparent in the lung at 14 and 28 days postinfection. This effect was not due to toxicity of Flt3L expressed in BCG, as BCG:Ct and BCG:Flt3L grew equally well in anexic culture (data not shown). To determine if the increased clearance of BCG:Flt3L in vivo reduced the virulence of the vaccine, immunodeficient RAG-1−/− mice were infected with either BCG:Flt3L or BCG:Ct and survival was monitored. RAG-1−/− mice survived approximately two times longer after infection with BCG:Flt3L than after infection with BCG:Ct (Fig. 6A). Infection of RAG-1−/− mice with the rBCG strains and analysis of early changes in the numbers of DC revealed a trend toward increased numbers of CD11c+ MHC-II+ cells in the DLNs after vaccination with BCG:Flt3L compared to the results obtained with BCG:Ct, which was not observed in the spleen (Fig. 6A and 6B). Together, these results indicate that secretion of Flt3L improves the safety profile of BCG without altering the protective efficacy against virulent M. tuberculosis infection.
FIG. 5.
Protective efficacy of BCG secreting Flt3L. Mice were immunized subcutaneously with 5 × 105 CFU of BCG:Flt3L or BCG:Ct, and 12 weeks after immunization they were challenged by the aerosol route with M. tuberculosis H37Rv. Four weeks postchallenge the bacterial loads, expressed as log10 CFU (means ± standard errors of the means), were analyzed in the (A) lungs and (B) spleens of mice. The statistical significance between naïve and rBCG-vaccinated groups (asterisk, P < 0.01) was analyzed by ANOVA. The data are representative of three individual experiments. Unv, unvaccinated mice. (C and D) For assessment of rBCG growth in vivo, mice were infected i.v. with 1 × 106 CFU of BCG:Flt3L (•) or BCG:Ct (○). At 1, 14, 28, and 56 days postinfection the bacterial loads were assessed in the lungs (C) and spleens (D). The statistical significance between groups (asterisk, P < 0.05) was analyzed by ANOVA.
FIG. 6.
Safety of BCG:Flt3L in immunodeficient mice. (A) RAG-1−/− mice were infected i.v. with 1 × 106 CFU of BCG:Flt3L (•) or BCG:Ct (○), and survival was monitored over time. The significance of differences in survival was determined by the Mantel-Cox log rank test (asterisk, P < 0.001). (B and C) RAG-1−/− mice were vaccinated subcutaneously with 5 × 105 CFU of rBCG strains, and the numbers of CD11c+ MHC-II+ cells in the DLNs (B) and spleens (C) of the vaccinated mice were determined by flow cytometry. Striped bars, unvaccinated mice; open bars, BCG:Ct-vaccinated mice; solid bars, BCG:Flt3L-vaccinated mice. There were no statistically significant differences between groups as determined by ANOVA. The data are representative of one of two individual experiments.
DISCUSSION
Delivery of molecules during vaccination that target important components of the protective host immune response is a powerful vaccine strategy that has been applied to the control of many infectious diseases (19, 30). DCs play a central role in priming immune responses and are particularly important in the generation of Th1-like responses required to combat intracellular infections (13). We considered the possibility that targeting DCs may be a particularly effective way to improve protective immunity to control mycobacterial infection. In this report we demonstrate that vaccine delivery of Flt3L, a well-characterized DC growth factor, could influence the protective efficacy against virulent M. tuberculosis infection in mice. The fusion consisting of Ftl3L and the M. tuberculosis Ag85B protein expanded DCs in vitro (Fig. 1), induced strong Th1-like T-cell responses in vivo, and displayed significant protective efficacy against aerosol infection with M. tuberculosis (Fig. 2). Fusion of Flt3L to protective antigens has also been shown to enhance immunogenicity in models of viral immunity (34) and tumor metastasis (16). It appears that fusion of Flt3L to the antigen is necessary for effective induction of immunity, as codelivery of DNA encoding M. tuberculosis HSP65 and Flt3L was not effective against M. tuberculosis infection (3), and codelivery of Flt3L and Ag85B did not significantly improve the immunogenicity of Ag85B in our model (data not shown). Interestingly, DNA encoding Flt3L alone afforded protection against M. tuberculosis infection (Fig. 2). This suggests that Flt3L can induce partial protection against infection, which is further enhanced by addition of a specific M. tuberculosis antigen. These results are in accordance with previous studies that demonstrated that pretreatment with Flt3L can induce protection against viral (36, 38), parasitic (7), and bacterial infections (12).
DNA-encoded Flt3L-Ag85B was a less potent inducer of DC differentiation in vitro than pFlt3L, and this may have been due to the lower levels of Flt3L-Ag85B detected in supernatants of DNA-transfected HEK 293 cells (Fig. 1). Reduced expression of the fusion protein compared to that of the single components was also apparent upon detection of Ag85B by Western blotting with anti-Ag85B polyclonal antibody, which demonstrated there were reduced levels of Ag85B in HEK 293 cells transfected with pFlt3L-Ag85B compared with the levels in pAg85B-transfected cells (data not shown). This suggests that sustained delivery of low levels of DNA-encoded Flt3L-Ag85B was sufficient to induce protective immune response, which was superior to that induced by delivery of higher levels of Flt3L or Ag85B alone. It is possible that the delivery of low levels of DNA-encoded Flt3L provides an optimal amount of DC expansion/activation, as it has been reported that high-dose Flt3L pretreatment can impair protective immunity to intracellular infections (1). While this report is in conflict with numerous studies that have demonstrated control of infection by Flt3L-induced DC expansion, it does suggest that the number of DCs must be properly regulated to adequately control infections. With this in mind, it would be particularly interesting to modify expression of the Flt3L-Ag85B fusion protein to determine if the protective effect of the subunit vaccine against TB can be further improved.
BCG has been widely used as a vehicle to deliver protective antigens and immunostimulatory agents to improve protection against TB (28). While the vaccine has been engineered to secrete immunostimulatory agents, such as cytokines (20, 25, 27) and, more recently, chemokines (33), we demonstrate here, for the first time, that the hemopoietic growth factor Flt3L can be expressed and secreted by a bacterial system in a functional form (Fig. 3). We hypothesized that BCG secreting Flt3L would act locally on DC precursors to increase DC numbers and improve antigen presentation and antimycobacterial T-cell responses. We observed an early increase in the number of DCs after BCG:Flt3L vaccination, which correlated with improved induction of IFN-γ-secreting cells, especially in lymph nodes draining the site of infection (Fig. 3). This further highlights the critical role that DCs play in the generation of antimycobacterial immunity (17). However, the increase in DC numbers and subsequent effect on immunogenicity were not sustained, and we did not observe increased IL-12 levels in cells from BCG-Flt3L-immunized mice (data not shown). This suggests that the presence of Flt3L did significantly alter IL-12 release by DCs in response to BCG or that the number of DCs was not increased enough to observe an effect on cytokine levels. Significant expansion of DC numbers in mice requires sustained delivery of relatively large amounts (10 μg/day) of soluble Flt3L (21). As BCG numbers rapidly decreased in mouse organs (Fig. 5) and the level of Flt3L secreted by BCG:Flt3L was low (Fig. 3), it is possible that reduced levels of Flt3L at later time points resulted in a reduced influence of BCG:Flt3L on DC numbers. It is not known if continual expansion of DCs would result in improved protective immunity against intracellular pathogens such as M. tuberculosis, and this could be addressed by developing BCG strains that express higher levels of Flt3L and/or by employing vectors that permit a more sustained mode of delivery.
New candidate TB vaccines have recently entered phase I clinical trials (35), and it is hoped that a new effective vaccine can be available for use within the next 10 years (40). Any new live vaccine to replace BCG would need to maintain the significant effect that the BCG vaccine has on preventing miliary and meningeal TB in young children and would ideally be suitable for use in immunocompromised individuals. BCG:Flt3L afforded protection similar to that provided by control BCG; however, the vaccine was less virulent in immunodeficient mice (Fig. 6). The reduced virulence correlated with the attenuated phenotype of BCG:Flt3L in the lungs of immunocompetent mice and a trend toward increased DC numbers in the DLNs of RAG-1−/− mice immunized with BCG:Flt3L (Fig. 6). This is in accordance with the capacity of Flt3L to expand DCs and influence immunity in RAG-1−/− mice (7, 38). These results suggest that manipulating components of host immunity may be a suitable strategy to improve the effectiveness of the BCG vaccine and to deliver new candidates to control TB in humans.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. J. Triccas is the recipient of an NHMRC Biomedical Career Development Award. U. Palendira and A. A Ryan are recipients of an Australian Postgraduate Award. The Ag85B protein was received as part of NIH NIAID contract HHSN266200400091C awarded to Colorado State University.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Alaniz, R. C., S. Sandall, E. K. Thomas, and C. B. Wilson. 2004. Increased dendritic cell numbers impair protective immunity to intracellular bacteria despite augmenting antigen-specific CD8+ T lymphocyte responses. J. Immunol. 172:3725-3735. [DOI] [PubMed] [Google Scholar]
- 2.Altare, F., A. Durandy, D. Lammas, J. F. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. [DOI] [PubMed] [Google Scholar]
- 3.Baek, K. M., S. Y. Ko, M. Lee, J. S. Lee, J. O. Kim, H. J. Ko, J. W. Lee, S. H. Lee, S. N. Cho, and C. Y. Kang. 2003. Comparative analysis of effects of cytokine gene adjuvants on DNA vaccination against Mycobacterium tuberculosis heat shock protein 65. Vaccine 21:3684-3689. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt, K., S. P. Hickman, and P. Salgame. 2004. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J. Immunol. 172:2748-2751. [DOI] [PubMed] [Google Scholar]
- 5.Britton, W. J., and U. Palendira. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34-45. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das, L., J. DeVecchio, and F. P. Heinzel. 2005. Fms-like tyrosine kinase 3-based immunoprophylaxis against infection is improved by adjuvant treatment with anti-interleukin-10 antibody. J. Infect. Dis. 192:693-702. [DOI] [PubMed] [Google Scholar]
- 8.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. van Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 9.Demangel, C., A. G. Bean, E. Martin, C. G. Feng, A. T. Kamath, and W. J. Britton. 1999. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis Bacillus Calmette Guerin-infected dendritic cells. Eur. J. Immunol. 29:1972-1979. [DOI] [PubMed] [Google Scholar]
- 10.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 11.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 12.Gregory, S. H., A. J. Sagnimeni, N. B. Zurowski, and A. W. Thomson. 2001. Flt3 ligand pretreatment promotes protective immunity to Listeria monocytogenes. Cytokine 13:202-208. [DOI] [PubMed] [Google Scholar]
- 13.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 14.Hickman, S. P., J. Chan, and P. Salgame. 2002. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J. Immunol. 168:4636-4642. [DOI] [PubMed] [Google Scholar]
- 15.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung, C. F., K. F. Hsu, W. F. Cheng, C. Y. Chai, L. He, M. Ling, and T. C. Wu. 2001. Enhancement of DNA vaccine potency by linkage of antigen gene to a gene encoding the extracellular domain of Fms-like tyrosine kinase 3-ligand. Cancer Res. 61:1080-1088. [PubMed] [Google Scholar]
- 17.Jiao, X., R. Lo-Man, P. Guermonprez, L. Fiette, E. Deriaud, S. Burgaud, B. Gicquel, N. Winter, and C. Leclerc. 2002. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J. Immunol. 168:1294-1301. [DOI] [PubMed] [Google Scholar]
- 18.Kamath, A. T., T. Hanke, H. Briscoe, and W. J. Britton. 1999. Co-immunization with DNA vaccines expressing granulocyte-macrophage colony-stimulating factor and mycobacterial secreted proteins enhances T-cell immunity, but not protective efficacy against Mycobacterium tuberculosis. Immunology 96:511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornbluth, R. S., and G. W. Stone. 2006. Immunostimulatory combinations: designing the next generation of vaccine adjuvants. J Leukoc. Biol. 80:1084-1102. [DOI] [PubMed] [Google Scholar]
- 20.Luo, Y., H. Yamada, X. Chen, A. A. Ryan, D. P. Evanoff, J. A. Triccas, and M. A. O'Donnell. 2004. Recombinant Mycobacterium bovis bacillus Calmette-Guerin (BCG) expressing mouse IL-18 augments Th1 immunity and macrophage cytotoxicity. Clin. Exp. Immunol. 137:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maraskovsky, E., K. Bresel, M. Teepe, and H. J. McKenna. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maraskovsky, E., E. Daro, E. Roux, M. Teepe, C. R. Maliszewski, J. Hoek, D. Caron, M. E. Lebsack, and H. J. McKenna. 2000. In vivo generation of human dendritic cells subsets by Flt3 ligand. Blood 96:878-884. [PubMed] [Google Scholar]
- 23.McKenna, H. J. 2001. Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr. Opin. Hematol. 8:149-154. [DOI] [PubMed] [Google Scholar]
- 24.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 25.Murray, P. J., A. Aldovini, and R. A. Young. 1996. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc. Natl. Acad. Sci. USA 93:934-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunn, P., B. Williams, K. Floyd, C. Dye, G. Elzinga, and M. Raviglione. 2005. Tuberculosis control in the era of HIV. Nat Rev. Immunol. 5:819-826. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell, M. A., A. Aldovini, R. B. Duda, H. Yang, A. Szilvasi, R. A. Young, and W. C. DeWolf. 1994. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect. Immun. 62:2508-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohara, N., and T. Yamada. 2001. Recombinant BCG vaccines. Vaccine 19:4089-4098. [DOI] [PubMed] [Google Scholar]
- 29.Palendira, U., A. T. Kamath, C. G. Feng, E. Martin, P. J. Chaplin, J. A. Triccas, and W. J. Britton. 2002. Coexpression of interleukin-12 chains by a self-splicing vector increases the protective cellular immune response of DNA and Mycobacterium bovis BCG vaccines against Mycobacterium tuberculosis. Infect. Immun. 70:1949-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11:S63-68. [DOI] [PubMed] [Google Scholar]
- 31.Pelicic, V., M. Jackson, J. M. Reyrat, W. R. Jacobs, Jr., B. Gicquel, and C. Guilhot. 1997. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 94:10955-10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche, P. W., J. A. Triccas, D. T. Avery, T. Fifis, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 170:1326-1330. [DOI] [PubMed] [Google Scholar]
- 33.Ryan, A. A., J. M. Spratt, W. J. Britton, and J. A. Triccas. 2007. Secretion of functional monocyte chemotactic protein 3 by recombinant Mycobacterium bovis BCG attenuates vaccine virulence and maintains protective efficacy against M. tuberculosis infection. Infect. Immun. 75:523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sailaja, G., S. Husain, B. P. Nayak, and A. M. Jabbar. 2003. Long-term maintenance of gp120-specific immune responses by genetic vaccination with the HIV-1 envelope genes linked to the gene encoding Flt-3 ligand. J. Immunol. 170:2496-2507. [DOI] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 36.Smith, J. R., A. M. Thackray, and R. Bujdoso. 2001. Reduced herpes simplex virus type 1 latency in Flt-3 ligand-treated mice is associated with enhanced numbers of natural killer and dendritic cells. Immunology 102:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spratt, J. M., A. A. Ryan, W. J. Britton, and J. A. Triccas. 2005. Epitope-tagging vectors for the expression and detection of recombinant proteins in mycobacteria. Plasmid 53:269-273. [DOI] [PubMed] [Google Scholar]
- 38.Vollstedt, S., M. Franchini, H. P. Hefti, B. Odermatt, M. O'Keeffe, G. Alber, B. Glanzmann, M. Riesen, M. Ackermann, and M. Suter. 2003. Flt3 ligand-treated neonatal mice have increased innate immunity against intracellular pathogens and efficiently control virus infections. J. Exp. Med. 197:575-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wozniak, T. M., A. A. Ryan, J. A. Triccas, and W. J. Britton. 2006. Plasmid interleukin-23 (IL-23), but not plasmid IL-27, enhances the protective efficacy of a DNA vaccine against Mycobacterium tuberculosis infection. Infect. Immun. 74:557-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young, D., and C. Dye. 2006. The development and impact of tuberculosis vaccines. Cell 124:683-687. [DOI] [PubMed] [Google Scholar]