Abstract
Galectin-3 (Gal-3) is a multifunctional β-galactoside-binding lectin that senses self-derived and microbial glycoconjugates. Although Gal-3 is important in immune reactions and host defense in some experimental models, the function of Gal-3 during helminthic diseases (e.g., schistosomiasis) is still elusive. We show that, compared to wild-type Schistosoma mansoni-infected mice, infected Gal-3−/− mice have a reduced number of T and B lymphocytes in the spleen, develop reduced liver granulomas at 7 weeks (acute phase) and 14 weeks (chronic phase) postinfection, and mount a biased cellular and humoral Th1 response. In an attempt to understand this latter phenomenon, we studied the role of endogenous Gal-3 in dendritic cells (DCs), the most potent antigen-presenting cells, both in vitro and in vivo. Although Gal-3 deficiency in DCs does not impact their differentiation and maturation processes, it greatly influences the strength (but not the nature) of the adaptive immune response that they trigger, suggesting that Gal-3 deficiency in some other cell types may be important during murine schistosomiasis. As a whole, this study implies that Gal-3 is a modulator of the immune/inflammatory responses during helminthic infection and reveals for the first time that Gal-3 expression in DCs is pivotal to control the magnitude of T-lymphocyte priming.
Mammalian lectins display important functions in the innate/acquired immune system by sensing self- and/or pathogen-derived glycoconjugates. Among these, galectins represent a β-galactoside-binding lectin family whose involvement in the regulation of host immune responses during physiological and pathological conditions, including inflammation, cancer, and infection (for reviews, see references 2, 32, 33, and 41-43), is emerging. Although they lack a classical signal sequence, galectins are present in extracellular fluid and on the cell surface and are also located inside the cells (for a review, see reference 22). Extracellular and intracellular galectins display numerous functions including cell adhesion, signaling, proliferation, differentiation, survival, and apoptosis (for a review, see reference 32). Among the 15 galectin members, galectin-3 (Gal-3, previously known as Mac-2) is expressed in many immunocompetent/inflammatory cells, including monocytes, dendritic cells (DCs), macrophages, eosinophils, mast cells, NK cells, and activated T and B cells (for reviews, see references 8, 16, and 48). Through its ability to bind polylactosamine structures [such as (Galβ1-4GlcNAc)n] on endogenous ligands (20, 28), Gal-3 intervenes in many cellular processes in vitro. In particular, it favors cell-cell (for instance DC-T cell) and cell-matrix glycoprotein adhesion (37), exerts chemotactic effects (47), controls cell proliferation (13, 58), and promotes phagocytosis by macrophages (19, 46, 53). Gal-3 also takes part in the control of T-cell and monocyte survival and activation. For instance, extracellular Gal-3, by associating with N-glycans on T-cell receptor (TCR) has been shown to down-modulate TCR responsiveness and to regulate the production of Th1 and Th2 cytokines by differentiated T cells (13, 35). On the other hand, intracellular Gal-3 can positively or negatively impact intracellular signaling pathways by regulating the activities of various kinases, including protein kinases C, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase (8). The role of Gal-3 in the promotion and control of inflammation and in the regulation of the immune response has been recently evaluated in different models. Gal-3 has been proposed to be a powerful proinflammatory signal in vitro (24, 27, 47, 57), and studies of Gal-3-deficient mice have provided support for the proinflammatory role of this lectin (10, 21, 59). For instance, the lack of Gal-3 protects mice against asthma reactions, an effect associated with an enhanced Th1 response in challenged animals (59). On the other hand, administration of Gal-3 (by means of gene therapy) inhibits asthmatic reactions (12, 34). These studies reveal opposite effects of the endogenous and exogenous Gal-3 and suggest that, according to its location (extracellular versus intracellular), Gal-3 differentially controls immune/inflammatory cell survival, migration, and cytokine release.
Along with its role in inflammation and immune responses in noninfectious conditions, Gal-3 can also sense certain microorganisms. Recently, it has been demonstrated that Gal-3 binds to multimeric LN (LacNAc) and LDN (LacdiNAc) forming GalNAcβ1-4GlcNAc motifs (53) as well as to other carbohydrate structures on glycoproteins and glycolipids (including lipopolysaccharides [LPS]) from many pathogens such as (myco)bacteria, protozoan parasites, and yeast (36). Through this activity, Gal-3 participates in the phagocytosis of some microorganisms by macrophages and triggers host responses to pathogens, at least in vitro (26, 46). Whether it also contributes to signaling events in accessory cells during in vivo infection is unclear. Likewise, its role in signaling pathways triggered by Toll-like receptors (TLRs), which represent key sensors in innate cells (for a review, see reference 23), is still elusive. The in vivo role of Gal-3 during infection has not been extensively studied. It appears to clear late Mycobacterium tuberculosis infection (5), to contribute to neutrophil recruitment at the site of Streptococcal pneumonia infection (49), and to exert an important role in innate immunity during Toxoplasma gondii infection (6). In the present report, to further investigate the role of Gal-3 in infection and innate or acquired immunity, we studied its role during murine schistosomiasis, a helminthic disease characterized by a dominant Th2 response triggered by the egg stage of the parasite (for a review, see reference 39). Recently, van den Berg et al. demonstrated that LDN motifs, which are expressed by Schistosoma mansoni eggs (29, 50), bind Gal-3 and suggested that this interaction may play an important role in the Th2-mediated inflammatory response that occurs in the liver (53). Support for this hypothesis has been recently provided by Van de Vijver and coworkers, who showed that beads that carry glycoconjugates with terminal LN or LDN elements gave rise to granulomas similar to the granulomatous lesions caused by schistosome eggs in a natural infection (54). Recently, although conflicting, two studies have addressed the role of endogenous Gal-3 during Schistosoma infection (7, 38). Here, in agreement with Oliveira et al. (38), we show that, relative to wild-type (WT) mice, S. mansoni-infected Gal-3-deficient mice develop reduced granuloma formation and have a dramatically decreased number of total lymphocytes in the spleen. Moreover, Gal-3 deficiency results in a more pronounced Th1 response in infected mice. In an attempt to understand this latter phenomenon, we studied the role of endogenous Gal-3 in DCs both in vitro and in vivo. We report that Gal-3 deficiency in DCs is important to control the priming of T lymphocytes but has no role in the Th1/Th2 balance of the immune response, suggesting that Gal-3 deficiency in some other cell types may be important during murine schistosomiasis.
MATERIALS AND METHODS
Animals.
Female 129 mice were purchased from Iffa-Credo (l'Arbresle, France). The generation of Gal-3-deficient (Gal-3−/−) 129 mice has been described by Colnot et al. (9). These animals show no morphological abnormalities and have no apparent phenotypes under standard pathogen-free conditions.
Reagents and Abs.
Monoclonal antibodies (MAbs) against CD3 (unconjugated or fluorescein isothiocyanate [FITC] conjugated), CD4 (unconjugated), Mac-1 (unconjugated), CD62L (biotin conjugated), CD11c (biotin or allophycocyanin [APC] conjugated), CD19 (APC conjugated), CD4 (FITC conjugated), CD25 (APC conjugated), and B220 (phycoerythrin conjugated) were purchased from Becton Dickinson (Le Pont de Claix, France). The 120G8 MAb was a gift from Asselin-Paturel (3). APC-conjugated streptavidin was from Clinisciences (Montrouge, France), and a CD4+ T-cell isolation kit and streptavidin-conjugated microbeads used for magnetic bead cell sorting purification were from Miltenyi Biotech (Paris, France). Biotinylated MAbs against murine immunoglobulin G2b (IgG2b) and IgG1 were purchased from Southern Biotechnologies (Birmingham, AL). Anti-Gal-3 MAb (clone SC-32790, with or without phycoerythrin conjugate) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-β-actin Ab and horseradish peroxidase-conjugated anti-mouse IgG were from Sigma Aldrich (Saint Quentin Fallavier, France). Pam3CSK4 was purchased from EMC Microcollections (Tuebingen, Germany), and poly(I·C) and ultrapure LPS (from Escherichia coli serotype 0111:B4) were from Cayla (Toulouse, France). Polyacrylamide (PAA)-coupled glycoconjugates LDN-PAA, LN-PAA, and glucitol-PAA (∼20% substitution) (FITC labeled or unlabeled) were from Lectinity (Lappeenranta, Finland). Lactose was from Sigma Aldrich.
Parasites and antigen preparation.
S. mansoni (Puerto Rican strain) cercariae were obtained from infected Biomphalaria glabrata snails (44). Schistosome eggs and soluble egg antigen (SEA) (52) were prepared as previously described (17).
Infection of mice and analysis of the parasitological and immunological parameters.
Eight-week-old female WT and Gal-3−/− mice were infected with 45 S. mansoni cercariae via the percutaneous route. Worm burdens were measured by liver perfusion 7 weeks postinfection (p.i.). At the time of perfusion, the small intestines and the livers were also collected for measurement of egg numbers deposited in these organs. Tissues were digested in 4% KOH as previously described (15). Records of the organ weights allowed the calculation of total tissue eggs per organ. Spleens and mesenteric lymph nodes (MLNs) were harvested 7 and 14 weeks p.i., and cells were stimulated with increasing doses of SEA for 3 days at 37°C. During the last 18 h, 0.5 μCi of [3H]thymidine/well was added. Production of gamma interferon (IFN-γ), interleukin-4 (IL-4), IL-5, IL-10, and IL-13 was measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, United Kingdom). To study the humoral response, mice were bled 7 and 14 weeks p.i., and the anti-SEA IgG1 and IgG2b titrations were determined for each mouse by ELISA (17). Serum titers are defined as the dilutions which give optical density readings at least twofold higher than the mean background of noninfected mouse serum.
Analysis of the egg-associated liver pathology in infected mice.
The egg-associated pathology was analyzed on the livers of mice 7 and 14 weeks p.i. in a double-blind fashion. Briefly, a small piece of liver from each mouse was fixed in Immunohistofix (Gentaur Molecular Products, Brussels, Belgium) for 2 to 3 days and embedded in wax. Then, 5-μm liver sections were prepared for Rojkind coloration to evaluate collagen deposition. The staining mix is made of a saturated solution of picric acid in distilled water containing 0.1% Fast Green FCF (Sigma-Aldrich, Saint Quentin Fallavier, France) and 0.1% Sirius red. After the staining, slides were washed in physiological water and mounted in Acrytol (Surgipath, Labonord, France) after dehydration. The diameters of granulomas surrounding single, mature, and viable eggs were measured using Metaview computing software (Roper Scientific, Evry, France), and the volume of each granuloma was calculated assuming a spherical shape. The proportion of eosinophils was determined by May-Grunwald-Giemsa staining and that of monocytes/macrophages and T lymphocytes was determined by immunohistochemistry using anti-Mac-1 and anti-CD4 MAbs, respectively. Collagen deposition in granulomas was visualized by Sirius red staining, and fibrosis was quantified using an arbitrary scale from 0 (no collagen deposition) to 3 (strong and tight collagen deposition all over the granuloma). For each parameter, ∼ 5 granulomas/mouse were examined.
Preparation and analysis of BM-DCs.
Bone marrow-derived DCs (BM-DCs) were generated from the BM of WT or Gal-3−/− 129 mice by culture in granulocyte-macrophage colony-stimulating factor-conditioned medium as previously described (1). DCs were used on day 14 of culture (>95% pure, as assessed by CD11c immunostaining). Gal-3 expression in BM-DCs was analyzed by Western blotting using anti-Gal-3 MAb (0.4 μg/ml). Phenotypic analysis was performed by flow cytometry using standard procedures. For endocytosis analysis, immature WT and Gal-3−/− BM-DCs (2 × 105 cells/well) were incubated in the presence of FITC-labeled glucitol-, LDN- or LN-conjugated PAA (50 μg/ml) or FITC-labeled dextran (10 μg/ml) for 2 h at 4°C or at 37°C to evaluate extracellular binding and specific endocytosis. Glycan endocytosis was then assessed by flow cytometry. In activation experiments, BM-DCs (1 × 106 cells/ml) were either cultured with live eggs (1/200 cells), Pam3CSK4 (500 ng/ml) plus poly(I·C) (2 μg/ml), or LPS (100 ng/ml) or were left untreated. In some experiments, LDN- or glucitol-conjugated PAA (50 μg/ml) was added 30 min before TLR agonists. After 18 to 20 h, culture supernatants were collected, and tumor necrosis factor alpha, IL-6, IP-10/CXCL10, and MIG/CXCL9 (where IP-10 is IFN-inducible protein 10 and MIG is monokine induced by IFN-γ) concentrations were measured by ELISA (R&D Systems).
Mixed leukocyte reaction (MLR).
WT and Gal-3−/− BM-DCs (3 × 106 cells/plate), either stimulated or unstimulated, were treated with mitomycin (25 μg/ml) for 30 min at 37°C, extensively washed, and cocultured at different ratios with purified naïve CD4+ T cells in a flat-bottom 96-well plate (1 × 105 T cells/well). Naive CD4+ T cells were obtained from BALB/c mice spleens by magnetic bead cell sorting purification, using a CD4+ T-cell isolation kit, followed by anti-CD62L MAb treatment and streptavidin-conjugated microbead purification (CD4+ CD62L+; purity of >95%). Five days later, cocultures were transferred into anti-CD3 MAb-bound plates for 48 h at 37°C. Culture supernatants were then harvested, and cytokine production was determined by ELISA. Proliferation was assessed by Alamar blue addition (20 μl/well), and the optical density was measured 24 h later, according to the manufacturer's protocol (Serotec, Cergy Saint-Christophe, France).
Immunization protocol and analysis of the immune response.
After overnight culture, keyhole limpet hemocyanin (KLH)-pulsed DCs, cultured in the presence or absence of schistosome eggs (1:200 DCs), were collected, washed, and administered at a dose of 1.5 × 106 cells intravenously in syngeneic naïve mice. Seven days later, splenic cells (5 × 105 cells/well in a flat-bottom 96-well plate) were harvested and restimulated with KLH or SEA for 72 h at 37°C.
Statistical analysis.
Results are expressed as the mean ± standard deviation (SD). All other P values were determined by using a Student's t test. P values of <0.05 were considered significant.
RESULTS
S. mansoni-infected Gal-3−/− mice have an impaired number of splenic B and T cells relative to WT infected mice.
Gal-3−/− and WT mice were percutaneously infected with S. mansoni, and 7 weeks later, the parasitological parameters were analyzed. As shown in Table 1, worm burdens (in both males and females) (data not shown) as well as tissue (liver and intestine) egg numbers were similar in the two groups, indicating that the lack of Gal-3 does not influence parasite survival and fecundity. This is consistent with recent results reporting no role of Gal-3 in the establishment of Schistosoma infection in mice (7, 38). Having established this, we next determined the frequency and the total number of B and T lymphocytes, as well as DCs, in the spleens of WT and Gal-3−/− infected mice. Relative to uninfected WT animals, we noticed no variations in the frequency or in the number of splenic B cells (CD3− CD19+) and T cells (CD3+ CD19−), including CD4+ CD25+ T cells in uninfected Gal-3−/− mice (Table 2). In S. mansoni-infected mice, although the frequency of these cells remained unchanged, except for T cells (decreased proportion compared to uninfected mice), their total number increased dramatically in WT but less in Gal-3−/− infected animals (∼ 40% reduction). These data are in agreement with the data from Oliveira et al., who reported a reduction in total lymphocyte counts in the spleen of S. mansoni-infected Gal-3-deficient mice (38). Analysis of both myeloid (CD11c+ 120G8−) and plasmacytoid (CD11c+ 120G8+) DCs did not show any significant variation between the two uninfected animal groups. Interestingly, in WT and Gal-3−/− infected mice, we observed a dramatic enhancement (∼ 2.2-fold to 3.5-fold) in the frequency and number (×9) of myeloid DCs. On the other hand, the number of plasmacytoid DCs remained unchanged during infection and was similar in both WT and Gal-3−/− mice. Thus, Gal-3 deficiency affects the number but not the proportion of both splenic T and B cells in S. mansoni-infected mice whereas it does not modulate the number or proportion of DCs.
TABLE 1.
Analysis of the worm burden and of the total tissue eggs in WT and Gal-3−/− infected female mice at 7 weeks p.i.a
| Sample source | Worm burden (no.) | No. of eggs/g of liver | No. of eggs/g of intestine |
|---|---|---|---|
| WT mice | 17.1 ± 7.5 | 776.8 ± 222.7 | 959.3 ± 309.0 |
| Gal-3−/− mice | 16.7 ± 2.8 | 796.2 ± 126.1 | 1063.5 ± 484.6 |
Results represent the mean number ± SD (8 mice per group). One representative experiment out of two is shown.
TABLE 2.
Analysis of the percentage and number of splenic cells in WT and Gal-3−/− infected micea
| Cell type | Splenic cell values in uninfected mice
|
Splenic cell values in S. mansoni-infected miceb
|
||||||
|---|---|---|---|---|---|---|---|---|
| WT
|
Gal-3−/−
|
WT
|
Gal-3−/−
|
|||||
| No. of cells (106/spleen) | % of cells | No. of cells (106/spleen) | % of cells | No. of cells (106/spleen) | % of cells | No. of cells (106/spleen) | % of cells | |
| CD3− CD19+ | 20.56 ± 8.19 | 39.56 ± 1.54 | 21.79 ± 5.34 | 35.14 ± 4.25 | 91.54 ± 7.36 | 39.75 ± 4.33 | 57.29 ± 4.45*** | 36.30 ± 2.51 |
| CD3+ CD19− | 22.58 ± 6.49 | 45.04 ± 5.69 | 27.42 ± 5.22 | 44.23 ± 4.61 | 70.46 ± 8.17 | 30.44 ± 2.49 | 44.48 ± 5.33** | 28.24 ± 3.70 |
| CD4+ CD25+ | 1.73 ± 0.90 | 3.23 ± 0.58 | 1.83 ± 0.72 | 2.95 ± 0.53 | 6.03 ± 0.65 | 2.62 ± 0.31 | 3.46 ± 0.21*** | 2.21 ± 0.42 |
| CD11c+ 120G8− | 0.45 ± 0.12 | 0.82 ± 0.04 | 0.40 ± 0.05 | 0.64 ± 0.23 | 4.11 ± 0.78 | 1.80 ± 0.44 | 3.58 ± 0.43 | 2.28 ± 0.4 |
| CD11c+ 120G8+ | 0.45 ± 0.03 | 0.83 ± 0.19 | 0.34 ± 0.25 | 0.55 ± 0.31 | 0.55 ± 0.13 | 0.24 ± 0.06 | 0.37 ± 0.15 | 0.23 ± 0.08 |
Results represent the mean percentage or number ± SD (4 mice per group). Values of infected mice are at 7 weeks p.i. One representative experiment out of two is shown.
Significant differences between WT and Gal-3−/− infected mice are indicated as follows:
P < 0.01
P < 0.005.
Infected Gal-3−/− mice develop a biased Th1 response compared to WT controls.
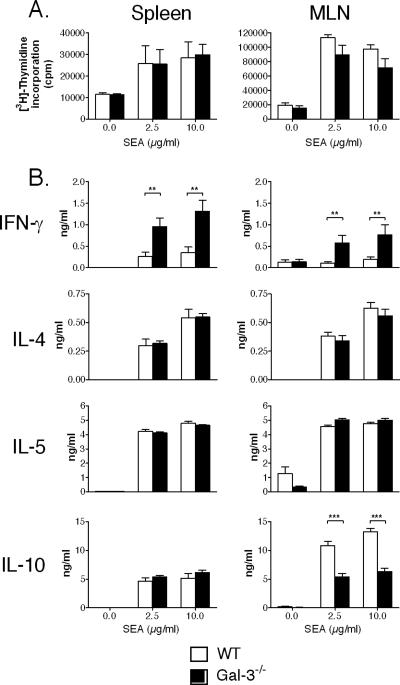
We next determined the nature of the cellular immune response in infected WT and Gal-3−/− mice. To this end, spleens and MLNs were removed 7 weeks p.i., and cells were restimulated with various doses of SEA. As seen in Fig. 1A, upon in vitro SEA challenge, splenic or MLN cells from WT and Gal-3−/− mice proliferate in a comparable manner. In contrast, major differences were observed in terms of cytokine synthesis. Interestingly, the production of the Th1 cytokine IFN-γ by SEA-stimulated splenic and MLN cells from Gal-3−/− infected mice was dramatically increased (∼4 to 6-fold) compared to WT mice (Fig. 1B). On the other hand, the production of the Th2-type cytokines IL-4, IL-5, and IL-13 (data not shown) was similar between Gal-3−/− and WT mice. Notably, although no significant differences were detected in splenic cells, the level of IL-10 was decreased in the supernatant of Gal-3−/− MLN cells.
FIG. 1.
Analysis of the cellular response in S. mansoni-infected WT and Gal-3−/− mice. Data reflect the proliferative response (A) and cytokine production (B) of spleen or MLN cells recovered from S. mansoni-infected WT or Gal-3−/− mice at 7 weeks p.i. Cells were restimulated with increasing doses of SEA (shown are 2.5 and 10 μg/ml) or were left unstimulated. Cytokine production and proliferation were measured after 3 days of culture. Results represent the means of triplicate cultures ± SDs (6 to 8 mice per group). One representative experiment out of two is shown. **, P < 0.01; ***, P < 0.005.
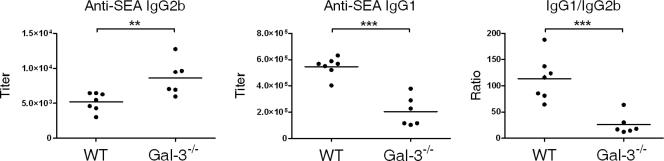
We then determined the titers of SEA-specific IgG1 (a marker of a Th2 response) and IgG2b (a marker of a Th1 response) in infected mice. Although the overall Ig levels were identical in both animal groups (data not shown), Gal-3−/− infected mice produced increased SEA-specific IgG2b (Fig. 2) and IgG2a (not shown), compared to control mice, in agreement with the enhanced production of IFN-γ shown in Fig. 1A. Although the level of Th2-type cytokines was not affected in these mice, Gal-3−/− infected mice produced less SEA-specific IgG1 (Fig. 2) than WT animals. Overall, this suggests that Gal-3 is important in the Th1/Th2 balance of the immune response during infection.
FIG. 2.
Analysis of the humoral response in S. mansoni-infected WT and Gal-3−/− mice. SEA-specific Ab isotype responses by S. mansoni-infected WT or Gal-3−/− mice at 7 weeks p.i. Individual IgG1 and IgG2b titers and the IgG1/IgG2b ratio are shown. The mean titers ± SDs (6 to 7 mice per group) are presented. One representative experiment out of two is shown. **, P < 0.01; ***, P < 0.005.
Gal-3 influences the development of liver granulomas in S. mansoni-infected mice.
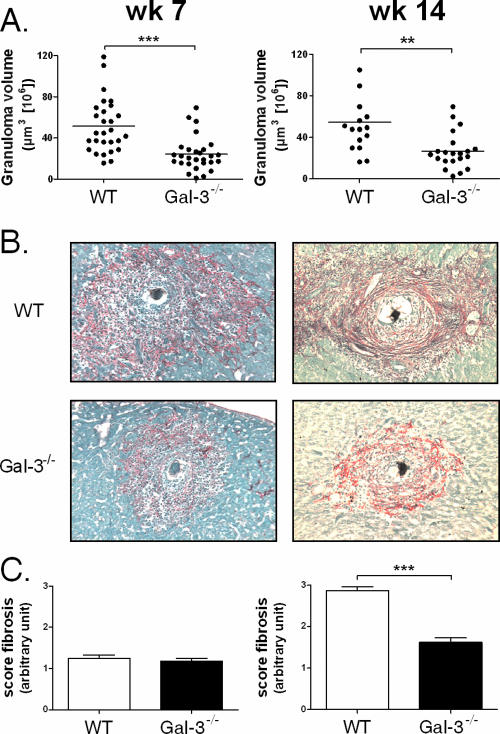
We then evaluated the influence of Gal-3 deficiency on the formation of liver granulomas during the acute (7 week) and chronic (14 week) phases of the disease. In Gal-3−/− infected mice, and relative to WT animals, the size of the granulomas surrounding eggs was significantly decreased at week 7 (Fig. 3A, left panel). However, analysis of the cellular composition of the granulomas indicated no significant difference in the frequency of eosinophils (∼75%), monocytes/macrophages (∼2%), and T lymphocytes (∼2%) between the two animal groups (not shown). Thus, during the acute phase of the disease, Gal-3 appears to contribute, in a quantitative but not qualitative manner, to the recruitment of immune/inflammatory cells into granulomas. Rojkind staining of liver sections showed no significant difference in the deposition of collagenous material and in fibrosis between the two animal groups at 7 weeks p.i. (Fig. 3B and C).
FIG. 3.
Analysis of the granulomatous response in S. mansoni-infected WT and Gal-3−/− mice. Rojkind staining of liver sections from S. mansoni-infected WT or Gal-3−/− mice at 7 weeks and 14 weeks p.i. is shown. (A) The volume of the granulomas is indicated at week 7 and week 14 p.i. for each animal group. **, P < 0.01; ***, P < 0.005. (B) Representative staining obtained at 7 weeks or 14 weeks p.i. (×100 magnification). (C) The fibrosis, visualized on Sirius red-stained sections, was quantified using an arbitrary scale from 0 to 3. ***, P < 0.005.
We next investigated the impact of Gal-3 deficiency during the chronic phase of the disease. The average size of granulomas in the Gal-3−/− infected mice was also significantly reduced at 14 weeks p.i relative to that in WT animals (Fig. 3A, right panel). As observed after 7 weeks p.i., no difference in the proportion of eosinophils, monocytes/macrophages, and T lymphocytes within the granulomas was noticed 14 weeks p.i. (not shown). Finally, as visualized by Rojkind staining, the collagenous network was significantly less compact in Gal-3-deficient mice than in WT animals (Fig. 3B and C). As a whole, Gal-3−/− infected mice develop smaller lesions at 7 and 14 weeks p.i and less severe hepatic fibrosis at 14 weeks p.i. than WT animals.
Gal-3 is functional in DCs but does not influence their differentiation or their maturation.
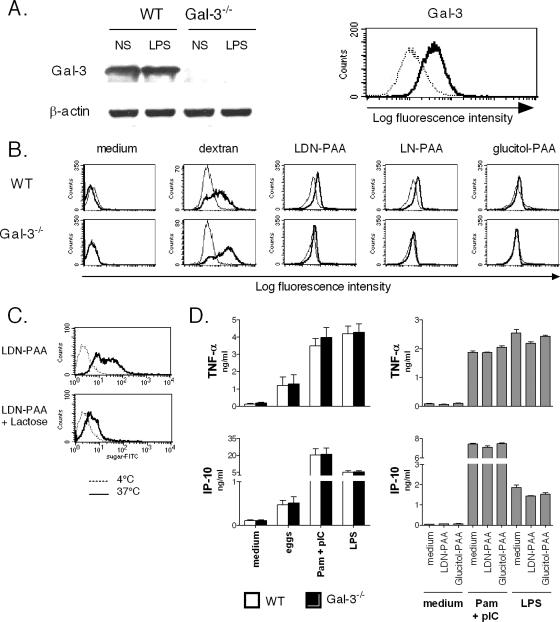
Since DCs play a key role in orchestrating immune responses (4), we next investigated the possibility that Gal-3 deficiency in DCs could, at least in part, explain the dysregulated immune response observed in Gal-3−/− infected mice. No difference in the yield of DCs, generated from either WT or Gal-3−/− BM progenitors, was found, suggesting that the lack of Gal-3 has no major consequences on cell death or survival during the differentiation process. As seen in Fig. 4A (left panel), Western blot analysis confirmed the presence of Gal-3 protein in DCs generated from WT but not Gal-3−/− BMs. Immunostaining revealed that Gal-3 is expressed on the surface of immature DCs (Fig. 4A, right panel). Of note, no substantial changes in Gal-3 expression were observed in DCs matured with the canonical TLR4 agonist LPS (Fig. 4A; also data not shown). We next investigated whether the lack of Gal-3 in DCs could impact their phenotype and functions. Fluorescence-activated cell sorting analysis revealed similar levels of mannose receptor, major histocompatibility complex class II, CD1d, CD54, and CD80 expression in immature WT and Gal-3−/− DCs (data not shown). To study the impact of Gal-3 deficiency on endocytosis, a key function of immature DCs, WT or Gal3−/− BM-DCs were exposed to FITC-labeled dextran, and internalization was followed by fluorescence-activated cell sorting analysis. As seen in Fig. 4B, Gal-3−/− DCs internalized dextran (at 37°C) as efficiently as the WT counterparts. To assess Gal-3-mediated endocytosis, FITC-labeled LDN-PAA and LN-PAA were utilized. FITC-labeled glucitol-PAA was used as a negative control. Compared to Gal-3-competent cells, Gal-3−/− DCs failed to internalize LDN-PAA and LN-PAA (Fig. 4B). Furthermore, pretreatment of WT DCs with lactose, a competitive inhibitor of Gal-3 binding, prevented LDN-PAA (Fig. 4C) and LN-PAA internalization (data not shown). These data indicate that Gal-3 expression on DCs mediates endocytosis of canonical Gal-3 ligands. On the other hand, Gal-3 deficiency does not influence the differentiation, the phenotype, or the Gal-3-independent endocytic functions of immature DCs.
FIG. 4.
(A) Expression of Gal-3 in immature BM-DCs. The left panel shows Western blot analysis of Gal-3 protein expression in DCs (5 × 105 cells/lane). In the right panel, gated CD11c+ DCs were analyzed for Gal-3 expression (bold line). The isotype control is indicated (thin line). (B) Endocytic function of Gal-3 in immature BM-DCs was assessed by flow cytometry. WT or Gal-3−/− BM-DCs were incubated with FITC-labeled dextran, LDN-PAA, LN-PAA, or glucitol-PAA for 2 h at 4°C (thin line) or 37°C (bold line). Endocytosis is displayed as an increase in fluorescence intensity at 37°C compared with 4°C. (C) WT DCs were either left untreated or were treated with lactose (5 mM) 30 min before exposure to FITC-labeled LDN-PAA. (D) Effects of Gal-3 deficiency on DC maturation (left panel). WT or Gal-3−/− DCs were incubated overnight with schistosome eggs, a combination of Pam3CSK4 plus poly(I·C), or LPS. WT DCs were left untreated or LDN-PAA or glucitol-PAA (50 μg/ml) was added 30 min before Pam3CSK4 plus poly(I·C) (Pam+pIC) or LPS stimulation (right panel). After an 18-h stimulation, quantification of secreted cytokines was performed by ELISA. The mean values ± SDs are presented (four experiments performed). TNF-α, tumor necrosis factor alpha.
Next, the ability of WT and Gal-3−/− DCs to mature in response to different stimuli, including whole parasite eggs and canonical TLR agonists, was studied. Since schistosome eggs have been shown to induce DC maturation through TLR-2 and TLR-3 (1), a combination of Pam3CSK4 (a TLR2 agonist) and poly(I·C) (a TLR3 agonist) was used, although this binary activation does not recapitulate the total stimulatory effects of the eggs. The TLR4 agonist LPS was also included in this study. Whatever the mode of stimulation, Gal-3−/− DCs, compared to WT cells, did not show any defect in their ability to up-regulate major histocompatibility complex class II, CD86, and CD40 (data not shown). Similarly, as seen in Fig. 4D (left panel) (also data not shown), mature WT and Gal-3−/− DCs produced equal amounts of the inflammatory cytokines tumor necrosis factor alpha and IL-6 as well as the chemokine IP-10/CXCL10 and MIG/CXCL9. Finally, we investigated the possibility that engagement of Gal-3 could modulate the maturation of DCs. To address this issue, WT DCs were pretreated with LDN-PAA (or glucitol-PAA as a control) 30 min before Pam3CSK4-poly(I·C) or LPS stimulation. LDN-PAA did not modulate the production of inflammatory cytokines or chemokines (Fig. 4D, right panel; also data not shown). In addition, LDN-PAA alone (without TLR agonists) did not trigger cytokine/chemokine production by DCs. Overall, Gal-3 deficiency in DCs or Gal-3 ligation does not cooperate or interfere with TLR signaling pathways.
Expression of Gal-3 in DCs is necessary to control, but not to polarize, the cytokine response of T lymphocytes.
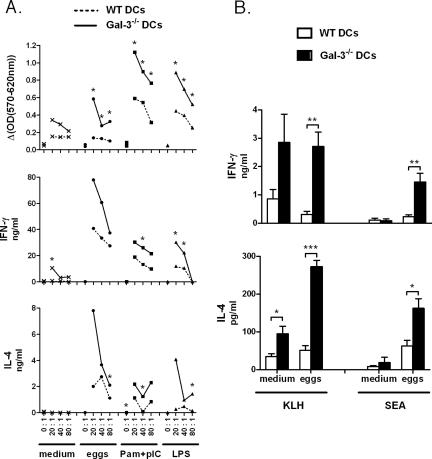
Next, we studied the impact of Gal-3 deficiency in DCs on the strength and on the nature of the T-cell response. To this end, we first compared the ability of Gal-3−/− and WT DCs, either immature or mature, to induce an MLR response. For this, DCs stimulated with schistosome eggs, Pam3CSK4-poly(I·C), or LPS or left untreated (medium) were cocultured at different ratios with purified naïve T cells isolated from WT BALB/c mice. As expected, compared to immature DCs, mature DCs induced a high level of T-cell proliferation (Fig. 5A). Interestingly, relative to WT counterparts, mature Gal-3−/− DCs induced increased proliferation as well as enhanced production of both IFN-γ and IL-4 by T cells (Fig. 5A) (as well as IL-5 and IL-10 [data not shown]). Analysis of the IFN-γ/IL-4 ratio (data not shown) revealed that Gal-3−/− DCs do not skew the immune response in either a Th1 or Th2 direction.
FIG. 5.
Effects of Gal-3 deficiency in DCs on the priming of T cells. (A) WT or Gal-3−/− BM-DCs were incubated for 18 h with medium, schistosome eggs, a combination of Pam3CSK4 plus poly(I·C), or LPS. MLR was carried out by coculturing DCs with naïve T cells prepared from the spleens of BALB/c mice. Different ratios of DCs to T cells were tested. For clarity, SD values are not shown. One representative experiment out of three is shown. *, P < 0.05. (B) WT or Gal-3−/− BM-DCs were incubated overnight with KLH with or without schistosome eggs. Cells were then injected intravenously into syngeneic mice, and 7 days later, splenic cells were restimulated with KLH or SEA. Supernatants were harvested 72 h later for cytokine production. In the absence of restimulation, no proliferation or cytokine production was observed. Results represent the means of triplicate cultures ± SDs. One representative experiment out of two is shown. *, P < 0.05; **, P < 0.01; ***, P < 0.005.
To investigate whether Gal-3−/− DCs prime T cells more efficiently than WT DCs in vivo, WT or Gal-3−/− DCs were pulsed overnight with KLH, in the presence or absence of schistosome eggs, and were then injected intravenously into naive syngeneic WT mice. Seven days later, whole splenic cells were restimulated with KLH or egg antigen (52). Of note, a higher number of splenic T lymphocytes were detected in mice that received Gal-3-deficient DCs (∼75% enhancement compared to mice that received WT DCs), while no difference in T-cell survival was observed (data not shown). After restimulation, spleen cells from mice that received Gal-3−/− DCs proliferated more vigorously (data not shown) and produced an increased level of cytokines (Fig. 5B). For instance, after KLH restimulation, administration of Gal-3−/− DCs (in particular, those sensitized with parasite eggs) induced higher amounts of IFN-γ and IL-4 (as well as IL-5 and IL-10 [data not shown]) than WT DCs. Finally, after SEA restimulation, Gal-3−/− DCs cultured with whole eggs induced higher levels of both IFN-γ and IL-4 by T cells. Notably, although all groups of mice contained an identical frequency of IFN-γ-positive T cells in the spleen (after CD3 or phorbol myristate acetate-ionomycin restimulation), the mean fluorescence intensity of the IFN-γ staining tended to be higher in mice that received Gal-3-deficient DCs (data not shown). Thus, it is likely that the increased production of cytokines in these mice is due to the increased number of T cells and to an enhanced cytokine production per cell number. To conclude, Gal-3 deficiency in DCs increases the T-cell cytokine response both in vitro and in vivo, without biasing the immune response toward a Th1 or Th2 direction.
DISCUSSION
The role of Gal-3 in the Th1/Th2 immune and inflammatory responses appears to vary according to the experimental models used (administration of Gal-3 versus Gal-3 null animals) (10, 13, 21, 59). To further investigate its role in these settings, we studied Gal-3-deficient mice with regard to their response to S. mansoni infection, which is characterized by a Th2 polarized immune response and inflammation in the liver. Because DCs are extremely potent in dictating the outcome of innate and acquired immune responses and since the functions of endogenous Gal-3 in DCs are presently unknown, we also attempted to determine its potential role in their maturation process and in their immunostimulatory and immunoregulatory properties.
Analysis of the parasitological parameters in infected WT and Gal-3−/− mice indicated no variation in the number of adult worms or in the number of eggs deposited in the tissues, confirming that parasites develop normally in infected Gal-3−/− mice (7, 38). This is in contrast to the Gal-3-induced direct antimicrobial (fungicidal) activity recently described (30). Analysis of spleen cell composition and numbers revealed a dramatic reduction in the number (but not in the frequency) of B and T cells (but not of DCs) in infected Gal-3−/− mice compared to WT controls. This effect may be due to differences in cell recruitment or proliferation in this immunological site between the two animal groups. Gal-3 has, indeed, been shown to exert potent chemotactic activities on many immune cell types and to play a part in their survival (8, 16), although in our setting we found no difference in the rate of T-cell survival between the different animal groups (data not shown). Analysis of the immune responses during the acute phase of the disease (7 weeks p.i.) clearly shows that during S. mansoni infection, Gal-3−/− mice mount an enhanced Th1-biased response, manifested by a higher level of IFN-γ and a decreased ratio of S. mansoni-specific IgG1 to IgG2b in serum (Fig. 2). Interestingly enough, this enhanced Th1-biased response persisted in Gal-3−/− infected mice at a more chronic time point (14 weeks p.i.) (data not shown). These data are in accordance with a recent study showing that Gal-3 deficiency leads to the development of a heightened Th1 response (and a decreased inflammation) in mice infected with T. gondii (6). The authors postulated that these effects are mediated, at least in part, by enhanced IL-12 production by DCs. During murine schistosomiasis, IL-12 production in DCs is difficult to evaluate, and we were unable to assess this parameter. Moreover, our data are also in line with those from Zuberi et al. (59) reporting that, in a murine model of asthma, Gal-3−/− mice develop a higher Th1 response (and a diminished inflammation in the lungs) than WT animals. We next investigated whether Gal-3 deficiency could lead to modulation of liver inflammation in infected mice. Recently, Van de Vijver et al. showed that, when coupled to inert beads, LN- and LDN-containing glycoconjugates induce granuloma formation in vivo, with similar features to schistosome egg-induced granulomas (54). Consistent with this finding and in agreement with Oliveira and coworkers (38), we here demonstrate that Gal-3 deficiency affects the size of liver granulomas but does not influence the proportion of immune cell types within the inflammatory foci. During the chronic phase of the disease, the livers of Gal-3-deficient mice were less damaged (not shown) and contained smaller lesions with a more diffuse network of collagen. Thus, endogenous Gal-3 plays a role in the pathology (lesion size and hepatic fibrosis) during murine schistosomiasis although whether this is due to endogenous and/or parasite-derived glycoconjugates is still unknown. Similarly, although we tend to favor the hypothesis that the reduced granuloma formation in Gal-3−/− animals is rather due to a more general defect in immune/inflammatory cell recruitment in inflamed sites in these mice, it is also possible that the Th1-biased response developed in these mice might play a part in the pathology (56).
We next investigated whether the observed amplified Th1 response in infected Gal-3−/− mice could be attributed to Gal-3 deficiency in DCs. The expression and the role of galectins in DCs have not been extensively studied, and their functions have been mainly investigated by treating cells with exogenously added recombinant protein. For instance, exogenous addition of Gal-1 (18, 31) or Gal-9 (11) promotes DC maturation and increases their immunostimulatory functions (increased Th1 response). Although artificial Gal-1 overexpression in DCs also results in similar effects (40), no study has addressed the role of endogenous galectins in DCs, and whether they could exert activities on DCs in a paracrine or autocrine manner is unknown. Similarly, no studies have addressed the function of Gal-3, which is known to exert many antagonistic functions compared to Gal-1 and Gal-9 (32, 45) in DCs. Gal-3 has been shown to be expressed in both human and mouse DCs (14, 51, 55). Here, we show that Gal-3 is expressed on the surface of BM-DCs and that it mediates the internalization of LN- and LDN-conjugated molecules in immature cells. Phenotypic analysis indicates that immature WT and Gal-3−/− BM-DCs display identical features, thus eliminating Gal-3 as a key factor in DC differentiation. This was confirmed in vivo by analyzing the number and the phenotype of splenic DCs in WT and Gal-3−/− mice (Table 2 and data not shown). Moreover, we showed that Gal-3 deficiency does not impact, in either a positive or a negative manner, the TLR-induced maturation of DCs. Moreover, Gal-3 ligation in the absence of other stimulation did not induce DC maturation in terms of phenotype (data not shown) and cytokine production (Fig. 4D), indicating that Gal-3 does not act as a danger signal in DCs. We next addressed the question of whether Gal-3 expression in DCs could modulate the priming and/or the differentiation of naïve T cells. Previous studies have shown that Gal-3 exerts potent effects on T-cell proliferation (25), apoptosis, and homeostasis (58). On the other hand, Gal-3 has also been reported to associate with specific N-glycans on the TCR complex to control TCR-mediated intracellular pathways in T lymphocytes (13, 35). This process appears to maintain the development of immune responses, but whether this is mediated through Gal-3 expression by DCs or by other cells (including T cells themselves) is unknown. Here, we show for the first time that Gal-3 expression in DCs is essential and sufficient to control the magnitude of T-cell priming both in vitro and in vivo, since Gal-3−/− DCs enhanced the proliferation and the cytokine release by T lymphocytes. In vivo, this effect was not due to an enhanced migration of DCs into the spleen or to an increased survival of T cells in this organ (not shown). Importantly, although it controls cytokine release by T cells, loss of Gal-3 expression by DCs does not modulate the Th1/Th2 balance of the immune response. This is in line with the nonessential role of Gal-3 in DC maturation (Fig. 4D), a complex process influencing the quality of the ensuing immune response. In our experimental infectious model, it is difficult to address the exact mechanisms behind the more pronounced Th1 bias observed in Gal-3-deficient animals. Although our data show that the lack of Gal-3 in DCs enhances the T-cell response (Fig. 5), it cannot fully explain our observations (similar Th2 response in WT and Gal-3−/− mice). Thus, it is likely that Gal-3 deficiency in other immune cell types is also important in this phenomenon.
Taken as a whole, this study reveals for the first time that Gal-3 expression in DCs plays a part in the control of the T-cell response but does not regulate the differentiation or maturation of DCs. This study also confirms, in an infectious model, that Gal-3 acts as an important regulator of immune and inflammatory responses.
Acknowledgments
We gratefully acknowledge C. Vendeville for her outstanding technical assistance. We thank R. Pierce (Inserm U547, Institut Pasteur de Lille) for critical reading of the manuscript.
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Pasteur Institute of Lille, and the University of Lille 2. This project was also supported by the Conseil Régional Nord Pas de Calais/Actions de Recherche Concertées d'initiative régionale and Fonds Européen de Développement Régional. L.B. was supported by a grant from the Ministère de l'Education Nationale de la Recherche et Technique, and F.V. was the recipient of a doctoral fellowship from l'Ecole Normale Supérieure de Lyon. C.V., P.G., T.J., and C.F. are supported by the Inserm, and F.T. was supported by the Centre National de la Recherche Scientifique.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Aksoy, E., C. S. Zouain, F. Vanhoutte, J. Fontaine, N. Pavelka, N. Thieblemont, F. Willems, P. Ricciardi-Castagnoli, M. Goldman, M. Capron, B. Ryffel, and F. Trottein. 2005. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 280:277-283. [DOI] [PubMed] [Google Scholar]
- 2.Almkvist, J., and A. Karlsson. 2004. Galectins as inflammatory mediators. Glycoconj. J. 19:575-581. [DOI] [PubMed] [Google Scholar]
- 3.Asselin-Paturel, C., G. Brizard, J. J. Pin, F. Briere, and G. Trinchieri. 2003. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 171:6466-6477. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Beatty, W. L., E. R. Rhoades, D. K. Hsu, F. T. Liu, and D. G. Russell. 2002. Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 4:167-176. [DOI] [PubMed] [Google Scholar]
- 6.Bernardes, E. S., N. M. Silva, L. P. Ruas, J. R. Mineo, A. M. Loyola, D. K. Hsu, F. T. Liu, R. Chammas, and M. C. Roque-Barreira. 2006. Toxoplasma gondii infection reveals a novel regulatory role for galectin-3 in the interface of innate and adaptive immunity. Am. J. Pathol. 168:1910-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickle, Q., and H. Helmby. 2007. Lack of galectin-3 involvement in murine intestinal nematode and schistosome infection. Parasite Immunol. 29:93-100. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. Y., F. T. Liu, and R. Y. Yang. 2005. Roles of galectin-3 in immune responses. Arch. Immunol. Ther. Exp. 53:497-504. [PubMed] [Google Scholar]
- 9.Colnot, C., D. Fowlis, M. A. Ripoche, I. Bouchaert, and F. Poirier. 1998. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev. Dyn. 211:306-313. [DOI] [PubMed] [Google Scholar]
- 10.Colnot, C., M. A. Ripoche, G. Milon, X. Montagutelli, P. R. Crocker, and F. Poirier. 1998. Maintenance of granulocyte numbers during acute peritonitis is defective in galectin-3-null mutant mice. Immunology 94:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai, S. Y., R. Nakagawa, A. Itoh, H. Murakami, Y. Kashio, H. Abe, S. Katoh, K. Kontani, M. Kihara, S. L. Zhang, T. Hata, T. Nakamura, A. Yamauchi, and M. Hirashima. 2005. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 175:2974-2981. [DOI] [PubMed] [Google Scholar]
- 12.del Pozo, V., M. Rojo, M. L. Rubio, I. Cortegano, B. Cardaba, S. Gallardo, M. Ortega, E. Civantos, E. Lopez, C. Martin-Mosquero, G. Peces-Barba, P. Palomino, N. Gonzalez-Mangado, and C. Lahoz. 2002. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through interleukin-5 gene downregulation. Am. J. Respir. Crit. Care Med. 166:732-737. [DOI] [PubMed] [Google Scholar]
- 13.Demetriou, M., M. Granovsky, S. Quaggin, and J. W. Dennis. 2001. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409:733-739. [DOI] [PubMed] [Google Scholar]
- 14.Dietz, A. B., P. A. Bulur, G. J. Knutson, R. Matasic, and S. Vuk-Pavlovic. 2000. Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem. Biophys. Res. Commun. 275:731-738. [DOI] [PubMed] [Google Scholar]
- 15.Doenhoff, M., R. Musallam, J. Bain, and A. McGregor. 1978. Studies on the host-parasite relationship in Schistosoma mansoni-infected mice: the immunological dependence of parasite egg excretion. Immunology 35:771-778. [PMC free article] [PubMed] [Google Scholar]
- 16.Dumic, J., S. Dabelic, and M. Flogel. 2006. Galectin-3: an open-ended story. Biochim. Biophys. Acta 1760:616-635. [DOI] [PubMed] [Google Scholar]
- 17.Faveeuw, C., V. Angeli, J. Fontaine, C. Maliszewski, A. Capron, L. Van Kaer, M. Moser, M. Capron, and F. Trottein. 2002. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J. Immunol. 169:906-912. [DOI] [PubMed] [Google Scholar]
- 18.Fulcher, J. A., S. T. Hashimi, E. L. Levroney, M. Pang, K. B. Gurney, L. G. Baum, and B. Lee. 2006. Galectin-1-matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J. Immunol. 177:216-226. [DOI] [PubMed] [Google Scholar]
- 19.Furtak, V., F. Hatcher, and J. Ochieng. 2001. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem. Biophys. Res. Commun. 289:845-850. [DOI] [PubMed] [Google Scholar]
- 20.Hirabayashi, J., T. Hashidate, Y. Arata, N. Nishi, T. Nakamura, M. Hirashima, T. Urashima, T. Oka, M. Futai, W. E. Muller, F. Yagi, and K. Kasai. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta 1572:232-254. [DOI] [PubMed] [Google Scholar]
- 21.Hsu, D. K., R. Y. Yang, Z. Pan, L. Yu, D. R. Salomon, W. P. Fung-Leung, and F. T. Liu. 2000. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 156:1073-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, R. C. 1999. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1473:172-185. [DOI] [PubMed] [Google Scholar]
- 23.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 24.Jeng, K. C., L. G. Frigeri, and F. T. Liu. 1994. An endogenous lectin, galectin-3 (epsilon BP/Mac-2), potentiates IL-1 production by human monocytes. Immunol. Lett. 42:113-116. [DOI] [PubMed] [Google Scholar]
- 25.Joo, H. G., P. S. Goedegebuure, N. Sadanaga, M. Nagoshi, W. von Bernstorff, and T. J. Eberlein. 2001. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J. Leukoc. Biol. 69:555-564. [PubMed] [Google Scholar]
- 26.Jouault, T., M. El Abed-El Behi, M. Martinez-Esparza, L. Breuilh, P. A. Trinel, M. Chamaillard, F. Trottein, and D. Poulain. 2006. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J. Immunol. 177:4679-4687. [DOI] [PubMed] [Google Scholar]
- 27.Karlsson, A., P. Follin, H. Leffler, and C. Dahlgren. 1998. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood 91:3430-3438. [PubMed] [Google Scholar]
- 28.Kasai, K., and J. Hirabayashi. 1996. Galectins: a family of animal lectins that decipher glycocodes. J. Biochem. (Tokyo) 119:1-8. [DOI] [PubMed] [Google Scholar]
- 29.Khoo, K. H., and A. Dell. 2001. Glycoconjugates from parasitic helminths: structure diversity and immunobiological implications. Adv. Exp. Med. Biol. 491:185-205. [DOI] [PubMed] [Google Scholar]
- 30.Kohatsu, L., D. K. Hsu, A. G. Jegalian, F. T. Liu, and L. G. Baum. 2006. Galectin-3 induces death of Candida species expressing specific beta-1,2-linked mannans. J. Immunol. 177:4718-4726. [DOI] [PubMed] [Google Scholar]
- 31.Levroney, E. L., H. C. Aguilar, J. A. Fulcher, L. Kohatsu, K. E. Pace, M. Pang, K. B. Gurney, L. G. Baum, and B. Lee. 2005. Novel innate immune functions for galectin-1: galectin-1 inhibits cell fusion by Nipah virus envelope glycoproteins and augments dendritic cell secretion of proinflammatory cytokines. J. Immunol. 175:413-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu, F. T. 2000. Galectins: a new family of regulators of inflammation. Clin. Immunol. 97:79-88. [DOI] [PubMed] [Google Scholar]
- 33.Liu, F. T., and G. A. Rabinovich. 2005. Galectins as modulators of tumour progression. Nat. Rev. Cancer 5:29-41. [DOI] [PubMed] [Google Scholar]
- 34.Lopez, E., V. del Pozo, T. Miguel, B. Sastre, C. Seoane, E. Civantos, E. Llanes, M. L. Baeza, P. Palomino, B. Cardaba, S. Gallardo, F. Manzarbeitia, J. M. Zubeldia, and C. Lahoz. 2006. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J. Immunol. 176:1943-1950. [DOI] [PubMed] [Google Scholar]
- 35.Morgan, R., G. Gao, J. Pawling, J. W. Dennis, M. Demetriou, and B. Li. 2004. N-acetylglucosaminyltransferase V (Mgat5)-mediated N-glycosylation negatively regulates Th1 cytokine production by T cells. J. Immunol. 173:7200-7208. [DOI] [PubMed] [Google Scholar]
- 36.Ochieng, J., V. Furtak, and P. Lukyanov. 2004. Extracellular functions of galectin-3. Glycoconj. J. 19:527-535. [DOI] [PubMed] [Google Scholar]
- 37.Ochieng, J., M. L. Leite-Browning, and P. Warfield. 1998. Regulation of cellular adhesion to extracellular matrix proteins by galectin-3. Biochem. Biophys. Res. Commun. 246:788-791. [DOI] [PubMed] [Google Scholar]
- 38.Oliveira, F. L., P. Frazao, R. Chammas, D. K. Hsu, F. T. Liu, R. Borojevic, C. M. Takiya, and M. C. El-Cheikh. 2007. Kinetics of mobilization and differentiation of lymphohematopoietic cells during experimental murine schistosomiasis in galectin-3-/- mice. J. Leukoc. Biol. 82:300-310. [DOI] [PubMed] [Google Scholar]
- 39.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 40.Perone, M. J., A. T. Larregina, W. J. Shufesky, G. D. Papworth, M. L. Sullivan, A. F. Zahorchak, D. B. Stolz, L. G. Baum, S. C. Watkins, A. W. Thomson, and A. E. Morelli. 2006. Transgenic galectin-1 induces maturation of dendritic cells that elicit contrasting responses in naive and activated T cells. J. Immunol. 176:7207-7220. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovich, G. A., L. G. Baum, N. Tinari, R. Paganelli, C. Natoli, F. T. Liu, and S. Iacobelli. 2002. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 23:313-320. [DOI] [PubMed] [Google Scholar]
- 42.Rabinovich, G. A., and A. Gruppi. 2005. Galectins as immunoregulators during infectious processes: from microbial invasion to the resolution of the disease. Parasite Immunol. 27:103-114. [DOI] [PubMed] [Google Scholar]
- 43.Rabinovich, G. A., N. Rubinstein, and M. A. Toscano. 2002. Role of galectins in inflammatory and immunomodulatory processes. Biochim. Biophys. Acta 1572:274-284. [DOI] [PubMed] [Google Scholar]
- 44.Ramalho-Pinto, F. J., G. Gazzinelli, R. E. Howells, T. A. Mota-Santos, E. A. Figueiredo, and J. Pellegrino. 1974. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp. Parasitol. 36:360-372. [DOI] [PubMed] [Google Scholar]
- 45.Rubinstein, N., J. M. Ilarregui, M. A. Toscano, and G. A. Rabinovich. 2004. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens 64:1-12. [DOI] [PubMed] [Google Scholar]
- 46.Sano, H., D. K. Hsu, J. R. Apgar, L. Yu, B. B. Sharma, I. Kuwabara, S. Izui, and F. T. Liu. 2003. Critical role of galectin-3 in phagocytosis by macrophages. J. Clin. Investig. 112:389-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sano, H., D. K. Hsu, L. Yu, J. R. Apgar, I. Kuwabara, T. Yamanaka, M. Hirashima, and F. T. Liu. 2000. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 165:2156-2164. [DOI] [PubMed] [Google Scholar]
- 48.Sato, S., and J. Nieminen. 2004. Seeing strangers or announcing “danger”: galectin-3 in two models of innate immunity. Glycoconj. J. 19:583-591. [DOI] [PubMed] [Google Scholar]
- 49.Sato, S., N. Ouellet, I. Pelletier, M. Simard, A. Rancourt, and M. G. Bergeron. 2002. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. J. Immunol. 168:1813-1822. [DOI] [PubMed] [Google Scholar]
- 50.Srivatsan, J., D. F. Smith, and R. D. Cummings. 1992. Schistosoma mansoni synthesizes novel biantennary Asn-linked oligosaccharides containing terminal beta-linked N-acetylgalactosamine. Glycobiology 2:445-452. [DOI] [PubMed] [Google Scholar]
- 51.Thery, C., M. Boussac, P. Veron, P. Ricciardi-Castagnoli, G. Raposo, J. Garin, and S. Amigorena. 2001. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166:7309-7318. [DOI] [PubMed] [Google Scholar]
- 52.Truong, M. J., V. Gruart, J. P. Kusnierz, J. P. Papin, S. Loiseau, A. Capron, and M. Capron. 1993. Human neutrophils express immunoglobulin E (IgE)-binding proteins (Mac-2/epsilon BP) of the S-type lectin family: role in IgE-dependent activation. J. Exp. Med. 177:243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Berg, T. K., H. Honing, N. Franke, A. van Remoortere, W. E. Schiphorst, F. T. Liu, A. M. Deelder, R. D. Cummings, C. H. Hokke, and I. van Die. 2004. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J. Immunol. 173:1902-1907. [DOI] [PubMed] [Google Scholar]
- 54.Van de Vijver, K. K., A. M. Deelder, W. Jacobs, E. A. Van Marck, and C. H. Hokke. 2006. LacdiNAc- and LacNAc-containing glycans induce granulomas in an in vivo model for schistosome egg-induced hepatic granuloma formation. Glycobiology 16:237-243. [DOI] [PubMed] [Google Scholar]
- 55.Vray, B., I. Camby, V. Vercruysse, T. Mijatovic, N. V. Bovin, P. Ricciardi-Castagnoli, H. Kaltner, I. Salmon, H. J. Gabius, and R. Kiss. 2004. Up-regulation of galectin-3 and its ligands by Trypanosoma cruzi infection with modulation of adhesion and migration of murine dendritic cells. Glycobiology 14:647-657. [DOI] [PubMed] [Google Scholar]
- 56.Wynn, T. A., R. W. Thompson, A. W. Cheever, and M. M. Mentink-Kane. 2004. Immunopathogenesis of schistosomiasis. Immunol. Rev. 201:156-167. [DOI] [PubMed] [Google Scholar]
- 57.Yamaoka, A., I. Kuwabara, L. G. Frigeri, and F. T. Liu. 1995. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. J. Immunol. 154:3479-3487. [PubMed] [Google Scholar]
- 58.Yang, R. Y., D. K. Hsu, and F. T. Liu. 1996. Expression of galectin-3 modulates T-cell growth and apoptosis. Proc. Natl. Acad. Sci. USA 93:6737-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuberi, R. I., D. K. Hsu, O. Kalayci, H. Y. Chen, H. K. Sheldon, L. Yu, J. R. Apgar, T. Kawakami, C. M. Lilly, and F. T. Liu. 2004. Critical role for galectin-3 in airway inflammation and bronchial hyperresponsiveness in a murine model of asthma. Am. J. Pathol. 165:2045-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]