Abstract
The gene annotated BAB2_1150 in the Brucella abortus 2308 genome sequence is predicted to encode a homolog of the well-characterized heme transporter ShuA of Shigella dysenteriae and accordingly has been given the designation bhuA (Brucella heme utilization). Phenotypic analysis of an isogenic bhuA mutant derived from B. abortus 2308 verified that there is a link between BhuA and the ability of the parent strain to use heme as an iron source in in vitro assays. Maximum expression of bhuA in B. abortus 2308 is observed during stationary phase when this strain in cultivated in low-iron minimal medium, and a comparison of the growth characteristics of the B. abortus bhuA mutant and 2308 in this medium suggested that heme serves as an important iron source for the parent strain during stationary phase. The B. abortus bhuA mutant HR1703 exhibits significant attenuation in cultured murine macrophages compared to strain 2308, and unlike its parent strain, the B. abortus bhuA mutant is unable to maintain a chronic spleen infection in experimentally infected BALB/c mice. These experimental findings suggest that heme and/or heme-containing proteins represent important iron sources for B. abortus 2308 during its residence in the mammalian host and that BhuA is required for efficient utilization of these iron sources.
The capacity of a bacterium to acquire sufficient amounts of iron is almost always linked to its ability to survive in its biological niche. Accomplishing this in the iron-restricted environment of a mammalian host represents a major virulence determinant in the case of bacterial pathogens (41). Indeed, pathogens often employ specialized transport machinery to counteract the iron restriction associated with the host innate immune defense.
Brucella abortus is a gram-negative zoonotic pathogen that causes abortion and infertility in ruminants and a chronic debilitating disease known as undulant fever in humans (1). Survival and replication of the brucellae within host macrophages are critical for virulence (38). These bacteria produce the monocatechol 2,3-dihydroxybenzoic acid (2,3-DHBA) (22) and a more complex 2,3-DHBA-based compound known as brucebactin (18) in response to iron limitation in vitro. Experimental evidence indicates that both of these compounds function as siderophores. Biochemical studies (23) and genome analysis (39) have also suggested that these are the only siderophores produced by the brucellae. Neither 2,3-DHBA nor brucebactin is required for the survival and replication of B. abortus 2308 in cultured murine macrophages or for wild-type virulence of this strain in experimentally infected BALB/c or C57BL6 mice (4, 18, 31). Consequently, the question of what iron source(s) the brucellae use during their prolonged residence in host macrophages during the chronic stage of infection remains unanswered (39).
Brucella melitensis 16M and B. abortus 2308 have been shown to utilize heme as an iron source in vitro (5, 11). Due to the central role of macrophages in heme recycling in mammals (10, 45) heme may represent a relevant iron source for the brucellae during residence in the macrophage (39). Other bacterial pathogens, including Shigella dysenteriae, Escherichia coli, Vibrio cholerae, Yersinia pestis, Staphylococcus aureus, Haemophilus influenzae, and Bordetella spp., produce heme transporters and have the capacity to use heme as an iron source. Loss of these heme transporters has a detrimental effect on the virulence of some of these pathogens in experimental animal models or cell lines, while in others loss of the heme transporter has little or no impact on virulence (26, 29, 35, 42, 46, 47, 48).
Analysis of the currently available Brucella genome sequences (9, 13, 19, 32) revealed the presence of a homolog of the shuA gene of S. dysenteriae (28). This gene encodes a TonB-dependent outer membrane protein involved in heme transport in S. dysenteriae. To determine if the Brucella ShuA homolog performs a similar function and to assess the relative importance of heme as an iron source for the brucellae, a derivative of virulent B. abortus 2308 lacking the ShuA homolog was constructed and evaluated to determine its phenotype in vitro and in the mouse model.
MATERIALS AND METHODS
Bacterial strains.
B. abortus 2308 and derivatives of this strain were cultivated on Schaedler agar supplemented with 5% defibrinated bovine blood (SBA) at 37°C with 5% CO2 or in brucella broth at 37°C with shaking unless otherwise noted. Low-iron minimal medium was prepared as previously described (22), and 50 μM FeCl3 was added to this medium to provide iron-replete growth conditions. Escherichia coli strain DH5α was used as a host strain for recombinant DNA procedures, and this strain was cultivated on tryptic soy agar at 37°C or in LB broth at 37°C with shaking. Growth media were supplemented with ampicillin (25 μg/ml for B. abortus and 100 μg/ml for E. coli DH5α), chloramphenicol (5 μg/ml for B. abortus and 30 μg/ml for E. coli DH5α), and/or kanamycin (45 μg/ml) as necessary. Brucella stock cultures were maintained in brucella broth supplemented with 25% glycerol, and E. coli stock cultures were maintained in LB supplemented with 25% glycerol at −80°C.
Construction of the B. abortus bhuA mutant.
Oligonucleotide primers (forward primer TGTTGAGCATGAAAACCAAA and reverse primer TATTGATTGACAGCAAATTG) and PCR were used to amplify a 3,279-bp fragment of genomic DNA from B. abortus 2308 encompassing the bhuA gene (BAB2_1150). This fragment was cloned into pGEM-T Easy (Promega) and digested with ClaI, which resulted in removal of 1,549 bp of the 1,983-bp bhuA coding region. The linearized plasmid was then treated with the Klenow fragment of DNA polymerase I and ligated with the chloramphenicol acetyltransferase (cat) gene from pBlue-CM2 (36). The resulting plasmid was then used in a gene replacement strategy (14) to construct an isogenic bhuA mutant from virulent B. abortus 2308. The genotype of a B. abortus bhuA mutant (designated HR1703) constructed in this manner was confirmed by PCR analysis of genomic DNA from this strain using bhuA-, cat-, and pGEM-specific primer sets. Crystal violet exclusion was used to verify that the B. abortus bhuA mutant retained the smooth lipopolysaccharide phenotype (3).
Attempts to use a pMR10-based plasmid (16) carrying a cloned copy of the intact bhuA gene for genetic complementation of the B. abortus bhuA mutant (see Results) were unsuccessful. Therefore, relying on the observation that bhuA is transcribed as a monocistronic gene in B. abortus 2308 (data not shown), the bhuA gene was reconstructed in HR1703 using the following procedures. A version of the pGEM-based plasmid containing the 3,280-bp DNA fragment bearing the intact bhuA gene was introduced into B. abortus HR1703 by electroporation. Transformants were first plated on SBA containing 25 μg/μl ampicillin to select for merodiploid derivatives of HR1703 containing both the cat-disrupted and intact versions of the bhuA locus. One of these transformants was then subcultured in brucella broth at 37°C with shaking and plated onto SBA. Following replica plating of the bacterial colonies obtained onto SBA and SBA containing 25 μg/ml ampicillin and 5 μg/ml chloramphenicol, colonies that grew on SBA but not on SBA supplemented with antibiotics were selected. Reconstruction of the bhuA locus in ampicillin- and chloramphenicol-sensitive derivatives of HR1703 derived in this manner was verified by PCR analysis of genomic DNA from these strains with bhuA-, cat-, and pGEM-specific primer sets. One of these derivatives was selected for further examination and designated HR1703RC. The smooth lipopolysaccharide phenotype of HR1703RC was verified by crystal violet dye exclusion.
Capacity of the B. abortus strains to use heme as an iron source in vitro.
Free iron was removed from the hemin stock solutions used for the iron source utilization assays using the procedure described by Staggs and Perry (43). To test for the capacity of hemin to serve as an iron source, B. abortus strains were grown on SBA for 48 h at 37°C with 5% CO2. Bacterial cells were harvested into phosphate-buffered saline (pH 7.2), and the optical density at 600 nm was adjusted to 0.15 (corresponding to 109 CFU/ml). One hundred-microliter portions of these bacterial cell suspensions were then added to 500-ml flasks containing 100 ml low-iron minimal medium, and the flasks were incubated in a water bath at 37°C with shaking at 250 rpm. Following 96 h of growth the optical densities at 600 nm of the bacterial cultures were adjusted to 0.15, and 100-μl portions of the bacterial cell suspensions were mixed with 3 ml trypic soy broth (TSB) containing 0.7% agar and 300 μM ethylenediaminediacetate (EDDA). The mixtures were overlaid onto plates with TSB containing 1.5% agar and 300 μM EDDA. Seven-millimeter sterile filter paper (Whatman no. 3) disks were placed onto the plates, 10 μl of a 2 mM solution of hemin, a 20 mM solution of hemin, or a 50 mM solution of FeCl3 was added to the filter disks, and the plates were incubated for 96 h at 37°C with 5% CO2. Following this incubation period, the diameter (in millimeters) of the zone of bacterial growth around each filter disk was measured and recorded.
Construction of a bhuA-lacZ fusion and β-galactosidase assays.
PCR with sequence-specific oligonucleotide primers (forward primer ATAAGGCGACCTTACCGAG and reverse primer GACGGTGGCAAGCAGAGA) was used to amplify a 445-bp fragment of genomic DNA from B. abortus 2308. This fragment, which contained approximately 260 bp upstream of the bhuA open reading frame, was cloned into the EcoRV site upstream of the promoterless lacZ gene in pMR15 (17). The resulting plasmid (designated pbhuA-lacZ) and pMR15 were introduced into B. abortus 2308 by electroporation. Derivatives of B. abortus 2308 carrying these plasmids were grown in the low-iron minimal medium described by López-Goñi et al. (22) with and without 50 μM FeCl3. β-Galactosidase production by these cultures was measured using the procedures described by Miller (27).
Relative quantification of specific transcript levels using real-time RT-PCR.
Total cellular RNA was isolated from B. abortus 2308 following growth in low-iron minimal medium and low-iron minimal medium supplemented with 50 μM FeCl3 using a modification of the cesium chloride-based method described by Martin et al. (25). Following harvest, the bacterial cells were resuspended in a buffer containing lysozyme (5 mg/ml lysozyme, 50 mM glucose, 25 mM Tris [pH 8.0], and 10 mM EDTA in diethyl pyrocarbonate-treated sterile distilled deionized water) prior to lysis with sodium dodecyl sulfate. After collection from the cesium chloride tubes, the RNA preparations were ethanol precipitated and treated with Turbo DNase (Ambion) following the manufacturer's instructions to remove residual contaminating DNA. The absence of DNA from the RNA preparations was confirmed by the failure of the gene amplification reactions to generate a product detectable by agarose gel electrophoresis in the absence of reverse transcriptase in reverse transcriptase PCR (RT-PCR). Concentrations of RNA in the samples were determined by measuring the absorbance at 260 nm.
An iScript cDNA synthesis kit (Bio-Rad) was used to amplify 40 ng of RNA from these preparations into cDNA for each preparation following the manufacturer's instructions and using the random primers supplied with the kit. The cDNA preparations were then used as the templates in real-time RT-PCR analysis (34) to evaluate the relative levels of gene-specific mRNA transcripts in the total cellular RNA preparations. Gene-specific oligonucleotide primers (bhuA forward primer 5′-CGTTTCTCAAGCGCGTGGAAGT-3′; bhuA reverse primer 5′-AGCGCATTAACGGTCTCGTAGC-3′; BAB1_0370 forward primer 5′-CGTGCTTGAAACCTATGACG-3′; BAB1_0370 reverse primer 5′-TGTTGATATCCATCAGCACCA-3′; rplL forward primer 5′-CAGAAGAAAAGACCGAATTCGA-3′; rplL reverse primer 5′-GCACTTCCTTGATCACGTTGAT-3′) were designed to produce products that were approximately 100 to 150 bp long. Primer binding efficiencies for the primer sets were determined using a standard curve and the Bio-Rad iCycler. The randomly primed cDNA preparations generated from the total cellular RNA preparations were used in reaction mixtures containing 25 μl iQ SYBR green Supermix (Bio-Rad), 1.5 μl each of 5 μM forward and reverse gene-specific primers, 21 μl distilled deionized water, and 1 μl cDNA template per well. The PCR conditions were one cycle of 95°C for 3 min (melting), followed by 35 cycles of 95°C for 10 s, 58°C for 15 s, and 72°C for 15 s (amplification). Fluorescence data were collected with a Bio-Rad iCycler during the elongation step. A melting curve analysis was performed after amplification to ensure that a single product was formed with the primer sets. Samples were assayed three times in triplicate with purified RNA without reverse transcriptase as a negative control on each reaction plate evaluated. The differences in the relative levels of the bhuA- and BAB1_0370-specific transcripts present in the RNA preparations obtained under different experimental conditions were calculated using methods described by Pfaffl (34), using the rplL gene as an internal standard. This gene (designated BAB1_1265 in the B. abortus 2308 genome) encodes the ribosomal L7/L12 protein, and its expression is constitutive in 2308 under the experimental conditions used here.
Survival and replication of the B. abortus strains in cultured murine macrophages.
A modification of the methods described by Gee et al. (16) was used to evaluate the capacity of the B. abortus strains to survive and replicate in cultured resident peritoneal macrophages obtained from BALB/c mice. Because unopsonized brucellae were used for these experiments, the multiplicity of infection was increased from 100:1 to 500:1 (ratio of bacteria to macrophages) to ensure uniform infection of the macrophage monolayer (B. H. Bellaire, personal communication). After addition of the bacteria, the cultured macrophages were subjected to centrifugation at 400 × g at 4°C for 10 min to optimize contact of the unopsonized brucellae with the host cell monolayer and synchronize the infection.
Experimental infection of BALB/c mice.
Six-week-old female BALB/c mice were infected via the peritoneal route with 5 × 104 CFU of B. abortus 2308, HR1703 (2308 bhuA), or HR1703RC (HR1703 bhuA+). The number of brucellae present per spleen in the infected animals was determined at 1, 4, and 8 weeks postinfection as described previously (16).
Statistical analysis.
All statistical analyses were performed using the Student two-tailed t test (40). P values of ≤0.05 were considered significant.
RESULTS
Identification of a putative heme transporter in B. abortus 2308.
Heme serves as an efficient iron source for both B. abortus 2308 (5) and B. melitensis 16M (11) in in vitro assays. Phenotypic analysis of a hemH mutant derived from B. abortus 2308 has also shown that the parental strain has the capacity to transport intact heme into the cytoplasm (2). Gram-negative bacteria that transport the intact heme molecule across both membranes generally rely upon TonB-dependent transporters to move this molecule across the outer membrane (33). A survey of the B. abortus 2308 genome sequence revealed only three genes predicted to encode TonB-dependent outer membrane iron transporters, and only one of these was predicted to have the characteristics expected of a heme transporter. These characteristics include amino acid sequence homology and colinearity with other well-characterized outer membrane heme transporters, the presence of amino acid motifs predicted to interact with TonB, and the presence of conserved His residues corresponding to the His-128 and His-461 residues required for heme transport mediated by the HemR protein of Yersinia enterocolitica (7). The gene annotated BAB2_1150 in the B. abortus 2308 genome sequence is predicted to encode a 661-amino-acid protein that shares considerable amino acid identity, similarity, and colinearity with other well-characterized bacterial TonB-dependent heme transporters, including the S. dysenteriae ShuA (18% identity and 32% similarity) (28), Plesiomonas shigelloides HugA (19% identity and 32% similarity) (20), Yersinia pestis HmuR (18% identity and 31% similarity) (46), Y. enterocolitica HemR (19% identity and 31% similarity) (44), Bradyrhizobium japonicum HmuR (18% identity and 30% similarity) (30), and Vibrio vulnificus HupA (19% identity and 30% similarity) (21) proteins. Amino acid residues 29 to 37, 619 to 624, 83 to 115, and 133 to 154 of the Brucella ShuA homolog display significant homology with the consensus TonB boxes 1, 2, 3, and 4 (24), respectively, that are characteristic of bacterial outer membrane heme transporters. More importantly, amino acid sequence alignments suggest that His-119 and His-448 of the Brucella ShuA homolog correspond to the His-128 and His-461 residues that are critical for heme transport by the Y. enterocolitica HemR protein (7) and are highly conserved in many other TonB-dependent outer membrane heme transporters of this class. Based on these features, the putative Brucella heme transporter was given the provisional designation BhuA (Brucella heme uptake A), and the corresponding gene was designated bhuA. Genes homologous to bhuA are also present in the genome sequences of B. abortus 9-941 (BruAb2_1126), B. melitensis 16M (BMEII0105), and Brucella suis 1330 (BRA1190). RT-PCR analysis of total RNA from B. abortus 2308 with primers specific for the bhuA coding region and flanking regions indicated that bhuA is monocistronic (data not shown).
The B. abortus bhuA mutant cannot use heme as an iron source in vitro.
The B. abortus bhuA mutant retained the ability to use FeCl3 (Table 1) and 2,3-DHBA (data not shown) as iron sources in an in vitro assay that employs a solid growth medium containing a chelator, but it was unable to use hemin as an iron source. In contrast, the parental 2308 strain and HR1703RC exhibited robust growth around disks containing hemin on the chelator plates (Table 1). These experimental findings support the proposition that BhuA serves as a TonB-dependent heme transporter.
TABLE 1.
Capacities of B. abortus 2308, HR1703 (2308 bhuA), and HR1703RC (HR1703 bhuA+) to use hemin and FeCl3 as iron sources
| Strain | Diam (mm) of zone of bacterial growth witha:
|
||
|---|---|---|---|
| 2 mM hemin | 20 mM hemin | FeCl3 | |
| 2308 | 22.3 ± 2.5b | 32.7 ± 1.2d | 52.7 ± 2.1 |
| HR1703 | NGc | NG | 28.33 ± 1.3 |
| HR1703RC | 19.7 ± 1.5d | 30.3 ± 1.5d | 51.7 ± 3.8 |
The diameters of the zones of bacterial growth on TSB supplemented with 300 μM EDDA around filter disks impregnated with 10 μl of a 2 mM solution of hemin, a 20 mM solution of hemin, or a 50 mM solution of FeCl3 were measured after 96 h of incubation at 37°C with 5% CO2. The values are the means ± standard deviations of the average zone sizes obtained from three separate experiments, and three separate determinations of growth were performed for each strain in each experiment.
P ≤ 0.01 for a comparison of HR1703 with 2308 and HR1703RC.
NG, no growth. No zone of bacterial growth was observed surrounding the disks impregnated with either concentration of hemin for B. abortus HR1703.
P ≤ 0.005 for a comparison of HR1703 with 2308 and HR1703RC.
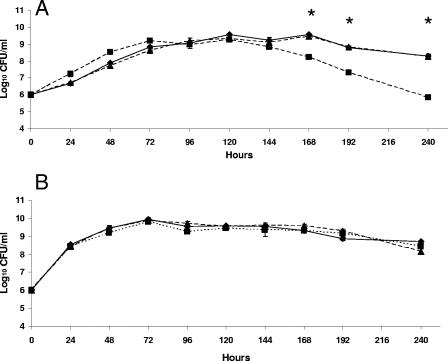
Although the B. abortus bhuA mutant was clearly able to use FeCl3 as an iron source on the chelator plates, this strain produced smaller zones of growth around disks containing FeCl3 than the parental 2308 strain or HR1703RC produced (Table 1). The physiologic basis for the smaller zone size is presently unknown, but this zone size does not appear to be the result of a generalized inability of the the B. abortus bhuA mutant to use FeCl3 as an iron source since addition of this compound to low-iron minimal medium allowed HR1703 to exhibit the same growth characteristics in this medium as 2308 and HR1703RC (Fig. 1B).
FIG. 1.
Growth and viability of B. abortus 2308 (diamonds), HR1703 (2308 bhuA) (squares), and HR1703RC (HR1703 bhuA+) (triangles) in (A) low-iron minimal medium and (B) low-iron minimal medium containing 50 μM FeCl3. The data are means and standard deviations for triplicate determinations from a single flask for each strain at each experimental time point in a single experiment. An asterisk indicates that the P value is <0.05 for a comparison of the data obtained for HR1703 and the data obtained for 2308 and HR1703RC. The data are representative of multiple (≥3) experiments from which equivalent results and statistical trends were obtained.
The B. abortus bhuA mutant exhibits a stationary-phase defect in iron acquisition.
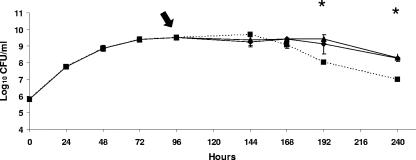
Although the B. abortus bhuA mutant HR1703 consistently demonstrated a slightly different growth pattern than B. abortus 2308 or HR1703RC during cultivation in low-iron minimal medium, all three of these strains reached stationary phase at approximately the same time postinoculation and at the same cell density (Fig. 1A). However, the B. abortus bhuA mutant began to exhibit an accelerated loss of viability after approximately 144 h of culture in low-iron minimal medium, but the parental 2308 strain and HR1703RC did not (Fig. 1A). In contrast, an accelerated loss of stationary-phase viability was not observed for the B. abortus bhuA mutant when this strain was grown in low-iron minimal medium supplemented with 50 μM FeCl3 at the time of inoculation (Fig. 1B). Addition of either FeCl3 (Fe3+) or Fe(NH4)2(SO4)2 (Fe2+) to cultures of the B. abortus bhuA mutant after 96 h of growth in low-iron minimal medium also prevented the loss of stationary-phase viability of this mutant (Fig. 2). These experimental findings suggest that BhuA plays an important role in stationary-phase iron acquisition in B. abortus 2308.
FIG. 2.
Growth and viability of the B. abortus bhuA mutant HR1703 in low-iron minimal medium (squares) or in this medium supplemented with 50 μM FeCl3 (diamonds) or 50 μM Fe(NH4)2(SO4)2 (triangles) at 96 h postinoculation. The arrow indicates the time of addition of FeCl3 and Fe(NH4)2(SO4)2 to the bacterial cultures. The data are means and standard deviations for triplicate determinations from a single flask for each strain at each experimental time point in a single experiment. An asterisk indicates that the P value is <0.05 for a comparison of the unsupplemented HR1703 culture and a culture of this strain supplemented with FeCl3 or Fe(NH4)2(SO4)2. The data are representative of multiple (≥3) experiments from which equivalent results and statistical trends were obtained.
The bhuA gene exhibits maximum expression in B. abortus 2308 during stationary phase.
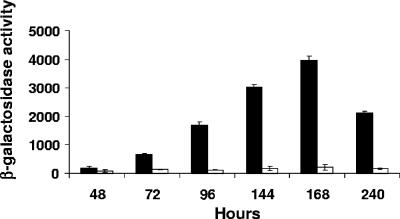
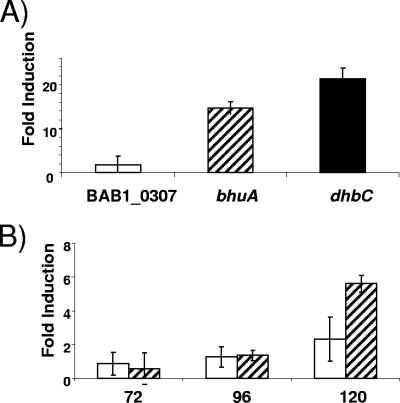
Bacterial genes involved in heme transport are typically expressed only under iron limitation conditions (33). Correspondingly, maximum levels of β-galactosidase production were observed in cultures of the B. abortus 2308 derivative carrying the plasmid-borne bhuA-lacZ fusion grown under iron-deprived conditions (Fig. 3), while growth under iron-replete conditions repressed β-galactosidase production by this strain. During growth under iron-deprived conditions, bhuA expression in B. abortus 2308 increases dramatically as this strain makes the transition into stationary phase. This profile of bhuA expression B. abortus 2308 was verified by real-time RT-PCR analysis of RNA obtained from cultures grown under iron-deprived and iron-replete conditions with bhuA-specific primers (Fig. 4). The dhbC gene was included as a positive control for the analysis shown in Fig. 4A because it has been experimentally demonstrated using both Northern blots and gene fusions that dhbC exhibits maximal expression in response to iron deprivation in B. abortus 2308 (6). Similarly, the gene designated BAB1_0370 in the B. abortus 2308 genome sequence displays neither iron repression (Fig. 4A) nor significant growth phase induction in this bacterium and thus serves as a useful negative control for the analyses shown in Fig. 4A and 4B. The pattern of iron-repressible and stationary-phase maximal bhuA expression shown in Fig. 3 and 4 further supports the proposition that BhuA plays an important role in stationary-phase iron acquisition in B. abortus 2308.
FIG. 3.
Expression of a bhuA-lacZ fusion in B. abortus 2308 during growth in low-iron minimal medium (filled bars) and low-iron minimal medium supplemented with 50 μM FeCl3 (open bars). β-Galactosidase activity is expressed on the y axis in Miller units (27). The data are means and standard deviations for triplicate determinations from a single culture for each strain at each experimental time point in a single experiment. The data are representative of multiple (≥3) experiments from which equivalent results and statistical trends were obtained. The β-galactosidase levels produced by the base pMR15 plasmid in B. abortus 2308 during growth in low-iron minimal medium or in this medium supplemented with 50 μM FeCl3 never exceeded 200 Miller units in the assays (data not shown).
FIG. 4.
Elevated levels of bhuA transcripts were present in B. abortus 2308 in response to (A) iron deprivation and (B) the transition into stationary phase during growth under iron-limiting conditions. The fold induction in response to iron deprivation represents the difference between the levels of bhuA, dhbC, and BAB1_0370 transcripts detected by real-time RT-PCR in RNA preparations from B. abortus 2308 cultures after 96 h of growth in low-iron minimal medium or low-iron minimal medium supplemented with 50 μM FeCl3. The open bars in panel B indicate the levels of BAB1_0370 transcripts, and the cross-hatched bars indicate the levels of bhuA transcripts detected by real-time RT-PCR in RNA preparations from B. abortus 2308 after 72, 96, and 120 h of growth in low-iron minimal medium compared to the levels of these transcripts detected in RNA preparations obtained from this strain after 48 h of cultivation in low-iron minimal medium. The data are means and standard deviations for triplicate determinations for each gene at each experimental time point in a single experiment. The data are representative of three separate experiments from which equivalent results were obtained.
B. abortus 2308 requires BhuA for maintenance of chronic spleen infection in experimentally infected BALB/c mice.
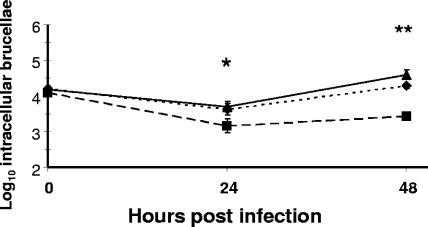
To evaluate the potential role of BhuA in pathogenesis and at the same time assess the relative importance of heme as an iron source for the brucellae during residence in a mammalian host, the virulence properties of B. abortus 2308, the bhuA mutant HR1703, and the HR1703 derivative carrying the reconstructed bhuA locus (HR1703RC) were examined in cultured murine macrophages and BALB/c mice. The B. abortus bhuA mutant exhibited significant attenuation compared to 2308 and HR1703RC in both macrophages (Fig. 5) and mice (Fig. 6). Considering the phenotype displayed by the B. abortus bhuA mutant during cultivation under iron-deprived conditions in vitro (e.g., accelerated loss of stationary-phase viability compared to 2308), it is noteworthy that equivalent numbers of 2308 and the bhuA mutant were recovered from the spleens of experimentally infected mice at 1 week postinfection, but accelerated clearance of the bhuA mutant from the spleens of these mice compared to that of 2308 began at some point between 1 and 4 weeks postinfection. These results suggest that B. abortus 2308 requires BhuA for maintenance of chronic spleen infection in BALB/c mice rather than for establishment of the infection.
FIG. 5.
Survival and replication of B. abortus 2308 (triangles), HR1703 (2308 bhuA) (squares), and HR1703RC (HR1703 bhuA+) (diamonds) in cultured resident peritoneal macrophages from BALB/c mice. The data are means and standard deviations for the number of intracellular brucellae recovered for each strain from three separate wells of cultured macrophages at each experimental time point in a single experiment. One asterisk indicates that the P value is <0.05 and two asterisks indicate that the P value is <0.01 for comparisons of the data obtained for HR1703 with the data obtained for 2308 and HR1703RC. The data are representative of multiple (≥3) experiments from which equivalent results and statistical trends were obtained.
FIG. 6.
Spleen colonization profiles for B. abortus 2308 (diamonds), HR1703 (2308 bhuA) (squares), and HR1703RC (HR1703 bhuA+) (triangles) in experimentally infected BALB/c mice. The data are means and standard deviations for the number of brucellae detected in the spleens of five mice infected with each strain at each experimental time point in a single experiment. Two asterisks indicate that the P value is <0.01 for comparisons of the data obtained for HR1703 with the data obtained for 2308 and HR1703RC.
DISCUSSION
There is a considerable flux of heme and heme-containing compounds through macrophages due to their central role in heme recycling in the host (10). When the macrophages engulf and degrade senescent erythrocytes (8), hemoglobin and heme are released into the macrophages. These phagocytes also work in concert with hemopexin and haptoglobin to scavenge heme and hemoglobin released from damaged cells before the concentrations of the latter compounds reach toxic levels (45). Consequently, it has been postulated that heme may repressent a biologically relevant iron source for the brucellae during their intracellular residence in host macrophages (39). The experimental findings presented here support the proposition that BhuA serves as the TonB-dependent outer membrane component of a heme transport system in B. abortus 2308. More importantly, the significant attenuation exhibited by the B. abortus bhuA mutant in cultured murine macrophages and experimentally infected mice indicates that heme serves as a critical iron source for this bacterium in vivo.
The link between BhuA and the maintenance of stationary-phase viability in B. abortus 2308 during cultivation under iron-deprived conditions is intriguing and potentially informative with regard to the relative importance of heme as an iron source for this bacterium. Laboratory studies (5, 11, 12, 22) and surveys of genome sequences (39) indicate that Brucella spp. possess multiple iron acquisition pathways that transport different iron sources, yet none of the other iron acquisition systems present in B. abortus 2308 has the capacity to compensate for the loss of BhuA during prolonged iron deprivation once this bacterium has made the transition into stationary-phase physiology. One possible explanation for these experimental findings is that the ability to transport heme allows B. abortus 2308 to incorporate the heme directly into the heme-containing proteins that play important roles in the maintenance of stationary-phase physiology, such as the type bd cytochromes (15). This would save the brucellae the energetic expense of having to synthesize the protoporphyrin IX backbone of heme and lessen the overall need for this bacterium to transport iron. The B. abortus bhuA mutant, in contrast, might be able to rely upon its other iron transport systems to meet its physiologic needs for iron during growth under iron-replete conditions. The experimental results presented here also indicate that the bhuA mutant can use these other iron transport systems to meets its physiological need for iron during exponential growth under iron-deprived conditions. However, these other iron transport systems do not appear to be able to supply the bhuA mutant with sufficient levels of iron to produce the full repertoire of iron-containing proteins needed for long-term survival during stationary phase when this strain is subjected to iron deprivation.
BhuA shares many of the features of TonB-dependent outer membrane heme transporters described in other bacteria, but this protein and its encoding gene also have a couple of unusual characteristics. Although BhuA possesses the conserved histidine residues that have been shown to be required for wild-type heme transport by Y. enterocolitica HemR (7) and are conserved in most of the bacterial TonB-dependent outer membrane heme transporters that have been described, BhuA does not have the conserved amino acid motifs FRAP and NPNL that are present in most of these proteins (33). The biological significance of the absence of these domains in Brucella BhuA is unknown. In many gram-negative bacteria that use heme as an iron source, the genes that encode the periplasmic binding protein-dependent ABC transporter that transports heme across the cytoplasmic membrane are also located in an operon with the gene that encodes the TonB-dependent outer membrane transporter. This does not appear to be the case in the Brucella spp. In B. abortus 2308 the bhuA gene is monocistronic, and the genes that are predicted to be involved in the transport of heme across the cytoplasmic membrane in this bacterium are located in an operon distant from the bhuA locus. Not surprisingly, the latter genes (annotated BAB2_0483 to BAB2_0485 in the B. abortus 2308 genome sequence) are homologs of the shuT, shuU, and shuV genes of S. dysenteriae (49). The same genetic organization (e.g., physical separation of bhuA and the shuT, shuU, and shuV homologs) is conserved in the genome sequences of B. abortus 9-941, B. melitensis 16M, and B. suis 1330. Whether or not the Brucella shuT, shuU, and shuV homologs function in heme transport is presently being evaluated.
Although the contributions of numerous bacterial heme transporters to virulence have been examined using a variety of animal models and cell cultures (26, 29, 35, 42, 46, 47, 48), few bacterial heme transporters exhibit a link to virulence as strong as the link between BhuA and virulence in B. abortus 2308 in the mouse model. In fact, the experimental findings reported here strongly suggest that heme represents a critical, and possibly indispensable, iron source for this bacterium during chronic infection. Iron acquisition mediated by BhuA also appears to be essential for the prolonged maintenance of stationary-phase viability when B. abortus 2308 is cultivated under iron-deprived conditions in vitro. It has been proposed that stationary-phase physiology plays an important role in allowing the brucellae to maintain long-term intracellular residence in host macrophages (37), and the phenotype exhibited by the B. abortus bhuA mutant supports this proposal. Consequently, gaining a better understanding of the physiologic basis for why heme represents such an important iron source for B. abortus 2308 during stationary phase should provide important insight into the mechanisms by which this bacterium produces disease in the host.
Acknowledgments
This work was supported by grants from the United States Department of Agriculture National Research Initiative Competitive Grants Program (grant 35204-12218) and the National Institute of Allergy and Infectious Diseases (grant AI 63516) to R.M.R.
We thank Bob Perry for his advice regarding the in vitro assays for hemin utilization.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 20 August 2007.
REFERENCES
- 1.Acha, P. N., and B. Szyfres. 1980. Zoonoses and communicable diseases common to man and animals, p. 28-45. Pan American Health Organization, Washington, DC.
- 2.Almirón, M., M. Martínez, N. Sanjuan, and R. A. Ugalde. 2001. Ferrochelatase is present in Brucella abortus and is critical for its intracellular survival and virulence. Infect. Immun. 69:6225-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. Institut National de la Recherche Agronomique, Paris, France.
- 4.Bellaire, B. H., P. H. Elzer, C. L. Baldwin, and R. M. Roop II. 1999. The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infect. Immun. 67:2615-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellaire, B. H. 2001. Ph.D. thesis. Louisiana State University Health Sciences Center, Shreveport.
- 6.Bellaire, B. H., P. E. Elzer, S. Hagius, J. Walker, C. L. Baldwin, and R. M. Roop II. 2003. Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydroxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infect. Immun. 71:1794-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracken, C. S., M. T. Baer, A. Abdur-Rashid, W. Helms, and I. Stojiljkovic. 1999. Use of hemin-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J. Bacteriol. 181:6063-6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bratosin, D., J. Mazurier, J. P. Tissier, J. Estaquier, J. J. Huart, J. C. Amiesen, D. Aminoff, and J. Montreuil. 1998. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. Biochimie 80:173-195. [DOI] [PubMed] [Google Scholar]
- 9.Chain, P. S. G., D. J. Comerci, M. E. Tomalsky, F. W. Larimer, S. A. Malfatti, L. M. Vergez, F. Aguero, M. L. Land, R. A. Ugalde, and E. Garcia. 2005. Whole-genome analyses of speciation events in pathogenic brucellae. Infect. Immun. 73:8353-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crichton, R. R., S. Wilmet, R. Legssyer, and R. J. Ward. 2002. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 91:9-18. [DOI] [PubMed] [Google Scholar]
- 11.Danese, I. 2001. Ph.D. thesis. Facultes Universitaires Notre-Dame de la Paix Namur, Namur, Belgium.
- 12.Danese, I., V. Haine, R.-M. Delrue, A. Tibor, P. Lestrate, O. Stevaux, P. Mertens, J.-Y. Paquet, J. Godfroid, X. de Bolle, and J.-J. Letesson. 2004. The Ton system, an ABC transporter, and a universally conserved GTPase are involved in iron utilization by Brucella melitensis 16M. Infect. Immun. 72:5783-5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.del Vecchio, V. G., V. Kapatral, R. J. Redkar, G. Patra, C. Mujer, T. Los, N. Ivanova, I. Anderson, A. Bahattacharyya, A. Lykidis, G. Reznik, L. Jablonski, N. Larsen, M. D'Souza, A. Bernal, M. Mazur, E. Goltsman, E. Selkov, P. H. Elzer, S. Hagius, D. O'Callaghan, J. J. Letesson, R. Haselkorn, N. Kyrpides, and R. Overbeek. 2002. The genome sequence of the facultative intracellular pathogen Brucella melitensis. Proc. Natl. Acad. Sci. USA 99:443-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elzer, P. H., R. W. Phillips, M. E. Kovach, K. M. Peterson, and R. M. Roop II. 1994. Characterization and genetic complementation of a Brucella abortus high-temperature-requirement A (htrA) deletion mutant. Infect. Immun. 62:4105-4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endley, S., D. McMurray, and T. A. Ficht. 2001. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 183:2454-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gee, J. M., M. W. Valderas, M. E. Kovach, V. K. Grippe, G. T. Robertson, W.-L. Ng, J. M. Richardson, M. E. Winkler, and R. M. Roop II. 2005. The Brucella abortus Cu/Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect. Immun. 73:2873-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gober, J. W., and L. Shapiro. 1992. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol. Biol. Cell 3:913-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González-Carreró, M. I., F. J. Sangari, J. Agüero, and J. M. García Lobo. 2002. Brucella abortus 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiology 148:353-360. [DOI] [PubMed] [Google Scholar]
- 19.Halling, S. M., B. D. Peterson-Burch, B. J. Bricker, R. L. Zuerner, Z. Zing, L.-L. Li, V. Kapur, D. P. Alt, and S. C. Olsen. 2005. Completion of the genome sequence of Brucella abortus and comparison to the highly similar genomes of Brucella melitensis and Brucella suis. J. Bacteriol. 187:2715-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson, D. P., E. E. Wyckoff, C. E. Rashidi, H. Verlei, and A. L. Oldham. 2001. Characterization of the Plesiomonas shigelloides genes encoding the heme iron utilization system. J. Bacteriol. 183:2715-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Litwin, C. M., and B. L. Byrne. 1998. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect. Immun. 66:3134-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Goñi, I., I. Moriyón, and J. B. Neilands. 1992. Identification of 2,3-dihydroxybenzoic acid as a Brucella abortus siderophore. Infect. Immun. 60:4496-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.López-Goñi, I., and I. Moriyón. 1995. Production of 2,3-dihydroxybenzoic acid by Brucella species. Curr. Microbiol. 31:291-293. [Google Scholar]
- 24.Lundrigan, M. D., and R. J. Kadner. 1986. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. J. Biol. Chem. 261:10797-10801. [PubMed] [Google Scholar]
- 25.Martin, D. W., M. J. Schurr, and V. Deretic. 1994. Analysis of promoters controlled by the putative sigma factor AlgU regulating conversion to mucoidy in Pseudomonas aeruginosa: relationship to σE and stress response. J. Bacteriol. 176:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mey, A. R., and S. M. Payne. 2001. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 42:835-849. [DOI] [PubMed] [Google Scholar]
- 27.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory. Cold Spring Harbor, NY.
- 28.Mills, M., and S. M. Payne. 1997. Identification of shuA, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shuA mutant. Infect. Immun. 65:5358-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, E. R., E. Sacco, A. Dickenson, D. J. Metzger, Y. Hu, P. E. Orndorff, and T. D. Connell. 2002. BhuR, a virulence-associated outer membrane protein of Bordetella avium, is required for the acquisition of iron from heme and hemoproteins. Infect. Immun. 70:5390-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nienaber, A., H. Hennecke, and H.-M. Fischer. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41:787-800. [DOI] [PubMed] [Google Scholar]
- 31.Parent, M. A., B. H. Bellaire, E. A. Murphy, R. M. Roop II, P. H. Elzer, and C. L. Baldwin. 2002. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (DHBA) facilitates intracellular survival of the bacteria. Microb. Pathog. 32:239-248. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen, I. T., R. Seshadri, K. E. Nelson, J. A. Eisen, J. F. Heidelberg, T. D. Read, R. J. Dodson, L. Umayam, L. M. Brinkac, M. J. Beanan, S. C. Daugherty, R. T. Deboy, A. S. Durkin, J. F. Dolonay, R. Madupu, W. C. Nelson, B. Ayodeji, M. Kraul, J. Shetty, J. Malek, S. E. Van Aken, S. Riedmuller, H. Tettelin, S. R. Gill, O. White, S. L. Salzberg, D. L. Hoover, L. E. Lindler, S. M. Halling, S. M. Boyle, and C. M. Fraser. 2002. The Brucella suis genome reveals fundamental similarities between animal and plant pathogens and symbionts. Proc. Natl. Acad. Sci. USA 99:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perkins-Balding, D., A. Rasmussen, and I. Stojiljkovic. 2004. Bacterial heme and hemoprotein receptors, p. 66-85. In J. H. Crosa, A. R. Mey, and S. M. Payne (ed.), Iron transport in bacteria. American Society for Microbiology, Washington, DC.
- 34.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves. S. A., A. G. Torres, and S. M. Payne. 2000. TonB is required for intracellular growth and virulence of Shigella dysenteriae. Infect. Immun. 68:6329-6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson, G. T., M. E. Kovach, C. A. Allen, T. A. Ficht, and R. M. Roop II. 2000. The Brucella abortus Lon functions as a generalized stress response protease and is required for wild-type virulence in BALB/c mice. Mol. Microbiol. 35:577-588. [DOI] [PubMed] [Google Scholar]
- 37.Roop, R. M., II, J. M. Gee, G. T. Robertson, J. M. Richardson, W.-L. Ng, and M. E. Winkler. 2003. Brucella stationary phase gene expression and virulence. Annu. Rev. Microbiol. 57:57-76. [DOI] [PubMed] [Google Scholar]
- 38.Roop, R. M., II, B. H. Bellaire, M. W. Valderas, and J. A. Cardelli. 2004. Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 52:621-630. [DOI] [PubMed] [Google Scholar]
- 39.Roop, R. M., II, B. H. Bellaire, E. Anderson, and J. T. Paulley. 2004. Iron metabolism in Brucella, p. 243-262. In I. López-Goñi and I. Moriyón (ed.), Brucella: molecular and cellular biology, Horizon Bioscience, Norfolk, United Kingdom.
- 40.Rosner, B. 2000. Fundamentals of biostatistics, 5th ed. Duxbury, Pacific Grove, CA.
- 41.Schaible, U. E., and S. H. E. Kaufmann. 2005. Iron and microbial infection. Nat. Rev. Microbiol. 3:946-953. [DOI] [PubMed] [Google Scholar]
- 42.Seale, T. W., D. J. Morton, P. W. Whitby, R. Wolf, S. D. Kosanke, T. M. VanWagoner, and T. L. Stull. 2006. Complex role of hemoglobin and hemoglobin-haptoglobin binding proteins in Haemophilus influenzae virulence in the infant rat model of invasive infection. Infect. Immun 74:6213-6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staggs, T. M., and R. D. Perry. 1991. Identification and cloning of a fur regulatory gene in Yersinia pestis. J. Bacteriol. 173:417-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stojiljkovic, I., and K. Hantke. 1992. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in gram-negative bacteria. EMBO J. 11:4359-4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taketani, S. 2005. Acquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J. Exp. Med. 205:297-318. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, J. M., H. A. Jones, and R. D. Perry. 1999. Molecular characterization of the hemin uptake locus (hmu) from Yersinia pestis and analysis of hmu mutants for hemin and hemoprotein utilization. Infect. Immun. 67:3879-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres, V. J., G. Pishchany, M. Humayun, O. Schneewind, and E. P. Skaar. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor require for heme iron utilization. J. Bacteriol. 188:8421-8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wyckoff, E. E., D. Duncan, A. G. Torres, M. Mills, and S. M. Payne. 1998. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol. Microbiol. 28:1139-1152. [DOI] [PubMed] [Google Scholar]