Abstract
Uropathogenic Escherichia coli (UPEC), the most frequent cause of urinary tract infection (UTI), is associated with an inflammatory response which includes the induction of cytokine/chemokine secretion by urothelial cells and neutrophil recruitment to the bladder. Recent studies indicate, however, that UPEC can evade the early activation of urothelial innate immune response in vitro. In this study, we report that infection with the prototypic UPEC strain NU14 suppresses tumor necrosis factor alpha (TNF-α)-mediated interleukin-8 (CXCL-8) and interleukin-6 (CXCL-6) secretion from urothelial cell cultures compared to infection with a type 1 piliated E. coli K-12 strain. Furthermore, examination of a panel of clinical E. coli isolates revealed that 15 of 17 strains also possessed the ability to suppress cytokine secretion. In a murine model of UTI, NU14 infection resulted in diminished levels of mRNAs encoding keratinocyte-derived chemokine, macrophage inflammatory peptide 2, and CXCL-6 in the bladder relative to infection with an E. coli K-12 strain. Furthermore, reduced stimulation of inflammatory chemokine production during NU14 infection correlated with decreased levels of bladder and urine myeloperoxidase and increased bacterial colonization. These data indicate that a broad phylogenetic range of clinical E. coli isolates, including UPEC, may evade the activation of innate immune response in the urinary tract, thereby providing a pathogenic advantage.
Urinary tract infections (UTI) are most frequently caused by an ascending colonization of the bladder and/or kidneys by Escherichia coli (9, 33). Infection of the urinary tract results in an inflammatory response characterized by increased levels of urinary cytokines and neutrophil influx (1, 13-15). The innate immune response to infection by uropathogenic E. coli (UPEC) depends upon activation of host pattern recognition receptors of the Toll-like receptor pathway, including Toll-like receptor 4 (TLR4) (5, 16, 37-39). Recognition of E. coli lipopolysaccharide (LPS) by urothelial cells that express TLR4 results in activation of the proinflammatory and prosurvival NF-κB pathway and secretion of chemokines/cytokines, including CXCL-6 and CXCL-8 (2). The resulting accumulation of inflammatory chemokines in the bladder mucosa and urine during UTI induces the recruitment of neutrophils, which leads to the clearance of bacteria and resolution of infection. Infection of the urinary tract by UPEC also induces adaptive immune responses characterized by humoral and cell-mediated responses which protect against future infection (42).
Many pathogenic bacterial species possess the ability to modulate the innate immune response to evade host defenses and promote colonization, including several that block the activation of the NF-κB pathway (7, 31, 32, 35, 36). We previously reported that UPEC strain NU14 blocks activation of the NF-κB pathway and thereby promotes apoptosis (26, 27). Insertional mutation of the genes encoding the periplasmic chaperone SurA or the LPS biosynthetic operons rfa or rfb abolished cytokine-suppressive activity of a UPEC isolate, suggesting that UPEC-specific modification of LPS prevents recognition by urothelial TLR4/CD14 or inhibits NF-κB activation (20). Similarly, E. coli strain RS218 (O18:K1:H7) was found to suppress the secretion of inflammatory chemokines by blocking the activation of the NF-κB and mitogen-activated protein kinase pathways in THP-1 monocytes (41).
Two potential mechanisms may underlie the suppression of the innate immune response by NU14: either NU14 fails to induce urothelial proinflammatory responses or NU14 actively modulates proinflammatory signals. We and others have shown that NU14 suppresses both TNF-α- and LPS-mediated NF-κB activation and interleukin-6 (IL-6) (CXCL-6) secretion in urothelial cell cultures (20, 27). NU14 can suppress LPS-mediated CXCL-6 secretion but not IL-1β-mediated CXCL-6 secretion from urothelial cell cultures (20). Additionally, UPEC can inhibit CXCL-6 secretion induced by a nonsuppressor K-12 strain from urothelial cultures in a mixed infection (20). The mechanism by which UPEC suppresses inflammatory responses in urothelial cultures is dependent upon a heat-labile factor, as suppression of LPS-induced NF-κB activation and suppression of nonsuppressor-strain-mediated CXCL-6 secretion were abolished by heat treatment of UPEC suppressor strains (20, 27).
Here, we report that the prototypic cystitis strain NU14 suppresses TNF-α-mediated activation of urothelial CXCL-8 secretion. We also show that this suppressive activity is broadly distributed across a panel of clinical isolates representing the different phylogenetic groups of E. coli. Furthermore, in a mouse model of UTI, the induction of keratinocyte-derived chemokine (KC), macrophage inflammatory peptide 2 (MIP-2), and CXCL-6 mRNA in the bladder was lower in NU14-infected mice than in mice infected with strain MG1655. Reduced cytokine/chemokine production also correlated with decreased recruitment of neutrophils into the bladders and urine of NU14-infected mice and increased bladder colonization by NU14.
MATERIALS AND METHODS
Bacterial strains and culture.
E. coli strains were cultured at 37°C in LB-Miller broth under static conditions for 48 h to promote the surface expression of type 1 pili (8). HB101, a nonpiliated K-12 strain, was transformed with the plasmid pWRS1-17 encoding type 1 pili and maintained in 40 μg/ml kanamycin (for convenience, this strain is referred to as “p1-17” in Fig. 1 through 3) (40). NU14 (O18:K1:H7) is a streptomycin-resistant E. coli strain isolated from a cystitis patient, and strain NU14-1 carries an insertional mutation in fimH, the gene encoding the adhesive subunit of type 1 pili (19, 28). Strain MG1655 is a laboratory strain of E. coli which is a type 1 piliated K-12 strain (6). Fecal and clinical strains were selected from the ECOR panel to represent a diversity of known virulence factor genes (21, 30). Type 1 pilus expression was confirmed by a mannose-sensitive hemagglutination assay using guinea pig erythrocytes (Cleveland Scientific, OH) (10, 19).
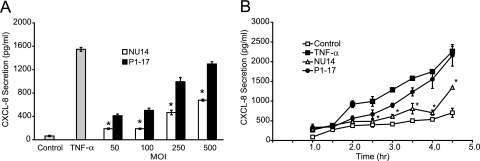
FIG. 1.
UPEC isolate NU14 induces less urothelial CXCL-8 secretion than a piliated lab strain. (A) TEU-1 cultures were incubated with media only (Control), 1.5 ng/ml recombinant human TNF-α, or increasing amounts of either NU14 or the type 1 piliated lab strain HB101/p1-17. Cells were incubated with either bacterial strain at an MOI of 50:1, 100:1, 250:1, or 500:1. Supernatants were collected after 4 h, and CXCL-8 secretion was determined by ELISA. NU14 induced significantly less CXCL-8 secretion than HB101/p1-17 at all MOI. (B) TEU-1 cells were incubated with media alone (Control), 1.5 ng/ml TNF-α, NU14, or HB101/p1-17 for various times (MOI of 250:1), and CXCL-8 secretion was determined by ELISA. TNF-α and HB101/p1-17 elicited increased CXCL-8 secretion relative to control or NU14 at 3 or more hours of incubation. Data represent the means ± standard deviations of three separate infections, and each experiment was performed in duplicate. Asterisks indicate statistically significant differences between NU14 and HB101/p1-17.
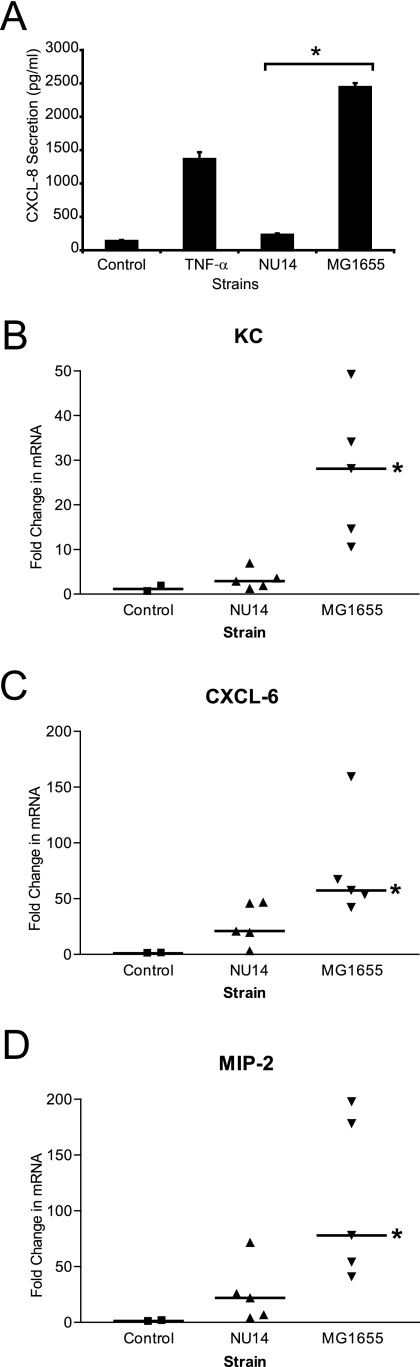
FIG. 3.
NU14 suppresses chemokine expression in murine UTI. (A) TEU-1 cells were treated with NU14 or MG1655 (MOI of 250:1). Following 4 h of culture, culture supernatants were harvested and CXCL-8 secretion was determined by ELISA. The asterisk indicates statistically significant differences between NU14 and MG1655. Error bars reflect standard deviations of triplicate samples. (B, C, and D) C57BL/6 mice (controls, n = 2; infected, n = 5) were catheterized, and 1 × 106 CFU of either NU14 or MG1655 was instilled into the bladder. After 4 h, the mice were sacrificed and the bladders were harvested for mRNA isolation. Quantitative real-time PCR was used to determine the changes in gene transcript levels by comparing the relative changes in KC (B), CXCL-6 (C), and MIP-2 (D) in infected mouse samples normalized to levels in uninfected controls. Asterisks indicate statistically significant differences between samples infected with NU14 and samples infected with MG1655.
Cell lines and culture.
The urothelial cell line TEU-1 was established by generating a primary epithelial culture from normal human ureter followed by immortalization as previously described (27). Once established, immortalized cells were maintained in a 5% CO2/37°C atmosphere in keratinocyte serum-free medium (KSFM) (Invitrogen) supplemented with bovine pituitary extract (50 mg/ml), epidermal growth factor (5 μg/liter), and penicillin-streptomycin. Tissue for this procedure was obtained in accordance with the guidelines of the Institutional Review Board of Northwestern University.
Cytokine secretion assays.
TEU-1 cells were cultured in six-well plates in KSFM without antibiotics approximately 16 h prior to infection with bacteria. Bacterial suspensions containing E. coli in antibiotic-free KSFM were added to each well, and cells were then returned to the incubator. Urothelial CXCL-8 secretion was determined following 4 h of infection with increasing multiplicities of infection (MOI) of 50:1, 100:1, 250:1, and 500:1 (bacterium:cell ratio) with NU14 or HB101/p1-17. In the time course experiments, cell culture supernatants were collected from cells incubated with either NU14 or HB101/p1-17 by use of an MOI of 250:1 every 30 min from 0 to 4.5 h. In the TNF-α suppression and clinical panel experiments, TEU-1 cells were infected using an MOI of 250:1, and sample supernatants were collected after 4 h. Where noted, media were supplemented with 1.5 ng/ml recombinant human TNF-α (Calbiochem/Biosource). Culture supernatants were cleared by centrifugation and assayed for secreted CXCL-8 or CXCL-6 by enzyme-linked immunosorbent assay (ELISA) (Pharmingen).
Mouse infection experiments.
The mouse infection model was adapted from previous studies (11, 18). Briefly, 6- to 8-week-old female C57BL/6 mice were instilled via transurethral catheter with a volume of 10 μl containing 1 × 106 CFU of the appropriate bacterial strain while under isoflurane anesthesia. This minimal inoculum volume prevents reflux into the kidneys (18). Bacterial inoculants were prepared from 40 ml of bacterial culture grown for 48 h in the presence of appropriate antibiotics. The bacterial suspensions were then harvested by centrifugation and resuspended and diluted to the final concentration in phosphate-buffered saline. For the colonization studies, 1 × 108 CFU of each strain was instilled into the bladder. Bladders were harvested 24 h postinfection, homogenized, and plated on eosin methylene blue agar with the appropriate antibiotics. All experiments were approved by the Animal Care and Use Committee at Northwestern University.
RNA isolation and quantitative real-time PCR.
Four hours postinoculation, mouse bladders were harvested and frozen in liquid nitrogen. Total mRNA was extracted from the bladders by using TRIzol (Invitrogen) according to the manufacturer's instructions. Reverse transcription of mRNA was performed using an iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. Quantitative real-time PCR was performed using iQ SYBR green supermix (Bio-Rad) with gene-specific primers for mouse L19 (forward, 5′-CCATGAGTATGCTCAGGCTTCAGA-3′; reverse, 5′-TACAGGCTGTGATACATGTGGCGA-3′), KC (forward, 5′-AGAAGACAGACTGCTCTGATGGCA-3′; reverse, 5′-TCCCACACATGTCCTCACCCTAAT-3′), CXCL-6 (forward, 5′-ATCCAGTTGCCTTCTTGGGACTGA-3′; reverse, 5′-TGGTACTCCAGAAGACCAGAGGAA-3′), and MIP-2 (forward, 5′-AACTGCGCTGTCAATGCCTGAAGA-3′; reverse, 5′-TCCAGGTCAGTTAGCCTTGCCTTT-3′) in an MJ Research Chromo 4 thermocycler. Change (n-fold) in mRNA abundance was determined using the 2−ΔΔCT method by normalizing cycle threshold (CT) values to each sample's CT values for the ribosomal protein L19 followed by normalization of CT to the average of two uninfected control mouse bladder CT values for the corresponding mRNA (29).
Neutrophil myeloperoxidase ELISA.
Mice were instilled with 1 × 106 CFU of either NU14 or MG1655. Eight hours postinfection, urine and bladders were harvested. Bladder homogenates and urine were prepared according to manufacturer's recommendations and assayed for neutrophil myeloperoxidase by ELISA (HyCult Biosciences).
Statistical analysis.
Results were compared with Student's t test or one-way analysis of variance followed by Bonferroni's multiple-comparison posttest using Prism software from GraphPad, Inc., as appropriate. Differences in the data were considered significant for P values less than or equal to 0.05.
RESULTS
UPEC isolate NU14 induces less CXCL-8 secretion from urothelial cell culture than a type 1 piliated K-12 strain.
We demonstrated previously that UPEC strain NU14 suppresses the activation of the proinflammatory NF-κB pathway, whereas the type 1 piliated laboratory strain HB101/pWRS1-17 does not (27). In order to determine if NU14 similarly modulates inflammatory chemokine secretion, we infected urothelial cell cultures with UPEC and K-12 strains and quantified IL-8 (CXCL-8) secretion by ELISA. Culture supernatants were harvested from TEU-1 urothelial cell cultures infected with NU14 or HB101/pWRS1-17 for 4 h at various MOI (Fig. 1A). Unstimulated urothelial cells secreted minimal CXCL-8 into the cell culture supernatant, while TNF-α, a known activator of NF-κB, induced robust secretion. Infection of urothelial cultures with HB101/pWRS1-17 resulted in a dose-dependent increase in CXCL-8 secretion. In contrast, NU14 induced significantly less CXCL-8 secretion than HB101/pWRS1-17 at all MOI (P ≤ 0.0001). We also monitored CXCL-8 secretion as a function of time in cultures infected with NU14 or HB101/p1-17 (Fig. 1B). Infection with NU14 induced significantly less CXCL-8 secretion than HB101/p1-17 or TNF-α from 150 min to 270 min (P ≤ 0.03). The magnitudes of CXCL-8 secretion from TEU-1 cells varied in response to the same stimuli between experiments (Fig. 1A and B, NU14 at 250:1 MOI and 4 h, respectively); however, these experiments consistently show that NU14 induces significantly lower levels of CXCL-8 secretion from urothelial cells than the nonsuppressor strains.
NU14 suppresses TNF-α-mediated CXCL-8 secretion from urothelial cultures.
To determine whether NU14 could also block TNF-α-induced CXCL-8 secretion, cell cultures were infected with NU14 in the presence of 1.5 ng/ml TNF-α. NU14-infected cultures secreted significantly less CXCL-8 than cells incubated with TNF-α alone (P ≤ 0.01). In contrast, HB101/p1-17 did not suppress TNF-α-mediated CXCL-8 secretion from urothelial cell cultures, suggesting that NU14 actively modulates cytokine secretion.
Multiple clinical E. coli isolates also suppressed CXCL-8 secretion from urothelial cells.
Genetic analyses of bacterial isolates from patients indicate that E. coli represents a diverse species capable of causing infection at many sites in the human body (34). To determine the extent to which modulation of urothelial CXCL-8 responses is shared by diverse E. coli strains, we examined a panel of strains selected from the ECOR reference collection (Table 1). Strains were chosen from the four major phylogenetic groups of E. coli, with an emphasis on the phylogenetic group B2, which is most commonly associated with community-acquired UTI and other extraintestinal infections (22). Specific strains were isolated from either human feces or patients with cystitis or pyelonephritis and were chosen so that the panel represented a diversity of known virulence factor genes (Table 1) (30). We compared the levels of secretion of CXCL-8 from urothelial cultures exposed to each of these 18 isolates. Secretion of CXCL-8 was induced by the type 1 piliated K-12 strain HB101/p1-17, as well as by clinical strains ECOR14 and ECOR51. NU14 and the other clinical isolates induced far less CXCL-8 secretion than HB101/p1-17 (P ≤ 0.01) (Fig. 2B).
TABLE 1.
Clinical and fecal E. coli isolates
| Isolatea | Originb | PGc | Result ford:
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSHA | ExPEC | CS | pap | sfa foc | focG | afa dra | iha | fim | hly | cnf | cdt | fyu | iroN | iut |
kpsMT
|
rfc | ibe | cva | traT | ompT | malX | ||||||
| IIp | IIb | III | K1 | ||||||||||||||||||||||||
| NU14 | C | B2 | +++ | + | + | + | + | − | − | − | + | + | + | − | + | + | − | + | + | − | − | + | + | − | + | + | + |
| 8 | F | A | + | + | + | − | − | − | − | + | + | − | − | − | + | − | + | + | + | − | − | − | − | − | − | + | − |
| 10 | F | A | ++ | − | + | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 11 | C | A | − | + | + | + | − | − | − | + | + | − | − | − | + | − | + | + | + | − | − | − | − | − | + | − | − |
| 13 | F | A | − | − | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 14 | P | A | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − |
| 51 | F | B2 | − | + | − | + | + | + | − | + | + | − | + | − | + | + | + | + | + | − | − | − | − | − | − | + | + |
| 53 | F | B2 | + | + | + | + | + | + | − | − | + | − | + | − | + | − | − | + | + | − | − | + | − | − | − | + | + |
| 54 | F | B2 | + | + | + | − | + | + | − | − | + | − | + | − | + | + | − | + | + | − | − | − | − | − | − | + | + |
| 59 | F | B2 | +++ | − | + | − | − | − | − | − | + | − | − | − | + | − | − | − | − | + | − | + | − | − | − | + | + |
| 60 | C | B2 | ++ | + | + | + | + | + | − | − | + | + | + | − | + | + | − | − | − | + | − | + | − | − | − | + | + |
| 61 | F | B2 | ++ | − | + | − | − | − | − | − | + | − | − | − | + | − | − | + | + | − | + | − | − | − | − | + | + |
| 62 | P | B2 | ++ | + | + | + | − | − | − | − | + | − | − | − | + | + | + | + | + | − | + | − | − | + | + | + | + |
| 63 | F | B2 | + | + | + | + | + | − | − | − | + | + | + | + | + | + | − | + | + | − | + | − | + | − | − | + | + |
| 64 | C | B2 | − | + | + | − | + | + | + | + | + | − | − | − | + | + | − | + | + | − | − | − | − | − | − | + | + |
| 40 | P | D | − | + | + | + | − | − | − | + | + | − | − | − | + | − | − | + | + | − | + | − | − | − | − | + | − |
| 41 | F | D | + | + | + | + | − | − | − | + | + | − | − | − | + | − | − | + | + | − | + | − | − | − | − | + | − |
| 49 | F | D | − | + | + | + | − | − | − | + | + | − | − | − | + | − | − | + | + | − | − | − | − | − | + | + | + |
| 50 | P | D | − | + | + | + | − | − | + | + | + | − | − | − | + | + | − | + | + | − | − | − | − | − | + | + | + |
F, feces; C, cystitis; P, pyelonephritis.
PG, phylogenetic group.
MSHA, mannose-sensitive hemagglutination (a measure of type 1 piliation) (−, exhibits no MSHA; +, relative MSHA levels, where + < ++ < +++); ExPEC +, classified as ExPEC; ExPEC −, not classified as ExPEC; CS, cytokine suppression (this study) (+, presence of CS; −, absence of CS). The remaining characteristics represent the presence (+) or absence (−) of genotypic markers identified by PCR: pap, P fimbria structural and adhesive subunits; sfa foc, S and F1C fimbriae; focG, F1C fimbriae; afa dra, Dr adhesins; iha, adhesin siderophore; fim, type 1 fimbriae; hly, hemolysin; cnf, cytotoxic necrotizing factor; cdtB, cytolethal distending toxin; fyu, yersiniabactin receptor; iroN, siderophore receptor; iut, aerobactin; kpsM II, group 2 capsule (K1 and K2 variants); kpsMT III, group 3 capsule; rfc, O4 lipopolysaccharide; ibe, invasion of brain endothelium; cva, colicin V; traT, serum resistance associated; ompT, outer membrane protease T; malX, pathogenicity island marker.
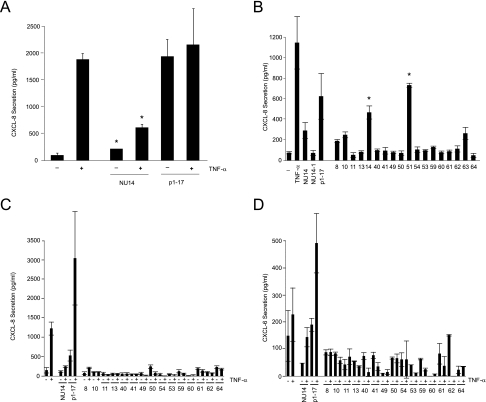
FIG. 2.
NU14 and clinical E. coli isolates suppress urothelial CXCL-8 and CXCL-6 secretion. (A) TEU-1 cells were treated with E. coli strain NU14 or HB101/p1-17 in the presence (+) or absence (−) of 1.5 ng/ml TNF-α. Following culture for 4 h, culture supernatants were harvested, and CXCL-8 secretion was measured by ELISA. (B) TEU-1 cells were treated for 4 h with TNF-α, NU14, HB101/p1-17, or clinical isolates 8 to 64 from the ECOR panel (MOI of 250:1), culture supernatants were harvested, and CXCL-8 secretion was measured by ELISA. (C and D) TEU-1 cells were treated for 4 h with E. coli strain NU14, HB101/p1-17, or various clinical isolates (8 to 64) in the presence (+) or absence (−) of 1.5 ng/ml TNF-α, and CXCL-8 (C) or CXCL-6 (D) secretion was measured by ELISA. Error bars reflect the standard deviations of triplicate samples. Asterisks indicate statistically significant differences between (A) TNF-α-treated and NU14-infected and (B) NU14- and ECOR strain 14- and 51-infected TEU-1 cells.
Poor stimulation of CXCL-8 secretion was not mediated by adherence via type 1 pili or due to the onset of apoptosis caused by expression of type 1 pili, for the isogenic FimH− mutant NU14-1 also elicited significantly reduced CXCL-8 secretion relative to HB101/p1-17 (Fig. 2B) (27). This is consistent with previous reports that NU14-1 also suppresses both TNF-α- and LPS-mediated NF-κB activation as well as LPS-induced CXCL-6 secretion (20, 27). Furthermore, ECOR strain piliation status did not correlate with suppression of chemokine secretion (Table 2).
TABLE 2.
Summary of modulation by E. coli
| Source or group | No. of strains exhibiting cytokine suppression/total no. of strains |
|---|---|
| Feces | 9/11 |
| Cystitis | 4/4 |
| Pyelonephritis | 3/4 |
| ECOR group A | 4/5 |
| ECOR group B2 | 8/10 |
| ECOR group D | 4/4 |
| Type 1 fimbria positive | 10/12 |
| Type 1 fimbria negative | 6/7 |
| Total | 17/19 |
To determine whether those ECOR strains which failed to induce urothelial CXCL-8 secretion also possessed the capacity to suppress proinflammatory signals, we evaluated inducing strains for the ability to suppress TNF-α-induced CXCL-8 secretion (Fig. 2C). All clinical isolates which failed to induce also suppressed TNF-α-mediated urothelial CXCL-8 secretion (Fig. 2C), consistent with our previous finding that NU14 suppressed NF-κB activation (27). We also measured the secretion of CXCL-6, another NF-κB-dependent cytokine released by urothelial cells in response to infection during UTI (20). All clinical strains which suppressed CXCL-8 secretion, including NU14, also suppressed TNF-α-mediated CXCL-6 release (Fig. 2D). In all, 16 of 18 ECOR panel isolates suppressed CXCL-8 release from urothelial cell cultures. No significant correlations were found between suppressive activity and clonal group, clinical origin, or known virulence factors (as identified by PCR) in the ECOR strains or NU14 (Tables 1 and 2). Furthermore, the ability to suppress chemokine secretion does not correlate with the extraintestinal pathogenic E. coli (ExPEC) classification, which is defined by the presence of two or more genes highly correlated with pathogenesis in vivo (Table 1). This suggests the presence of novel virulence factors which mediate suppression of urothelial chemokine secretion.
Infection with UPEC results in less chemokine mRNAs in a mouse model of UTI.
Suppression of CXCL-8 secretion by multiple clinical E. coli isolates in vitro suggests a pathogenic mechanism for modulating host inflammatory responses. To determine whether infection with UPEC results in reduced activation of innate immune responses in vivo, we utilized a murine UTI model. The mouse genome does not contain any direct homologues of the human CXCL-8 gene. Instead, the C-X-C family chemokines MIP-2 and KC play similar physiological functions in the mouse and are induced during experimental murine UTI (12). We compared the levels of induction of the NF-κB-dependent inflammatory chemokine/cytokine KC, CXCL-6, and MIP-2 mRNAs in the mouse bladder following infection with either NU14 or the K-12 strain MG1655. For in vivo experiments, MG1655 was used as a representative nonsuppressor strain instead of the laboratory strain HB101/p1-17 because MG1655 is a well-characterized isolate that encodes type 1 pili from a chromosomal locus rather than on a plasmid that could be lost in vivo in the absence of selection. MG1655, as with HB101/p1-17, induced robust CXCL-8 secretion from urothelial cell cultures and failed to inhibit TNF-α-mediated CXCL-8 secretion (Fig. 3A). At 4 h after infection, bladders of infected mice were harvested for RNA extraction. Reverse transcription and quantitative real-time PCR were used to quantify expression of KC, CXCL-6, and MIP-2 during infection. Infection of the mouse bladder with NU14 resulted in reduced induction of the mRNAs encoding KC, CXCL-6, and MIP-2 relative to infection with MG1655 (Fig. 3B to D) (P = 0.009, P = 0.05, and P < 0.04, respectively).
Infection with NU14 resulted in reduced urine myeloperoxidase.
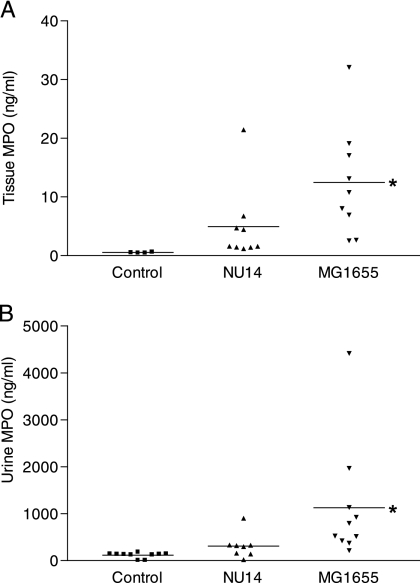
To determine whether modulation of bladder KC, CXCL-6, and MIP-2 mRNAs by NU14 resulted in functional differences in neutrophil recruitment to the bladder, we infected mice with either NU14 or MG1655 and quantified myeloperoxidase in bladder homogenates and urine by ELISA (Fig. 4A and B). Eight hours after infection, bladder homogenates and urine from mice challenged with MG1655 contained significantly increased levels of myeloperoxidase (12.48 ± 3.117 and 1,128 ± 398.7 ng/ml, respectively) compared to levels for untreated control mice (0.5443 ± 0.03504 and 116.4 ± 18.22 ng/ml, respectively) (P > 0.05) (Fig. 4A and B). Bladder homogenates and urine from NU14-infected mice showed no significant increase in myeloperoxidase (4.960 ± 2.162 and 311.7 ± 93.36 ng/ml, respectively) relative to levels for untreated mice, suggesting reduced or delayed neutrophil recruitment during infection (P < 0.05) (Fig. 4A and B). Reduced neutrophil recruitment into the bladder tissue and urine also correlated with reduced levels of chemokine mRNAs in the bladders of mice infected with NU14 compared to levels for the MG1655-infected counterparts (Fig. 3B to D). The finding that NU14 infection resulted in lower bladder and urine myeloperoxidase levels suggests that modulation of urothelial chemokine secretion is targeted at the level of neutrophil migration into the bladder and urine. These data also suggest that UPEC modulates the urothelial inflammatory response in vivo.
FIG. 4.
Quantitation of myeloperoxidase (MPO) in mouse urine and bladder homogenates during experimental UTI by ELISA. Infection with NU14 results in less MPO in mouse bladder homogenates and urine than infection with MG1655. Mice were infected with 1 × 106 CFU NU14 or MG1655 via catheter (for bladder experiments, four control mice and nine infected mice; for urine experiments, 10 control mice, eight NU14-infected mice, and 10 MG1655-infected mice). Urine samples were collected, and the bladders were removed and homogenized 6 h postinfection. Bladder (A) and urine (B) MPO levels were determined by ELISA. Asterisks indicate statistically significant differences between uninfected controls and samples infected with strain MG1655.
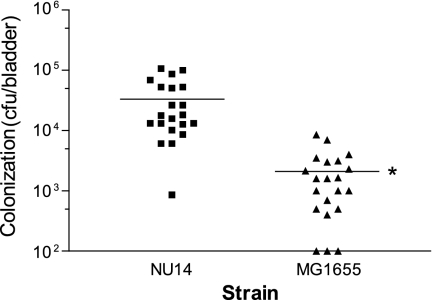
NU14 infection results in higher bacterial colonization than infection with MG1655.
We compared levels of colonization of the bladder by strains NU14 and MG1665 in a murine model of UTI. Mice were infected with either NU14 or MG1655 via transurethral catheter. After 24 h, the bladders of mice infected with UPEC strain NU14 retained approximately 16-fold more bacteria (33,200 ± 7,011 versus 2,090 ± 486.6 CFU) than mice infected with strain MG1655 (Fig. 5). Therefore, the increased bladder colonization by NU14 relative to MG1655 correlates with the ability to modulate innate immune responses in vivo.
FIG. 5.
Infection with NU14 results in greater bladder colonization. Female C57BL/6 mice (n = 21) were catheterized and instilled with 1 × 108 CFU of NU14 or MG1655 via transuretheral catheter. After 24 h, the mice were sacrificed, and the bladders were homogenized and plated to determine colonization. The asterisk indicates a statistically significant difference between samples infected with NU14 and samples infected with MG1655 (P = 0.0001).
DISCUSSION
In this study, we examined the actions of the prototypic UPEC isolate NU14 and a panel of diverse E. coli strains on urothelial cytokine secretion. We found that, in contrast to a laboratory strain, NU14 elicited a reduced CXCL-8 response and suppressed urothelial secretion of CXCL-8 in response to stimulation with TNF-α. We also tested a panel of diverse E. coli strains representing clonal groups commonly associated with UTI and including cystitis, pyelonephritis, and fecal isolates and found that the majority of strains in the panel failed to induce urothelial CXCL-8 and were also capable of inhibiting TNF-α-induced CXCL-8 and CXCL-6 secretion. The ability of UPEC and other clinical E. coli strains to inhibit inflammatory chemokine secretion in vitro suggests that the capacity to modulate immune responses is common among pathogenic E. coli strains.
Recently, transposon mutagenesis of the UPEC strain UTI89 revealed that suppression of chemokine/cytokine secretion is mediated by the LPS biosynthetic operons rfa and rfb along with the periplasmic membrane chaperone SurA in vitro (20). These results suggest that modification to the bacterial LPS core O antigen may play a role in suppression of TLR4-mediated cytokine secretion. Salmonella species are known to modify the O antigen, thereby escaping TLR4/CD14 activation of the NF-κB pathways, suggesting the possibility of a conserved mechanism between these closely related pathogens (17).
Similarly, Selvaraj and Prasadarao found that RS218, a strain homologous to and of the same serotype as UPEC isolate NU14 (22), was able to suppress chemokine/cytokine secretion from human monocytes (41). These investigators also found that suppression of chemokine secretion was heat labile and dependent upon expression of OmpA and IbeA. The cellular mechanisms underlying suppression by RS218 in monocytes and by NU14 in urothelial cells both involve decreased IκB phosphorylation (27, 41). These similarities suggest that NU14 and RS218 target similar cellular pathways in monocytes and urothelial cells to inhibit NF-κB activation and that this suppression requires several components of the bacterial outer membrane.
The stimulation of urothelial chemokine secretion, whether by E. coli or by TNF-α, LPS, or IL-1β, occurs in an NF-κB-dependent manner through signaling pathways that result in IκB phosphorylation. UPEC suppression of these NF-κB stimuli suggests that the cellular target of suppression occurs either at a common signaling pathway component upstream of IκB phosphorylation or through activation of an inhibitory pathway which is capable of inhibiting IκB phosphorylation by these stimuli. Future studies to identify the precise host cell target(s) of the NF-κB-suppressive activity of UPEC and other clinical E. coli isolates may also shed light upon the identities of the bacterial effectors and their mechanisms of action.
We also report that UPEC can suppress inflammatory responses in a murine model of early stages in UTI. Our experimental observations suggest that UPEC inhibits the transcription of inflammatory chemokines in the bladder relative to the nonpathogenic K-12 strain MG1655. Inhibition of the NF-κB-regulated KC, MIP-2, and CXCL-6 genes was determined using whole-bladder samples. Thus, it is possible that along with urothelial cells, other cell types, such as neutrophils, may be targets of UPEC suppression of chemokine secretion. Another clinical UPEC isolate, 1177, induces MIP-2 secretion from urothelial cells in a mouse model of UTI (12). Peak MIP-2 secretion, however, originated from recruited neutrophils, which required MIP-2 to cross the urothelial cell layer into the bladder lumen (12). Our data are consistent with a requirement for MIP-2 secretion in neutrophil recruitment into the urine, as indicated by the correlation between reduced MIP-2 levels in the bladders and urine of mice infected with NU14 and low myeloperoxidase levels relative to levels in mice infected with MG1655.
Ultimately, UPEC does stimulate inflammatory responses in the urinary tract. Infection with UPEC results in net secretion of CXCL-8 from urothelial cells, but the kinetics and magnitude of this induction are diminished relative to levels following infection with the same dose of nonsuppressor E. coli strains. Our models of early events in the pathogenesis of UTI by UPEC indicate that modulation of urothelial innate immune responses may be important in delaying or diminishing neutrophil influx to the bladder lumen. Modulation of chemokine secretion may allow additional time for invasion of urothelial cells by UPEC, where UPEC has been shown to form intracellular bacterial communities (IBC) in a mouse model of UTI (23). IBC may provide an early refuge from phagocytosis by neutrophils and later serve as latent reservoirs which are a potential source of recurrent UTI (3, 4, 23-25). This ability may underlie the increased colonization of the mouse bladder by strain NU14 compared to colonization by MG1655, which is unable to form IBC.
In summary, we demonstrated that clinical E. coli isolates possess the capacity to modulate urothelial cytokine expression in vitro. This capacity is shared among strains from diverse phylogenetic groups and different clinical origins. In vivo infection with NU14 results in reduced induction of inflammatory chemokines and cytokines, which correlates with decreased recruitment of neutrophils into the bladder and urine and increased bacterial colonization in a mouse model of UTI. Modulation of cytokine secretion is independent of the presence of type 1 pili and 21 other known virulence factors, suggesting that novel virulence factors mediate pathogenesis in the urinary tract by ExPEC.
Acknowledgments
We thank Fred Blattner and Guy Plunkett III for providing RS218 sequence data from the University of Wisconsin E. coli Genome Project (http://www.genome.wisc.edu) prior to publication. All experiments were performed in accordance with the guidelines of the Internal Review Board of Northwestern University and the Animal Care and Use Committee.
This work was supported by NIDDK award R01 DK04648 (A.J.S.).
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 27 August 2007.
REFERENCES
- 1.Agace, W. W., S. R. Hedges, M. Ceska, and C. Svanborg. 1993. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Investig. 92:780-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, G. G., S. M. Martin, and S. J. Hultgren. 2004. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect. 6:1094-1101. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 5.Backhed, F., L. Meijer, S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell. Microbiol. 4:493-501. [DOI] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Collier-Hyams, L. S., H. Zeng, J. Sun, A. D. Tomlinson, Z. Q. Bao, H. Chen, J. L. Madara, K. Orth, and A. S. Neish. 2002. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J. Immunol. 169:2846-2850. [DOI] [PubMed] [Google Scholar]
- 8.Duguid, J. P., and D. C. Old. 1980. Adhesive properties of Enterobacteriaceae, p. 185-217. In E. H. Beachey (ed.), Bacterial adherence, vol. 6. Chapman and Hall, London, United Kingdom. [Google Scholar]
- 9.Foxman, B., and P. Brown. 2003. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect. Dis. Clin. N. Am. 17:227-241. [DOI] [PubMed] [Google Scholar]
- 10.Gaffney, R. A., M. F. Venegas, C. Kanerva, E. L. Navas, B. E. Anderson, J. L. Duncan, and A. J. Schaeffer. 1995. Effect of vaginal fluid on adherence of type 1 piliated Escherichia coli to epithelial cells. J. Infect. Dis. 172:1528-1535. [DOI] [PubMed] [Google Scholar]
- 11.Godaly, G., B. Frendeus, A. Proudfoot, M. Svensson, P. Klemm, and C. Svanborg. 1998. Role of fimbriae-mediated adherence for neutrophil migration across Escherichia coli-infected epithelial cell layers. Mol. Microbiol. 30:725-735. [DOI] [PubMed] [Google Scholar]
- 12.Hang, L., M. Haraoka, W. W. Agace, H. Leffler, M. Burdick, R. Strieter, and C. Svanborg. 1999. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J. Immunol. 162:3037-3044. [PubMed] [Google Scholar]
- 13.Haraoka, M., L. Hang, B. Frendeus, G. Godaly, M. Burdick, R. Strieter, and C. Svanborg. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180:1220-1229. [DOI] [PubMed] [Google Scholar]
- 14.Hedges, S., P. Anderson, G. Lidin-Janson, P. de Man, and C. Svanborg. 1991. Interleukin-6 response to deliberate colonization of the human urinary tract with gram-negative bacteria. Infect. Immun. 59:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedges, S., M. Svensson, and C. Svanborg. 1992. Interleukin-6 response of epithelial cell lines to bacterial stimulation in vitro. Infect. Immun. 60:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedlund, M., B. Frendeus, C. Wachtler, L. Hang, H. Fischer, and C. Svanborg. 2001. Type 1 fimbriae deliver an LPS- and TLR4-dependent activation signal to CD14-negative cells. Mol. Microbiol. 39:542-552. [DOI] [PubMed] [Google Scholar]
- 17.Hoare, A., M. Bittner, J. Carter, S. Alvarez, M. Zaldivar, D. Bravo, M. A. Valvano, and I. Contreras. 2006. The outer core lipopolysaccharide of Salmonella enterica serovar Typhi is required for bacterial entry into epithelial cells. Infect. Immun. 74:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins, W. J., J. E. Elkahwaji, D. M. Heisey, and C. J. Ott. 2003. Inheritance of susceptibility to induced Escherichia coli bladder and kidney infections in female C3H/HeJ mice. J. Infect. Dis. 187:418-423. [DOI] [PubMed] [Google Scholar]
- 19.Hultgren, S. J., W. R. Schwan, A. J. Schaeffer, and J. L. Duncan. 1986. Regulation of production of type 1 pili among urinary tract isolates of Escherichia coli. Infect. Immun. 54:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunstad, D. A., S. S. Justice, C. S. Hung, S. R. Lauer, and S. J. Hultgren. 2005. Suppression of bladder epithelial cytokine responses by uropathogenic Escherichia coli. Infect. Immun. 73:3999-4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, J. R., S. J. Weissman, A. L. Stell, E. Trintchina, D. E. Dykhuizen, and E. V. Sokurenko. 2001. Clonal and pathotypic analysis of archetypal Escherichia coli cystitis isolate NU14. J. Infect. Dis. 184:1556-1565. [DOI] [PubMed] [Google Scholar]
- 23.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Justice, S. S., D. A. Hunstad, P. C. Seed, and S. J. Hultgren. 2006. Filamentation by Escherichia coli subverts innate defenses during urinary tract infection. Proc. Natl. Acad. Sci. USA 103:19884-19889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Justice, S. S., S. R. Lauer, S. J. Hultgren, and D. A. Hunstad. 2006. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 74:4793-4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klumpp, D. J., M. T. Rycyk, M. C. Chen, P. Thumbikat, S. Sengupta, and A. J. Schaeffer. 2006. Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect. Immun. 74:5106-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klumpp, D. J., A. C. Weiser, S. Sengupta, S. G. Forrestal, R. A. Batler, and A. J. Schaeffer. 2001. Uropathogenic Escherichia coli potentiates type 1 pilus-induced apoptosis by suppressing NF-κB. Infect. Immun. 69:6689-6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 29.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 30.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orth, K., L. E. Palmer, Z. Q. Bao, S. Stewart, A. E. Rudolph, J. B. Bliska, and J. E. Dixon. 1999. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285:1920-1923. [DOI] [PubMed] [Google Scholar]
- 32.Orth, K., Z. Xu, M. B. Mudgett, Z. Q. Bao, L. E. Palmer, J. B. Bliska, W. F. Mangel, B. Staskawicz, and J. E. Dixon. 2000. Disruption of signaling by Yersinia effector YopJ, a ubiquitin-like protein protease. Science 290:1594-1597. [DOI] [PubMed] [Google Scholar]
- 33.Ronald, A. R., L. E. Nicolle, E. Stamm, J. Krieger, J. Warren, A. Schaeffer, K. G. Naber, T. M. Hooton, J. Johnson, S. Chambers, and V. Andriole. 2001. Urinary tract infection in adults: research priorities and strategies. Int. J. Antimicrob. Agents 17:343-348. [DOI] [PubMed] [Google Scholar]
- 34.Russo, T. A., and J. R. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect. 5:449-456. [DOI] [PubMed] [Google Scholar]
- 35.Schesser, K., J. M. Dukuzumuremyi, C. Cilio, S. Borg, T. S. Wallis, S. Pettersson, and E. E. Galyov. 2000. The Salmonella YopJ-homologue AvrA does not possess YopJ-like activity. Microb. Pathog. 28:59-70. [DOI] [PubMed] [Google Scholar]
- 36.Schesser, K., A. K. Spiik, J. M. Dukuzumuremyi, M. F. Neurath, S. Pettersson, and H. Wolf-Watz. 1998. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28:1067-1079. [DOI] [PubMed] [Google Scholar]
- 37.Schilling, J. D., S. M. Martin, C. S. Hung, R. G. Lorenz, and S. J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schilling, J. D., S. M. Martin, D. A. Hunstad, K. P. Patel, M. A. Mulvey, S. S. Justice, R. G. Lorenz, and S. J. Hultgren. 2003. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 71:1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schilling, J. D., M. A. Mulvey, C. D. Vincent, R. G. Lorenz, and S. J. Hultgren. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148-1155. [DOI] [PubMed] [Google Scholar]
- 40.Schwan, W. R., H. S. Seifert, and J. L. Duncan. 1992. Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J. Bacteriol. 174:2367-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvaraj, S. K., and N. V. Prasadarao. 2005. Escherichia coli K1 inhibits proinflammatory cytokine induction in monocytes by preventing NF-kappaB activation. J. Leukoc. Biol. 78:544-554. [DOI] [PubMed] [Google Scholar]
- 42.Thumbikat, P., C. Waltenbaugh, A. J. Schaeffer, and D. J. Klumpp. 2006. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 176:3080-3086. [DOI] [PubMed] [Google Scholar]