Abstract
Borrelia burgdorferi CspZ (TIGR open reading frame designation, BBH06) is part of a functionally related group of proteins that bind one or more members of the factor H (FH) protein family. In this report we assess the conservation, distribution, properties, and ligand binding abilities of CspZ from the three main Borrelia species associated with Lyme disease infections in humans. CspZ (also referred to as BbCRASP-2 in the literature) was found to be highly conserved at the intraspecies level but divergent at the interspecies level. All CspZ orthologs that originated from B. burgdorferi isolates bound FH from a diverse group of mammals. In contrast, CspZ derived from B. garinii and B. afzelii did not. Regardless of the Borrelia species of origin, all CspZ proteins tested bound to unknown ∼60-kDa serum proteins produced by different mammals. To further define the molecular basis for the differential binding of CspZ orthologs to host proteins, DNA sequence, truncation, and site-directed mutagenesis analyses were performed. DNA sequence analyses revealed that B. garinii and B. afzelii CspZ orthologs possess a 64-amino-acid N-terminal domain that is absent from B. burgdorferi CspZ. However, binding analyses of recombinant proteins revealed that this domain does not in and of itself influence ligand binding properties. Truncation and mutagenesis analyses further revealed that the key determinants required for ligand binding are discontinuous and that the presentation of the ligand binding pocket is dependent on alpha helices with high coiled-coil formation probability. The data presented here provide insight into the molecular basis of CspZ-ligand interactions and suggest that CspZ orthologs from diverse Borrelia species can contribute to the host-pathogen interaction through their interaction with serum proteins.
The binding of negative regulators of the complement cascade such as factor H (FH), FH-like protein 1 (FHL-1), and C4b binding protein has been demonstrated to be an important virulence mechanism for numerous pathogens (reviewed in reference 19). A correlation between this phenotype and serum resistance has been demonstrated for the Lyme disease spirochetes and several other organisms (6, 19). The binding of FH and FHL-1 is thought to contribute to pathogenesis by facilitating evasion of complement and opsonophagocytosis (2, 4, 11, 32). Several of the FH or FHL-1 binding proteins (FHBPs) produced by spirochetes have been identified (11, 13, 17, 24). They include Borrelia hermsii FhbA (13-15), Treponema denticola FhbB (24), and CspZ (BBH06), CspA (BBA68), and the OspE paralogs (BBL39, BBN38, and BBP38) of the Lyme disease spirochetes (3, 6, 9, 11, 29).
The CspZ protein, which is the focus of this report, is the most recent FHBP of the Lyme disease spirochetes to be identified. It has also been referred to as BbCRASP-2 in the literature (9). Among the Lyme disease spirochetes, B. burgdorferi displays the most complex FH binding phenotype, producing up to five different FHBPs belonging to three different protein families. B. afzelii and B. garinii, which are generally more sensitive to complement (5, 20, 40), produce fewer FHBPs. Some FHBP-encoding genes are environmentally regulated (10, 26, 31, 39), and the proteins they encode have been shown to have differing binding specificities for members of the FH protein family (19, 24, 34). Recent work has demonstrated that FHBPs can also bind to a series of serum proteins (16, 26, 35). These observations suggest that the FHBPs may contribute to Borrelia pathogenesis through multiple virulence mechanisms.
In B. burgdorferi B31MI, cspZ is carried on a 28-kb linear plasmid designated lp28-3 (8). The distribution and conservation of cspZ among pathogenic species of the B. burgdorferi sensu lato complex have not been assessed, and little is known regarding the ligand binding abilities of CspZ proteins from diverse Borrelia species. In addition, the determinants required for ligand binding have not been fully identified. The goals of this study were to address voids in our current understanding of the molecular basis of the CspZ-ligand interaction and the contribution of CspZ to the pathogenesis of diverse Borrelia species.
MATERIALS AND METHODS
Bacterial isolates and cultivation and infection of mice.
Table 1 lists and describes the species/isolates that were analyzed as part of this report. All strains were cultivated in modified Barbour-Stoenner-Kelley (BSK-H) complete medium (Sigma) at 33°C in sealed bottles in CO2 incubators (3% CO2). Bacteria were harvested by centrifugation and gently washed with phosphate-buffered saline (PBS). To generate infection serum, mice (C3H/HeJ; Jackson Labs) were infected with B. burgdorferi B31MI by intradermal inoculation as previously described (7). Serum was harvested from the infected mice at week 6 postinoculation. Blots were screened using a 1:1,000 dilution of infection serum in blocking buffer (1% PBS, 0.2% Tween, 5% Carnation nonfat dry milk), and antibody binding was detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) (1:40,000; Pierce).
TABLE 1.
Borrelia isolates used in this study
| Species/isolate | Geographic origin | Sourcea |
|---|---|---|
| B. burgdorferi | ||
| 297 | Connecticut | Human CSF |
| 3028 | Texas | Human skin |
| B31 | New York | I. scapularis |
| CA7 | California | I. pacificus |
| CA9 | California | I. pacificus |
| JD1 | Massachusetts | I. scapularis |
| LP4 | Connecticut | Human EM |
| LP5 | Connecticut | Human EM |
| LP7 | Connecticut | Human EM |
| T2 | United States | I. scapularis |
| Veery | Connecticut | Veery bird |
| B. afzelii | ||
| ECM1 | Sweden | Human skin |
| IP21 | Russia | I. persulcatus |
| UM01 | Sweden | Human skin |
| VS461 | Switzerland | I. scapularis |
| B. garinii | ||
| 20047 | France | I. ricinus |
| B-4/87 | Norway | I. ricinus |
| FRG | Germany | I. ricinus |
| IP89 | Russia | I. persulcatus |
| PBi | Germany | I. ricinus |
| VS492 | Switzerland | I. ricinus |
| B. coriaceae | California | O. coriaceus |
CSF, cerebrospinal fluid; EM, erythema migrans; I. scapularis, Ixodes scapularis; I. pacificus, Ixodes pacificus; I. persulcatus, Ixodes persulcatus; I. ricinus, Ixodes ricinus; O. coriaceus, Ornithodoros coriaceus.
SDS-PAGE, immunoblotting, and FH/FHL-1 ALBI analyses.
Borrelia cell lysates and recombinant proteins (r-proteins) were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane as previously described (23). FH and/or FHL-1 binding to membrane-immobilized proteins was assessed using the affinity ligand binding immunoblot (ALBI) assay approach (referred to as the FH ALBI assay). The membranes were incubated in blocking buffer for 1 h, followed by incubation with purified human FH (5 ng μl−1; Calbiochem) or r-FHL-1 (2.5 ng μl−1; Abnova) in blocking buffer for 1 h. Membranes were washed with PBS-Tween and incubated with goat anti-human FH (1:1,000; Calbiochem) in blocking buffer for 1 h. Detection was through HRP-conjugated rabbit anti-goat IgG (1:40,000; Pierce) and chemiluminescence.
PCR, generation of r-proteins, and site-directed mutagenesis.
The r-proteins were generated using a PCR-based approach. PCR was performed using GoTaq polymerase (Promega) and standard methods. As needed, PCR amplicons were purified from agarose gels by use of a QIAquick gel extraction kit (QIAGEN). To allow for the production of r-protein, amplicons were generated using primers (Table 2) with tail sequences designed for annealing to the predigested pET series of vectors by use of ligase-independent cloning (LIC) methodologies. The pET46-Ek/LIC and pET32-Ek/LIC vectors (Novagen) were used for the generation of His-tagged and S-tagged/His-tagged proteins, respectively. Site-directed mutagenesis was conducted as previously described using a mutagenic primer approach (28). The production of r-proteins by Escherichia coli was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the proteins were purified using nickel chromatography. Protein purity and equal loading were assessed by screening with mouse anti-His tag antibodies (1:5,000; Novagen). Detection of bound antibodies was through goat anti-mouse IgG HRP conjugate at 1:40,000.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| BbCspZ-LIC-F | GACGACGACAAGATTGATGTTAGTAGATTAAATCAGAGAAATATTAATGAG |
| BbCspZ-LIC-R | GAGGAGAAGCCCGGTCTATAATAAAGTTTGCTTAATAGCTTTATAAGCC |
| BgCspZ-21-F | CATATGTTAGACAGAGGGCGAAATGATTTAAATCAG |
| BgCspZ-6-F | GAATATATTTTAGCTATAATATCTTTATTTTTTATTATGTCTTG |
| BgCspZ-280-R | CTTAATAGCTTTATAGGCCTCCTCAAGTTC |
| BbCspZcc1m-F | GACGACGACAAGATTGATGTTATGAGATTAAATCAGAGAAATACTAATGAGCGTAAAAT |
| BbCspZ-cc1m-R | CAACAAAAATTTTACGCTCATTAGTATTTCTCTG |
| BbCspZ-cc2m-F | GTTAATAAGTCTAAGGAGCGTGAAAAGATTATAG |
| BbCspZ-cc2m-R | CTATAATCTTTTCACGCTCCTTAGACTTATTAAC |
| BbCspZ-40-236-F | GACGACAAGATTTATTATTCTATAAAATTAGACGCTATTTATAACGAATGTAC |
| BbCspZ-60-236-F | GACGACAAGATTATGACTTATTCGGAAGGTACATTTTCTGATCAAAG |
| BbCspZ-80-236-F | GACGACAAGATTTTTAAAAAAGACAATAAAATTGTTAATAAGTTTAAGGAG |
| BbCspZ-100-236-F | GACGACAAGATTTACAAACCTATGTTTTTAAGTAAATTAATTGATGATTTTG |
| BbCspZ-120-236-F | GACGACAAGATTGTAGATAATGATGTGTCTAATGCCAGACATG |
Generation of antiserum.
To generate antiserum, cspZ was amplified from B. burgdorferi B31MI and annealed into the pET46-Ek/LIC vector, and r-protein was produced as described above. The purified protein was then used to inoculate C3H/HeJ mice with 50 μg r-protein in Freund's complete adjuvant for the first injection and in incomplete Freund adjuvant for boosts (two boosts 2 weeks apart). At week 6, the mice were euthanized and the blood was recovered. Serum was collected from blood by centrifuging for 5 min at 14,000 × g after a 3-h incubation at room temperature (RT). The specificity of the antiserum for CspZ was confirmed through immunoblot analyses.
ELISA.
Enzyme-linked immunosorbent assay (ELISA) analyses were conducted using 96-well plates (Costar 3590; Corning) coated with 1 μg per well of r-protein in carbonate buffer (pH 9.6; 16 h at 4°C). The r-proteins used in these analyses harbor the N-terminal S tag. After protein immobilization, the plates were incubated in blocking buffer for 1 h and washed with PBS-Tween, and purified human FH was added (5 ng μl−1; 1 h; RT). The binding of FH was assessed using goat anti-human FH antiserum (1:1,000; 1 h; RT) and rabbit anti-goat IgG HRP-conjugated secondary antibody (1:40,000). To verify immobilization of the r-proteins in the wells, a triplicate set of wells was screened with HRP-conjugated S-protein (1:40,000). A 1-Step Turbo TMB ELISA kit (Pierce) served as the substrate. The reaction was stopped by the addition of 1 M sulfuric acid. The A450 value was read in an ELISA plate reader (ELx 808; Biotek).
DNA sequence analysis and computer-assisted protein structure predictions.
The sequences of select amplicons and all DNA constructs used to generate r-proteins were determined on a fee-for-service basis by MWG Biotech. Predictive protein structure analyses were conducted using programs available on the Protein Sequence Analysis (PSA) server (38, 42) and the Rosetta program. Coiled-coil formation probability was assessed using the COILS algorithm. Sequence alignments were conducted using CLUSTAL.
PFGE and Southern blot hybridization analysis.
Pulsed-field gel electrophoresis (PFGE) reagents and procedures were as previously described (15). In brief, for PFGE analyses, agarose plugs containing 2 × 108 bacterial cells were prepared, and the DNA was separated using the Bio-Rad contour-clamped homogenous electric field mapper system (1% genetic technology grade agarose gels; 0.5× Tris-boric acid-EDTA buffer; 14°C). The DNA was transferred onto Hybond-N membrane by use of the VacuGene vacuum blotting system (Pharmacia) and fixed to the membrane by UV cross-linking. The membrane was blocked in hybridization buffer (5× Denhardt's solution, 6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.5% SDS) at 60°C for 1 h, followed by hybridization with a radiolabeled PCR-generated cspZ amplicon from B. burgdorferi B31MI at 60°C for 16 h. The amplicon was radioactively labeled using the Prime-A-Gene labeling system (Promega) with [α-32P]dATP (6,000 Ci mmol−1). Blots were washed with 2× SSC, 0.5% SDS, and 0.2× SSC, 0.5% SDS at 55°C for 1 h each. Detection was through exposure to X-ray film (Phenix) at −70°C for 2 h.
Reverse affinity ligand binding immunoblot analyses.
To determine if r-proteins can bind to FH and or other serum proteins from different animals, a “reverse ALBI” approach was employed as previously described (16). In brief, serum samples from 15 different animals were obtained from Valley Biomedical and fractionated by SDS-PAGE under nonreducing conditions. The serum proteins were then transferred to membranes as described above. The membranes were screened with the appropriate S-tagged r-protein at a concentration of 10 μg ml−1 in blocking buffer. Unbound protein was removed by washing and bound protein detected using HRP-conjugated S-protein (1:40,000).
RESULTS
Analysis of the distribution of CspZ among diverse Lyme disease spirochete isolates.
To screen for cspZ among diverse Lyme disease isolates, PCR analyses were performed. The PCR primers were designed based on the B. burgdorferi B31 and B. garinii PBi cspZ sequences (8). All isolates were screened with both primer sets. The B31-derived primers amplified cspZ from most B. burgdorferi isolates and from a small subset of B. garinii and B. afzelii isolates. The B. garinii PBi-derived primer set amplified cspZ from a subset of B. garinii and B. afzelii but not B. burgdorferi isolates (data not shown). While the PCR analysis suggests that cspZ is not universal, it was detected in a majority of strains. It is noteworthy that the amplicons derived from B. garinii and B. afzelii were approximately 150 bp larger than those obtained from B. burgdorferi. DNA sequence analyses confirmed that this size polymorphism is due to an insertion present at the 5′ end of the B. garinii and B. afzelii cspZ gene sequences that is absent from B. burgdorferi. The significance of this insertion is discussed in detail below.
Southern hybridization analysis of cspZ.
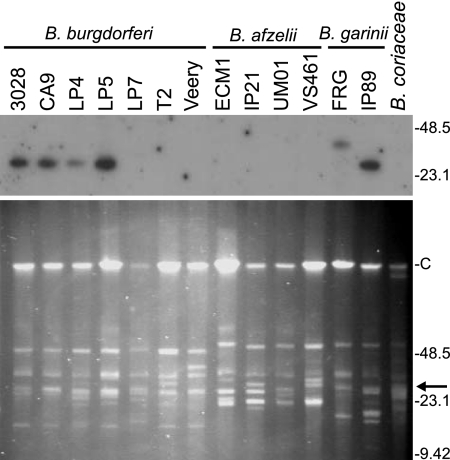
To determine if the inability to PCR amplify cspZ from some strains was due to the absence of the gene (as opposed to sequence variation within primer binding sites), Southern hybridization analysis of DNA fractionated by PFGE was performed (Fig. 1). The DNA was hybridized with a radiolabeled cspZ amplicon derived from B. burgdorferi B31MI. A hybridizing plasmid was detected in all B. burgdorferi isolates that were PCR positive for cspZ. Upon prolonged exposure, a hybridizing plasmid of 28 kb was also detected in isolate LP7. The weak detection of a hybridizing plasmid in this isolate presumably indicates that only a portion of the LP7 population carries lp28-3. The apparent absence of cspZ from the B. burgdorferi isolates T2 and Veery and from the PCR-positive B. afzelii isolates ECM1 and UM01 suggests that lp28-3 has been lost from or is carried by only a minor subset of the total population. The loss of lp28-3 and other plasmids during in vitro cultivation and during passage through mice has been well documented (21, 27, 33, 36).
FIG. 1.
PFGE and cspZ hybridization analyses of diverse Lyme disease spirochete isolates. All methods were as described in the text. The plasmid content of each isolate was visually assessed through ethidium bromide staining of the fractionated DNA (bottom). The DNA was then transferred to a membrane and hybridized with a radiolabeled cspZ amplicon derived from B. burgdorferi B31MI (top). Molecular size markers (in kilobases) are indicated in each panel. The letter “C” indicates the migration position of the 960-kb linear plasmid. The arrow in the lower panel indicates the approximate migration position of the hybridizing plasmid.
Comparative sequence analysis of CspZ: identification of species-linked polymorphisms.
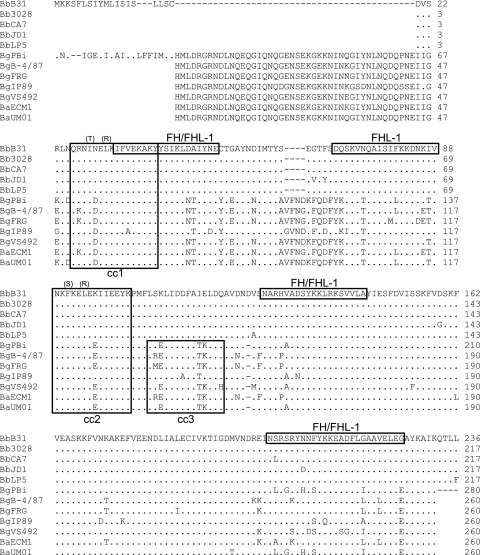
To identify polymorphisms that could influence ligand binding, 10 cspZ amplicons were cloned and sequenced, and the deduced amino acid sequences were aligned (Fig. 2). Amino acid similarity and identity values are presented in Table 3. CspZ is highly conserved among B. burgdorferi isolates (identity, ≥97.2%). A greater degree of intraspecies sequence divergence was observed for B. garinii (81.9 to 96.9%) and B. afzelii (90.8%) strains. Sequence analyses also revealed several species-linked polymorphisms. B. afzelii cspZ and B. garinii cspZ encode an N-terminal domain that is absent from all B. burgdorferi CspZ sequences. This N-terminal extension contains a leader sequence and a consensus lipidation motif. Note that in the sequence analyses conducted in this report, the signal peptide sequence was not determined for some strains, and hence the length of the N-terminal extension among isolates may vary slightly from the 64-amino-acid (aa) domain present in the B. garinii PBi isolate. A second insertion of 4 aa is also present in B. garinii and B. afzelii CspZ sequences, and B. burgdorferi harbors a C-terminal extension of 4 residues. Analyses described below assess the possible impact of some of these polymorphisms on ligand binding capability.
FIG. 2.
Amino acid sequence alignment of CspZ from diverse Lyme disease isolates. The nucleotide sequences of cspZ from 12 isolates (indicated to the left) were translated and aligned (with B. burgdorferi B31MI CspZ serving as the reference sequence). Blank spaces indicate regions for which sequence was not directly determined and hence do not indicate actual gaps. Gaps introduced by alignment are indicated by dashes, and residues identical to those in the reference sequence are indicated by periods. Putative FH and/or FHL-1 binding domains previously postulated for the B31MI CspZ-derived sequence are indicated by boxing of the reference sequence (9). Note that the data presented in this report do not in most cases support the designation of these linear sequence elements as serving as direct interaction sites for either FH or FHL-1. Predicted coiled-coil domains are indicated by boxing, and each is sequentially numbered (cc1, cc2, or cc3). Amino acid substitutions introduced into B. burgdorferi B31MI CspZ through site-directed mutagenesis are indicated in parentheses above the targeted residues. Abbreviations: Bb, B. burgdorferi; Bg, B. garinii; Ba, B. afzelii.
TABLE 3.
Amino acid similarity and identity values for CspZ among Lyme disease isolates
| Isolate | CspZ amino acid similarity/identity (%) to:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. burgdorferi
|
B. garinii
|
B. afzelii
|
||||||||||
| B31 | 3028 | CA7 | JD1 | LP5 | PBi | B-4/87 | FRG | IP89 | VS492 | ECM1 | UM01 | |
| B. burgdorferi | ||||||||||||
| B31 | 100 | 99.5 | 98.2 | 99.1 | 68.3 | 66.8 | 66.8 | 71.7 | 67.2 | 66.4 | 67.9 | |
| 3028 | 100 | 99.5 | 98.2 | 99.1 | 68.3 | 66.8 | 66.8 | 71.7 | 67.2 | 66.4 | 67.9 | |
| CA7 | 99.5 | 99.5 | 97.7 | 98.6 | 68.7 | 66.4 | 66.4 | 71.3 | 66.8 | 66.4 | 68.3 | |
| JD1 | 99.1 | 99.1 | 98.6 | 97.2 | 67.5 | 65.3 | 65.3 | 70.9 | 66 | 64.9 | 67.2 | |
| LP5 | 99.5 | 99.5 | 99.1 | 98.6 | 67.9 | 66.4 | 66.4 | 71.3 | 67.2 | 66 | 67.5 | |
| B. garinii | ||||||||||||
| PBi | 74.6 | 74.6 | 75 | 73.8 | 74.2 | 91.2 | 90.4 | 85.8 | 93.8 | 91.2 | 99.6 | |
| B-4/87 | 74.6 | 74.6 | 74.2 | 73.8 | 74.2 | 96.5 | 96.9 | 82.3 | 91.2 | 98.8 | 90.8 | |
| FRG | 74.6 | 74.6 | 74.2 | 73.8 | 74.2 | 96.2 | 99.2 | 81.9 | 90.8 | 96.5 | 90 | |
| IP89 | 75.8 | 75.8 | 75.4 | 75.4 | 75.4 | 92.7 | 90.8 | 90.4 | 84.2 | 81.9 | 85.4 | |
| VS492 | 73.8 | 73.8 | 73.5 | 73.5 | 73.5 | 98.1 | 96.2 | 95.8 | 92.3 | 90.8 | 93.5 | |
| B. afzelii | ||||||||||||
| ECM1 | 74.6 | 74.6 | 74.2 | 73.8 | 74.2 | 96.5 | 100 | 99.2 | 90.8 | 96.2 | 90.8 | |
| UM01 | 74.2 | 74.2 | 74.6 | 73.5 | 73.8 | 99.6 | 96.2 | 95.8 | 92.3 | 97.7 | 96.2 | |
Analysis of FH binding to CspZ orthologs from diverse Lyme disease spirochete isolates.
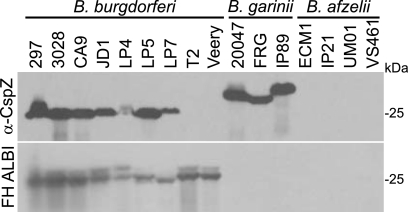
CspZ orthologs produced by geographically diverse isolates representing the three major human Lyme disease spirochete species were tested for their ability to bind FH. First, to verify CspZ production, immunoblots of cell lysates were screened with anti-CspZ antiserum generated in mice by use of His-tagged r-CspZ derived from B. burgdorferi B31MI (Fig. 3). The antiserum was determined to be highly specific and nonreactive with unrelated His-tagged proteins (not shown). The immunoblot analyses revealed that seven of nine B. burgdorferi and three of three B. garinii isolates tested produced CspZ. However, an immunoreactive protein was not detected in any B. afzelii isolate. To determine if the immunoreactive proteins display FH binding ability, an identical immunoblot was screened using the FH ALBI assay. Strong FH binding to proteins in the 25- to 27-kDa size range in all B. burgdorferi isolates was observed. FH binding in B. burgdorferi isolates T2 and Veery, which did not produce CspZ, is through CspA (26.6 kDa). However, FH binding to a protein in the CspZ size range was not observed for any B. afzelii or B. garinii isolate. The data suggest that in spite of relatively high homology, the CspZ orthologs of B. afzelii and B. garinii lack FH binding ability. This observation is consistent with that reported by McDowell et al., who demonstrated that the majority of B. afzelii and B. garinii isolates do not produce an FHBP in the 25- to 27-kDa size range (29).
FIG. 3.
Immunoblot and FH ALBI assay analyses of diverse Lyme disease spirochete isolates. Cell lysates derived from a representative panel of 16 isolates (species identity indicated above each group) were fractionated by SDS-PAGE, transferred to membranes, and screened with anti-CspZ (α-CspZ) antiserum or tested for FH binding using the FH ALBI assay (as indicated to the left). All procedures were as described in the text. Purified human FH served as the FH source.
Analysis of ligand binding to r-CspZ derived from different Borrelia species.
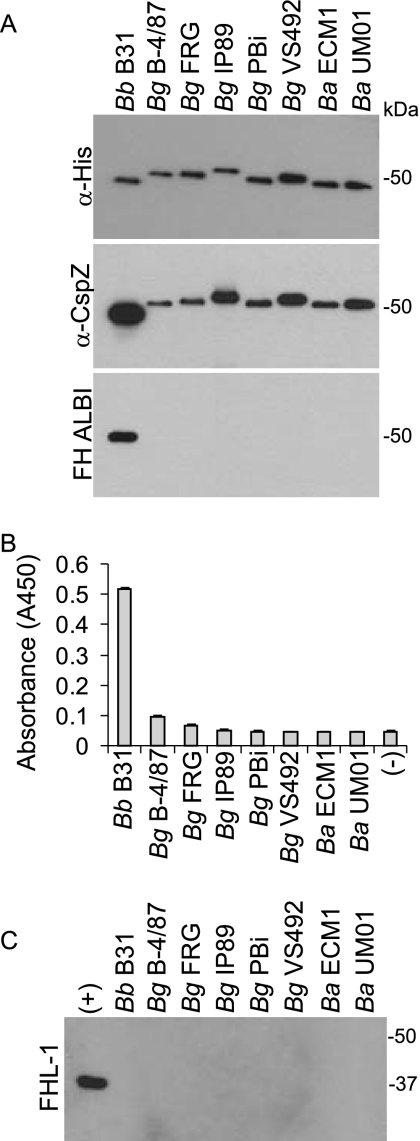
The data above indicate that CspZ orthologs of B. garinii lack FH binding ability. To investigate this further, r-CspZ proteins were generated and tested for FH binding using the ALBI assay. As controls, blots were screened with anti-His and anti-CspZ antisera (Fig. 4A). Only CspZ from B. burgdorferi bound FH. This result was confirmed through ELISA analyses (Fig. 4B). The data presented here are consistent with the binding analysis conducted using whole-cell lysates, which indicate that the CspZ orthologs of B. garinii and B. afzelii do not have FH binding ability.
FIG. 4.
Analysis of FH and FHL-1 binding by r-CspZ orthologs. r-CspZ proteins were generated using the gene sequences from the strains indicated above each lane. (A) Equal loading of the proteins was assessed through immunoblot analyses using anti-His antiserum, and relative antigenic relatedness was assessed using anti-B31MI CspZ antiserum. The abilities of the r-proteins to bind FH were assessed using the FH ALBI assay (A) and through ELISA (B). In both cases, purified human FH served as the source of FH. ELISAs were performed as described in the text, with S- and His-tagged r-Hpk1 from B. burgdorferi B31MI (TIGR open reading frame designation, BB0420) serving as the negative control. Equivalent immobilizations of the r-proteins were confirmed using HRP-conjugated S-protein. ELISAs were performed in triplicate, and the data are presented as the average A450 readings, with error bars representing standard deviation. (C) The ability of the r-proteins to bind r-FHL-1 was assessed using the ALBI format and r-FhbA from B. hermsii. Abbreviations: Bb, B. burgdorferi; Bg, B. garinii; Ba, B. afzelii; α-, anti-.
It has been reported that B. burgdorferi CspZ can bind both FH and FHL-1 (9). While B. garinii and B. afzelii CspZ orthologs lack FH binding ability, the abilities of these proteins to bind FHL-1 had not been tested. FHL-1 binding was assessed using the ALBI approach and r-FHL-1. In contrast to what was seen in an earlier report (9), we did not observe detectable binding of FHL-1 to any CspZ ortholog, including those derived from B. burgdorferi, which readily bound FH. The positive control for these analyses consisted of r-FhbA derived from B. hermsii, a relapsing fever spirochete. FhbA binds both FH and FHL-1, and consistent with that, binding of FHL-1 to FhbA was readily observed using the ALBI approach (14). Possible explanations for this discrepancy in data relating to FHL-1 binding are discussed below.
Analysis of the CspZ determinants required for the binding of FH and infection-induced antibody.
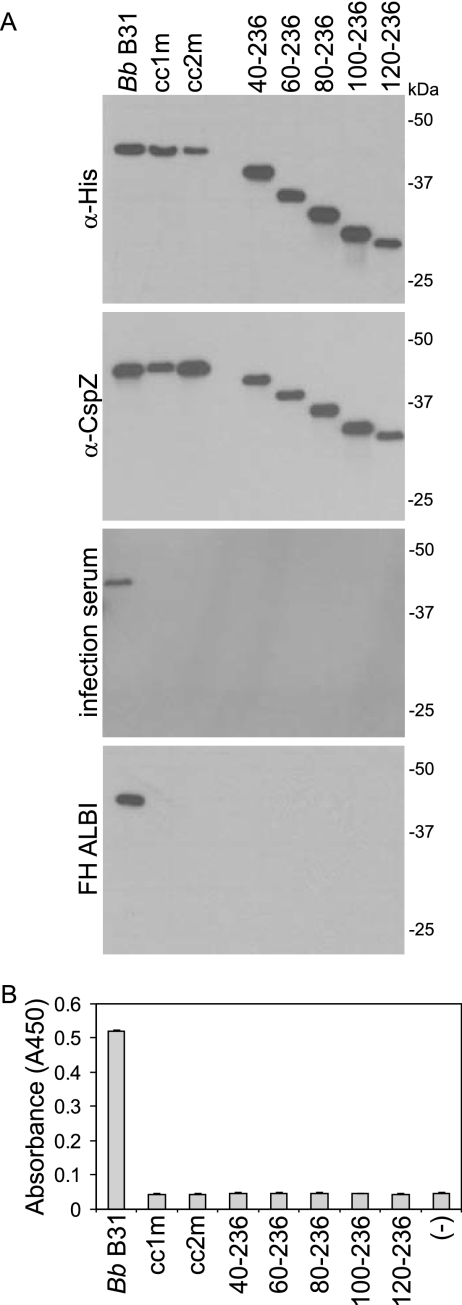
The ligand binding activity of FHBPs has been demonstrated to be highly sensitive to truncation and disruption of specific structural elements (1, 17, 25, 28, 30). A previous study demonstrated that the FH binding ability of CspZ can be eliminated by a C-terminal truncation of 16 aa (9). To determine if the N-terminal domain of CspZ is also involved in ligand binding, a series of N-terminal truncations was generated. These proteins were tested for ligand binding using immunoblotting, ALBI, and ELISA approaches. The loading and integrity of each r-protein were demonstrated by screening blots with anti-His and anti-CspZ antisera (Fig. 5A). In all assay formats, deletion of the first 20 residues beyond the leader peptide completely abolished FH and infection antibody binding (Fig. 5A and B). We previously demonstrated that independent N- and C-terminal truncations of the OspE proteins of the Lyme disease spirochetes also abolished the binding of both FH and antibody elicited to these proteins during experimental infection with B. burgdorferi (30). The data presented here provide additional support for the hypothesis that the ligand binding site(s) and epitopes presented during infection are conformationally defined and discontinuous.
FIG. 5.
Identification of CspZ determinants required for the interaction with FH/FHL-1 and antibody elicited during infection. To identify the determinants of CspZ that are involved in the binding of CspZ to FH/FHL-1 and infection-induced antibody, a series of r-proteins was generated with site-directed mutations or as truncations. All proteins were generated using the B. burgdorferi B31MI-derived sequence. The full-length protein is labeled as Bb B31. Proteins with a decreased probability of coiled-coil formation either in cc1 or in cc2 were generated by site-directed mutagenesis and designated cc1m or cc2m, respectively. The truncations are designated by the amino acid regions that they span. (A) Equivalent loading was demonstrated using anti-His (α-His) and anti-CspZ (α-CspZ) antisera. The immunoreactivity of the proteins with anti-CspZ antibody that develops during infection of mice with B. burgdorferi B31MI was assessed by immunoblotting. FH binding was assessed using the ALBI (A) and ELISA (B) formats. Recombinant Hpk1 (BB0420) served as the negative control in the ELISA, and error bars represent standard deviation. All methods were as described in the text.
Earlier analyses have demonstrated that internal structural domains are required for the proper presentation of the ligand binding sites of the FHBPs CspA and OspE (25, 28, 30). A strong correlation between coiled-coil formation probability and FH, FHL-1, and infection antibody binding has been shown. It has been demonstrated that site-directed substitutions for OspE and CspA that specifically decreased coiled-coil formation probability also abolished ligand binding. In contrast, substitutions that altered the primary sequence of the coiled-coil defining heptad repeat sequence (a to g), but that did not influence coiled-coil formation probability, had no impact on ligand binding. The coiled-coil heptad repeat motif can vary in sequence but always contains residues at the a and d positions that are nonpolar, while the e and g position residues may be charged. This periodicity of nonpolar residues allows for two or more alpha helices to participate in strong hydrophobic interactions and form coiled coils. Analyses of the CspZ sequences with the COILS program revealed that all orthologs have two domains with high predicted probability of coiled-coil formation (designated cc1 and cc2; Fig. 2). To determine if the coiled-coil elements of CspZ are directly or indirectly involved in ligand binding, site-directed substitutions were introduced. The residues to be targeted were identified through computer-assisted analyses using the COILS algorithm (22). Two CspZ variants were generated in which amino acid substitutions in the a and d positions were introduced into cc1 (I29T, L32R) and cc2 (F91S, L94R). These mutants were designated cc1m and cc2m, respectively. The site-directed r-CspZ proteins were then tested for their abilities to bind FH using ALBI and ELISA approaches. In addition, the proteins were tested for recognition by infection-induced antibody through immunoblotting. Both of the coiled-coil domains were determined to be required for FH and infection antibody binding. These data support a growing body of evidence that supports the hypothesis that the formation and presentation of the binding pocket involve hydrophobic interactions mediated by coiled-coil domains (14, 25, 28, 30).
Analysis of the interaction of CspZ with FH and other serum proteins from diverse animals.
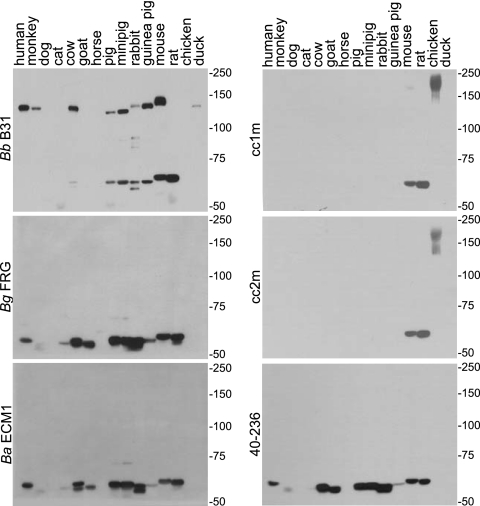
It has been postulated that the ability to bind FH and other serum proteins derived from different mammalian hosts could have implications regarding the permissive host range of the Lyme disease spirochetes (37). The OspE paralogs of the Lyme disease spirochetes and the FhbA protein of B. hermsii bind to FH produced by several mammals as well as to a series of unknown serum proteins (16). These unknown serum proteins belong to two different size classes of either ∼60 or ∼75 kDa. In contrast to FhbA and OspE, CspA has a highly restricted FH binding range (human only) but can bind to other serum proteins from a wider range of mammals (26). To assess the FH and serum protein binding range of CspZ from each of the major Lyme disease spirochete species, sera from 15 different animals were fractionated, blotted, and screened with full-length forms of r-CspZ. The r-proteins were generated based on the CspZ sequences of B. burgdorferi B31, B. garinii FRG, and B. afzelii ECM1 (Fig. 6). In addition, as a means of assessing the specificity of the interaction, blots were also screened with truncated and coiled-coil mutants of the B. burgdorferi B31MI CspZ protein. The specific approach employed in these analyses has been referred to as a reverse ALBI assay. It differs from the ALBI assay described above in that the serum samples from each animal are separated using nonreducing conditions, immunoblotted, and then screened with S-tagged r-proteins. The use of S-tagged proteins allows the binding of the r-proteins to be detected using an antibody-independent approach (i.e., HRP-conjugated S-protein). This is critical, as antibody-based detection approaches result in high signal-to-noise ratio due to the cross-reactivity of the anti-IgG secondary antiserum with IgG from diverse mammals. Focusing first on the binding patterns of the full-length forms of CspZ, B. burgdorferi B31 r-CspZ bound to FH (150 kDa) from 9 of the 15 samples tested. It also bound to the ∼60-kDa serum proteins in 7 of 15 serum samples derived from different mammalian species. In contrast, CspZ from B. afzelii and B. garinii did not bind to FH from any animals tested but did bind to the ∼60-kDa serum proteins produced by 10 of 15 of the animals tested. The data presented here suggest that while the B. garinii and B. afzelii CspZ orthologs may not contribute to complement evasion through FH binding, they may be involved in the host-pathogen interaction through the binding of other serum proteins.
FIG. 6.
Differential interaction of CspZ variants and mutants with FH and other serum proteins from diverse mammals. Reverse ALBI assays were performed as previously described (16) (summarized in the text). Serum samples from the animals indicated above each lane were fractionated under nonreducing conditions and the proteins transferred to membranes. The membranes were incubated with the S-tagged r-proteins indicated to the left of each panel. Abbreviations: Bb, B. burgdorferi; Bg, B. garinii; Ba, B. afzelii.
To determine if the inability of CspZ from B. afzelii and B. garinii to bind FH might be due to interference by the unique N-terminal domain present in these proteins, a variant of the B. garinii FRG CspZ protein was generated that lacked this N-terminal extension. As with the full-length protein, this truncated variant did not bind to FH, indicating that the presence of the N-terminal domain does not in and of itself prevent FH binding (data not shown). Hence, the differing abilities of CspZ proteins from different Lyme disease spirochete species to bind FH appear to be due to polymorphisms in other regions of the protein.
Binding of CspZ coiled-coil mutants and truncation variants to FH and other serum proteins was also assessed using the reverse ALBI approach. Consistent with the ALBI assays, the CspZ coiled-coil mutants did not bind to FH, nor did they bind to the ∼60-kDa serum protein(s) from most mammals. Interestingly, these proteins retained their ability to bind to serum proteins specifically produced by mice and rats. In contrast to the coiled-coil mutants, the N-terminal truncation variant lost FH binding ability but retained the ability to bind to the ∼60-kDa serum protein. This observation indicates that while there is some commonality in the FH and serum protein binding sites, there are also unique and separable determinants involved in the binding of these different ligands.
DISCUSSION
Borrelia burgdorferi sensu lato complex isolates produce a variable number of FHBPs, and consistent with this, complement sensitivity also varies among species. B. burgdorferi, which produces the largest repertoire of FHBPs, is generally more complement resistant than B. afzelii and B. garinii (5, 20, 40). The relative contribution of each specific FHBP in complement evasion and pathogenesis has not been fully defined. The most recent FHBP to be identified in B. burgdorferi is CspZ (open reading frame designation, BBH06). Prior to this research, it was unknown if CspZ orthologs are produced by other Lyme disease spirochete species and if they have similar functional activities. The initial goals of this report were to assess the distribution and expression of CspZ in the three major species of Lyme disease spirochetes and to further define the molecular basis of the interaction of this protein with FH and other host proteins.
The data presented above demonstrate that cspZ is carried and expressed by the majority of B. burgdorferi isolates. The gene is less widely distributed among B. garinii isolates (five of nine tested), and whether it is present in B. afzelii is not entirely clear. While PCR analyses detected a cspZ sequence in some B. afzelii strains, this gene sequence was highly similar to that of B. garinii. In addition, the gene was not detected through hybridization, and an immunoreactive protein was not detected by immunoblotting. It is plausible that the gene amplified from B. afzelii originated from a minor subpopulation of B. garinii present in these unclonal isolate populations. The B. afzelii isolates originated from human skin biopsy specimens from patients in Europe, where coinfection with multiple Borrelia species is common. Alternatively, it is possible that the B. afzelii populations analyzed are in the process of losing lp28-3. While lp28-3 is widely carried by Lyme disease spirochete strains, it is not universal (27). In any event, it is clear that the majority of B. burgdorferi strains carry the gene and that CspZ orthologs are produced by some B. garinii isolates.
Comparative sequence analyses revealed that cspZ is highly conserved within a species but is divergent between species. Most notable was the detection of a polymorphism encoding a 64-aa domain that is present at the N terminus of the B. garinii CspZ protein but absent from B. burgdorferi. This domain was not detected elsewhere in the B. burgdorferi and B. garinii genomes by BLAST searches, suggesting that the N-terminal “insertion” did not originate through a recombination event with an existing Borrelia gene.
While immunoblot analyses detected a protein that is antigenically related to CspZ in B. burgdorferi and B. garinii isolates, FH ALBI assays revealed that the B. garinii protein does not bind FH or FHL-1. To confirm this finding, a series of r-CspZ proteins derived from B. burgdorferi and B. garinii was generated and tested for FH and FHL-1 binding using ALBI and ELISA formats. The positive control for these analyses consisted of r-FhbA, a B. hermsii protein that binds both FH and FHL-1 (14). r-CspZ from B. burgdorferi but not that from B. garinii bound FH. To determine if the inability of B. garinii CspZ to bind FH was due to interference by the 64-aa N-terminal domain, r-CspZ (B. garinii FRG) proteins with and without this domain were generated. Neither protein bound FH (not shown), indicating that the N-terminal extension is not the basis for the lack of FH binding. All of the r-proteins described above were also tested for FHL-1 binding. With the exception of the positive control (B. hermsii FhbA), FHL-1 binding to r-CspZ proteins was not observed. Hartmann et al. previously reported that CspZ can bind both FH and FHL-1 (9). The basis for this discrepancy in FHL-1 binding is unclear.
Several studies have sought to identify the molecular determinants involved in FH and FHL-1 binding (1, 6, 13, 18, 24, 30). In addition, the molecular basis of the interaction of the FHBPs with infection-induced antibody has also been investigated (15, 30). Significantly different conclusions have been reached regarding the nature of the FH/FHL-1 interaction with Borrelia FHBPs (1, 9, 14, 16, 17, 25, 28, 30, 41). Based on studies using peptides, it has been suggested that FH and FHL-1 bind to specific linear sequence elements of CspZ and several other spirochetal FHBPs. Hartmann et al. concluded that within CspZ there are three linear binding sites for FH and FHL-1 and an additional site that specifically binds FHL-1 (9). However, in the same study it was demonstrated that CspZ truncations that lack the C-terminal 16-aa residues but retain the internal putative linear binding sites do not bind FH or FHL-1. Hence, the impact of the truncations seems inconsistent with the data indicating that CspZ-derived peptides alone can bind FH and/or FHL-1. Several earlier analyses have demonstrated that independent N- and C-terminal truncations of spirochetal FHBPs lead to a complete loss of ligand binding, even though the proteins retain the putative linear binding determinants (14, 15, 25, 28, 30). Based on these observations, we postulated that ligand binding occurs not through linear elements but rather through conformationally defined, discontinuous binding sites. In an effort to reconcile these differences in interpretation, we extended the binding studies of Hartmann et al. by generating N-terminal truncations and site-directed mutants of CspZ (9). Deletion of the first 20 N-terminal residues beyond the leader peptide abolished FH binding. This truncation variant fully retained three of the four putative FHL-1 binding sites and two of the three FH binding sites that were putatively identified by Hartmann et al. (9). In addition, the introduction of substitutions in regions outside of the putative linear binding sites through site-directed mutagenesis also abolished FH binding. The substitutions that were introduced were designed to disrupt putative coiled-coil domains. Coiled coils are defined by a heptad repeat sequence, (a-g)n, in which the a and d residues are nonpolar and the e and g residues are charged. The parallel or antiparallel interaction of two or more alpha helices that harbor the heptad repeat domain can result in highly stable inter- or intramolecular hydrophobic interactions that are highly resistant to thermal and chemical disruption. Each CspZ coiled-coil domain was independently mutated by substitution of a and d position residues (a total of two residues per mutant) with charged or polar residues. Computer predictions indicated that these substitutions decrease the probability of coiled-coil formation for cc1 from 53 to 0.4% and the probability of cc2 formation from 79 to 1.3%. It is noteworthy that in both mutants a Leu was substituted in each putative coiled coil. Leu residues have been demonstrated to be critical for the self-assembly of vaccinia virus envelope protein A27L (12). Substitution of Leu residues at the a and/or d position of the coiled-coil heptad repeat negatively impacted the oligomerization, structural stability, and biological activity of A27L. The loss of FH/FHL-1 binding ability by the specific and limited substitutions introduced into CspZ provides additional support for the hypothesis that the binding sites are not linear but are conformationally defined, with widely separated domains of the proteins involved in the presentation and formation of the ligand binding pocket.
FHBPs may contribute to the virulence and ecology of the Borrelia in several different ways. It has been demonstrated that some FHBPs can bind to FH derived from different mammalian hosts, while others bind in a species-specific manner (16, 26). The differential binding to FH from diverse mammals could influence immune evasion capabilities in a species-specific fashion and thus determine the host range and enzootic cycles of the Lyme disease spirochetes. In addition, the binding of borrelial FHBPs to additional serum proteins has been demonstrated (16, 26, 35). While the additional serum proteins bound by the Lyme disease spirochetes have not been identified, the ability to bind additional ligands suggests that there may be important functions for the FHBPs beyond the binding of complement regulators. In this study, we investigated the ability of CspZ to bind to FH and other serum proteins from several animal species. Binding was assessed using a reverse ALBI assay. This assay, which is conducted in a manner different from that for a standard ALBI assay, is described in detail above. Using this assay, it was found that CspZ derived from B. burgdorferi bound to FH and a set of ∼60-kDa serum proteins from a wide range of animals. While B. garinii CspZ-derived proteins do not bind FH, they do bind to the ∼60-kDa serum proteins. The specific nature of this interaction is supported by the fact that as few as two amino acid substitutions, as in the coiled-coil mutants, abolish ligand binding. In contrast, N-terminal truncation variants of CspZ lost FH binding but not the ability to bind other serum proteins. The demonstration that the FH and serum protein binding activities are separable has important implications for future studies designed to specifically determine the contribution of each interaction in pathogenesis. It may be possible to introduce FHBP genes that have site-directed mutations that selectively abrogate the binding of each individual ligand into borrelial strains. Studies are now under way to pursue this goal.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 10 September 2007.
REFERENCES
- 1.Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z. Z. Cheng, T. S. Jokiranta, I. J. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo, A., T. Meri, L. Rämö, T. S. Jokiranta, T. Heikkilä, I. J. T. Seppälä, J. Oksi, M. Viljanen, and S. Meri. 2001. Complement evasion by Borrelia burgdorferi: serum-resistant strains promote C3b inactivation. Infect. Immun. 69:3685-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa, R., H. Komatsuzawa, T. Kawai, S. Yamada, R. B. Goncalves, S. Izumi, T. Fujiwara, Y. Nakano, H. Shiba, M. A. Taubman, H. Kurihara, and M. Sugai. 2003. Outer membrane protein 100, a versatile virulence factor of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 50:1125-1139. [DOI] [PubMed] [Google Scholar]
- 5.Bhide, M. R., M. Travnicek, M. Levkutova, J. Culik, V. Revajova, and M. Levkut. 2005. Sensitivity of Borrelia genospecies to serum complement from different animals and human: a host-pathogen relationship. FEMS Immunol. Med. Microbiol. 43:165-172. [DOI] [PubMed] [Google Scholar]
- 6.Brooks, C. S., S. R. Vuppala, A. M. Jett, A. Alitalo, S. Meri, and D. R. Akins. 2005. Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J. Immunol. 175:3299-3308. [DOI] [PubMed] [Google Scholar]
- 7.Earnhart, C. G., E. L. Buckles, J. S. Dumler, and R. T. Marconi. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC antibody response. Infect. Immun. 73:7869-7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser, C., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischman, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann, K., C. Corvey, C. Skerka, M. Kirschfink, M. Karas, V. Brade, J. C. Miller, B. Stevenson, R. Wallich, P. F. Zipfel, and P. Kraiczy. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol. Microbiol. 61:1220-1236. [DOI] [PubMed] [Google Scholar]
- 10.Hefty, P. S., C. S. Brooks, A. M. Jett, G. L. White, S. K. Wikel, R. C. Kennedy, and D. R. Akins. 2002. OspE-related, OspF-related, and Elp lipoproteins are immunogenic in baboons experimentally infected with Borrelia burgdorferi and in human Lyme disease patients. J. Clin. Microbiol. 40:4256-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellwage, J., T. Meri, T. Heikkila, A. Alitalo, J. Panelius, P. Lahdenne, I. J. T. Seppala, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427-8435. [DOI] [PubMed] [Google Scholar]
- 12.Ho, Y., J. C. Hsiao, M. H. Yang, C. S. Chung, Y. C. Peng, T. H. Lin, W. Chang, and D. L. Tzou. 2005. The oligomeric structure of vaccinia viral envelope protein A27L is essential for binding to heparin and heparan sulfates on cell surfaces: a structural and functional approach using site-specific mutagenesis. J. Mol. Biol. 349:1060-1071. [DOI] [PubMed] [Google Scholar]
- 13.Hovis, K., J. V. McDowell, L. Griffin, and R. T. Marconi. 2004. Identification and characterization of a linear plasmid encoded factor H-binding protein (FhbA) of the relapsing fever spirochete Borrelia hermsii. J. Bacteriol. 186:2612-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hovis, K. M., J. P. Jones, T. Sadlon, G. Raval, D. L. Gordon, and R. T. Marconi. 2006. Molecular analyses of the interaction of Borrelia hermsii FhbA with the complement regulatory proteins factor H and factor H-like protein 1. Infect. Immun. 74:2007-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovis, K. M., M. E. Schriefer, S. Bahlani, and R. T. Marconi. 2006. Immunological and molecular analyses of the Borrelia hermsii factor H and factor H-like protein 1 binding protein, FhbA: demonstration of its utility as a diagnostic marker and epidemiological tool for tick-borne relapsing fever. Infect. Immun. 74:4519-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovis, K. M., E. Tran, C. M. Sundy, E. Buckles, J. V. McDowell, and R. T. Marconi. 2006. Selective binding of Borrelia burgdorferi B31MI OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraiczy, P., J. Hellwage, C. Skerka, M. Kirschfink, V. Brade, P. F. Zipfel, and R. Wallich. 2003. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur. J. Immunol. 33:697-707. [DOI] [PubMed] [Google Scholar]
- 18.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 31:1674-1684. [DOI] [PubMed] [Google Scholar]
- 19.Kraiczy, P., and R. Würzner. 2006. Complement escape of human pathogenic bacteria by acquisition of complement regulators. Mol. Immunol. 43:31-44. [DOI] [PubMed] [Google Scholar]
- 20.Kurtenbach, K., H. S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with the loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lupas, A., M. Van Dyke, and J. Stock. 1991. Predicting coiled coils from protein sequences. Science 252:1162-1164. [DOI] [PubMed] [Google Scholar]
- 23.Marconi, R. T., M. E. Konkel, and C. F. Garon. 1993. Variability of osp genes and gene products among species of Lyme disease spirochetes. Infect. Immun. 61:2611-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDowell, J. V., J. Frederick, L. Stamm, and R. T. Marconi. 2007. Identification of the gene encoding the FhbB protein of Treponema denticola, a highly unique factor H-like protein 1 binding protein. Infect. Immun. 75:1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDowell, J. V., M. E. Harlin, E. A. Rogers, and R. T. Marconi. 2005. Putative coiled-coil structural elements of the BBA68 protein of the Lyme disease spirochetes are required for formation of its factor H binding site. J. Bacteriol. 187:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDowell, J. V., K. M. Hovis, H. Zhang, E. Tran, J. Lankford, and R. T. Marconi. 2006. Evidence that the BBA68 protein (BbCRASP-1) of the Lyme disease spirochetes does not contribute to factor H-mediated immune evasion in humans and other animals. Infect. Immun. 74:3030-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDowell, J. V., S. Y. Sung, M. Labandeira-Rey, J. T. Skare, and R. T. Marconi. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect. Immun. 69:3670-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDowell, J. V., J. Wolfgang, L. Senty, C. M. Sundy, M. J. Noto, and R. T. Marconi. 2004. Demonstration of the involvement of outer surface protein E coiled-coil structural domains and higher order structural elements in the binding of infection-induced antibody and the complement-regulatory protein, factor H. J. Immunol. 173:7471-7480. [DOI] [PubMed] [Google Scholar]
- 29.McDowell, J. V., J. Wolfgang, E. Tran, M. S. Metts, D. Hamilton, and R. T. Marconi. 2003. Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect. Immun. 71:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metts, S., J. V. McDowell, M. Theisen, P. R. Hansen, and R. T. Marconi. 2003. Analysis of the OspE determinants involved in the binding of factor H and OspE-targeting antibodies during Borrelia burgdorferi infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. G. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandiripally, V., L. Wei, C. Skerka, P. F. Zipfel, and D. Cue. 2003. Recruitment of complement factor H-like protein 1 promotes intracellular invasion by group A streptococci. Infect. Immun. 71:7119-7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 97:13865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossmann, E., V. Kitiratschky, H. Hofmann, P. Kraiczy, M. M. Simon, and R. Wallich. 2006. Borrelia burgdorferi complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes is expressed in humans and induces antibody responses restricted to nondenatured structural determinants. Infect. Immun. 74:7024-7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossmann, E., P. Kraiczy, P. Herzberger, C. Skerka, M. Kirschfink, M. M. Simon, P. F. Zipfel, and R. Wallich. 2007. Dual binding specificity of a Borrelia hermsii-associated complement regulator-acquiring surface protein for factor H and plasminogen discloses a putative virulence factor of relapsing fever spirochetes. J. Immunol. 178:7292-7301. [DOI] [PubMed] [Google Scholar]
- 36.Schwan, T. G., W. Burgdorfer, and C. F. Garon. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stultz, C. M., J. V. White, and T. F. Smith. 1993. Structural analysis based on state-space modeling. Protein Sci. 2:305-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokarz, R., J. Anderton, L. Katona, and J. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Dam, A. P., A. Oei, R. Jaspars, C. Fijen, B. Wilske, L. Spanjaard, and J. Dankert. 1997. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect. Immun. 65:1228-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallich, R., J. Pattathu, V. Kitiratschky, C. Brenner, P. F. Zipfel, V. Brade, M. M. Simon, and P. Kraiczy. 2005. Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochetes Borrelia afzelii and Borrelia garinii. Infect. Immun. 73:2351-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, J. V., C. M. Stultz, and T. F. Smith. 1994. Protein classification by stochastic modeling and optimal filtering of amino-acid sequences. Math. Biosci. 119:35-75. [DOI] [PubMed] [Google Scholar]