Abstract
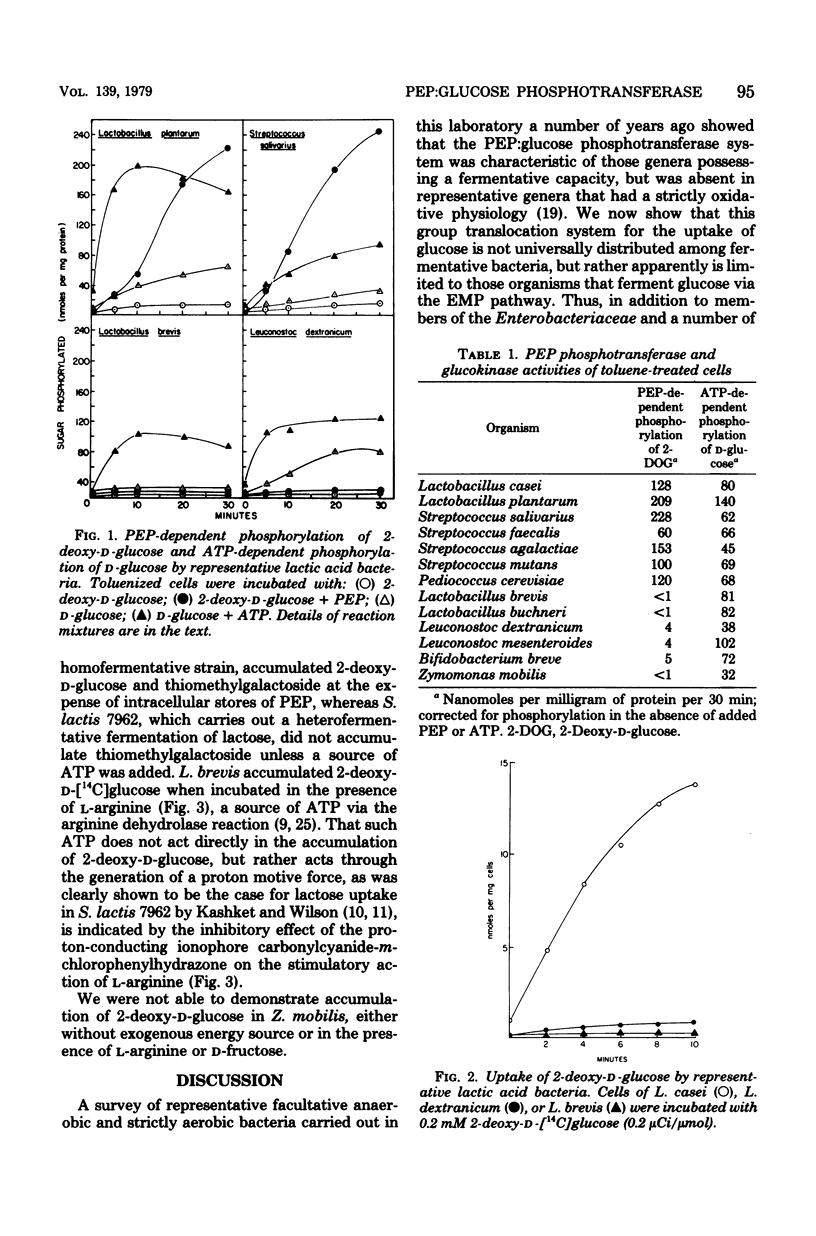
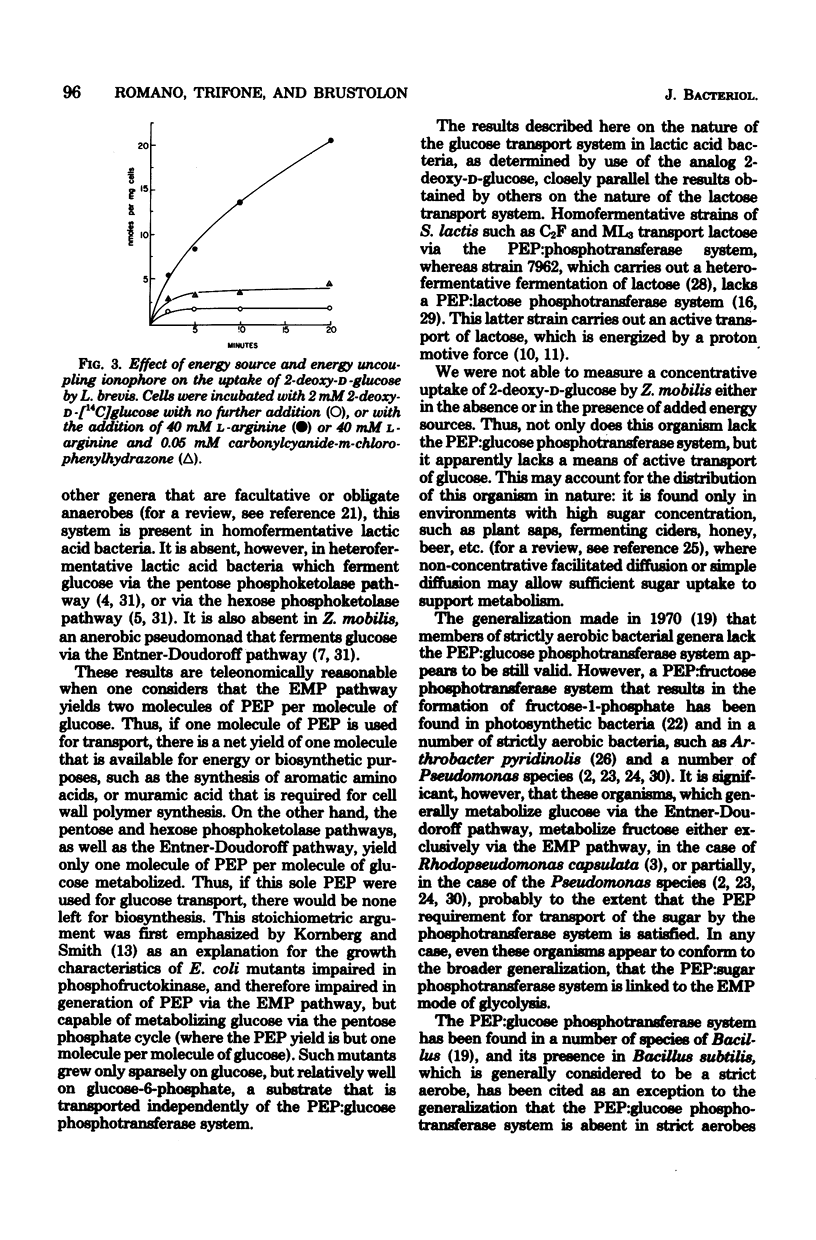
A number of selected fermentative bacteria were surveyed for the presence of the phosphoenolpyruvate:glucose phosphotransferase system, with particular attention to those organisms which ferment glucose by pathways other than the Embden-Meyerhof-Parnas pathway. The phosphoenolpyruvate:glusoe phosphotransferase system was found in all homofermentative lactic acid bacteria tested that ferment glucose via the Embden-Meyerhof-Parnas pathway, but in none of a group of heterofermentative species of Lactobacillus or Leuconostoc, which ferment glucose via the phosphoketolase pathway. A phosphoenolpyruvate:glucose phosphotransferase system was also absent in Zymomonas mobilis, which ferments glucose via an anaerobic Entner-Doudoroff pathway. It thus appears that the phosphotransferase mode of glucose transport is limited to bacteria with the Embden-Meyerhof-Parnas mode of glucose fermentation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Baumann L. Catabolism of D-fructose and D-ribose by Pseudomonas doudoroffii. I. Physiological studies and mutant analysis. Arch Microbiol. 1975 Nov 7;105(3):225–240. doi: 10.1007/BF00447141. [DOI] [PubMed] [Google Scholar]
- Conrad R., Schlegel H. G. Different pathways for fructose and glucose utilization in Rhodopseudomonas capsulata and demonstration of 1-phosphofructokinase in phototrophic bacteria. Biochim Biophys Acta. 1974 Jul 17;358(1):221–225. doi: 10.1016/0005-2744(74)90273-3. [DOI] [PubMed] [Google Scholar]
- DeMOSS R. D., BARD R. C., GUNSALUS I. C. The mechanism of the heterolactic fermentation; a new route of ethanol formation. J Bacteriol. 1951 Oct;62(4):499–511. doi: 10.1128/jb.62.4.499-511.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBS M., DEMOSS R. D. Anaerobic dissimilation of C14-labeled glucose and fructose by Pseudomonas lindneri. J Biol Chem. 1954 Apr;207(2):689–694. [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. J. PATHWAYS OF GLUCOSE CATABOLISM IN BACILLUS SUBTILIS. J Bacteriol. 1963 Aug;86:303–311. doi: 10.1128/jb.86.2.303-311.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., Ghosh D. Probable role of a membrane-bound phosphoenolpyruvate-hexose phosphotransferase system of Escherichia coli in the permeation of sugars. Indian J Biochem. 1968 Jun;5(2):49–52. [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Role of phosphofructokinase in the utilization of glucose by Escherichia coli. Nature. 1970 Jul 4;227(5253):44–46. doi: 10.1038/227044a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Richey D. P., Lin E. C. Importance of facilitated diffusion for effective utilization of glycerol by Escherichia coli. J Bacteriol. 1972 Nov;112(2):784–790. doi: 10.1128/jb.112.2.784-790.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Roseman S. Phosphoenolpyruvate-dependent fructose phosphorylation in photosynthetic bacteria. J Biol Chem. 1971 Dec 25;246(24):7819–7821. [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L., Berman S. M., Cánovas J. L., Berman R. H. Pathways of D-fructose catabolism in species of Pseudomonas. Arch Microbiol. 1977 Feb 4;112(1):49–55. doi: 10.1007/BF00446653. [DOI] [PubMed] [Google Scholar]
- Sawyer M. H., Baumann P., Baumann L. Pathways of D-fructose and D-glucose catabolism in marine species of Alcaligenes, Pseudomonas marina, and Alteromonas communis. Arch Microbiol. 1977 Mar 1;112(2):169–172. doi: 10.1007/BF00429331. [DOI] [PubMed] [Google Scholar]
- Sobel M. E., Krulwich T. A. Metabolism of D-fructose by Arthrobacter pyridinolis. J Bacteriol. 1973 Feb;113(2):907–913. doi: 10.1128/jb.113.2.907-913.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Thomas T. D. Phosphoenolpyruvate and 2-phosphoglycerate: endogenous energy source(s) for sugar accumulation by starved cells of Streptococcus lactis. J Bacteriol. 1977 May;130(2):583–595. doi: 10.1128/jb.130.2.583-595.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijken J. P., Quayle J. R. Fructose metabolism in four Pseudomonas species. Arch Microbiol. 1977 Sep 28;114(3):281–286. doi: 10.1007/BF00446874. [DOI] [PubMed] [Google Scholar]
- de Vries W., Stouthamer A. H. Pathway of glucose fermentation in relation to the taxonomy of bifidobacteria. J Bacteriol. 1967 Feb;93(2):574–576. doi: 10.1128/jb.93.2.574-576.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



