Abstract
Immunity to pneumococcal colonization in mice by exposure to live or killed pneumococci has been shown to be antibody independent but dependent on CD4+ T cells. Here we show that intranasal immunization with pneumococcal proteins (pneumococcal surface protein C, adhesin A, and a pneumolysoid) can elicit a similar mechanism of protection. Colonization could be significantly reduced in mice congenitally deficient in immunoglobulins after intranasal immunization with this mixture of proteins; conversely, the depletion of CD4+ T cells in immunized wild-type mice at the time of challenge eliminated the protection afforded by immunization. Overall, our results show that intranasal immunization with a mixture of pneumococcal proteins protects against colonization in an antibody-independent, CD4+ T-cell-dependent manner.
Almost 1 million children in the developing world die of infections due to Streptococcus pneumoniae (pneumococcus) each year (23). The success of passive immunization and polysaccharide-based vaccines for the prevention of colonization and/or disease has clearly demonstrated the importance of capsular antibodies in controlling pneumococcal disease and colonization. Furthermore, studies in animals (17) and in humans (10, 11) clearly demonstrate that these antibodies can protect against nasopharyngeal (NP) pneumococcal colonization, which precedes pneumococcal disease (3). The importance of this effect has recently become clear and has paralleled what was learned after universal immunization with Haemophilus influenzae type b vaccine: it has been estimated that the conjugate vaccine in the United States has prevented more than twice as many cases of invasive pneumococcal disease through indirect effects on pneumococcal transmission (i.e., herd immunity) as through its direct effect of protecting vaccinated children (9).
Protection by anticapsular antibody is limited by its serotype specificity, which has led several investigators to evaluate whether pneumococcal colonization can also be prevented by immunization with conserved antigens. In particular, several pneumococcal proteins have been evaluated as vaccine candidates in animal models of pneumococcal colonization by either the parenteral or the mucosal route (1, 4, 6-8, 19, 20). Mucosal immunization with some of these proteins in particular has been shown to elicit systemic and mucosal antibodies and to confer protection against pneumococcal disease and colonization (4, 6, 21, 24). The logical assumption has been made that a combination of systemic and mucosal antibodies elicited by such an immunization is responsible for the protection against colonization. To our knowledge, however, this causal association has never been formally tested.
Our group has been evaluating two mucosal vaccine candidates based on noncapsular antigens: a whole-cell vaccine (WCV) consisting of killed unencapsulated bacteria and a vaccine containing the cell wall polysaccharide (C-Ps), which is present in all pneumococcal strains. Intranasal immunization with either of these two antigens confers antibody-independent, CD4+ T-cell-dependent protection against pneumococcal colonization (16, 18). In both cases, we have also gathered evidence implicating the cytokine interleukin-17A (IL-17A) (16; unpublished data) which implies that CD4+ TH17A-producing T cells are likely responsible for protection.
Following these studies, we wished to test the hypothesis that, similar to the WCV or C-Ps, protection derived from intranasal immunization with purified pneumococcal proteins is dependent on CD4+ T cells and independent of antibody. To this end, we evaluated the mechanism of protection that is elicited by mucosal administration of three proteins that were previously shown to block colonization upon immunization by this route.
MATERIALS AND METHODS
Immunogens and bacterial strains.
Pneumococcal surface protein C (PspC) and surface adhesin A (PsaA) were prepared as described previously (4, 22). PdT, a derivative of pneumolysin carrying three amino acid substitutions (W433F, D385N, and C428G) which render the molecule nontoxic but do not interfere with TLR4-mediated inflammatory properties, was also described previously (14). The protein vaccine (3P-CT) consisted of a mixture of these three proteins (PspC, 5 μg/dose; PsaA, 5 μg/dose; and PdT, 1.8 μg/dose) with cholera toxin (CT) as an adjuvant (1 μg/dose). The WCV was derived from strain RX1AL-, a capsule- and autolysin-negative mutant, prepared as described previously (15); the final WCV mixture contained 108 (killed) CFU of this strain plus 1 μg of CT (List Biological Laboratories, Campbell, CA) per 10-μl dose. Control mice were immunized with 1 μg of CT in 10 μl saline. Pneumococcal challenge was performed with strain 0603, a serotype 6B clinical strain (15). Frozen mid-log-phase aliquots were thawed and diluted to ∼106 CFU/10 μl of intranasal inoculum for challenge.
Animal models.
To assess the efficacy of the protein mixture in the prevention of pneumococcal colonization, groups of 8 to 12 C57BL/6J mice (female; age, 6 weeks; Jackson Laboratories, Bar Harbor, ME) were randomized by cage to receive 3P-CT, WCV-CT, or CT alone as previously described (15). Inoculations were given three times at weekly intervals. Three weeks after the third immunization, serum samples were obtained from anesthetized mice. One week after collection of the sera, the mice were challenged intranasally with ∼106 CFU of strain 0603. At 1 week after challenge, the mice were euthanized by CO2 inhalation; an upper respiratory wash was done by instilling sterile, nonbacteriostatic saline retrograde through the transected trachea and collecting the first six drops (about 0.1 ml) from the nostrils. An animal was considered colonized if at least 1 CFU/100 μl of wash fluid was detected.
To test the role of antibody in protection, C57BL/6J μMT−/− mice (B6.129S2-Igh-6tm1Cgn/J, in which B-cell development is blocked at the pro-B stage [13]) were obtained from Jackson Laboratories. The mice were randomized by cage to receive 3P-CT, WCV-CT, or CT alone. Immunization and challenge were delivered as described above.
To further test the role of various T-cell subsets in protection at the time of challenge, groups of wild-type C57BL/6 mice targeted for T-cell depletion were first immunized with 3P-CT or CT using the same protocol given above. One group of mice (n = 16) received intranasal CT, and three groups received 3P-CT (n = 12 to 16 for each group). To evaluate the effect of depleting either CD4+ or CD8+ T cells, two of the individual groups of mice that received 3P-CT had 1 mg of antibodies administered intraperitoneally 1 day before, 1 day after, and 4 days after challenge. Mice were given rat anti-mouse CD4 monoclonal immunoglobulin G (IgG2b) (purified from hybridoma GK1.5; American Type Culture Collection [ATCC], Manassas, VA) or rat anti-mouse CD8 monoclonal IgG2a (purified from hybridoma 53-6.72; ATCC) or they received no antibody treatment. Overall, >95% of CD4+ or CD8+ T cells, respectively, were depleted from the spleens of the treated animals, as assessed by flow cytometry (data not shown). No reduction in CD8+ T cells in the spleen was observed in mice given anti-CD4+ antibody as assessed by flow cytometry; similarly, anti-CD8+ antibody treatment did not reduce CD4+ T cells in the spleen (data not shown).
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were performed on serum samples collected prior to exposure to the challenge strain. Ninety-six-well ELISA plates were coated overnight with one of three pneumococcal proteins (PspC, PsaA, or PdT, 1 μg/ml). Following blocking in phosphate-buffered saline-Tween (0.05%) with 5% fetal calf serum (or phosphate-buffered saline-0.05% casein in the case of PdT-coated plates), dilutions of serum were added and incubated at room temperature for 2 h. Plates were washed and secondary antibody to mouse Ig was added and incubated at room temperature for 1 h. The plates were washed and developed with SureBlue TMB microwell peroxidase substrate (KPL, Gaithersburg, MD).
Measurement of IL-17A secretion by splenocytes.
Cellular suspensions of splenocytes from immunized and control mice were obtained by passing spleens of mice euthanized after pneumococcal challenge through a 70-μm cell strainer (BD Biosciences, Bedford, MA). After washing and removal of red blood cells by hemolysis, cells were plated into 24-well tissue culture plates at a concentration of 105 cells/well in 500 μl of Dulbecco's modified Eagle's medium (BioWhittaker, Walkersville, MD) containing 10% low-endotoxin defined fetal bovine serum (HyClone, Logan, UT) and ciprofloxacin (10 μg/ml; gift from Miles Pharmaceuticals). Following 72 h of stimulation with PsaA, PspC, or PdT (all at 10 μg/ml), supernatants were collected following centrifugation and stored at −80°C until being analyzed by ELISA (R&D Systems, Minneapolis, MN) for their IL-17A concentrations. The supernatants were analyzed in duplicate and read against a standard, following directions provided by the manufacturer.
Statistical analysis.
Comparisons of ELISA values, colonization densities, and IL-17A production levels between groups were evaluated using the Mann-Whitney U test. For all comparisons, P < 0.05 was considered to represent statistical significance.
RESULTS AND DISCUSSION
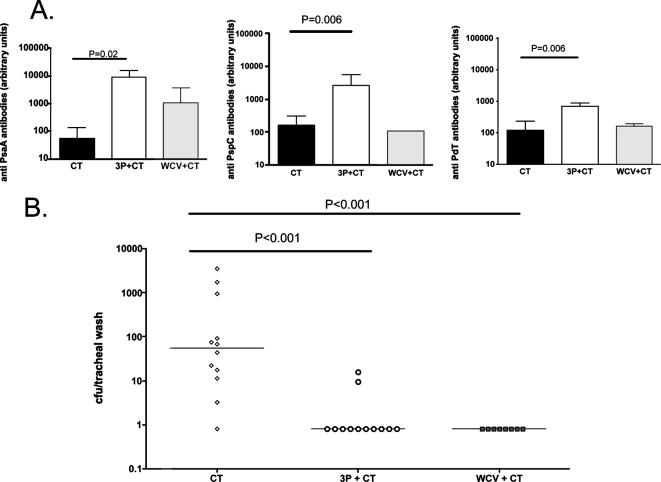
C57BL/6 mice were intranasally immunized three times at 1-week intervals with adjuvant alone (CT), WCV-CT, or 3P-CT as described above. Three weeks after the last immunization and prior to challenge, blood samples were obtained from randomly selected mice (n = 4 to 7 per group) from each group and analyzed by ELISA for the presence of antibodies directed against the individual proteins. As shown in Fig. 1A, mice immunized with 3P-CT had significantly higher serum antibody concentrations against all three proteins than mice that received CT alone (results of comparison of anti-PsaA, -PspC, or -PdT antibody concentrations were P, 0.02; P, 0.006; and P, 0.006, respectively, by Mann-Whitney U test). While mice immunized with WCV had, in general, higher levels of antibodies to the three proteins than controls, these differences did not reach statistical significance (P, 0.11; P, 0.68; and P. 0.88 for PsaA, PspC, and PdT, respectively, versus CT alone by Mann-Whitney U test). Immunization with the protein mixture resulted in significant protection against nasopharyngeal colonization: mice immunized with 3P-CT had significantly lower densities of pneumococcal colonization with strain 0603 than control mice that received CT alone (Fig. 1B); there was no difference in density of colonization between mice that received 3P-CT and mice that received the WCV (P > 0.5).
FIG. 1.
Immunogenicity and protection against NP challenge by intranasal immunization with 3P-CT in wild-type mice. (A) Serum antibody concentrations in response to proteins PsaA, PspC, and PdT in immunized mice. Serum antibody concentrations in response to these proteins were measured in C57BL/6 mice that were immunized intranasally with CT alone, 3P-CT, or WCV-CT. Serum samples were obtained 3 weeks after the last immunizations. Antibody concentrations were determined by ELISA; median concentration and interquartile range are shown. (B) Results of nasopharyngeal challenge of immunized mice. Mice immunized as described above were challenged with strain 0603 4 weeks after the last immunization. Tracheal aspirates obtained 1 week later were serially diluted and plated for quantification of colonization. Density of colonization of each individual mouse is shown; lines represent the median densities of colonization.
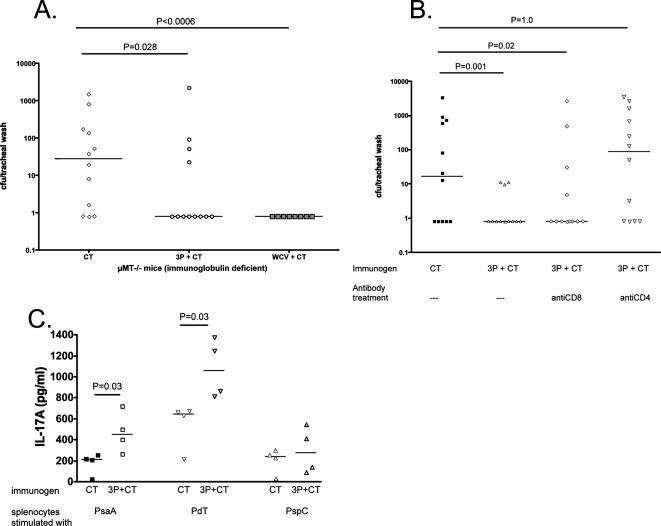
We have previously reported that intranasal immunization with WCV or C-Ps results in CD4+-dependent, antibody-independent protection against colonization (16, 18). To evaluate whether antibody-independent protection can also be elicited by a protein mixture, we immunized μMT−/− mice three times at 1-week intervals with adjuvant (CT) alone, 3P-CT, or WCV-CT as a positive control. Four weeks after the last immunization, mice were challenged intranasally with strain 0603; tracheal washes were obtained 1 week later. As shown in Fig. 2A, a significant reduction in NP colonization was observed in mice that received either the protein mixture or the WCV compared to colonization in mice that received the adjuvant alone, indicating that protection can be established in the absence of antibodies.
FIG. 2.
Antibody independence and CD4+ T-cell dependence of protection against NP challenge by 3P-CT. (A) Nasopharyngeal challenge of immunized μMT (Ig deficient) mice with strain 0603. μMT mice were immunized with CT, 3P-CT, or WCV-CT and subsequently challenged as described in the text. Density of colonization of each mouse is shown; lines represent median densities of colonization. Mice immunized with 3P-CT or WCV-CT had significantly lower densities of colonization than mice that received CT alone. (B) Effect on protection against NP challenge of CD4+ or CD8+ T-cell depletion of immunized wild-type mice. C57BL/6 mice immunized with 3P-CT received anti-CD4+ or anti-CD8+ T-cell antibodies at the time of challenge. Whereas mice that received anti-CD8+ antibodies were significantly protected against colonization, the administration of anti-CD4+ antibodies abolished protection by the 3P-CT. Density of colonization of each mouse is shown; lines represent median densities of colonization. (C) Measurement of IL-17A secretion by splenocytes. Splenocytes from immunized and control mice were stimulated with individual proteins for 3 days as described in the text; supernatants were collected and assayed for IL-17A concentration by ELISA.
By contrast, when wild-type C57BL/6 mice previously immunized with 3P-CT were depleted of CD4+ T cells by treatment with anti-CD4+ antibodies around the time of challenge, the protection associated with immunization was lost. The depletion of CD8+ T cells with anti-CD8+ antibodies had no effect upon protection. Together, these results show that protection is critically dependent on the presence of specific CD4+ T cells at the time of challenge (Fig. 2B). Finally, we evaluated the response of splenocytes from immunized (n = 4; 2 wild-type and 2 μMT−/− mice) versus control mice (n = 4; 2 wild-type and 2 μMT−/− mice) when stimulated with individual proteins (Fig. 2C). For two of these proteins, the IL-17A response was significantly higher in immunized mice than in controls (P was 0.03 for both PsaA and PdT). These data therefore indicate that, as is the case with the WCV and C-Ps, intranasal immunization with this protein mixture induces CD4+ T-cell-dependent and antibody-independent protection against NP colonization. We also show that mice develop protein-specific IL-17A responses following immunization; whether this cytokine is playing a role in protection merits further investigation.
Several investigators have previously demonstrated that mucosally administered protein antigens elicit systemic antibodies and also confer protection against invasive disease and/or carriage (2, 4, 6, 21, 24). Overall, our data show that, while antibodies are indeed elicited by intranasal immunization with a mixture of proteins (and more so than when the immunogen consists of whole killed bacteria), they are not required for the observed protection against NP colonization. These data are consistent with our previously published results regarding WCV or C-Ps or immunity following exposure to live pneumococci, but also extend them to immunity following exposure to proteins. Thus, intranasal immunization with purified pneumococcal proteins results in antibody-independent, CD4+-dependent immunity to carriage.
There have been limited studies on T-cell responses to pneumococcal antigens in humans to date. As an example, human T-cell responses to PspA following natural exposure have been reported (5), but the role of CD4+ T cells (independent of antibody) in protection against human colonization and/or disease had not specifically been investigated. Recently, Zhang et al. reported a negative association between CD4+ T-cell responses to pneumolysin and the likelihood of pneumococcal colonization in young children undergoing adenoidectomy (25). It remains to be shown, however, whether such CD4+ T-cell responses represent a mechanism whereby humans gradually become more resistant to pneumococcal carriage (or perhaps tend to carry strains for shorter periods of time (12). In conclusion, the data presented in this report lend further support to the hypothesis that antigen-specific CD4+ T-cell responses can confer protection against pneumococcal colonization. Studies are ongoing to evaluate whether such responses, and in particular IL-17A responses, can be detected in humans and whether they are associated with protection against pneumococcal colonization and possibly also against invasive disease following colonization.
Acknowledgments
We gratefully acknowledge support from the Pamela and Jack Egan Fund. R.M. is supported by grants from the National Institutes of Health (AI067737 and AI06601) and a grant from PATH. M.L. and C.M.T. are supported by grant 5 R01 AI048935 from the National Institutes of Health.
We thank P. Anderson for helpful discussions and suggestions during the course of this work and Pat Coan for expert technical assistance.
Editor: A. Camilli
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Alexander, J. E., R. A. Lock, C. C. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arulanandam, B. P., J. M. Lynch, D. E. Briles, S. Hollingshead, and D. W. Metzger. 2001. Intranasal vaccination with pneumococcal surface protein A and interleukin-12 augments antibody-mediated opsonization and protective immunity against Streptococcus pneumoniae infection. Infect. Immun. 69:6718-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baril, L., J. Dietemann, M. Essevaz-Roulet, L. Beniguel, P. Coan, D. E. Briles, B. Guy, and G. Cozon. 2006. Pneumococcal surface protein A (PspA) is effective at eliciting T cell-mediated responses during invasive pneumococcal disease in adults. Clin. Exp. Immunol. 145:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles, D. E., S. K. Hollingshead, G. S. Nabors, J. C. Paton, and A. Brooks-Walter. 2000. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine 19(Suppl. 1):S87-S95. [DOI] [PubMed] [Google Scholar]
- 8.Briles, D. E., S. K. Hollingshead, J. C. Paton, E. W. Ades, L. Novak, F. W. van Ginkel, and W. H. Benjamin, Jr. 2003. Immunizations with pneumococcal surface protein A and pneumolysin are protective against pneumonia in a murine model of pulmonary infection with Streptococcus pneumoniae. J. Infect. Dis. 188:339-348. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2005. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998-2003. Morb. Mortal. Wkly. Rep. 54:893-897. [PubMed] [Google Scholar]
- 10.Dagan, R., N. Givon-Lavi, D. Fraser, M. Lipsitch, G. R. Siber, and R. Kohberger. 2005. Serum serotype-specific pneumococcal anticapsular immunoglobulin G concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J. Infect. Dis. 192:367-376. [DOI] [PubMed] [Google Scholar]
- 11.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387-393. [DOI] [PubMed] [Google Scholar]
- 12.Högberg, L., P. Geli, H. Ringberg, E. Melander, M. Lipsitch, and K. Ekdahl. 2007. Age- and serogroup-related differences in the observed duration of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 45:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 14.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malley, R., M. Lipsitch, A. Stack, R. Saladino, G. Fleisher, S. Pelton, C. Thompson, D. E. Briles, and P. Anderson. 2001. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by encapsulated pneumococci. Infect. Immun. 69:4870-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malley, R., A. Srivastava, M. Lipsitch, C. M. Thompson, C. Watkins, A. Tzianabos, and P. W. Anderson. 2006. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect. Immun. 74:2187-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malley, R., A. M. Stack, M. L. Ferretti, C. M. Thompson, and R. A. Saladino. 1998. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J. Infect. Dis. 178:878-882. [DOI] [PubMed] [Google Scholar]
- 18.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton, J. C., R. A. Lock, C. J. Lee, J. P. Li, A. M. Berry, T. J. Mitchell, P. W. Andrew, D. Hansman, and G. J. Boulnois. 1991. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect. Immun. 59:2297-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pimenta, F. C., E. N. Miyaji, A. P. Areas, M. L. Oliveira, A. L. de Andrade, P. L. Ho, S. K. Hollingshead, and L. C. Leite. 2006. Intranasal immunization with the cholera toxin B subunit-pneumococcal surface antigen A fusion protein induces protection against colonization with Streptococcus pneumoniae and has negligible impact on the nasopharyngeal and oral microbiota of mice. Infect. Immun. 74:4939-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero-Steiner, S., J. Caba, G. Rajam, T. Langley, A. Floyd, S. E. Johnson, J. S. Sampson, G. M. Carlone, and E. W. Ades. 2006. Adherence of recombinant pneumococcal surface adhesin A (rPsaA)-coated particles to human nasopharnygeal epithelial cells for the evaluation of anti-PsaA functional antibodies. Vaccine 24:3224-3231. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. 2006. State of the art of new vaccines: research and development. www.who.int/vaccines-documents/DocsPDF06/814.pdf.
- 24.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 25.Zhang, Q., L. Bagrade, J. Bernatoniene, E. Clarke, J. C. Paton, T. J. Mitchell, D. A. Nunez, and A. Finn. 2007. Low CD4 T cell immunity to pneumolysin is associated with nasopharyngeal carriage of pneumococci in children. J. Infect. Dis. 195:1194-1202. [DOI] [PubMed] [Google Scholar]