Abstract
The enolase protein of the human malarial parasite Plasmodium falciparum has recently been characterized. Apart from its glycolytic function, enolase has also been shown to possess antigenic properties and to be present on the cell wall of certain invasive organisms, such as Candida albicans. In order to assess whether enolase of P. falciparum is also antigenic, sera from residents of a region of Eastern India where malaria is endemic were tested against the recombinant P. falciparum enolase (r-Pfen) protein. About 96% of immune adult sera samples reacted with r-Pfen over and above the seronegative controls. Rabbit anti-r-Pfen antibodies inhibited the growth of in vitro cultures of P. falciparum. Mice immunized with r-Pfen showed protection against a challenge with the 17XL lethal strain of the mouse malarial parasite Plasmodium yoelii. The antibodies raised against r-Pfen were specific for Plasmodium and did not react to the host tissues. Immunofluorescence as well as electron microscopic examinations revealed localization of the enolase protein on the merozoite cell surface. These observations establish malaria enolase to be a potential protective antigen.
Malaria continues to be a life-threatening infectious disease in the tropical world. Despite tremendous efforts to control the malaria epidemic, current prophylaxis and drug treatments are proving insufficient. The extensive spreading of drug-resistant Plasmodium strains as well as insecticide-resistant mosquitoes makes it urgent to develop an effective malaria vaccine. Long years of antigen identification and characterization have yielded many potential vaccine candidates, but developing an effective malaria vaccine has remained an incredibly difficult challenge (17). It has been observed that immunity to the disease develops gradually, after many attacks and over many years, in adults living in areas where malaria is endemic (2). The successful passive transfer of this immunity by injecting antibodies from malaria-immune persons to children susceptible to malaria has demonstrated that antibodies alone can trigger protection (5, 9, 58). These experiments have worked across geographical borders, as immunoglobulin G (IgG) from malaria-immune West Africans have cured East Africans as well as Thai malaria patients (5). The nature of this immunity is poorly understood at the molecular level. However, attempts have been made to identify antigens, the humoral response against which leads to protection. Seroepidemiological studies have identified several specific malarial blood-stage antigens including ring-infected erythrocyte surface antigen (10), apical membrane antigen (53), and PfP0, a conserved ribosomal protein (7, 18, 24), as protective antigens.
Enolase has been reported to be present on the cell surface of several organisms (38). It is also considered to be a major immunostimulatory protein in the case of visceral leishmaniasis (19). Enolase has been demonstrated to play a protective role in Candida albicans infection (31, 41, 55). Recently, it has also been identified as a potential vaccine candidate in Chlamydia pneumoniae infection (13). The single enolase gene of Plasmodium falciparum has been reported to possess certain plant-like features (42). We have recently expressed the recombinant P. falciparum enolase protein (r-Pfen) and studied its enzymatic properties (36). There has been one report regarding the presence of anti-enolase antibodies among malaria patients (45). Therefore, we decided to examine the immunogenic and protective properties of Pfen. In this paper, we report the prevalence of anti-enolase antibodies in sera of immune adults resident in regions of India where malaria is endemic, the surface localization of enolase protein on the merozoites, the growth-inhibitory properties of anti-enolase antibodies, and the protective capacity of Pfen in mice challenged with the lethal 17XL strain of the mouse malaria parasite Plasmodium yoelii.
MATERIALS AND METHODS
Human serum samples.
Human serum samples were collected from healthy adult residents and symptomatic children living in areas of the Phulbani district, Orissa, Eastern India, where P. falciparum is endemic, as described earlier (24). The criteria used for the set of healthy adults were the following: (i) permanent residency of the area, (ii) established record of suffering from symptomatic malaria earlier in childhood, and (iii) lack of clinical symptoms of malaria for a minimum of the previous 3 years. These parameters were determined through an extensive questionnaire and also from records of the primary health care center (24). From this sample set, 24 samples were selected and tested for reactivity with r-Pfen protein. The sex ratio of the adult sample set was matched (11 samples from males and 13 from females), and samples represented adults 16 to 65 years old. Most of the adult samples (67%) were from people 25 to 50 years old. This distribution reflected the whole collection and was similar to a profile presented earlier (24). Six serum samples from symptomatic children were collected from the same area. The age range of the children was 2 to 7 years, and the samples were matched for the sex ratio (3 from males and 3 from females). Upon examination of thick blood smears, 3 out of 24 (12.5%) adult samples and 3 out of 6 (50%) samples from children showed the presence of P. falciparum rings and gametocytes. The samples were collected with appropriate ethical clearance after obtaining subject consent. Approximately 0.1 to 1.0 ml of blood was collected using heparinized capillaries or tubes, and plasma samples were prepared. As seronegative controls, 34 Caucasian serum samples (kind gifts from Chris King, United States, and Pierre Druilhe, France) were used.
r-Pfen protein preparation and ELISA.
A His-tagged fusion r-Pfen protein was generated using the vector pQE30 (QIAGEN, Hilden, Germany), induced and purified from Escherichia coli using protocols described earlier (36). About 200 ng of antigen was used to coat microtiter plates (Nunc, Roskilde, Denmark), as reported earlier (24), for probing with human serum from Orissa. For comparing the reactivity of rabbit anti-r-Pfen antiserum against r-Pfen and rabbit muscle enolase (RMen), both of these proteins were coated in equimolar quantities (100 μl of 0.03 μM). After blocking, the samples were treated with human serum (1:250 dilution) or rabbit serum samples at dilutions of 1:100 and 1:1,000 in phosphate-buffered saline (PBS). An enzyme-linked immunosorbent assay (ELISA) was performed using horseradish peroxidase-conjugated anti-human and anti-mouse IgG at a 1:2,000 dilution.
Maintenance of Plasmodium and parasite protein preparation.
Asexual stages of P. falciparum strain 3D7 parasites were cultured in vitro as described earlier (18). Briefly, the parasites were maintained at 5% hematocrit in complete RPMI medium (RPMI medium plus 0.5% albumax) (GIBCO BRL, NY) at 37°C. The cultures were grown in the presence of a calibrated gas mixture (5% CO2, 2% O2, with the balance N2) in sealed flasks. P. yoelii strain 17XL was maintained by passaging asexual stages through Swiss mice by intraperitoneal injections. Parasitemia was monitored by making periodic peripheral thin smears stained with Giemsa (Sigma Chemical Co., St. Louis, MO) or Field stain (Biolab Diagnostics, Tarapur, India).
Parasite protein extraction.
P. falciparum culture was allowed to reach ∼5 to 7% parasitemia; cultures were then harvested and washed with incomplete RPMI solution. For P. yoelii parasite extract, mice were infected with P. yoelii strain 17XL and were allowed to reach 20 to 40% parasitemia. At this stage, 1 to 2 ml of blood was collected in equal volumes of anticoagulant containing 136 mM glucose, 42 mM citric acid, and 75 mM sodium citrate. Red blood cells (RBCs) were pelleted and washed with PBS. Infected erythrocytes from both types of parasites were then treated with 0.05% saponin (Sigma Chemical Co., St. Louis, MO) for 10 min at 4°C to release the parasites from the host erythrocyte membrane (20). The parasite pellets were then sonicated in PBS containing 1% Triton X-100 and cocktail protease inhibitor (Roche Applied Science, Indianapolis, IN). The supernatant was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis.
Protein extraction from lymphocytes and liver.
Human blood was collected with the anticoagulant EDTA, and the lymphocytes were separated by centrifugation at 600 × g for 15 min at 4°C on a Histopaque 1077 density gradient (Sigma Diagnostics, Inc.). The buffy coat was used as the source of human leukocytes. Crude protein extract of the leukocytes was obtained by lysing the cells in a buffer containing 1% Triton X-100 in PBS in the presence of pepstatin (1 μg/ml) and leupeptin (1 μg/ml) (Sigma Chemical Co., St. Louis, MO). For liver protein extraction, liver was dissected from the mouse, and the tissue was homogenized in the presence of pepstatin (1 μg/ml) and leupeptin (1 μg/ml) and centrifuged. Supernatants from both the preparations were used for SDS-PAGE and immunoblot analysis.
Growth inhibition assay.
Asexual stages of P. falciparum 3D7 parasites were cultured in vitro as described earlier (6). An invasion-blocking assay was performed in triplicate in 96-well sterile tissue culture plates (Nunc, Roskilde, Denmark) in a total culture volume of 200 μl. P. falciparum culture, synchronized by repeated sorbitol treatment at a parasitemia level of 1 to 2% with 5% hematocrit, was incubated with different preparations and dilutions of rabbit anti-enolase antibodies. Two rabbits were immunized with r-Pfen as described earlier (36), and the sera were pooled for this assay. Rabbit antiserum was used at dilutions of 1:200 and 1:500, and the preimmune antiserum was used at a dilution of 1:100. IgG fractions were also purified from immunized rabbits and their preimmune sera using protein A columns and were used at a final concentration of 1 mg/ml each. RBC smears were made at different time points (24, 48, and 60 h postsynchronization) and stained with Giemsa (Sigma Chemical Co., St. Louis, MO), and parasitemia was measured by microscopic counting. All the treatments were done in triplicate, and at least three sets of 2,000 RBCs were counted for each treatment at every time point. Results of the growth inhibition assay were statistically analyzed using one-way analysis of variance (GraphPad InStat, San Diego, CA). The means were considered statistically significantly different if the P values were <0.05.
Indirect IFA.
An immunofluorescence assay (IFA) was performed at room temperature with the air-dried blood smears. The slides were fixed with 4% formaldehyde in PBS for 10 min, washed five times, permeabilized with 0.25% Triton X-100 in PBS for 10 min, washed, fixed with 3% bovine serum albumin-PBS for 45 min, and incubated for 1 h with various antibodies. Anti-r-Pfen antiserum was used at a dilution of 1:200. AlexaFluor 568-conjugated anti-rabbit IgG and AlexaFluor 488-conjugated anti-mouse IgG (Molecular Probes, NJ) were used as secondary antibodies at dilutions of 1:500. All antibody dilutions were made in 1% bovine serum albumin-PBS. Parasite nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, NJ) at a final concentration of 1 μg/ml.
Vaccination studies with r-Pfen in mice.
Experiments were performed on three groups of 8-week-old male Swiss mice (five mice per group). Mice were injected intraperitoneally with r-Pfen emulsified in Freund's adjuvant at 21-day intervals (the first injection was 100 μg of r-Pfen in complete Freund's adjuvant, followed by 50 μg for the two boosters in incomplete Freund's adjuvant). In one control group, mice were injected in parallel with a recombinant Drosophila odorant binding protein OSF (as an irrelevant His-tagged protein control) emulsified in complete Freund's adjuvant. The other control group received no injections. After three immunizations, the antibody titers against r-Pfen were monitored. Mice having anti-r-Pfen antibody titers greater than 1:300,000 were then challenged with the lethal strain of P. yoelii (strain 17XL; 106 parasites per mouse), and parasitemia was monitored daily. Results of the parasitemia profiles on days 4, 5, and 6 were statistically analyzed using one-way analysis of variance (GraphPad InStat, San Diego, CA).
Solution IFA.
Intact merozoites for the IFA were prepared as described previously (7). Mice were infected with P. yoelii strain 17XL and were allowed to develop to ∼50 to 60% parasitemia, with the majority of parasites in segmented schizont stage. Infected blood was incubated at room temperature for 5 to 6 h in RPMI 1640 medium with 10% albumax. The liberated merozoites were harvested at 4°C, washed, and resuspended in complete RPMI 1640 medium (∼107 merozoites/ml). All the subsequent steps were also carried out on ice. Merozoites were incubated with rabbit anti-enolase antiserum (1:50 dilution in complete RPMI 1640 medium) for 30 min on ice. These samples were then washed three times with incomplete RPMI medium, resuspended at a 1:500 dilution of AlexaFluor 488-conjugated anti-rabbit IgG for 30 min in complete RPMI 1640 medium, and washed again seven to eight times. Parasite nuclei were stained with DAPI at a final concentration of 1 μg/ml. Merozoites were washed and mounted under glass coverslips in 5 μl of Vectashield mounting medium (Vector Laboratories, CA) on glass slides. The slides were observed under a Nikon microscope using a 100× phase-contrast objective.
For trypan blue staining of the merozoites, 0.1 ml of merozoites (∼105 cells per ml) was suspended in incomplete RPMI medium and stained with 20 μl of 0.4% trypan blue (Sigma Chemical Co., St. Louis, MO). The suspension was mixed thoroughly and allowed to stand for 5 min at room temperature. These cells were then observed under a microscope.
Immunoelectron microscopy.
Preparations of P. falciparum-containing RBCs and human leukocytes were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, PA) in 0.25 M HEPES (pH 7.4) for 1 h at room temperature and then in 8% paraformaldehyde in the same buffer overnight at 4°C. Cells were infiltrated, frozen, and sectioned as described previously (14). The sections were immunolabeled with mouse anti-r-Pfen antibodies (1:100 in PBS-1% fish skin gelatin) and then with anti-mouse IgG antibodies, followed directly by 15-nm protein A gold particles (Department of Cell Biology, Medical School, Utrecht University, The Netherlands) before examination with a Philips CM120 electron microscope (Eindhoven, The Netherlands) under 80 kV.
RESULTS
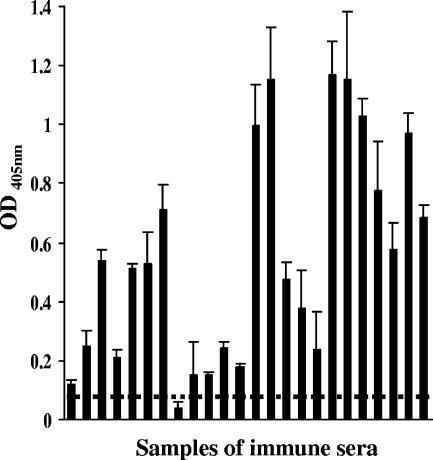
A His-tagged r-Pfen fusion protein was generated using protocols described earlier (36). An ELISA was carried out with r-Pfen as the substrate, using serum from adults and children from Orissa, Eastern India, along with control serum samples from Caucasians who had not been exposed to malaria. The histogram presented in Fig. 1 shows the individual reactivity of 24 serum samples from malaria-immune adults. The average values of the reactivity of the 34 serum samples from Caucasians and the 6 serum samples from children were 0.078 ± 0.007 and 0.088 ± 0.019, respectively. Using the Caucasian serum average reactivity plus 3 standard deviations (SDs) as the cutoff value, it was observed that 96% of immune adults showed reactivity to r-Pfen protein. Of the six samples from children from Orissa, none showed reactivity above the cutoff value of the average of the Caucasian sera plus 3 SDs (data not shown). These results demonstrated that anti-enolase antibodies were prevalent among malaria-immune adults resident in Eastern India.
FIG. 1.
Reactivity of r-Pfen (200 ng) with 24 serum samples collected from immune adults resident in the Phulbani district of Orissa where malaria is endemic. The horizontal line represents the cutoff determined by the mean reactivity plus 3 SDs determined from 34 normal Caucasian serum samples. OD, optical density.
Immunoblotting, growth inhibition assays, and indirect IFAs.
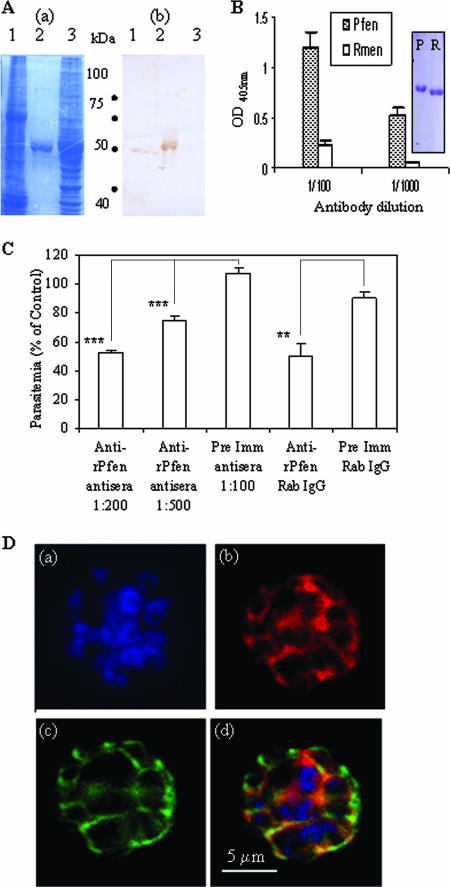
In order to test whether antibodies against parasite enolase can interfere with parasite growth, it was decided to test the specificity of the antibodies raised in rabbits against r-Pfen. Whole-cell extracts prepared from P. falciparum and from human erythrocytes and purified r-Pfen were subjected to SDS-PAGE and blotted on a membrane, and the blot was treated with rabbit anti-r-Pfen antibodies (Fig. 2A). The rabbit antiserum did not react with human erythrocyte proteins although it recognized the recombinant 50-kDa r-Pfen protein. Among the proteins in the whole-cell extract from P. falciparum, the antiserum recognized a single protein with a molecular mass of ∼49 kDa (Fig. 2A). The size matches well with the expected size of Pfen (48.6 kDa). To test the specificity of the serum to native protein, an ELISA was carried out using r-Pfen and commercially available rabbit enolase protein (Fig. 2B). The Coomassie-stained SDS gel shows that the Plasmodium and mammalian enolase proteins used for the ELISA were pure proteins (Fig. 2B). The ELISA and immunoblotting data established the specificity of the rabbit anti-r-Pfen antibodies toward Pfen.
FIG. 2.
(A) SDS-gel electrophoresis of ∼100 μg of cell lysates from P. falciparum and noninfected RBCs (lanes 1 and 3) and ∼5 μg of purified r-Pfen protein (lane 2) was followed by Coomassie blue staining (a) and immunoblotting using rabbit anti-enolase antisera (1:800 dilution) (b). (B) ELISA. Reactivity of equimolar amounts (100 μl of 30 nM solutions) of r-Pfen and RMen, checked with rabbit anti-r-Pfen antiserum at 1:100 and 1:1,000 dilutions. Inset shows the SDS-PAGE analysis and Coomassie blue staining of 10 μl of 30 μM stock solutions of r-Pfen (P) and RMen (R), which were used for the ELISAs. OD, optical density. (C) Effect of polyclonal rabbit antibodies on in vitro growth of synchronized cultures of P. falciparum. Synchronized cultures were treated with rabbit anti-r-Pfen antiserum at dilutions of 1:200 and 1:500 and with rabbit preimmune (Pre Imm) antiserum at a 1:100 dilution. IgG purified from rabbit anti-r-Pfen and preimmune serum was used at a final concentration of 1 mg/ml each. Parasitemia is shown as a percentage of the untreated control. **, P < 0.01; ***, P < 0.001. (D) IFA of the schizont stage of P. falciparum using DAPI (a), rabbit anti-r-Pfen antiserum (1:200) with secondary anti-rabbit IgG conjugated with AlexaFluor 568 (b), and mouse anti-MSP-1 antibodies (1:100) with secondary anti-mouse IgG conjugated with AlexaFluor 488 (c). An overlay of the images from frames a, b, and c is shown in frame d.
A growth inhibition assay was then performed using the asexual stages of P. falciparum parasites (Fig. 2C). The synchronized cultures were examined through development of the trophozoite (around 24 h), schizont (around 48 h), and subsequent ring (>48 h) stages in the presence of anti-r-Pfen antibodies. No significant difference in parasitemia levels was observed during the trophozoite and schizont stages. For instance, the culture treated with purified anti-r-Pfen IgG showed parasitemia levels of 1.25% ± 0.07% and 1.39% ± 0.09% versus the control parasitemia levels of 1.62% ± 0.30% and 1.61% ± 0.24% at 24 and 48 h, respectively. However, significant differences were observed at 60 h in the numbers of freshly infected ring stages in the presence of anti-r-Pfen antibodies (Fig. 2C). While the percent parasitemia with anti-r-Pfen IgG remained 1.24% ± 0.11%, the control reached 2.63% ± 0.30%, showing about 50% inhibition with purified IgG. The amount of purified IgGs required for 50% inhibition was much greater than that of serum at a 1:200 dilution. This suggests that other classes of Igs may also contribute to growth inhibition. In our attempts to raise monoclonal antibodies against Pfen, we observed that several of the parent clones secreted the IgM class of antibodies (37). These results demonstrate that the antibodies against Pfen inhibit the growth of the parasite. Since the inhibition was observed specifically at the ring stage, it can be surmised that the antibodies interfered with the invasion of merozoites into fresh red cells.
These results also indicate that enolase is likely to be expressed on the surface of P. falciparum merozoites. Therefore, we studied the localization of enolase in mature segmented schizonts of P. falciparum along with a known merozoite surface marker, merozoite surface protein 1 (MSP-1) (Fig. 2D). An indirect IFA showed that in addition to the cytoplasmic presence of enolase, the typical beehive pattern of a surface protein was also observed when P. falciparum schizonts were stained with anti-enolase antibodies, and there was significant colocalization of enolase with MSP-1 protein (Fig. 2D). These results support the view that enolase is localized on the surface of P. falciparum merozoites.
Vaccination studies with r-Pfen in mice.
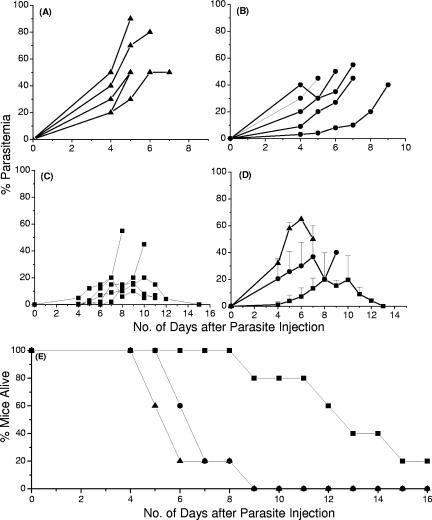
Next, we wanted to evaluate the ability of the Pfen to protect mice against a challenge with mouse malaria parasites. Since P. falciparum and P. yoelii enolases share 90% sequence homology and since antibodies to r-Pfen cross-react with P. yoelii enolase in all the erythrocytic stages of the parasite (37), we examined the effect of immunization of mice with r-Pfen, followed by a challenge with lethal P. yoelii strain 17XL. Figure 3A to C show the parasitemia profile of each mouse from the three groups over a period of 15 days after the P. yoelii challenge. All the control mice and the mice immunized with the irrelevant His-tagged protein developed a high degree of parasitemia (>17% on average) by day 4 postchallenge (Fig. 3A and B), whereas r-Pfen-immunized mice showed <1% parasitemia at that time point (Fig. 3C and D). Figure 3D shows the average parasitemia values for each treatment. The highest average parasitemia values were 70% and 40% for nonimmunized mice and mice injected with irrelevant His-tagged protein, respectively. However, among the mice immunized with r-Pfen, there was significant delay in the increase in parasitemia, and the highest average parasitemia was about 20% on day 8 postchallenge. The averages of these groups were compared using one-way analysis of variance, which showed that the mice immunized with enolase were significantly protected (P < 0.01) for days 4, 5, and 6, while there were no significant differences seen between the control and the group immunized with irrelevant His-tagged protein (P > 0.05). Figure 3E shows the survival profile of the mice. The immunized mice also had a significantly longer survival period. The reduction in parasitemia was found to correlate with the antibody titer present in each mouse. The sera from mice immunized with the His-tagged OSF protein showed no cross-reactivity to the His-tagged r-Pfen (data not shown), indicating that the protection was specific for r-Pfen. These results establish partial protection of the mice upon vaccination with the Plasmodium enolase protein. It is documented that combinations of different parasite strains with different mouse strains give different susceptibility patterns of malaria (47) and that the protection depends on not only the immunity to a specific antigen but also various other factors such as diet (1). Our results with enolase are similar to those obtained with other malaria candidate antigens and P. yoelii lethal strain combinations (7, 32, 59).
FIG. 3.
Vaccination study using r-Pfen as an immunogen. Groups of five mice each were either nonimmunized (▴) (A), or immunized with an irrelevant His-tagged protein (Drosophila odorant binding protein OSF) (•) (B) or r-Pfen (▪) (C). The parasitemia profile of each mouse is shown in panels A to C. The average parasitemia and survival pattern for each group of mice are shown in panels D and E.
Specificity of enolase as an antigen and surface localization.
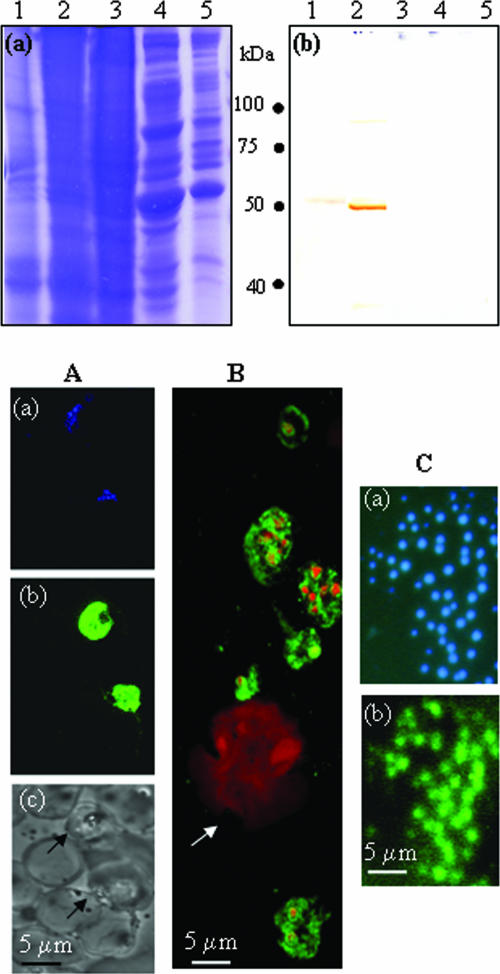
In order to use Plasmodium enolase as a vaccine candidate, the immune response has to be specific for the antigen, and the enolase protein must be present on the surface of P. yoelii merozoites. The specificity of the antibodies raised in r-Pfen-immunized mice was examined against P. yoelii and P. falciparum as well as murine and human cells (Fig. 4). An immunoblot assay showed specific recognition of the serum with both P. falciparum and P. yoelii proteins at the expected molecular sizes of 48.6 and 50.3 kDa (Fig. 4, top panel, b). Neither the murine liver and leukocyte preparations nor the human leukocyte extract exhibited any reactivity with serum from immunized mice, demonstrating the specificity of the antibodies generated in mice toward the parasite enolase protein.
FIG. 4.
(Top panel) Specificity of polyclonal antibodies raised against r-Pfen in mouse: ∼100 μg of proteins from the crude extract of P. yoelii (lane 1), P. falciparum (lane 2), mouse liver (lane 3), human (lane 4), and mouse leukocytes (lane 5) was analyzed on a 12% SDS gel followed by Coomassie blue staining (a) or Western blotting using mouse anti-r-Pfen antisera at 1:800 dilution (b). (Bottom panel) IFA of permeabilized P. falciparum-infected and noninfected human red cells (A), P. yoelii-infected mouse blood smear (B), and nonpermeabilized free P. yoelii merozoites with anti-r-Pfen antiserum (C). Panel A shows the same field of P. falciparum-infected erythrocytes stained with DAPI (blue) (a) and with mouse anti-r-Pfen antiserum (green) (b) and a bright-field image of the area showing infected (arrow) and uninfected erythrocytes (c). (B) Merged confocal image of P. yoelii-infected mouse blood smear stained with DAPI (red) and mouse anti-r-Pfen antiserum (green). The arrow indicates a monocyte. (C) Solution IFA of free P. yoelii merozoites treated with DAPI (a) or rabbit anti-r-Pfen antiserum followed by goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (b). Each antiserum was used at a 1:50 dilution.
P. falciparum- and P. yoelii-infected blood cells, permeabilized with Triton X-100, were also examined by IFA. This assay not only determined the specificity of anti-r-Pfen antibodies but also assesses the localization of parasite enolase at different stages of infection (Fig. 4, bottom panel). No staining of Plasmodium enolase was seen in the red cell compartment of the infected erythrocytes, nor was any staining observed with uninfected mammalian erythrocytes (Fig. 4, bottom panel, A and B) or with mouse monocytes (Fig. 4, bottom panel, B). Dominant cytoplasmic localization of enolase was observed in all the erythrocytic substages: the rings, trophozoites, and schizonts (Fig. 4, bottom panel, A and B). There was also some evidence of localization within the nucleus in some of these stages (Fig. 4, bottom panel, B). Since permeabilized cell preparations cannot resolve the issue of surface localization unequivocally, a solution IFA was performed on freshly prepared nonpermeabilized merozoites from P. yoelii to assess the presence of enolase on the surface of the merozoites (Fig. 4, bottom panel, C). Distinct anti-enolase signal was observed on the free merozoites (Fig. 4, bottom panel, C), whereas no signal was observed with preimmune serum (data not shown). The accepted criterion for viability of merozoites is their ability to invade red cells in culture. Since such an assay is not possible for rodent species, we decided to try trypan blue staining to assess the permeability of the dye in fixed merozoites. It was found that trypan blue did not stain the merozoites either with or without fixation with 1% formaldehyde. Thus, trypan blue does not appear to be an exclusion criterion for P. yoelii merozoites. Therefore, in order to assess the presence of enolase on the merozoite surface, we decided to carry out immunoelectron microscopy (IEM).
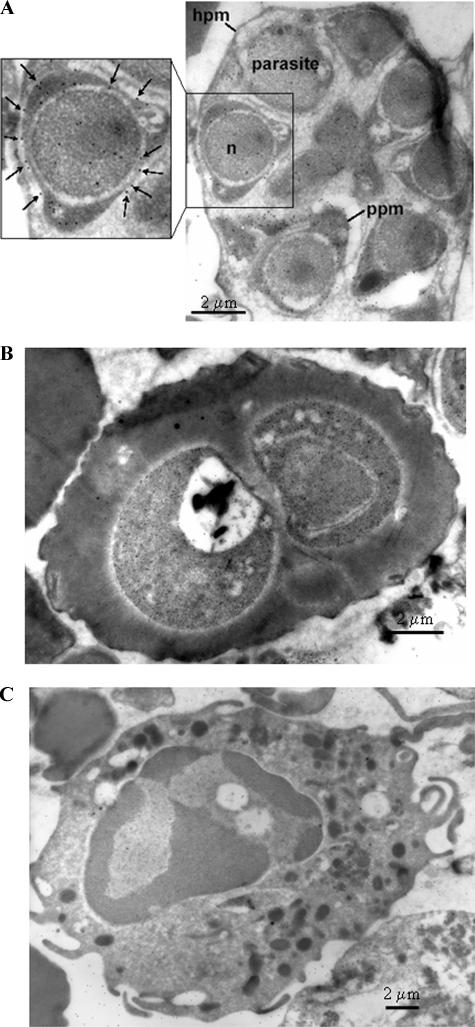
IEM was carried out on human leukocytes as well as on various stages of P. falciparum-infected erythrocytes (Fig. 5). Figures 5A and B show IEM images of P. falciparum segmented schizont and young trophozoite stages, respectively, while Fig. 5C shows an IEM image of a human leukocyte. There was an absence of anti-r-Pfen activity in the human leukocyte or the red cell compartment of the infected erythrocyte, confirming the specificity of anti-enolase antibodies. While the surface localization of enolase at the trophozoite stage is not unequivocal, the presence of enolase on the parasite cell surface (marked with arrows) in the segmented schizonts is evident. The localization of enolase on the parasite plasma membrane is seen on several of the segmented merozoites, whereas neither the RBC cytoplasm nor the RBC plasma membrane of the infected cells shows any reactivity (Fig. 5A and B). The presence of enolase in the parasite cytoplasm and the nucleus is also observed.
FIG. 5.
IEM image using mouse anti-r-Pfen antiserum at a 1:100 dilution with a P. falciparum-infected red cell at segmented schizont stage (A), a P. falciparum-infected cell at young trophozoite stage (B), and a human leukocyte (C). In panel A, the presence of enolase on the parasite plasma membrane (ppm) is marked with arrows. Hpm, host cell plasma membrane; n, nucleus.
DISCUSSION
In this paper we have demonstrated that the Plasmodium enolase protein is immunogenic and is present on the cell surface of merozoites and that anti-enolase antibodies can protect against the malarial parasite. The in vitro parasite growth inhibition observed in the P. falciparum culture is unlikely to be due to the inhibition of the glycolytic activity of enolase, since the rabbit anti-enolase antibodies did not inhibit the enzymatic activity of enolase (data not shown). Thus, the observed growth inhibition may be due either to the direct disruption of some nonglycolytic, but surface-related, function(s) of Plasmodium enolase or to indirect effects of antibody binding to the merozoite surface. Protective anti-r-Pfen antibodies raised in animals were specific for Plasmodium. These reacted with the enolase from both human and murine Plasmodium species but did not cross-react with the host proteins. Thus, Pfen possesses the properties suitable for a potential candidate malaria vaccine antigen.
Our results show that anti-enolase antibodies are widespread among adult residents of the area of Eastern India where malaria is endemic. We observed that a high frequency of adults (96%) tested positive for enolase while none of the samples from children tested positive, indicating that there is an age correlation with the buildup of anti-enolase antibodies. Of the adult population examined, only 12.5% of the immune adults showed the presence of parasites through examination of blood smears, but 96% were positive for anti-enolase antibodies. This difference could be due to either the persistence of anti-enolase antibodies after the clearance of parasites or the lack of sensitivity of the blood smear to detect low levels of parasites present in immune adults. The sensitive methods of PCRs detect low levels of the parasite in about a threefold higher frequency in the population (28). It would be useful to employ such methods in order to establish if anti-enolase antibodies may be used for detection of parasites, as has been previously suggested (45). It is also possible that the high frequency of anti-enolase antibodies may be due to cross-reactivities with anti-enolases induced by exposure to other pathogens such as Candida (41), Chlamydia (13), and Leishmania (19).
Antigenic variation and allelic polymorphisms render the malaria vaccine candidate antigens ineffective for use as a long-lived and broadly available vaccine. In rodent malaria challenge studies, two leading vaccine candidates, MSP-119 (44) and AMA-1 (apical membrane antigen 1) (11), were found to protect only from homologous, but not heterologous, challenge. In several other studies, protection has been documented to apply across a limited number of strains (11, 27). A subunit vaccine with chimeric antigens composed of multiple epitopes from different antigens of P. falciparum is thought to be an ideal way to address these difficulties. However, a recent study showed that variants of MSP-1 can mutually turn off CD4+ T cells, implying that the inclusion of multiple allelic variants in a vaccine may be detrimental to both the initial priming as well as the in vivo restimulation of preexisting effector T cells (23). For malaria vaccine candidates, it is often the conserved subdomains and regions that have shown promising protective effects, for example, region II of the circumsporozoite protein (8) and the thrombospondin-related anonymous protein (49). Thus, candidate antigens conserved across different strains of Plasmodium may serve as better candidates, as long as these are immunogenic and do not cross-react with human proteins. r-Pfen is perhaps one such candidate. Although the Plasmodium enolase protein shows considerable homology (∼68% identity and ∼78% homology) with the human orthologue, it possesses certain novel plant-like features (42), and there are distinct structural differences (35). The lack of cross-reactivity of anti-r-Pfen with host enolases has been demonstrated in this paper through immunoblotting, IFAs, and electron microscopic examinations.
Antibodies against human enolase protein have been detected in certain autoimmune disorders such as Hashimoto's encephalopathy (16, 33), retinopathy (26, 60), rheumatoid arthritis (21, 46), systemic sclerosis (29), autoimmune premature ovarian failure (51), relapsing polychondritis (52), and lung adenocarcinoma (54). Detection of anti-enolase antibodies has been suggested as a means of specific diagnosis for some of these disorders (16, 51, 54, 60). Although the pathological significance of these anti-enolase antibodies is not very clear, one has to exercise caution in the advocacy of Pfen as a malaria vaccine candidate and use only those regions of the protein that are protective and also very specific for the parasite. Epitope mapping of protective antibodies, as well as comparative structural analysis of host and parasite enolase proteins, will help toward defining such domain(s) on the parasite enolase protein. We are working toward identification of the protective parasite-specific epitopes of enolase. Monoclonal antibodies have been generated by immunizing mice with r-Pfen. Two of the Plasmodium-specific clones obtained mapped at the N-terminal 165 aminoacyl region of r-Pfen, which contains the plant-specific peptide insert (37). These studies indicate that such parasite-specific epitopes can be identified.
Presence of enolase on the surface of a cell has been reported in some mammalian cell lines, such as the U937 monocytoid line (30), neutrophils, T cells, B cells, peripheral blood monocytes (43), and human brain tumor cells (56, 57). Neither the function(s) nor the regulation of the surface-localized enolase is understood at present. Cell surface association of enolase is also reported in the case of pathogens such as Streptococci (3, 39, 40), C. albicans (31, 41, 55), and other bacteria (4, 15, 48). Recently, different experimental approaches such as genetic, cellular biology, and proteomic have been used to show that yeast enolase can reach the cell surface (25). Secretion of enolase and its involvement in the invasion process have been reported for an apicomplexan avian parasite, Eimeria tenella (22). In Plasmodium, enolase is present on the surface as well as in the nucleus, as observed through IFA and IEM data presented in this paper. Our biochemical analyses of subcellular fractions of P. yoelii have also provided similar results (34). The nuclear presence of enolase has also been reported in the closely related apicomplexan Toxoplasma gondii. However, the significance of such translocation is not yet understood (12). Increasingly, metabolic enzymes are being reported to have diverse functions (50). Clearly, the housekeeping proteins are regulated and utilized for other functions, perhaps when glycolysis is not the main objective of the cell, such as in the invasive merozoite stage. What determines the translocation of enolase to regions other than the cytoplasm, as well as the function(s) of enolase protein at these locations, remains to be elucidated.
In this paper we have shown that a housekeeping enolase protein of Plasmodium can protect mice against malaria. An effective malaria vaccine has been difficult to achieve for several reasons, predominant among which are antigenic variation and diversity. An additional problem is the emerging resistance of the parasite to most antimalarial drugs. Thus, it may be worthwhile to evaluate enolase, a protein critical to the survival of the parasite, for the immunoprophylactic and immunotherapeutic control of malaria.
Acknowledgments
We are indebted to Nirbhay Kumar of Johns Hopkins University for help with parasite cultures for IEM and to the Malaria Research and Reference Reagent Resource for MSP-1 antibodies. We are also grateful to B. Ravindran, Chris King, and Pierre Druilhe for the human serum samples. We thank Marc Pypaert and the technical personnel in the Yale Center for Cell and Molecular Imaging for excellent assistance in electron microscopy.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 4 September 2007.
REFERENCES
- 1.Ariyasinghe, A., S. R. Morshed, M. K. Mannoor, H. Y. Bakir, H. Kawamura, C. Miyaji, T. Nagura, T. Kawamura, H. Watanabe, H. Sekikawa, and T. Abo. 2006. Protection against malaria due to innate immunity enhanced by low-protein diet. J. Parasitol. 92:531-538. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K. 1995. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today 11:105-111. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann, S., M. Rohde, G. S. Chhatwal, and S. Hammerschmidt. 2001. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol. Microbiol. 40:1273-1287. [DOI] [PubMed] [Google Scholar]
- 4.Bernal, D., J. E. de la Rubia, A. M. Carrasco-Abad, R. Toledo, S. Mas-Coma, and A. Marcilla. 2004. Identification of enolase as a plasminogen-binding protein in excretory-secretory products of Fasciola hepatica. FEBS Lett. 563:203-206. [DOI] [PubMed] [Google Scholar]
- 5.Bouharoun-Tayoun, H., C. Oeuvray, F. Lunel, and P. Druilhe. 1995. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182:409-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee, S., S. Singh, R. Sohoni, V. Kattige, C. Deshpande, S. Chiplunkar, N. Kumar, and S. Sharma. 2000. Characterization of domains of the phosphoriboprotein P0 of Plasmodium falciparum. Mol. Biochem. Parasitol. 107:143-154. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee, S., S. Singh, R. Sohoni, N. J. Singh, A. Vaidya, C. Long, and S. Sharma. 2000. Antibodies against ribosomal phosphoprotein P0 of Plasmodium falciparum protect mice against challenge with Plasmodium yoelii. Infect. Immun. 68:4312-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee, S., M. Wery, P. Sharma, and V. S. Chauhan. 1995. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect. Immun. 63:4375-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen, S., I. A. McGregor, and S. Carrington. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733-737. [DOI] [PubMed] [Google Scholar]
- 10.Coppel, R. L., A. F. Cowman, R. F. Anders, A. E. Bianco, R. B. Saint, K. R. Lingelbach, D. J. Kemp, and G. V. Brown. 1984. Immune sera recognize on erythrocytes Plasmodium falciparum antigen composed of repeated amino acid sequences. Nature 310:789-792. [DOI] [PubMed] [Google Scholar]
- 11.Crewther, P. E., M. L. Matthew, R. H. Flegg, and R. F. Anders. 1996. Protective immune responses to apical membrane antigen 1 of Plasmodium chabaudi involve recognition of strain-specific epitopes. Infect. Immun. 64:3310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson, D. J., S. F. Parmley, and S. Tomavo. 2002. Evidence for nuclear localisation of two stage-specific isoenzymes of enolase in Toxoplasma gondii correlates with active parasite replication. Int. J. Parasitol. 32:1399-1410. [DOI] [PubMed] [Google Scholar]
- 13.Finco, O., A. Bonci, M. Agnusdei, M. Scarselli, R. Petracca, N. Norais, G. Ferrari, I. Garaguso, M. Donati, V. Sambri, R. Cevenini, G. Ratti, and G. Grandi. 2005. Identification of new potential vaccine candidates against Chlamydia pneumoniae by multiple screenings. Vaccine 23:1178-1188. [DOI] [PubMed] [Google Scholar]
- 14.Folsch, H., M. Pypaert, P. Schu, and I. Mellman. 2001. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 152:595-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, D., and A. G. Smulian. 2001. Plasminogen-binding activity of enolase in the opportunistic pathogen Pneumocystis carinii. Med. Mycol. 39:495-507. [DOI] [PubMed] [Google Scholar]
- 16.Fujii, A., M. Yoneda, T. Ito, O. Yamamura, S. Satomi, H. Higa, A. Kimura, M. Suzuki, M. Yamashita, T. Yuasa, H. Suzuki, and M. Kuriyama. 2005. Autoantibodies against the amino terminal of alpha-enolase are a useful diagnostic marker of Hashimoto's encephalopathy. J. Neuroimmunol. 162:130-136. [DOI] [PubMed] [Google Scholar]
- 17.Girard, M. P., Z. H. Reed, M. Friede, and M. P. Kieny. 2007. A review of human vaccine research and development: malaria. Vaccine 25:1567-1580. [DOI] [PubMed] [Google Scholar]
- 18.Goswami, A., S. Singh, V. D. Redkar, and S. Sharma. 1997. Characterization of P0, a ribosomal phosphoprotein of Plasmodium falciparum. Antibody against amino-terminal domain inhibits parasite growth. J. Biol. Chem. 272:12138-12143. [DOI] [PubMed] [Google Scholar]
- 19.Gupta, S. K., B. S. Sisodia, S. Sinha, K. Hajela, S. Naik, A. K. Shasany, and A. Dube. 2007. Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 7:816-823. [DOI] [PubMed] [Google Scholar]
- 20.Hiller, N. L., T. Akompong, J. S. Morrow, A. A. Holder, and K. Haldar. 2003. Identification of a stomatin orthologue in vacuoles induced in human erythrocytes by malaria parasites. A role for microbial raft proteins in apicomplexan vacuole biogenesis. J. Biol. Chem. 278:48413-48421. [DOI] [PubMed] [Google Scholar]
- 21.Kinloch, A., V. Tatzer, R. Wait, D. Peston, K. Lundberg, P. Donatien, D. Moyes, P. C. Taylor, and P. J. Venables. 2005. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 7:R1421-R1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labbe, M., M. Peroval, C. Bourdieu, F. Girard-Misguich, and P. Pery. 2006. Eimeria tenella enolase and pyruvate kinase: a likely role in glycolysis and in others functions. Int. J. Parasitol. 36:1443-1452. [DOI] [PubMed] [Google Scholar]
- 23.Lee, E. A., K. L. Flanagan, G. Minigo, W. H. Reece, R. Bailey, M. Pinder, A. V. Hill, and M. Plebanski. 2006. Dimorphic Plasmodium falciparum merozoite surface protein-1 epitopes turn off memory T cells and interfere with T cell priming. Eur. J. Immunol. 36:1168-1178. [DOI] [PubMed] [Google Scholar]
- 24.Lobo, C. A., S. K. Kar, B. Ravindran, L. Kabilan, and S. Sharma. 1994. Novel proteins of Plasmodium falciparum identified by differential immunoscreening using immune and patient sera. Infect. Immun. 62:651-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Villar, E., L. Monteoliva, M. R. Larsen, E. Sachon, M. Shabaz, M. Pardo, J. Pla, C. Gil, P. Roepstorff, and C. Nombela. 2006. Genetic and proteomic evidences support the localization of yeast enolase in the cell surface. Proteomics 6(Suppl. 1):S107—S118. [DOI] [PubMed] [Google Scholar]
- 26.Magrys, A., T. Anekonda, G. Ren, and G. Adamus. 2007. The Role of anti-alpha-enolase autoantibodies in pathogenicity of autoimmune-mediated retinopathy. J. Clin. Immunol. 27:181-192. [DOI] [PubMed] [Google Scholar]
- 27.Majarian, W. R., T. M. Daly, W. P. Weidanz, and C. A. Long. 1984. Passive immunization against murine malaria with an IgG3 monoclonal antibody. J. Immunol. 132:3131-3137. [PubMed] [Google Scholar]
- 28.Malhotra, I., P. Mungai, E. Muchiri, J. Ouma, S. Sharma, J. W. Kazura, and C. L. King. 2005. Distinct Th1- and Th2-type prenatal cytokine responses to Plasmodium falciparum erythrocyte invasion ligands. Infect. Immun. 73:3462-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massabki, P. S., N. P. Silva, D. M. Lourenco, and L. E. Andrade. 2003. Neuron specific enolase concentration is increased in serum and decreased in platelets of patients with active systemic sclerosis. J. Rheumatol. 30:2606-2612. [PubMed] [Google Scholar]
- 30.Miles, L. A., C. M. Dahlberg, J. Plescia, J. Felez, K. Kato, and E. F. Plow. 1991. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry 30:1682-1691. [DOI] [PubMed] [Google Scholar]
- 31.Montagnoli, C., S. Sandini, A. Bacci, L. Romani, and R. La Valle. 2004. Immunogenicity and protective effect of recombinant enolase of Candida albicans in a murine model of systemic candidiasis. Med. Mycol. 42:319-324. [DOI] [PubMed] [Google Scholar]
- 32.Narum, D. L., S. A. Ogun, A. H. Batchelor, and A. A. Holder. 2006. Passive immunization with a multicomponent vaccine against conserved domains of apical membrane antigen 1 and 235-kilodalton rhoptry proteins protects mice against Plasmodium yoelii blood-stage challenge infection. Infect. Immun. 74:5529-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochi, H., I. Horiuchi, N. Araki, T. Toda, T. Araki, K. Sato, H. Murai, M. Osoegawa, T. Yamada, K. Okamura, T. Ogino, K. Mizumoto, H. Yamashita, H. Saya, and J. Kira. 2002. Proteomic analysis of human brain identifies alpha-enolase as a novel autoantigen in Hashimoto's encephalopathy. FEBS Lett. 528:197-202. [DOI] [PubMed] [Google Scholar]
- 34.Pal-Bhowmick, I., H. K. Vora, and G. K. Jarori. 2007. Sub-cellular localization and post-translational modifications of enolase in Plasmodium yoelli suggest moonlighting function(s). Malaria J. 6:45. doi: 10.1186/1475-2875-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal-Bhowmick, I., S. Krishnan, and G. K. Jarori. 2007. Differential susceptibility of Plasmodium falciparum versus yeast and mammalian enolases to dissociation into active monomers. FEBS J. 274:1932-1945. [DOI] [PubMed] [Google Scholar]
- 36.Pal-Bhowmick, I., K. Sadagopan, H. K. Vora, A. Sehgal, S. Sharma, and G. K. Jarori. 2004. Cloning, over-expression, purification and characterization of Plasmodium falciparum enolase. Eur. J. Biochem. 271:4845-4854. [DOI] [PubMed] [Google Scholar]
- 37.Pal-Bhowmick, I., H. K. Vora, J. Roy, S. Sharma, and G. K. Jarori. 2006. Generation and characterisation of monoclonal antibodies specific to Plasmodium falciparum enolase. J. Vector Borne Dis. 43:43-52. [PubMed] [Google Scholar]
- 38.Pancholi, V. 2001. Multifunctional alpha-enolase: its role in diseases. Cell Mol. Life Sci. 58:902-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pancholi, V., and V. A. Fischetti. 1998. α-Enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J. Biol. Chem. 273:14503-14515. [DOI] [PubMed] [Google Scholar]
- 40.Pancholi, V., P. Fontan, and H. Jin. 2003. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb. Pathog. 35:293-303. [DOI] [PubMed] [Google Scholar]
- 41.Pitarch, A., A. Jimenez, C. Nombela, and C. Gil. 2006. Decoding serological response to Candida cell wall immunome into novel diagnostic, prognostic, and therapeutic candidates for systemic candidiasis by proteomic and bioinformatic analyses. Mol. Cell Proteomics 5:79-96. [DOI] [PubMed] [Google Scholar]
- 42.Read, M., K. E. Hicks, P. F. Sims, and J. E. Hyde. 1994. Molecular characterisation of the enolase gene from the human malaria parasite Plasmodium falciparum. Evidence for ancestry within a photosynthetic lineage. Eur. J. Biochem. 220:513-520. [DOI] [PubMed] [Google Scholar]
- 43.Redlitz, A., B. J. Fowler, E. F. Plow, and L. A. Miles. 1995. The role of an enolase-related molecule in plasminogen binding to cells. Eur. J. Biochem. 227:407-415. [DOI] [PubMed] [Google Scholar]
- 44.Renia, L., I. T. Ling, M. Marussig, F. Miltgen, A. A. Holder, and D. Mazier. 1997. Immunization with a recombinant C-terminal fragment of Plasmodium yoelii merozoite surface protein 1 protects mice against homologous but not heterologous P. yoelii sporozoite challenge. Infect. Immun. 65:4419-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato, K., S. Kano, Y. Matsumoto, R. Glanarongran, S. Krudsood, S. Looareesuwan, M. Aikawa, and M. Suzuki. 2000. Application of yeast enolase as antigen for immunodiagnosis of malaria. Southeast Asian J. Trop. Med. Public Health 31(Suppl. 1):79-84. [PubMed] [Google Scholar]
- 46.Saulot, V., O. Vittecoq, R. Charlionet, P. Fardellone, C. Lange, L. Marvin, N. Machour, X. Le Loet, D. Gilbert, and F. Tron. 2002. Presence of autoantibodies to the glycolytic enzyme alpha-enolase in sera from patients with early rheumatoid arthritis. Arthritis Rheum. 46:1196-1201. [DOI] [PubMed] [Google Scholar]
- 47.Sayles, P. C., and D. L. Wassom. 1988. Immunoregulation in murine malaria. Susceptibility of inbred mice to infection with Plasmodium yoelii depends on the dynamic interplay of host and parasite genes. J. Immunol. 141:241-248. [PubMed] [Google Scholar]
- 48.Schaumburg, J., O. Diekmann, P. Hagendorff, S. Bergmann, M. Rohde, S. Hammerschmidt, L. Jansch, J. Wehland, and U. Karst. 2004. The cell wall subproteome of Listeria monocytogenes. Proteomics 4:2991-3006. [DOI] [PubMed] [Google Scholar]
- 49.Sharma, P., A. Bharadwaj, V. K. Bhasin, V. N. Sailaja, and V. S. Chauhan. 1996. Antibodies to a conserved-motif peptide sequence of the Plasmodium falciparum thrombospondin-related anonymous protein and circumsporozoite protein recognize a 78-kilodalton protein in the asexual blood stages of the parasite and inhibit merozoite invasion in vitro. Infect. Immun. 64:2172-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sriram, G., J. A. Martinez, E. R. McCabe, J. C. Liao, and K. M. Dipple. 2005. Single-gene disorders: what role could moonlighting enzymes play? Am. J. Hum. Genet. 76:911-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sundblad, V., L. Bussmann, V. A. Chiauzzi, V. Pancholi, and E. H. Charreau. 2006. Alpha-enolase: a novel autoantigen in patients with premature ovarian failure. Clin. Endocrinol. 65:745-751. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka, Y., M. Nakamura, T. Matsui, N. Iizuka, H. Kondo, S. Tohma, K. Masuko, K. Yudoh, H. Nakamura, K. Nishioka, I. Koizuka, and T. Kato. 2006. Proteomic surveillance of autoantigens in relapsing polychondritis. Microbiol. Immunol. 50:117-126. [DOI] [PubMed] [Google Scholar]
- 53.Thomas, A. W., J. F. Trape, C. Rogier, A. Goncalves, V. E. Rosario, and D. L. Narum. 1994. High prevalence of natural antibodies against Plasmodium falciparum 83-kilodalton apical membrane antigen (PF83/AMA-1) as detected by capture-enzyme-linked immunosorbent assay using full-length baculovirus recombinant PF83/AMA-1. Am. J. Trop. Med. Hyg. 51:730-740. [DOI] [PubMed] [Google Scholar]
- 54.Ueda, K. 2005. Proteome analysis of autoantibodies in sera of patients with cancer. Rinsho Byori 53:437-445. [In Japanese.] [PubMed] [Google Scholar]
- 55.van Deventer, H. J., W. H. Goessens, A. J. van Vliet, and H. A. Verbrugh. 1996. Anti-enolase antibodies partially protective against systemic candidiasis in mice. Clin. Microbiol. Infect 2:36-43. [DOI] [PubMed] [Google Scholar]
- 56.Vinores, S. A., M. M. Herman, and L. J. Rubinstein. 1986. Electron-immunocytochemical localization of neuron-specific enolase in cytoplasm and on membranes of primary and metastatic cerebral tumours and on glial filaments of glioma cells. Histopathology 10:891-908. [DOI] [PubMed] [Google Scholar]
- 57.Vinores, S. A., M. M. Herman, and L. J. Rubinstein. 1987. Localization of neuron-specific (gamma gamma) enolase in proliferating (supportive and neoplastic) Schwann cells. An immunohisto- and electron-immunocyto-chemical study of ganglioneuroblastoma and schwannomas. Histochem. J. 19:438-448. [DOI] [PubMed] [Google Scholar]
- 58.Voller, A., D. I. Green, and W. H. Richards. 1973. Cross immunity studies with East and West African strains of Plasmodium falciparum in owl monkeys (Aotus trivirgatus). J. Trop. Med. Hyg. 76:135-139. [PubMed] [Google Scholar]
- 59.Wang, T., H. Fujioka, J. A. Drazba, and T. Y. Sam-Yellowe. 2006. Rhop-3 protein conservation among Plasmodium species and induced protection against lethal P. yoelii and P. berghei challenge. Parasitol. Res. 99:238-252. [DOI] [PubMed] [Google Scholar]
- 60.Weleber, R. G., R. C. Watzke, W. T. Shults, K. M. Trzupek, J. R. Heckenlively, R. A. Egan, and G. Adamus. 2005. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am. J. Ophthalmol. 139:780-794. [DOI] [PubMed] [Google Scholar]