Abstract
We have identified acid sphingomyelinase (ASM) as an important player in the early and late anti-Salmonella activity of macrophages. A functional ASM participated in the killing activity of macrophages against wild-type Salmonella enterica serovar Typhimurium. The role of ASM in early macrophage killing of Salmonella appears to be linked to an active NADPH phagocyte oxidase enzymatic complex, since the flavoprotein inhibitor diphenyleneiodonium not only blocked a productive respiratory burst but also abrogated the survival advantage of Salmonella in macrophages lacking ASM. Lack of ASM activity also increased the intracellular survival of an isogenic ΔspiC::FRT Salmonella strain deficient in a translocator and effector of the Salmonella pathogenicity island 2 (SPI2) type III secretion system, suggesting that the antimicrobial activity associated with ASM is manifested regardless of the SPI2 status of the bacteria. Constitutively expressed ASM is responsible for the role that this lipid-metabolizing hydrolase plays in the innate host defense of macrophages against Salmonella. Accordingly, the ASM activity and intracellular concentration and composition of ceramide, gangliosides, and neutral sphingolipids did not increase upon Salmonella infection. Salmonella triggered, nonetheless, a significant increase in the secreted fraction of ASM. Collectively, these findings have elucidated a novel role for constitutive ASM in the anti-Salmonella activity of murine macrophages.
Infection with Salmonella enterica serovar Typhimurium is a major global health problem. In the United States alone, it is estimated that over a million people contract nontyphoidal salmonellosis every year (46). Most clinical presentations of nontyphoidal Salmonella infections are manifested as gastroenteritis that resolves spontaneously without antibiotic treatment. In immunocompromised individuals, nontyphoidal Salmonella infections can become life threatening. The incidence of Salmonella serovar Typhimurium disseminating extraintestinally has increased with the onset of the human immunodeficiency virus epidemic. In parts of Africa where human immunodeficiency virus is endemic, 37 to 50% of blood cultures are positive for nontyphoidal Salmonella and infections with Salmonella serovar Typhimurium can be connected with mortality rates as high as 80% (11, 18).
The diverse stages of salmonellosis reflect the parasitic relationship between Salmonella and host mononuclear phagocytes (28, 29, 43, 45). Mononuclear phagocytes translocate Salmonella across the intestinal epithelium, disseminate the bacterium extraintestinally, and support intracellular replication (7, 40). Salmonella resides in macrophages within phagosomes that establish contact with recycling and early endosomes and the trans-Golgi network but avoid maturation along terminal stages of the degradative pathway (10, 20, 24, 31). The nonfusogenic intracellular nature of Salmonella (1), a significant part of its virulence characteristics, is intimately associated with the Salmonella pathogenicity island 2 (SPI2) type III secretion system that translocates effector proteins across the bacterial cell envelope and the phagosomal membrane into the host cell cytoplasm (16, 25, 27, 34). By modulating phagosomal maturation, SPI2 helps Salmonella avoid contact with the hydrolytic enzymes of lysosomes and reduces contact with reactive oxygen and nitrogen species (ROS and RNS, respectively) (3, 35, 44). Nonetheless, phagocytes constitute a critical component in resistance to Salmonella. Investigations using immunodeficient mice have shown that macrophages exploit early Toll-like receptor 4 signaling and NRAMP1-dependent mechanisms in resistance to systemic salmonellosis (28, 43). Within the first few hours following phagocytosis, ROS generated by the NADPH phagocyte oxidase enzymatic complex kill many of the internalized Salmonella bacteria, while RNS generated by the inducible nitric oxide synthase (iNOS) expressed in response to gamma interferon control the replication of this intracellular pathogen later in the infection (23, 24, 41).
Lipid-mediated signaling initiated by neutral or acid sphingomyelinases (ASMs) is increasingly recognized as a critical component in diverse cellular processes, including innate immunity. In particular, ASM, which hydrolyzes sphingomyelin to ceramide in lipid rafts and endocytic vacuoles (19), has recently been associated with host defense against Listeria monocytogenes and Pseudomonas aeruginosa (13, 36). The increased susceptibility of ASM-deficient mice to Listeria correlates with the reduced listericidal activity of their macrophages. Likewise, mice lacking ASM are hypersusceptible to Salmonella serovar Typhimurium (36); however, the anti-Salmonella capacity of macrophages lacking ASM is currently unknown. Herein, macrophages from ASM-deficient mice were used to determine whether the lipid-metabolizing activity of ASM plays a role during the early and late anti-Salmonella defenses of macrophages.
MATERIALS AND METHODS
Mice.
Pairs of heterozygous ASM+/− mice bred in an sv129 × BL/6 background (17) were kept in our animal facility according to Institutional Animal Care and Use Committee guidelines. DNA isolated from mouse tails was used as a template for PCR amplification in a reaction mixture containing DNA polymerase (CLP, San Diego, CA), deoxynucleoside triphosphates, a common forward primer (5′-AGCCGTGTCCTCTTCCTTAC-3′), and reverse primers specific for wild-type and mutant ASM alleles (5′-CGAGACTGTTGCCAGACATC-3′ and 5′-GGCTACCCGTGATATTGCTG-3′, respectively). PCRs were performed for 30 cycles of 93°C for 1 min, 58°C for 1 min, and 72°C for 1 min, followed by a final extension for 5 min at 72°C. The mutant (∼500-bp) and wild-type (∼300-bp) alleles were visualized following agarose gel electrophoresis and ethidium bromide staining. Homozygous mice bearing a wild-type or ASM-deficient allele were used for experimentation.
Macrophages.
Peritoneal macrophages were harvested from ASM−/− mice or their wild-type ASM+/+ littermates 4 days after intraperitoneal inoculation of 1 mg/ml sodium periodate (Sigma). Peritoneal exudate cells were resuspended in RPMI 1640 containing 2 mM l-glutamine, 15 mM HEPES, 1 mM sodium pyruvate (all from Sigma), and 10% heat-inactivated fetal bovine serum (Cambrex) (RPMI+ medium). Cells were plated at densities of 3 × 105 cells/well onto 8-well Permanox chamber slides (Nalge Nunc International), 105 cells/well in 96-well Microlite-2 plates (Thermo Labsystems), 106 cells/well in 24-well plates, and 2 × 106 cells/well in 6-well tissue culture plates (Falcon) for killing assays, superoxide measurement, ASM activity analysis, and sphingolipid analysis, respectively. The macrophages were cultured for 16 h at 37°C in the presence of 100 U·ml−1/100 mg·ml−1 of penicillin/streptomycin (Cellgro) prior to Salmonella infection. The antibiotics were removed before infection as previously described (24, 41, 42).
Macrophage killing assays.
Macrophage killing assays were performed by following a modified version of published protocols (2, 4, 5, 24, 26, 42). Wild-type Salmonella enterica serovar Typhimurium strain 14028s or its ΔspiC::FRT isogenic strain, AV0201 (24), were grown overnight in LB medium. Bacteria opsonized for 20 min at 37°C in 10% normal mouse serum were added to ASM−/− or ASM+/+ macrophages at a multiplicity of infection (MOI) of 10. Extracellular bacteria were removed from the monolayers after 35 min of challenge by washing them with prewarmed RPMI+ medium containing 6 μg/ml of gentamicin (Sigma). At the time of infection, selected groups of cells were treated with 10 μM diphenyleneiodonium (DPI; Sigma), an inhibitor of both NADPH oxidase and NOS, or 1 mM of the NOS inhibitor N-monomethyl-l-arginine (MMLA; Sigma). Salmonella-infected macrophages were lysed at the times after challenge indicated in the figures. The intracellular bacteria recovered at various points after infection were enumerated on LB agar plates. The results are expressed as percent survival calculated according to the equation (number of CFU at time n/number of CFU at time zero) × 100 (42).
Phagocytosis.
Salmonella bacteria grown overnight in LB medium were incubated with 1 mg/ml fluorescein isothiocyanate (FITC; Sigma) in Dulbecco's phosphate-buffered saline (PBS; Sigma) for 30 min at 37°C. The cultures were washed three times with Dulbecco's PBS to eliminate free FITC, and the bacteria were opsonized with normal mouse serum prior to the macrophage challenge as described above. Peritoneal macrophages from ASM+/+ and ASM−/− mice plated at a density of 3 × 105 cells on glass coverslips placed in six-well tissue culture plates were challenged with FITC-labeled Salmonella bacteria at an MOI of 20 for 30 min at 37°C in a 5% CO2 incubator. After most cell-free Salmonella bacteria were washed out with prewarmed PBS, 0.2% trypan blue prepared in PBS was added for 1 min to quench the fluorescence of extracellular FITC-labeled Salmonella. The specimens were washed in PBS, and the cells were fixed with 2% paraformaldehyde. The coverslips were mounted on glass slides using Vectashield with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories, Burlingame, CA). The coverslips were examined with a Zeiss fluorescence microscope equipped with a charge-coupled-device camera controlled by the SlideBook deconvolution image-processing software (Intelligent Imaging Innovations, Denver, CO).
Nitrite measurement.
The concentration of nitrite (NO2−) produced by Salmonella-infected macrophages 20 h postchallenge was estimated spectrophotometrically at 550 nm in a VersaMax microplate reader (Molecular Devices) after culture supernatants were mixed with an equal volume of Griess reagent (0.5% sulfanilamide and 0.05% N-1-naphthylethylenediamide hydrochloride in 2.5% phosphoric acid). The NO2− concentration was calculated by regression analysis using a NaNO2 standard curve.
Superoxide measurement.
Macrophages were infected with Salmonella bacteria at an MOI of 10. The monolayers were washed after 15 min, and the medium was replaced with RPMI+ medium containing 6 μg/ml gentamicin and 12.5 μM of the superoxide probe lucigenin (41). The flavoprotein inhibitor DPI was added to selected groups of macrophages at a final concentration of 10 μM. Chemiluminescence was recorded at 37°C on an LMax luminometer (Molecular Devices) at 5-min intervals for 1 h with an integration time of 4 s.
ASM activity.
Macrophage ASM activity was determined from cellular extracts by following the method described by Loidl et al. (22). Selected groups of macrophages were challenged with wild-type or spiC-deficient Salmonella bacteria at an MOI of 10 as described above. To inhibit NADPH oxidase and iNOS activity, 10 μM DPI was added at the time of Salmonella infection. To assess intracellular ASM activity, control or Salmonella-infected macrophages were resuspended in 50 mM sodium acetate buffer, pH 5.0, containing 0.2% Triton X-100 and 1 mM EDTA. Cellular extracts were obtained after three 10-s cycles of homogenization at 4°C using a sonic dismembrator (Fisher) equipped with a microtip probe. Cellular debris was removed by centrifugation at 13,000 rpm at 4°C for 10 min in an ultracentrifuge (Sorvall, Newton, CT). Secreted ASM activity was determined as described by Schissel et al. (32). To assay for secreted ASM activity, ASM+/+ or ASM−/− macrophages were challenged with Salmonella or Escherichia coli strain O311 as described above. Supernatants from uninfected, E. coli-infected, or Salmonella-infected macrophages were collected over 4-h intervals. Because serum contains ASM activity, the cells were incubated in serum-free RPMI+ medium supplemented with 1 ml/100 ml Nutridoma-SP (Sigma) during the 0- to 4-h or 4- to 8-h collection periods. The supernatants were centrifuged at 12,000 × g to remove cellular debris, concentrated ∼10-fold in a Microcon centrifugal filter tube (Millipore) with a molecular weight cutoff of 30,000, and resuspended in 50 mM sodium acetate, pH 5.0, containing 0.2% Triton X-100 and 0.1 mM ZnCl2. Cellular lysates and supernatants were incubated for 60 min at 37°C with 100 pmol of 7-nitro-2,1,3-benzoxadiazol-4-yl (NBD)-sphingomyelin (Invitrogen, Carlsbad, CA) prepared as a 20 μM stock solution in 50 mM sodium acetate, pH 5.0, containing 2% Triton X-100. Enzymatic production of NBD-ceramide was stopped by extracting the lipids from the cellular lysates or supernatants in chloroform-methanol-PBS (1:1:0.9). Following centrifugation at 820 × g for 5 min, the organic phase containing NBD-labeled lipids was removed, dried over N2, and resuspended in chloroform-methanol (1:1). Samples were loaded onto silica gel 60-impregnated high-performance thin-layer chromatography (TLC) plates (Fisher) and resolved two times with 50 ml chloroform-methanol-acetic acid (190:9:1). NBD-ceramide was visualized in a Molecular Imager FX (Bio-Rad) using a 488-nm excitation wavelength and a 530-nm emission wavelength. The amount of ceramide was quantified by regression analysis using a standard curve prepared with NBD-ceramide. The limits of detection of NBD-labeled lipids using this TLC method are approximately 1 fmol.
Macrophage sphingolipid analysis.
Total sphingolipids were extracted from control or Salmonella-challenged, periodate-elicited macrophages isolated from ASM+/+ or ASM−/− mice as described by van Echten-Deckert (37). Selected groups of macrophages were washed in antibiotic-free RPMI+ medium and challenged for 35 min with opsonized wild-type or spiC-deficient Salmonella bacteria at an MOI of 10. The macrophages were washed, scraped, and resuspended in ice-cold PBS and centrifuged and resuspended in sterile water. Cellular membranes were disrupted using a sonic dismembrator (Fisher) with a microtip probe, and lipids were extracted in chloroform-methanol-water-pyridine (60:30:6:1) at 37°C while shaking the samples at 300 rpm overnight. Denatured proteins were removed by passing the samples through cotton wadding. The lipids were dried in a RotaVAP (Büchi, Switzerland) and resuspended in methanol, and contaminating phospholipids were removed by alkaline methanolysis (0.1 M NaOH in methanol) by shaking the samples at 37°C for 2 h at 200 rpm. Following neutralization with 0.1 M acetic acid in methanol, the lipids were dried under N2 and resuspended in methanol. After the addition of 300 mM ammonium acetate, each sample was loaded onto a PrepSep C18 column (Fisher) previously prepared by washing with chloroform-methanol (2:1), methanol, and chloroform-methanol-0.1 M KCl (3:48:47). The lipids were desalted in the column with water, eluted in methanol followed by chloroform-methanol (1:1), dried under N2, and resuspended in chloroform-methanol (1:1). Samples were loaded onto silica gel 60-impregnated high-performance TLC plates and resolved once with chloroform-methanol-aqueous 0.22% CaCl2 (45:26:6) for sphingolipid separation. After drying, the plates were sprayed with 0.1 mg/ml primuline (Sigma-Aldrich) prepared in acetone-water (80:20), and the sphingolipid banding was visualized in a Molecular Imager FX (Bio-Rad) as described above. Neutral glycosphingolipids (cerebrosides, lactosyl ceramide, ceramide trihexoside, and globoside), sphingolipid mix (cerebrosides, sulfatides, and sphingomyelin), or mixed gangliosides (GD1a, GM1, GD1b, and GT1b with minor amounts of GQ1b, GM2, GD3, and GD2) (all from Matreya, Pleasant Gap, PA) were used as standards.
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM) or standard deviations. SEM is defined as the standard deviation/√n, in which n is the number of independent observations. An unpaired, two-tailed Student's t test was used for statistical analysis for comparison of the means from two groups. To determine statistical significance between multiple comparisons, two-way analyses of variance (ANOVAs) were performed, followed by a Bonferroni posttest. Data were considered statistically significant when P was <0.05.
RESULTS
ASM enhances the anti-Salmonella activity of macrophages.
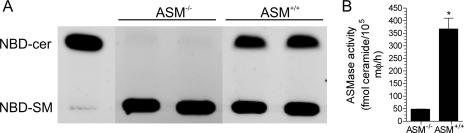
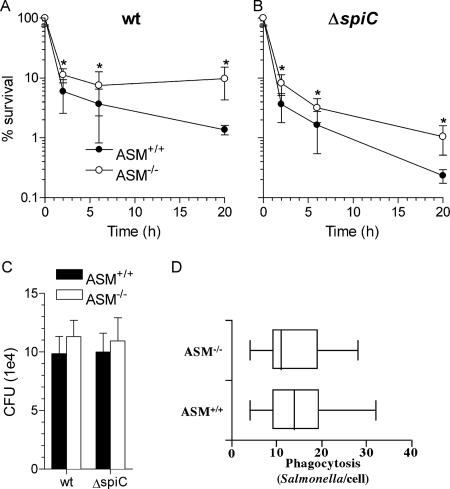
The phenotypes of ASM−/− and ASM+/+ macrophages were confirmed by assessing ASM activity from cellular lysates. As expected, extracts from ASM-deficient macrophages sustained limited enzymatic synthesis of NBD-ceramide from NBD-sphingomyelin (Fig. 1A). In contrast, resting ASM+/+ macrophages harbored an ASM activity of 360 fmol ceramide/105 cells/h (Fig. 1A and B). ASM-proficient and -deficient macrophages were used to measure the contribution of ASM to the anti-Salmonella activity of macrophages. The survival of wild-type Salmonella was increased twofold (P < 0.05) in ASM-deficient macrophages at 2 h postinfection (Fig. 2A). By 20 h, the bacterial burden was about fivefold greater (P < 0.05) in ASM-deficient macrophages than in ASM+/+ congenic controls. Together, these data indicate that ASM contributes to both the early and late anti-Salmonella activity of macrophages. The SPI2 type III secretion system enhances the intracellular survival of Salmonella within macrophages by reducing contact with lysosomes and NADPH oxidase- and iNOS-containing vesicles (3, 35, 38). To determine if SPI2 antagonizes the antimicrobial effects of ASM, macrophages were infected with Salmonella strain AV0201 carrying a mutation in spiC that renders SPI2 secretion afunctional (8, 35, 47). As expected (9, 39, 44), ΔspiC::FRT Salmonella bacteria exhibited early and late hypersusceptibility to the antimicrobial actions of ASM+/+ macrophages compared to wild-type Salmonella (Fig. 2A and B). Similar to wild-type controls, spiC-deficient Salmonella reached about a fivefold higher bacterial load in ASM-deficient macrophages than in ASM-proficient controls 20 h after infection (Fig. 2B). However, the absence of ASM did not restore the intracellular survival of ΔspiC::FRT bacteria to wild-type levels. These data suggest that SPI2 does not antagonize the antimicrobial activity associated with ASM. The survival advantage of Salmonella in ASM−/− macrophages cannot be explained by altered bacterial internalization (14), because wild-type and ΔspiC::FRT Salmonella bacteria were recovered in similar numbers after 35 min of infection, regardless of the ASM status of the phagocytes (Fig. 2C). The phagocytic capacity of ASM+/+ and ASM−/− macrophages was estimated by enumerating the number of intracellular FITC-labeled Salmonella bacteria following 30 min of bacterial challenge, and the intracellular bacterial load was quantified by scoring the number of bacteria per macrophage. These data revealed no significant differences (P = 0.7) in the abilities of ASM+/+ and ASM−/− macrophages to phagocytize Salmonella bacteria (Fig. 2D).
FIG. 1.
ASM enzymatic activity of murine macrophages. (A) Peritoneal macrophages isolated from ASM−/− and ASM+/+ mice were assessed for ASM activity by incubating cellular lysates with NBD-sphingomyelin (NBD-SM) for 60 min at 37°C. Enzymatic production of NBD-ceramide (NBD-cer) was visualized following high-performance TLC of lipid extracts. NBD-ceramide (1 pmol) was loaded as a control (lane 1). (B) The amount of ceramide produced by intracellular ASM was quantified by regression analysis using an NBD-ceramide standard curve. The results are expressed as fmol ceramide/105 macrophages (mφ)/h (mean ± standard deviation) (n = 3). *, P < 0.05, compared to ASM−/− control by unpaired, two-way t test.
FIG. 2.
Intracellular survival of Salmonella is enhanced in macrophages lacking ASM. The antimicrobial activities of macrophages from ASM+/+ and ASM−/− mice against wild-type (wt) (A) and spiC-deficient (B) Salmonella bacteria were monitored over time. Percent survival is expressed as the mean ± standard deviation (n = 6 to 10). Logarithmically transformed data were analyzed by two-way ANOVA. *, P < 0.05 for comparison of ASM+/+ and ASM−/− antimicrobial activities as determined by a Bonferroni posttest. (C) Internalization of bacteria by ASM+/+ and ASM−/− macrophages was estimated by recording the number of bacteria capable of forming a colony following 35 min of infection. Data are expressed as mean numbers of CFU ± SEM (n = 16). (D) Phagocytic indices were calculated for ASM+/+ and ASM−/− macrophages by enumerating the number of intracellular FITC-labeled Salmonella bacteria per macrophage by fluorescence microscopy. A total of 50 to 60 macrophages were analyzed from six separate slides per group. The numbers of bacteria per macrophage are represented in a box-and-whisker plot as median, intraquartile, and total ranges.
ASM-mediated macrophage anti-Salmonella activity is dependent upon production of ROS.
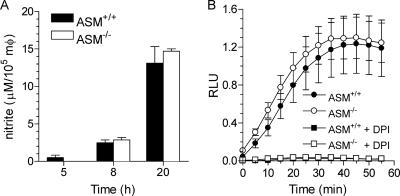
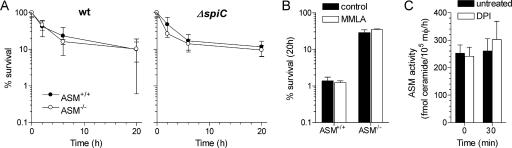
Because ASM optimizes the early and late stages of anti-Salmonella activity, we tested whether ASM activity may influence the production of ROS and RNS. Macrophages lacking ASM generated the same amounts of NO2− as wild-type controls (Fig. 3A). ASM-proficient and -deficient macrophages generated similar amounts of ROS 1 h after Salmonella infection (Fig. 3B). The addition of the flavoprotein inhibitor DPI completely abolished the respiratory burst (Fig. 3B). Inhibitors of the oxidative burst and synthesis of NO were used to determine whether the ASM-dependent killing is related to NADPH oxidase or iNOS activity. DPI abolished the ASM-dependent killing (Fig. 4A). The effects of DPI seemed to be related to inhibition of NADPH oxidase activity, since the NOS inhibitor MMLA, which reduced the NO2− produced by Salmonella-infected macrophages to levels lower than 1 μM, had no effect (P = 0.65) on ASM-dependent killing of Salmonella bacteria (Fig. 4B). The inhibitory effects of DPI cannot be explained by the inhibition of ASM activity either, since untreated and DPI-treated macrophages had similar ASM activities (Fig. 4C).
FIG. 3.
Generation of ROS and RNS by Salmonella-infected macrophages is independent of ASM. (A) The generation of RNS by Salmonella-infected macrophages from ASM+/+ and ASM−/− mice was determined by measuring the accumulation of nitrite in supernatants at the indicated times. Data are expressed as μM nitrite/105 macrophages (mφ) (mean ± SEM). (B) The oxidative burst of ASM-proficient or -deficient macrophages was measured as lucigenin-dependent chemiluminescence after Salmonella challenge. Selected samples were treated with 10 μM DPI at the time of the infection. Results are expressed as mean relative luminescence units (RLU) ± SEM. The data are from three to six independent observations.
FIG. 4.
ASM-mediated anti-Salmonella activity of macrophages depends on a functional NADPH oxidase. (A) The effect that the flavoprotein inhibitor DPI has on the survival of wild-type (wt) and spiC-deficient Salmonella was studied in macrophages from ASM+/+ and ASM−/− mice. Data are expressed as mean percent survival ± standard deviation. (B) The effect of the NOS inhibitor MMLA on the survival of Salmonella in ASM+/+ and ASM−/− macrophages was determined after 20 h of infection. DPI and MMLA were added to the macrophages at the time of the infection. Data are expressed as mean percent survival ± SEM. (C) Mean ASM activity ± SEM was determined for untreated or DPI-treated ASM+/+ macrophages (mφ) prior to (0 min) or 30 min following challenge with Salmonella. The data are from four to seven independent observations.
Salmonella infection reduces ASM activity.
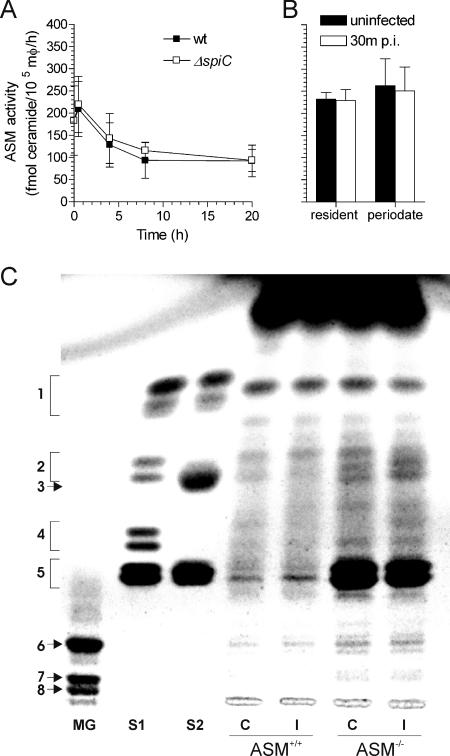
The kinetics of ASM activity were recorded upon Salmonella challenge. ASM activity peaked 30 min after infection (Fig. 5A), although this increase was not significantly different (P > 0.5) from that of uninfected controls. Overall ASM activity decreased after a few hours of infection and was independent of the SPI2 status of the bacteria (Fig. 5A). Since the elicitation process could potentially mask the effects that Salmonella infection may have upon the sphingomyelin pathway, the ASM activities of periodate-elicited and resident macrophages were compared. The ASM activities of Salmonella-infected and uninfected controls in both populations of phagocytes were similar (Fig. 5B). Together, these data suggest that constitutive expression of ASM optimizes the anti-Salmonella activity of macrophages.
FIG. 5.
Salmonella infection decreases constitutive ASM activity of macrophages. (A) Intracellular ASM activity was assessed at the indicated times following challenge of periodate-elicited macrophages (mφ) with wild-type (wt) or spiC-deficient (n = 4 to 6) Salmonella. (B) Comparison of the ASM activities of resident and periodate-elicited Salmonella-infected ASM+/+ macrophages (n = 4 to 8). ASM activities are expressed as mean fmol ceramide/105 macrophages/h ± standard deviation. p.i., postinfection. (C) Total sphingolipids extracted from control (lanes C) or Salmonella-infected (lanes I) ASM+/+ or ASM−/− macrophages were visualized following separation by high-performance TLC. Mixed gangliosides GM1 (6), GD1a (7), and GD1b (8) (lane MG); neutral glycosphingolipids cerebroside (1), lactosyl ceramide (2), ceramide trihexoside (4), and globoside (5) (lane S1); and sphingolipid mix of cerebrosides (1), sulfatides (3), and sphingomyelin (5) (lane S2) were used as standards.
The contents of high-order sphingolipids generated from ceramide in ASM+/+ and ASM−/− macrophages were compared. The sphingolipid profile of ASM+/+ macrophages (Fig. 5C, lane 1) included cerebrosides, gangliosides, and sphingomyelin. The patterns of gangliosides, sphingomyelin, and neutral sphingolipids were unchanged in ASM+/+ macrophages after 30 min of Salmonella infection. Compared to the results with ASM+/+ macrophages, a large amount of lipid comigrating with sphingomyelin accumulated in ASM−/− macrophages. Sphingolipids were also extracted from 5 × 107 J774 macrophage-like cells that were infected with either wild-type or spiC-deficient Salmonella. Consistent with the results obtained for wild-type bacteria in primary macrophages, the sphingolipid profiles obtained from J774 cells at 0, 4, and 20 h postinfection were similar to those of uninfected controls and were unaffected by the presence of a functional SPI2 (not shown).
Salmonella infection increases the amount of ASM secreted extracellularly by macrophages.
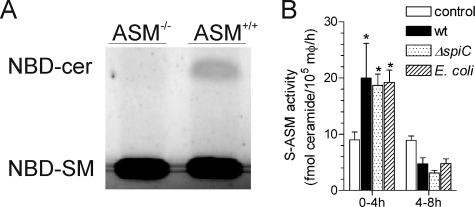
Differential trafficking gives rise to the segregation of ASM in lysosomal and secretory fractions (32). We tested whether Salmonella infection may lead to a redistribution of ASM in macrophages. Culture supernatants harvested from ASM−/− or ASM+/+ macrophages were assayed for ASM enzymatic activity by monitoring the hydrolysis of NBD-sphingomyelin to NBD-ceramide. Macrophage supernatants contained sphingomyelinase enzymatic activity, reflecting a functional ASM (Fig. 6A). Interestingly, secreted ASM accumulated to significantly higher levels in infected cellular supernatants over 4 h than in uninfected controls (Fig. 6B). The increase in ASM activity from infected macrophages represents about 1/10 of the ASM activity lost from endosomal fractions during the first 4 h following Salmonella challenge. The increase in secreted ASM activity was, however, transitory, since it dropped below baseline levels at later times of the infection (Fig. 6B). The increase in secretory ASM following Salmonella infection cannot be explained by a previously observed subversion of the exocytic pathway by SPI2 effectors (20), because wild-type and spiC-deficient Salmonella bacteria induced similar secretions of ASM. Nor are the increased secretory ASM activities of macrophages specific to Salmonella infection, as E. coli induced similar patterns of ASM secretion (Fig. 6B).
FIG. 6.
Infection of macrophages with Salmonella stimulates extracellular ASM secretion. (A) Secreted ASM activity of ASM−/− or ASM+/+ macrophages was assessed in cellular supernatants by TLC as hydrolysis of NBD-sphingomyelin (NBD-SM) to NBD-ceramide (NBD-cer). (B) Secreted ASM (S-ASM) from uninfected controls and E. coli- or Salmonella-challenged ASM+/+ macrophages (mφ) was quantified in supernatants after 4 h of accumulation. The data are expressed as the mean ± standard deviation (n = 4). Statistical analysis of untransformed data was performed with a two-way ANOVA. *, P < 0.05, compared to uninfected controls as determined by a Bonferroni posttest.
DISCUSSION
We present the first characterization of lysosomal and secreted ASM activities following the infection of macrophages with Salmonella. Most macrophage ASM was found to reside intracellularly, likely reflecting enzymatic activity of the lysosomal fractions. Remarkably, lysosomal ASM activity was reduced by almost 100 fmol/105 macrophages/h a few hours after infection, coinciding with a surge of 10 fmol/105 macrophages/h ASM activity in the secreted fraction. These dynamic changes in the cellular distribution of ASM activity in Salmonella-infected macrophages were neither associated with global changes in sphingolipid profiles nor required for bacterial uptake (Fig. 2C), as has been shown for other pathogens (12, 14). Rather, ASM was associated with the stimulation of the early innate host defenses of macrophages against Salmonella. Together, these findings have uncovered a role for constitutive ASM in the anti-Salmonella activity of macrophages.
The contribution of ROS to defense against Salmonella is manifested by the increased incidence of salmonellosis in individuals harboring a cadre of defective variants in the cytosolic or membrane components of the NADPH oxidase complex (21). The respiratory burst dominates the early innate defenses of macrophages against Salmonella (23, 41). Two independent lines of experimentation suggest that the involvement of ASM in the anti-Salmonella activity of macrophages is linked to the NADPH oxidase function. First, ASM-mediated killing was evident as early as 2 h after infection, during a period in which the lack of the membrane gp91phox subunit of the NADPH phagocyte oxidase completely abrogates intracellular killing of Salmonella (41). Second, the NADPH oxidase inhibitor DPI not only increased the number of Salmonella bacteria recovered from macrophages but also abolished the differences in killing between ASM+/+ and ASM−/− phagocytes. The inhibitory effects associated with DPI appear to be specific to the NADPH oxidase enzymatic complex since specific inhibition of iNOS did not abrogate the ASM-dependent phenotype. Collectively, these findings indicate that ASM optimizes early macrophage killing of Salmonella by acting synergistically with a functional NADPH oxidase.
The synergism of ASM and the respiratory burst appears to be independent of stimulation of NADPH oxidase activity by mediators of the sphingomyelin pathway. In accord with previous data examining ROS production by Listeria-infected phagocytes (36), ASM-deficient macrophages sustained a normal respiratory burst in response to Salmonella. Stimulation of K+ fluxes into phagosomes that promote the activation of proteases from the proteoglycan matrix in the lysosomal lumen (33) may provide an intriguing scenario for the association between ASM and ROS-mediated killing. In view of this, ASM has been shown to activate the lysosomal aspartyl-protease cathepsin D (15). Alternatively, ASM may target ROS production to the Salmonella phagosome. It should be noted that an important fraction of the NADPH oxidase-mediated anti-Salmonella activity of macrophages, likely representing conventional ROS-mediated cytotoxicity, occurs in the absence of the sphingomyelin pathway.
The SPI2 type III secretion system has been associated with inhibition of both lysosomal fusion and trafficking of NADPH oxidase- or iNOS-containing vesicles (3, 35, 38). More recently, a functional SPI2 type III secretion system has been associated with the segregation of Salmonella phagosomes along the exocytic pathway and the acquisition of ceramide (20). It is tempting to imagine that the extracellular flux of ASM seen in Salmonella-infected macrophages is associated with the SPI2-mediated segregation of Salmonella bacteria along the exocytic pathway. However, several lines of evidence indicate that this scenario is incorrect. On the one hand, ASM contributed equally to the killing of wild-type and spiC-deficient Salmonella bacteria. On the other hand, the overall makeup of sphingolipids and the amount of secreted ASM in Salmonella-infected macrophages were unaffected by the SPI2 status of the bacteria. More-likely possibilities for the enhanced secreted ASM activity seen shortly after Salmonella infection include the export of ASM to the outer leaflet of the host cell membrane triggered during the initial contact of host cells and pathogen. Fusion of lysosomal vesicles with the plasma membrane during phagosomal biogenesis (6, 30) may deliver secreted ASM to the nascent phagosome. The fact that E. coli also triggered an early surge in the extracellular secretion of ASM activity further supports the idea that at least part of the cellular redistribution of ASM is activated during phagocytosis. Extracellular secretion of ASM in response to two enterobacterial species suggests that the role of ASM uncovered here is not specific to Salmonella but may be required for the bactericidal activity of murine macrophages against a variety of pathogenic microorganisms.
In summary, our data have elucidated a hitherto unknown role for ASM in the anti-Salmonella arsenal of professional phagocytes. Future experimentation will be required to determine the origin of the ASM enzymatic activity secreted extracellularly in response to Salmonella and to elucidate the association of ASM with the anti-Salmonella activity of the NADPH phagocyte oxidase.
Acknowledgments
Support of this work was provided by the National Institutes of Health (AI54959, AI053213, AI07447, and RR16082).
We are grateful to R. Kolesnick at the Memorial Sloan-Kettering Cancer Center, New York, NY, for providing breeding pairs of the ASM+/− mice.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 13 August 2007.
REFERENCES
- 1.Buchmeier, N. A., and F. Heffron. 1991. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 59:2232-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier, N. A., and F. Heffron. 1989. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect. Immun. 57:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirillo, D. M., R. H. Valdivia, D. M. Monack, and S. Falkow. 1998. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol. Microbiol. 30:175-188. [DOI] [PubMed] [Google Scholar]
- 5.De Groote, M. A., U. A. Ochsner, M. U. Shiloh, C. Nathan, J. M. McCord, M. C. Dinauer, S. J. Libby, A. Vazquez-Torres, Y. Xu, and F. C. Fang. 1997. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 94:13997-14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desjardins, M., N. N. Nzala, R. Corsini, and C. Rondeau. 1997. Maturation of phagosomes is accompanied by changes in their fusion properties and size-selective acquisition of solute materials from endosomes. J. Cell Sci. 110:2303-2314. [DOI] [PubMed] [Google Scholar]
- 7.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman, J. A., C. Rappl, V. Kuhle, M. Hensel, and S. I. Miller. 2002. SpiC is required for translocation of Salmonella pathogenicity island 2 effectors and secretion of translocon proteins SseB and SseC. J. Bacteriol. 184:4971-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallois, A., J. R. Klein, L. A. Allen, B. D. Jones, and W. M. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 10.Geddes, K., M. Worley, G. Niemann, and F. Heffron. 2005. Identification of new secreted effectors in Salmonella enterica serovar Typhimurium. Infect. Immun. 73:6260-6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, S. M., A. L. Walsh, E. M. Molyneux, A. J. Phiri, and M. E. Molyneux. 2000. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans. R. Soc. Trop. Med. Hyg. 94:310-314. [DOI] [PubMed] [Google Scholar]
- 12.Grassmé, H., E. Gulbins, B. Brenner, K. Ferlinz, K. Sandhoff, K. Harzer, F. Lang, and T. F. Meyer. 1997. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91:605-615. [DOI] [PubMed] [Google Scholar]
- 13.Grassmé, H., V. Jendrossek, A. Riehle, G. von Kürthy, J. Berger, H. Schwarz, M. Weller, R. Kolesnick, and E. Gulbins. 2003. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat. Med. 9:322-330. [DOI] [PubMed] [Google Scholar]
- 14.Hauck, C. R., H. Grassme, J. Bock, V. Jendrossek, K. Ferlinz, T. F. Meyer, and E. Gulbins. 2000. Acid sphingomyelinase is involved in CEACAM receptor-mediated phagocytosis of Neisseria gonorrhoeae. FEBS Lett. 478:260-266. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich, M., M. Wickel, W. Schneider-Brachert, C. Sandberg, J. Gahr, R. Schwandner, T. Weber, P. Saftig, C. Peters, J. Brunner, M. Kronke, and S. Schutze. 1999. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 18:5252-5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 17.Horinouchi, K., S. Erlich, D. P. Perl, K. Ferlinz, C. L. Bisgaier, K. Sandhoff, R. J. Desnick, C. L. Stewart, and E. H. Schuchman. 1995. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat. Genet. 10:288-293. [DOI] [PubMed] [Google Scholar]
- 18.Kankwatira, A. M., G. A. Mwafulirwa, and M. A. Gordon. 2004. Non-typhoidal salmonella bacteraemia—an under-recognized feature of AIDS in African adults. Trop. Dr. 34:198-200. [DOI] [PubMed] [Google Scholar]
- 19.Kolesnick, R. N., and M. Kronke. 1998. Regulation of ceramide production and apoptosis. Annu. Rev. Physiol. 60:643-665. [DOI] [PubMed] [Google Scholar]
- 20.Kuhle, V., G. L. Abrahams, and M. Hensel. 2006. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic 7:716-730. [DOI] [PubMed] [Google Scholar]
- 21.Liese, J., S. Kloos, V. Jendrossek, T. Petropoulou, U. Wintergerst, G. Notheis, M. Gahr, and B. H. Belohradsky. 2000. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. J. Pediatr. 137:687-693. [DOI] [PubMed] [Google Scholar]
- 22.Loidl, A., R. Claus, H. P. Deigner, and A. Hermetter. 2002. High-precision fluorescence assay for sphingomyelinase activity of isolated enzymes and cell lysates. J. Lipid Res. 43:815-823. [PubMed] [Google Scholar]
- 23.Mastroeni, P., A. Vazquez-Torres, F. C. Fang, Y. Xu, S. Khan, C. E. Hormaeche, and G. Dougan. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 192:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCollister, B. D., T. J. Bourret, R. Gill, J. Jones-Carson, and A. Vazquez-Torres. 2005. Repression of SPI2 transcription by nitric oxide-producing, IFNγ-activated macrophages promotes maturation of Salmonella phagosomes. J. Exp. Med. 202:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miao, E. A., M. Brittnacher, A. Haraga, R. L. Jeng, M. D. Welch, and S. I. Miller. 2003. Salmonella effectors translocated across the vacuolar membrane interact with the actin cytoskeleton. Mol. Microbiol. 48:401-415. [DOI] [PubMed] [Google Scholar]
- 26.Miller, S. I., and J. J. Mekalanos. 1990. Constitutive expression of the PhoP regulon attenuates Salmonella virulence and survival within macrophages. J. Bacteriol. 172:2485-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochman, H., F. C. Soncini, F. Solomon, and E. A. Groisman. 1996. Identification of a pathogenicity island required for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA 93:7800-7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plant, J., and A. A. Glynn. 1974. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature 248:345-347. [DOI] [PubMed] [Google Scholar]
- 29.Richter-Dahlfors, A., A. M. Buchan, and B. B. Finlay. 1997. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J. Exp. Med. 186:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez, A., P. Webster, J. Ortego, and N. W. Andrews. 1997. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137:93-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salcedo, S. P., and D. W. Holden. 2003. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 22:5003-5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schissel, S. L., X. Jiang, J. Tweedie-Hardman, T. Jeong, E. H. Camejo, J. Najib, J. H. Rapp, K. J. Williams, and I. Tabas. 1998. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J. Biol. Chem. 273:2738-2746. [DOI] [PubMed] [Google Scholar]
- 33.Segal, A. W. 2005. How neutrophils kill microbes. Annu. Rev. Immunol. 23:197-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchiya, K., M. A. Barbieri, K. Funato, A. H. Shah, P. D. Stahl, and E. A. Groisman. 1999. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J. 18:3924-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utermöhlen, O., U. Karow, J. Lohler, and M. Kronke. 2003. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J. Immunol. 170:2621-2628. [DOI] [PubMed] [Google Scholar]
- 37.van Echten-Deckert, G. 2000. Sphingolipid extraction and analysis by thin-layer chromatography. Methods Enzymol. 312:64-79. [DOI] [PubMed] [Google Scholar]
- 38.Vázquez-Torres, A., and F. C. Fang. 2001. Salmonella evasion of the NADPH phagocyte oxidase. Microbes Infect. 3:1313-1320. [DOI] [PubMed] [Google Scholar]
- 39.Vazquez-Torres, A., G. Fantuzzi, C. K. Edwards III, C. A. Dinarello, and F. C. Fang. 2001. Defective localization of the NADPH phagocyte oxidase to Salmonella-containing phagosomes in tumor necrosis factor p55 receptor-deficient macrophages. Proc. Natl. Acad. Sci. USA 98:2561-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vazquez-Torres, A., T. Stevanin, J. Jones-Carson, M. Castor, R. Read, and F. C. Fang. Analysis of NO-dependent antimicrobial actions of macrophages and mice. Methods Enzymol., in press. [DOI] [PMC free article] [PubMed]
- 43.Vazquez-Torres, A., B. A. Vallance, M. A. Bergman, B. B. Finlay, B. T. Cookson, J. Jones-Carson, and F. C. Fang. 2004. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 172:6202-6208. [DOI] [PubMed] [Google Scholar]
- 44.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. W. Holden, S. M. Lucia, M. C. Dinauer, P. Mastroeni, and F. C. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 45.Wijburg, O. L., C. P. Simmons, N. van Rooijen, and R. A. Strugnell. 2000. Dual role for macrophages in vivo in pathogenesis and control of murine Salmonella enterica var. Typhimurium infections. Eur. J. Immunol. 30:944-953. [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization. April 2005, revision date. Drug-resistant Salmonella. Fact sheet no. 139. http://www.who.int/mediacentre/factsheets/fs139/en/.
- 47.Yu, X. J., J. Ruiz-Albert, K. E. Unsworth, S. Garvis, M. Liu, and D. W. Holden. 2002. SpiC is required for secretion of Salmonella Pathogenicity Island 2 type III secretion system proteins. Cell. Microbiol. 4:531-540. [DOI] [PubMed] [Google Scholar]