Abstract
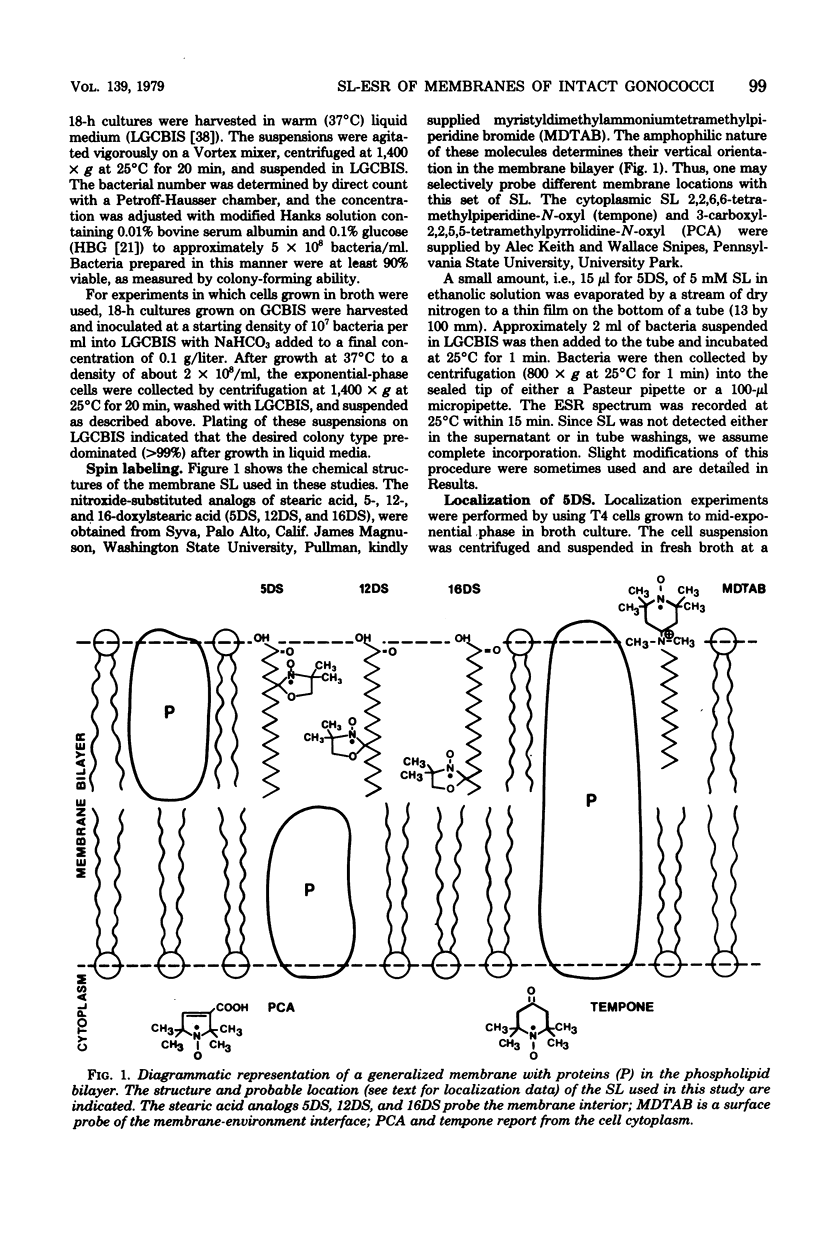
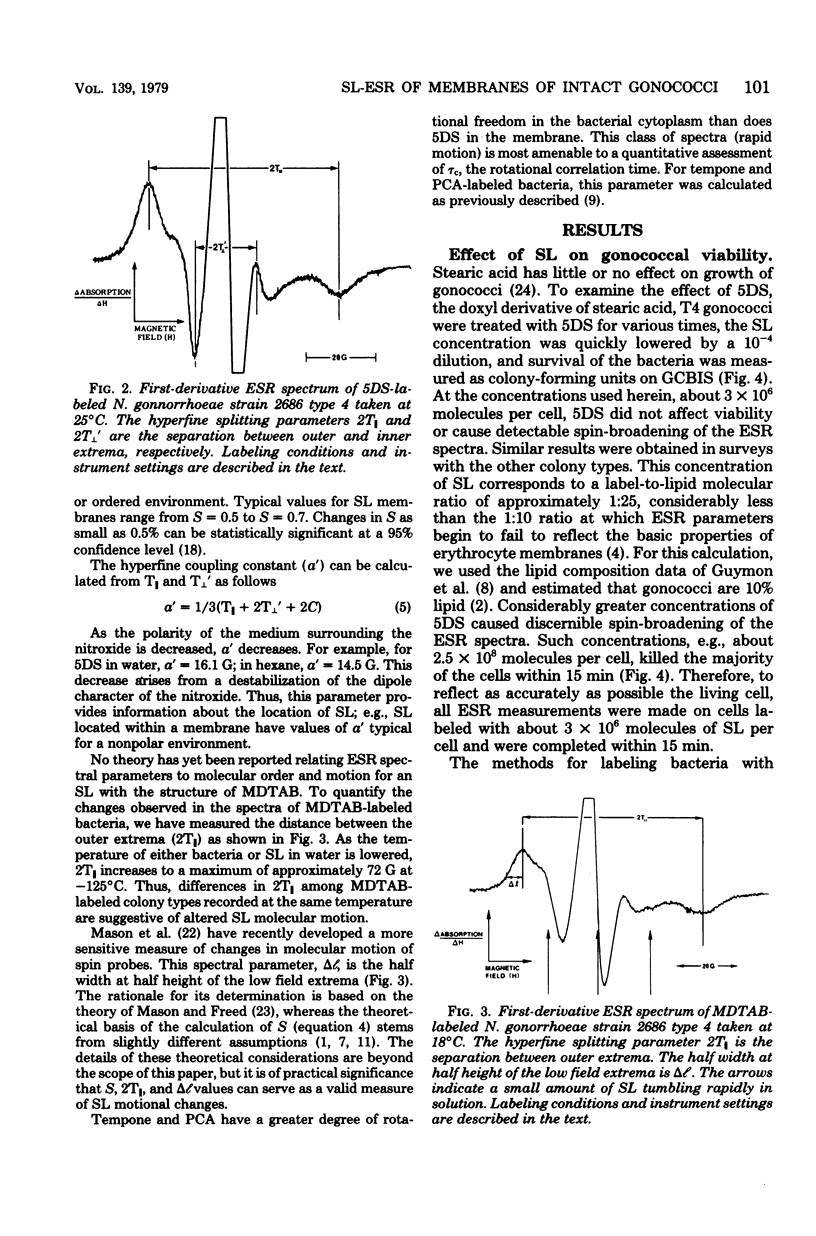
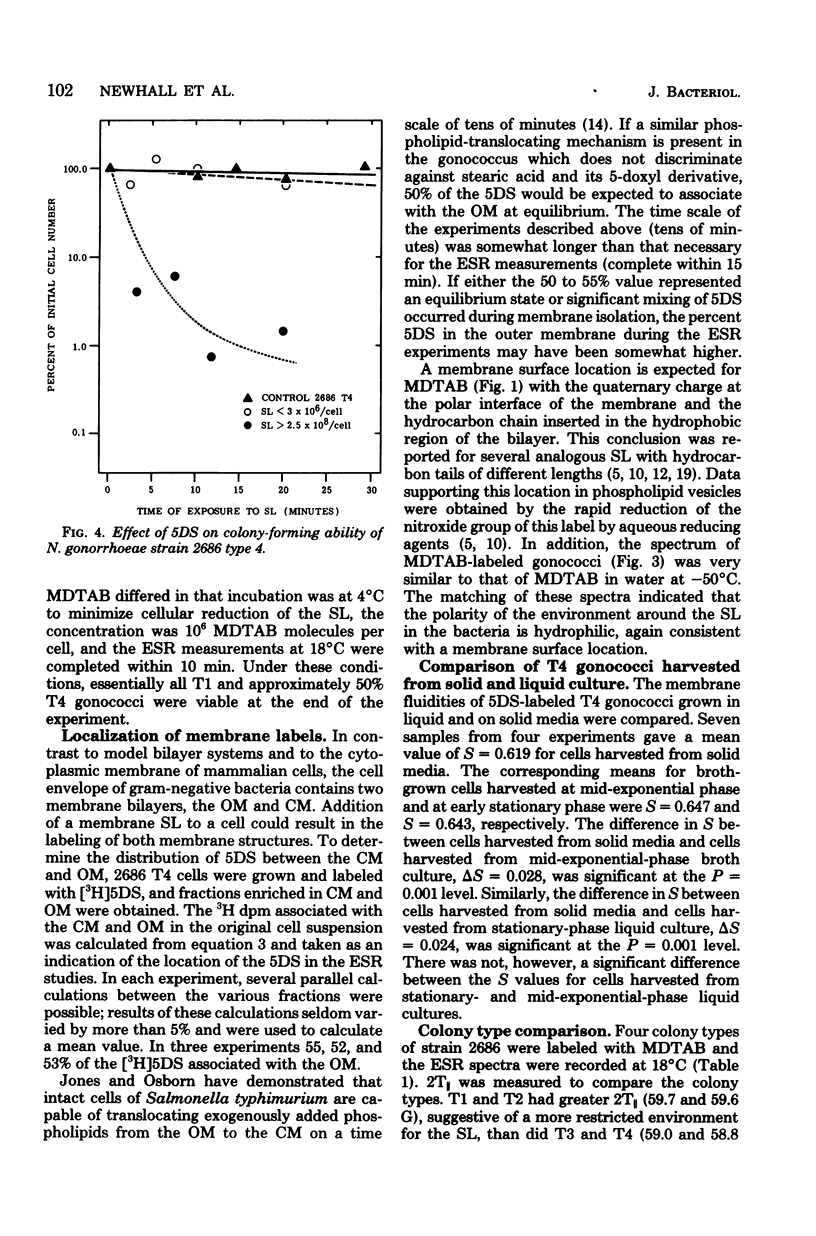
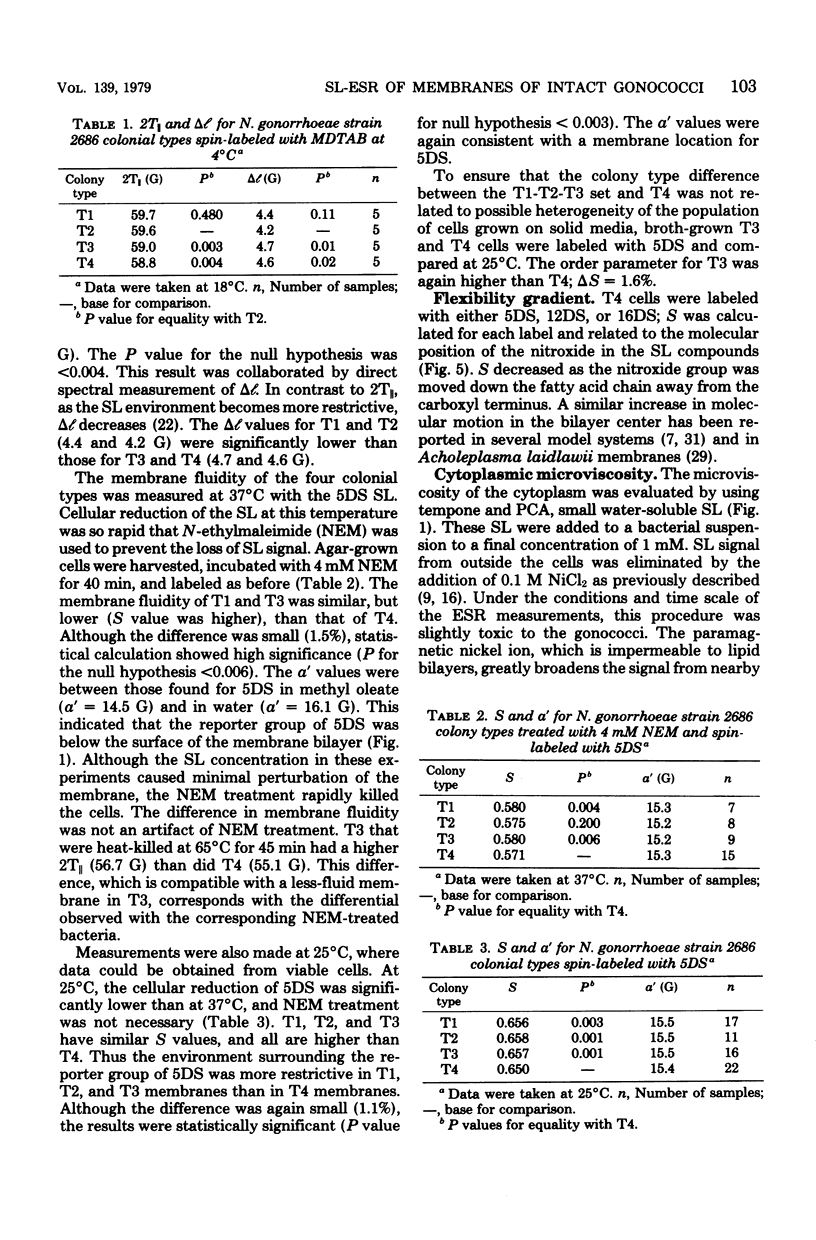
Spin-label electron spin resonance was used to characterize the microenvironment around spin probes which localize (i) in membranes, (ii) at the membrane surface, or (iii) in the cytoplasm of living Neisseria gonorrhoeae. Four colony types (T1, T2, T3, and T4) of gonococci were compared on the basis of the electron spin resonance parameters 2T parallel to, S (order parameter), and tau c (microviscosity). The concentration of spin label used had little or no effect on viability. T1 and T2 gonococci were found to have a more restricted environment for molecular motion of a membrane surface spin label than did T3 and T4. The membrane fluidity, as measured by a membrane lipid spin label, of T4 (S = 0.571) was significantly greater than that of T1 or T3 (S = 0.580). This difference was detected at 37 degrees C, at 25 degrees C, in agar-grown bacteria, and in exponential-phase cells. Studies using spin labels which probe different levels of the membrane indicated the presence of a membrane flexibility gradient. Cytoplasmic spin-label studies indicated that the cytoplasm of all gonococcal colony types was three to five times more viscous than water.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe J. L., Wlodkowski T. J. Lipids of Branhamella catarrhalis and Neisseria gonorrhoeae. J Bacteriol. 1976 Jul;127(1):168–178. doi: 10.1128/jb.127.1.168-178.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttefield D. A., Whisnant C. C., Chesnut D. B. On the use of the spin labeling technique in the study of erythrocyte membranes. Biochim Biophys Acta. 1976 Apr 5;426(4):697–702. doi: 10.1016/0005-2736(76)90134-6. [DOI] [PubMed] [Google Scholar]
- Castle J. D., Hubbell W. L. Estimation of membrane surface potential and charge density from the phase equilibrium of a paramagnetic amphiphile. Biochemistry. 1976 Nov 2;15(22):4818–4831. doi: 10.1021/bi00667a011. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Guymon L. F., Walstad D. L., Sparling P. F. Cell envelope alterations in antibiotic-sensitive and-resistant strains of Neisseria gonorrhoeae. J Bacteriol. 1978 Oct;136(1):391–401. doi: 10.1128/jb.136.1.391-401.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak R. A., Kleinhans F. W., Ochs S. The viscosity of mammalian nerve axoplasm measured by electron spin resonance. J Physiol. 1976 Dec;263(2):115–137. doi: 10.1113/jphysiol.1976.sp011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Motion of steroid spin labels in membranes. Proc Natl Acad Sci U S A. 1969 May;63(1):16–22. doi: 10.1073/pnas.63.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., Metcalfe J. C., Metcalfe S. M., McConnell H. M. The interaction of small molecules with spin-labelled erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):415–427. doi: 10.1016/0005-2736(70)90219-1. [DOI] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. C., Osborn M. J. Translocation of phospholipids between the outer and inner membranes of Salmonella typhimurium. J Biol Chem. 1977 Oct 25;252(20):7405–7412. [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Canonico P. G., Caspary W. J. Electron spin resonance studies of spin-labeled mammalian cells by detection of surface-membrane signals. Proc Natl Acad Sci U S A. 1973 Jan;70(1):66–70. doi: 10.1073/pnas.70.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. D., Snipes W. Viscosity of cellular protoplasm. Science. 1974 Feb 15;183(4125):666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- Kury P. G., McConnell M. Regulation of Membrane Flexibility in Human Erythrocytes. Biochemistry. 1975 Jul;14(13):2798–2803. doi: 10.1021/bi00684a002. [DOI] [PubMed] [Google Scholar]
- Lepock J. R., Morse P. D., 2nd, Mehlhorn R. J., Hammerstedt R. H., Snipes W., Keith A. D. Spin labels for cell surfaces. FEBS Lett. 1975 Dec 1;60(1):185–189. doi: 10.1016/0014-5793(75)80448-0. [DOI] [PubMed] [Google Scholar]
- MARTIN S. P., GREEN R. Methods for the study of surviving leukocytes: A. Preparation of cell suspension. Methods Med Res. 1958;7:136–138. [PubMed] [Google Scholar]
- Mason R. P., Giavedoni E. B., Dalmasso A. P. Complement-induced decrease in membrane mobility: introducing a more sensitive index of spin-label motion. Biochemistry. 1977 Mar 22;16(6):1196–1201. doi: 10.1021/bi00625a026. [DOI] [PubMed] [Google Scholar]
- Miller R. D., Brown K. E., Morse S. A. Inhibitory action of fatty acids on the growth of Neisseria gonorrhoeae. Infect Immun. 1977 Aug;17(2):303–312. doi: 10.1128/iai.17.2.303-312.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Ruhlig M., Snipes W., Keith A. D. A spin-label study of the viscosity profile of sarcoplasmic reticular vesicles. Arch Biochem Biophys. 1975 May;168(1):40–56. doi: 10.1016/0003-9861(75)90226-x. [DOI] [PubMed] [Google Scholar]
- Perry M. B., Daoust V. The lipopolysaccharides of Neisseria gonorrhoeae colony types 1 and 4. Can J Biochem. 1975 May;53(5):623–629. doi: 10.1139/o75-084. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. S., Fulbright R. S., Eads M. E., Sawyer W. D. Ethylenediaminetetraacetic acid-sensitive antiphagocytic activity of Neisseria gonorrhoeae. Infect Immun. 1977 Mar;15(3):817–827. doi: 10.1128/iai.15.3.817-827.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottem S., Hubbell W. L., Hayflick L., McConnell H. M. Motion of fatty acid spin labels in the plasma membrane of mycoplasma. Biochim Biophys Acta. 1970;219(1):104–113. doi: 10.1016/0005-2736(70)90065-9. [DOI] [PubMed] [Google Scholar]
- Sachs F., Latorre R. Cytoplasmic solvent structure of single barnacle muscle cells studied by electron spin resonance. Biophys J. 1974 Apr;14(4):316–326. doi: 10.1016/S0006-3495(74)85918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J., Axel F., Limacher H. Molecular architecture of bilayer membranes. Ann N Y Acad Sci. 1973 Dec 31;222:588–596. doi: 10.1111/j.1749-6632.1973.tb15289.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Phospholipids and fatty acids of Neisseria gonorrhoeae. J Bacteriol. 1975 Nov;124(2):713–717. doi: 10.1128/jb.124.2.713-717.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



