Abstract
We compared the age profiles of infection and specific antibody intensities in two communities with different transmission levels in East Africa to examine the contribution of humoral responses to human immunity to the vector-borne helminth Wuchereria bancrofti. The worm intensities were higher and exhibited a nonlinear age pattern in a high-transmission community, Masaika, in contrast to the low but linearly increasing age infection profile observed for a low-transmission community, Kingwede. The mean levels of specific immunoglobulin G1 (IgG1), IgG2, IgG4, and IgE were also higher in Masaika, but intriguingly, the IgG3 response was higher in Kingwede. The age-antibody patterns differed in the two communities but in a manner apparently contrary to a role in acquired immunity when the data were assessed using simple correlation methods. By contrast, multivariate analyses showed that the antibody response to infection may be classified into three types and that two of these types, a IgG3-type response and a response measuring a trade-off in host production of IgG4 and IgG3 versus production of IgG1, IgG2, and IgE, had a negative effect on Wuchereria circulating antigen levels in a manner that supported a role for these responses in the generation of acquired immunity to infection. Mathematical modeling supported the conclusions drawn from empirical data analyses that variations in both transmission and worm intensity can explain community differences in the age profiles and impacts of these antibody response types. This study showed that parasite-specific antibody responses may be associated with the generation of acquired immunity to human filarial infection but in a form which is dependent on worm transmission intensity and interactions between immune components.
Despite the existence of numerous studies describing the immune responses of individual hosts putatively exposed to different stages of infection, the role of acquired immunity in regulating the infection burden of the important mosquito-borne filarial parasite Wuchereria bancrofti in humans continues to evoke controversy (21). Indirect evidence from analysis and modeling of epidemiological infection data at the community level suggests the operation of this immunity (24), but interpretation of this type of data is fraught with difficulty (6, 10, 48, 49). This is especially a problem in the case of filariasis, where the analysis is further constrained because of the use of diverse blood sampling methods for determining infection status and levels in different studies (24).
A number of previous workers have examined humoral immune responses to filariasis in areas where this infection is endemic, with the objective of more directly investigating the role of acquired immunity in shaping the epidemiology of infection. These studies unambiguously showed that W. bancrofti infection can induce strong antibody responses (4, 29, 32, 42, 47), but protective responses have not been conclusively identified yet. Recent theoretical analysis and evidence from other helminth infections (3, 9, 27, 28, 35, 36) suggest that one reason for this situation in the study of filariasis could be the paucity of studies that have used an immunoepidemiological perspective to investigate the role of acquired immunity in influencing parasitic infection patterns in host communities in areas where the organism is endemic. In particular, this perspective, which attempts to link observed individual host immune responses to epidemiological patterns, has shown how observation of an increasingly negative correlation between the levels of an immune response and the intensities of infection with increasing host age could indicate a protective role for the response being examined (10, 27, 48-50).
One difficulty in interpreting epidemiological age correlations between specific immune responses and parasite infection levels, however, is that these variables may be related to both age and exposure, which makes distinguishing between purely age effects and exposure-driven gain of protective immunity in immunoepidemiological investigations problematic (6, 27). Recent theoretical work has suggested that protective immunity in lymphatic filariasis may be dependent on the community transmission intensity, such that acquired immunity is manifest only in areas where there is higher transmission (24). Taken together, these observations suggest that (i) age-dependent associations between immune response levels and infection intensities can be expected to vary for communities with different mean transmission intensities and (ii) that taking a comparative immunoepidemiological approach to assessing age copatterns for communities in which transmission intensity differs is necessary for identifying and evaluating the role of protective immunity in regulating filarial infection in humans (3, 14, 16, 17, 24, 25, 27, 28, 48).
We present here results from one such comparative immunoepidemiological analysis in which we focused on comparing observed age relationships between filarial specific antibody responses and W. bancrofti intensity in a community with low parasite transmission intensity in coastal East Africa with the relationships observed in a community in the same region with a higher transmission intensity (25, 39, 43). One feature of the analyses reported here was the use of a combined empirical data analysis and mathematical modeling approach for investigating mechanisms that may underlie the observed differences in the age patterns of parasite-specific antibody responses between communities exposed to different transmission pressures (24, 25, 50, 51). We also contrasted the use of univariate and multivariate statistical methods in the empirical analyses of the data to distinguish between single and combined effects of the antibodies examined in regulating W. bancrofti infection. Our results are discussed below in terms of both the role of humoral responses in the generation of immunity to this important tropical parasitic disease and the design and analysis of studies for investigating acquired immunity to parasitic infections in human communities.
MATERIALS AND METHODS
Study population.
The study was conducted in two broadly ethnically, socioeconomically, and nutritionally comparable communities with no previous history of mass antifilaria interventions, which are located approximately 80 km apart in the same East African area where W. bancrofti is endemic, namely Masaika village in Pangani District (Tanga Region) of Tanzania and Kingwede village in Kwale District (Coast Province) of Kenya. The primary design involved collection of samples and data for determining individual host infection and antibody levels in order to quantify, model, and compare age copatterns of these variables between the two communities. Individuals from both communities were also examined for chronic clinical status; however, the outcomes were not taken into account in the present analyses because of our previous finding that disease manifestations did not significantly affect antifilaria antibody responses (13). A detailed description of the study communities has been given elsewhere (43), together with findings from clinical, microfilaria (mf), and circulating filarial antigen (CFA) examinations. Briefly, in Masaika and Kingwede there were 950 and 1,013 inhabitants who were 1 year old or older; the overall mf prevalence and the overall CFA prevalence in these individuals were 25.5 and 52.2%, respectively, in Masaika and 2.9% and 16.4%, respectively, in Kingwede. Thus, the prevalence of lymphatic filariasis was higher in Masaika than in Kingwede, a fact that was reflected in a much higher level of transmission in Masaika than in Kingwede (the annual transmission potentials were 92.9 and 6.4, respectively, during the year preceding the surveys reported here [39]). The study was approved by the Medical Research Coordinating Committee of the National Institute for Medical Research, Tanzania, the Kenyatta National Hospital Ethical and Research Committee, Kenya, and the Central Scientific-Ethical Committee, Denmark.
Parasitological examination.
Briefly, blood sampling for parasitological examination started at 2100 h due to the nocturnal mf periodicity in the study area. From each individual, 100 μl of finger prick blood was collected in a heparinized capillary tube and transferred to a tube with 1 ml of 3% acetic acid. Specimens were later examined in a counting chamber with a microscope, and the number of mf/ml was recorded, as described previously (43).
Preparation of serum.
Immediately after finger prick blood sampling, 5 ml of venous blood was collected in plain Vacutainer tubes. Serum was separated by centrifugation after overnight clotting in a refrigerator, and sodium azide was added to a concentration of 15 mM as a preservative. Serum was initially frozen at −20°C in the field and was later stored at −80°C in the main laboratory until use. Before further handling and testing of sera, 3 μl/ml tri-N-butyl phosphate (catalog no. T-4908; Sigma) and 10 μl/ml Tween 80 (catalog no. P-1754; Sigma) were added to eliminate lipid-coated virus (33).
CFA.
Serum specimens were examined for the presence of CFA by using a TropBio enzyme-linked immunosorbent assay kit (catalog no. 03-010-01; TropBio Ltd. Pty., Townsville, Australia). The test was performed according to the procedures recommended by the manufacturer and described previously (41). Serum specimens with optical density values of ≥standard 2 (≥32 antigen units) were considered positive for CFA, and specimens with optical density values of ≥standard 7 were assigned a fixed value of 32,000 CFA units.
Measurement of filaria-specific antibodies.
Sera were examined for the presence of filaria-specific immunoglobulin G1 (IgG1), IgG2, IgG3, IgG4, and IgE antibodies with an enzyme-linked immunosorbent assay. The methods used for preparation of antigen from Brugia pahangi adult worms recovered from experimentally infected jirds (Meriones unguiculatus) and for conducting the specific antibody assays have been described in detail previously (13).
Data analysis.
Only data from individuals who were examined for the presence of mf, CFA, and all the filaria-specific antibodies selected for investigation in this study (IgG1, IgG2, IgG3, IgG4, and IgE) were included in the data analysis. All response data (mf, CFA, and specific antibody levels) were log10 transformed (x + 1) to approximate normally distributed residuals before statistical analyses were performed. Description and comparison of age-infection and antibody level patterns within and between the two communities were done by fitting generalized linear models (GLM) with Gaussian distributed errors, using age and community as independent variables and log-transformed mf, CFA, or specific antibody values as responses. Variations in the shape of the age-intensity profiles for each of these responses between the two communities were determined by testing for significance of the age and community interaction term (22). Sequential sums of squares were used in calculating the F values for significance testing (27). We assessed epidemiological age associations between the antibody responses and mf or CFA intensities using two complementary approaches. The first approach was based on univariate statistical methods that essentially attempted to evaluate the effects of one antibody at a time to determine the role that each antibody played independently in regulating Wuchereria infection. This was accomplished via calculation of Pearson partial correlations between infection intensity and antibody variables within five age classes constructed to reflect the age structures of the two study communities (35), with tests for assessing heterogeneity in correlation coefficients between age classes carried out using methods described by Sokal and Rohlf (44). This was followed by an attempt to evaluate all the antibody effects simultaneously to determine their joint contributions to host regulation of worm intensity. This analysis was performed not only to obviate the statistical problems associated with multiple testing of numerous explanatory immunological variables (e.g., the five different antibody types) and with multicolinearity or correlation between multiple explanatory variables (45) but also in recognition of the growing belief that joint effects between multiple immunological parameters are likely to determine the outcome of parasitic infection (2, 15, 26, 40). Multivariate analysis was carried out in this study by performing a principal component regression analysis (23). Essentially, this method entailed reducing the multivariate correlated antibody response data into main independent composite components first and then assessing and comparing the relationship between the derived two or three major components and infection intensity in the young age class (<30 years) versus the older age class (≥30 years) in each community, using individual host scores for each of these components as explanatory variables in linear regressions with log CFA levels as the response (5).
Mathematical modeling.
We constructed a simple mathematical model using the results and insights gained from the empirical data analyses (described below) to investigate and illustrate how transmission and adult worm intensity variables interacting at the individual host level may generate and hence explain the observed age patterns of specific antifilaria antibody responses in communities in which the organism is endemic with different transmission levels. Given the empirical results regarding the main categories or types of antibody-age response observed in the data (see below), we modeled three major antibody response patterns: (i) a pattern perhaps embodying the observed IgG4 response (antibody response type 1), (ii) a pattern likely shown by the observed IgG3 response (antibody response type 2), and (iii) a pattern perhaps indicated by the IgG1, IgG2, and IgE responses (antibody response type 3). The specific structure of the model, the set of coupled differential equations describing the model, and the parameter values used in the simulations carried out in this report are described in detail in the Appendix.
RESULTS
Demographic and infection age profiles of the populations examined.
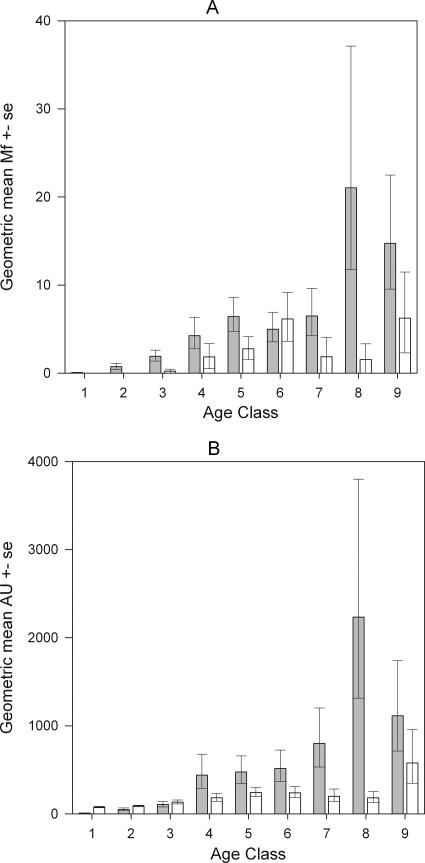
Masaika had a total registered population of 950 individuals who were 1 year old or older, and 817 (86.0%) of these individuals were examined for the presence of mf, CFA, and filaria-specific IgG1, IgG2, IgG3, IgG4, and IgE antibodies (Table 1). Similarly, in Kingwede, a total of 1,013 individuals who were 1 year old or older were registered, and 762 (75.2%) of these individuals were examined for the presence of mf, CFA, and for the filaria-specific antibodies mentioned above (Table 1). Slightly higher proportions of inhabitants were examined for the presence of parasitological and immune variables in Masaika than in Kingwede for all the age groups examined. The filarial infection prevalence, however, whether measured by mf status or measured by CFA status, was markedly higher in Masaika than in Kingwede in all these age groups (Table 1). Figure 1 shows the corresponding age-related profiles for mf and CFA geometric mean intensities of the two populations examined and indicates that there was a similarly higher age-specific parasite burden in Masaika than in Kingwde. The results of the GLM analysis carried out with these data, however, also indicated that there were significant differences in the rate of increase with age for these infection variables between the two communities (Table 2) (the age × community terms were significant for both infection variables); specifically, the intensity of mf or CFA appeared to increase linearly with age in Kingwede but showed some nonlinearity characterized by a steady rise with an apparent subsequent decline in the oldest age class in Masaika (Fig. 1).
TABLE 1.
Age-stratified characteristics of the populations in Masaika and Kingwede examined for the presence of filaria-specific antibodies (IgG1, IgG2, IgG3, IgG4, and IgE)
| Ages (yr) | Masaika
|
Kingwede
|
||||||
|---|---|---|---|---|---|---|---|---|
| Total no. | No. examined (% coverage) | No. mf positive (%) | No. CFA positive (%) | Total no. | No. examined (% coverage) | No. mf positive (%) | No. CFA positive (%) | |
| 1-4 | 99 | 71 (71.7) | 1 (1.4) | 6 (8.5) | 163 | 105 (64.4) | 0 (0) | 3 (2.8) |
| 5-9 | 111 | 100 (90.1) | 8 (8.0) | 30 (30.0) | 154 | 129 (83.8) | 0 (0) | 9 (7.0) |
| 10-14 | 139 | 126 (90.6) | 23 (18.3) | 59 (46.8) | 157 | 138 (87.9) | 1 (0.7) | 19 (13.7) |
| 15-19 | 95 | 78 (82.1) | 20 (25.6) | 45 (57.7) | 102 | 77 (75.5) | 2 (2.6) | 15 (19.5) |
| 20-29 | 162 | 138 (85.2) | 46 (33.3) | 83 (60.1) | 179 | 121 (67.6) | 6 (5.0) | 29 (23.8) |
| 30-39 | 138 | 120 (87.0) | 34 (28.3) | 74 (61.7) | 104 | 86 (82.7) | 8 (9.3) | 21 (24.4) |
| 40-49 | 83 | 74 (89.2) | 27 (36.5) | 53 (71.6) | 58 | 35 (60.3) | 1 (2.9) | 8 (22.9) |
| 50-59 | 45 | 41 (91.1) | 19 (46.3) | 32 (78.0) | 52 | 40 (76.9) | 1 (2.5) | 8 (20.0) |
| ≥60 | 78 | 69 (88.5) | 30 (43.5) | 47 (68.1) | 44 | 31 (70.5) | 3 (9.4) | 13 (40.6) |
| Total | 950 | 817 (86.0) | 208 (25.5) | 429 (52.5) | 1,013 | 762 (75.2) | 22 (2.9) | 125 (16.4) |
FIG. 1.
Age-specific intensities of (A) mf counts (Mf) and (B) CFA units (AU) observed in different host age classes in Masaika (filled bars) and Kingwede (open bars). The bars indicate geometric means, while the error bars indicate the standard errors of the means. The values for mf counts and CFA units for Kingwede were multiplied by 10 to enable clearer comparisons with the markedly higher infection intensity values obtained for individuals in Masaika. Age class 1, 1 to 4 years; class 2, 5 to 9 years; class 3, 10 to 14 years; class 4, 15 to 19 years; class 5, 20 to 29 years; class 6, 30 to 39 years; class 7, 40 to 49 years; class 8, 50 to 59 years; and class 9, ≥60 years.
TABLE 2.
Statistical results from Gaussian GLM fits for assessing the effects of community and age on infection and antibody levels: F values and their levels of significance for the main effects of community and age and the community-age interaction effect
| Infection variable and isotype |
F valuea
|
||
|---|---|---|---|
| Community | Age | Community-age interaction | |
| mf | 13.04 (H > L)* | 55.76 (+)* | 24.83* |
| CFA | 45.88 (H > L)* | 116.53 (+)* | 22.87* |
| IgG1 | 96.63 (H > L)* | 49.42 (−)* | 3.517 |
| IgG2 | 271.83 (H > L)* | 0.11 | 21.64* |
| IgG3 | 71.55 (L > H)* | 29.06 (+)* | 30.51* |
| IgG4 | 146.39 (H > L)* | 27.48 (+)* | 2.33 |
| IgE | 27.02 (H > L)* | 0.55 | 7.66* |
H, high-transmission community (Masaika); L, low-transmission community (Kingwede); >, levels greater in one community than in the other; +, significant positive relationship between age and infection or antibody levels; −, negative relationship between age and infection or antibody levels; *, P < 0.001 (in all cases n = 1,579). Values for all the response variables were log10 transformed prior to analysis.
Age-specific antibody profiles in Masaika and Kingwede.
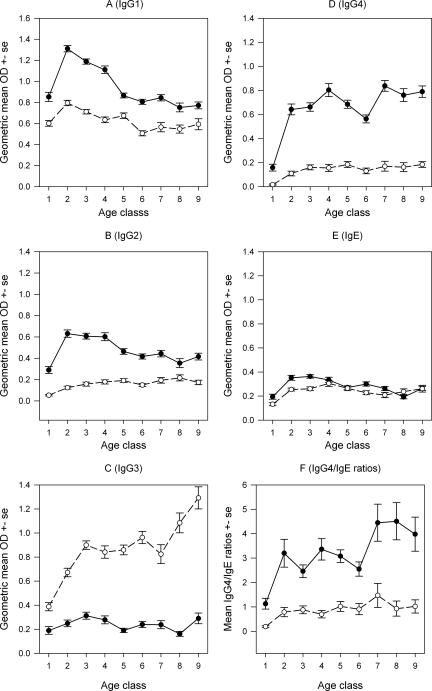
The specific IgG1, IgG2, IgG3, IgG4, and IgE antibody levels observed for different ages in both Masaika and Kingwede are shown in Fig. 2. Age significantly affected the amounts of all the specific antibodies examined in Masaika, but it appeared to have a significant effect on specific IgG1, IgG3, and IgG4 production but not on IgG2 and IgE levels in Kingwede (indicated by a lack of significance for the age term in contrast to a significant effect for the community × age term for these antibodies in the GLM results shown in Table 2). The mean levels of specific IgG1, IgG2, IgG4, and apparently IgE were significantly higher in Masaika than in Kingwede, indicating the positive influence of transmission intensity on the generation of these antibodies (Fig. 2 and Table 2) (P < 0.001 for the community term in each case), but the opposite was true for the specific IgG3 response. The mean antibody levels of this isotype were markedly higher in all age classes in the low-transmission Kingwede village than in Masaika (Fig. 2 and Table 2). In Masaika, the mean specific IgG1, IgG2, and IgE levels increased in the youngest age groups and then declined gradually with age. This differed from the observed specific IgG3 and IgG4 age responses; while the mean levels of IgG3 were generally low and changed only slightly with increasing age, the mean levels of specific IgG4 increased with age and reached a plateau in the older age groups, apparently mirroring the corresponding age pattern of infection intensities (whether measured by mf or CFA) (Fig. 1). In Kingwede, the mean levels of specific IgG1 and IgG4 appeared to follow a pattern similar to that seen in Masaika (which is supported by the lack of significance for the age × community term in the GLM model results in Table 2). In comparison, the age-specific mean levels of IgG2, IgG3, and to a lesser extent IgE in this community were quite different from the levels seen in Masaika (Fig. 2), as shown by the significant age × community terms in Table 2. Thus, in contrast to the patterns observed in Masaika, in Kingwede, while the level of specific IgG3 increased linearly with age, the mean levels of both IgG2 and IgE increased with age and reached a plateau in the older age classes (Fig. 2).
FIG. 2.
Age-associated geometric mean filaria-specific IgG1, IgG2, IgG3, IgG4, and IgE levels in Masaika (•) and Kingwede (○). Panel F shows the mean IgG4/IgE ratios obtained for each age class in the two study communities. The error bars indicate standard errors of the means. Age classes are defined in the legend for Fig. 1.
Profile of IgG4/IgE ratios in relation to age.
The age-specific mean IgG4/IgE ratios for all the individuals examined in Masaika and Kingwede are shown in Fig. 2F. The ratios were higher in Masaika than in Kingwede for all age groups (Gaussian GLM assessing the effects of community and age, F = 35.82, df = 1,402, and P < 0.001 for the effect of community). Furthermore, in each community, contrary to expectations, this ratio was again lowest in the youngest age group and appeared to increase with host age (GLM as described above, F = 21.32, df = 1,239, and P < 0.001 for the effect of age), and the increase was significantly greater in the high-transmission community (Masaika) than in Kingwede (GLM, F = 4.41, df = 1,50, and P = 0.035 for a community × age effect).
Age associations between individual antibody levels and mf or CFA intensities.
The results describing the individual or univariate associations between infection intensity and W. bancrofti-specific antibody levels in relation to the ages of individuals from the two study communities, based on the age-stratified Pearson product moment partial correlation test, are shown in Tables 3 and 4. This test determines the correlation between host infection intensity and a specific antibody level after any joint effects due to the responses of the other antibody isotypes examined are controlled for (13). Hence, it essentially examines the independent effects of each antibody in regulating parasite infection intensity. Correlations were determined for five discrete age classes, chosen both to give adequate sample sizes within each age group and to provide a clear reflection of the study populations in each community (compare Table 1 with Tables 3 and 4), and are shown for specific antibody associations with both mf- and CFA-based infection variables in the case of Masaika and only for CFA levels (see above) for Kingwede. In Masaika (Table 3), the values for the estimated partial correlations indicate that there was a broadly negative association between specific IgG1, IgG2, IgG3, and IgE responses and filarial infection intensity (either mf or CFA) in all age classes examined, with the strength of the negative association rising from the youngest age class and then declining among the older age groups. This pattern, however, was significant only for the specific IgG1 response to either of the worm intensity variables examined (the test of heterogeneity of correlation coefficients was significant for both mf and CFA [Table 3]), although, as expected (given that antibodies in this study were measured against adult worm antigens), there was some indication of a more prominent negative association between specific IgG3 and IgE levels and CFA intensity than between these antibody levels and mf levels. By contrast, specific IgG4 intensities were positively correlated with infection intensity in all age classes, with no apparent age patterns, although again the response was found to be more prominent in relation to CFA than in relation to mf intensity (Table 3). Although the estimated correlations were lower and the age patterns were more variable, a similar pattern of a decreasing negative association between CFA intensity and specific IgG1, IgG2, IgG3, and IgE with increasing mean host age was also observed for Kingwede (Table 4), with the notable difference that this pattern was found to be significant for IgG3 and IgE by comparison with the corresponding findings for these antibodies in Masaika (Table 3). The IgG4 levels and CFA intensity were again positively correlated for all age classes, and there was some indication that the strength of association may increase with mean host age (Table 4).
TABLE 3.
Partial correlation coefficients for filaria-specific antibody levels and individual host infection intensities in different age classes of the examined population in Masaika
| Ages (yr) | n | % Positive | Partial correlation coefficienta
|
||||
|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | IgE | |||
| Age association with microfilaremia intensity | |||||||
| 1-9 | 171 | 5.3 | −0.099 | −0.028 | −0.074 | 0.265** | −0.071 |
| 10-19 | 204 | 22.5 | −0.392*** | −0.003 | −0.027 | 0.157* | −0.071 |
| 20-39 | 258 | 31.0 | −0.240*** | −0.233*** | −0.082 | 0.270*** | −0.059 |
| 40-59 | 111 | 40.0 | −0.249** | −0.245*** | 0.069 | 0.367*** | −0.019 |
| ≥60 | 69 | 43.5 | −0.152 | −0.189 | −0.069 | 0.288* | 0.016 |
| All | 817 | −0.286*** | −0.128*** | −0.034 | 0.312*** | −0.044 | |
| χ2b | 9.86* | 8.74 | 2.01 | 3.94 | 0.57 | ||
| Age association with antigenemia levels | |||||||
| 1-9 | 171 | 21.1 | 0.008 | −0.130 | −0.110 | 0.537*** | −0.133 |
| 10-19 | 204 | 51.0 | −0.275** | −0.096 | −0.064 | 0.533*** | −0.273** |
| 20-39 | 258 | 60.9 | −0.314** | −0.179** | −0.121 | 0.497*** | −0.140* |
| 40-59 | 111 | 73.9 | −0.221* | −0.227* | −0.006 | 0.495*** | −0.183 |
| ≥60 | 69 | 68.1 | −0.068 | −0.234 | −0.205 | 0.453*** | −0.063 |
| All | 817 | −0.266*** | −0.134*** | −0.085* | 0.558*** | −0.157*** | |
| χ2b | 13.76** | 2.02 | 2.11 | 0.92 | 3.64 | ||
***, P < 0.001; **, P < 0.01; *, P < 0.05.
Test for homogeneity of correlation coefficients.
TABLE 4.
Partial correlation coefficients for filaria-specific antibody and individual host CFA levels in different age classes of the examined population in Kingwede
| Ages (yr) | n | % Positive | Partial correlation coefficienta
|
||||
|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgG3 | IgG4 | IgE | |||
| 1-9 | 234 | 5.1 | −0.049 | 0.129 | −0.014 | 0.202** | 0.026 |
| 10-19 | 215 | 15.8 | 0.133 | −0.143* | 0.046 | 0.465** | −0.222* |
| 20-39 | 207 | 24.2 | −0.115 | 0.028 | −0.164* | 0.510*** | −0.055 |
| 40-59 | 75 | 21.3 | −0.076 | −0.148 | −0.043 | 0.334** | −0.063 |
| ≥60 | 31 | 41.9 | −0.012 | 0.032 | −0.280 | 0.750*** | 0.455* |
| All | 762 | −0.072 | −0.042 | −0.031 | 0.461*** | −0.071 | |
| χ2b | 7.29 | 9.42 | 10.02* | 32.51*** | 26.38*** | ||
***, P < 0.001; **, P < 0.01; *, P < 0.05.
Test for homogeneity of correlation coefficients.
Multivariate patterns in the specific antibody response to infection.
The results of a principal component regression analysis (5, 23, 46) of the antibody-CFA level data are presented in Table 5 and indicate that three major composite variables may explain >85% of the total variation in these data. Principal component 1 was mainly reflective of IgG4 activity and accounted for 42% of the total variation in the data, while principal component 2 was primarily reflective of the IgG3 response and accounted for 32% of the total variation observed (Table 5). By contrast, principal component 3 appeared to measure a trade-off in the combined levels of IgG4 and apparently IgG3 on the one hand and IgG1 and IgG2 and to a lesser extent IgE levels on the other hand in the host antibody response, with the negative correlation between these two response types (coefficients with opposite signs [Table 5]), suggesting that a higher level of specific IgG1, IgG2, and IgE production in response to filarial antigens by individuals is countered by generation of less IgG4 and IgG3 in the same individuals.
TABLE 5.
Results of the principal component analysis of the filaria-specific antibody data
| Component | Importance of derived principal components
|
Loadings to componentsa
|
||||||
|---|---|---|---|---|---|---|---|---|
| SD | Proportion of variance | Cumulative proportion | Log IgG1 | Log IgG2 | Log IgG3 | Log IgG4 | Log IgE | |
| 1 | 0.198 | 0.416 | 0.416 | 0.355 | 0.364 | −0.482 | 0.710 | |
| 2 | 0.174 | 0.321 | 0.737 | 0.352 | 0.301 | 0.843 | 0.229 | 0.150 |
| 3 | 0.110 | 0.128 | 0.865 | 0.663 | 0.274 | −0.172 | −0.640 | 0.154 |
| 4 | 0.088 | 0.081 | 0.946 | |||||
| 5 | 0.072 | 0.054 | 1.000 | |||||
Bold type indicates the response variables contributing the most to each individual principal component (see text).
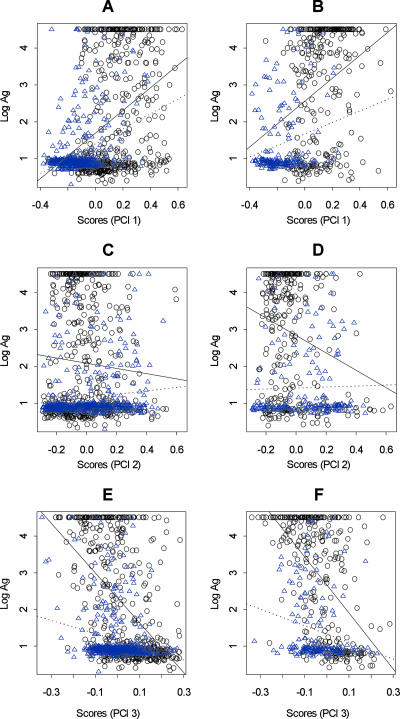
To quantify the relationship between community exposure intensity and host filarial infection intensity (as measured by CFA levels) and these three principal components, we regressed the observed CFA levels on the scores obtained for each of these components for each individual from the two communities. To assess if associations between these immune responses were significantly associated with CFA levels in the younger or older individuals, the regressions were carried out and the results were compared for two broad host age classes: individuals less than 30 years old and individuals 30 years old and older. The results are shown in Fig. 3 and Table 6 and indicate that there were significant, although contrasting, overall linear relationships between CFA levels and each principal component in the two study communities. Thus, while the CFA level was found to be positively correlated with the IgG4-dominated response represented by principal component 1 in both communities irrespective of the host age (Fig. 3A and B and Table 6), the relationship with CFA showed contrasting trends in the case of the IgG3-dominated response, as represented by principal component 2 (Fig. 3C and D). In Masaika, the intensity of host infection appeared to be negatively correlated with principal component 2 scores or levels of IgG3, but this was found to be significant only for older hosts. In contrast, this relationship showed the opposite trend irrespective of the host age for the data from Kingwede (Fig. 3C and D and Table 6). Thus, in this community, increasing IgG3 levels in the host were associated with a higher level of circulating W. bancrofti antigen. The relationship between infection intensity and scores derived from component 3, however, was significantly negative for both communities (Fig. 3E and F and Table 6). This indicates that increasing levels of combined specific IgG1, IgG2, and IgE responses or decreasing levels of particularly the specific IgG4 response in individuals were associated with reduced filarial antigen levels, and the magnitude of this effect appeared to be greater in the community with higher transmission (Table 6). There is some indication that the strength of these effects was also higher for older individuals, especially in Masaika (Fig. 3E and F and Table 6).
FIG. 3.
Relationship between individual host intensity of CFA and scores for the first three principal components (PCI) in hosts who were less than 30 years old (A, C, and E) and hosts who were 30 years old or older (B, D, and F) in Masaika (○) and Kingwede (▵). The y axes show the log CFA values, while the x axes indicate individual host scores contributing to principal components 1 (A and B), 2 (C and D). and 3 (E and F) (see text). The lines represent the linear regression model predictions of the relationship between log CFA values and the corresponding principal component scores for hosts from Masaika (solid lines) and Kingwede (dotted lines). The relationship was positive between infection intensity and principal component 1 scores but negative in the case of the relationships for principal component 3 in both communities. For principal component 2, the association was negative for Masaika but positive for Kingwede. Most associations were significant; the only exception was the relationship between CFA and principal component 2, where the observed negative association was significant only in individuals who were 30 years old or older in Masaika (Table 6).
TABLE 6.
Coefficients of linear regression of host CFA intensity (log values) on the first three principal component scores
| Parameter | Ages (yr) | Value | SE | t value | P value |
|---|---|---|---|---|---|
| Masaika | |||||
| Principal component | <30 | 3.0743 | 0.3619 | 8.4948 | <0.0001 |
| 1 scores | ≥30 | 3.0944 | 0.5717 | 5.4123 | <0.0001 |
| Principal component | <30 | −0.7121 | 0.4329 | −1.6451 | 0.1006a |
| 2 scores | ≥30 | −2.3616 | 0.5661 | −4.1719 | <.0001 |
| Principal component | <30 | −6.7891 | 0.4719 | −14.3875 | <0.0001 |
| 3 scores | ≥30 | −7.6294 | 0.6558 | −11.6331 | <0.0001 |
| Kingwede | |||||
| Principal component | <30 | 2.0250 | 0.2279 | 8.8871 | <0.0001 |
| 1 scores | ≥30 | 1.5553 | 0.5476 | 2.8405 | 0.005 |
| Principal component | <30 | 0.3160 | 0.1651 | 1.9152 | 0.0559a |
| 2 scores | ≥30 | 0.1339 | 0.3723 | 0.3596 | 0.7196a |
| Principal component | <30 | −1.8053 | 0.3307 | −5.4583 | <0.0001 |
| 3 scores | ≥30 | −2.2684 | 0.8130 | −2.7901 | 0.005 |
Not significant.
Mathematical modeling of age patterns of filaria-specific antibody responses.
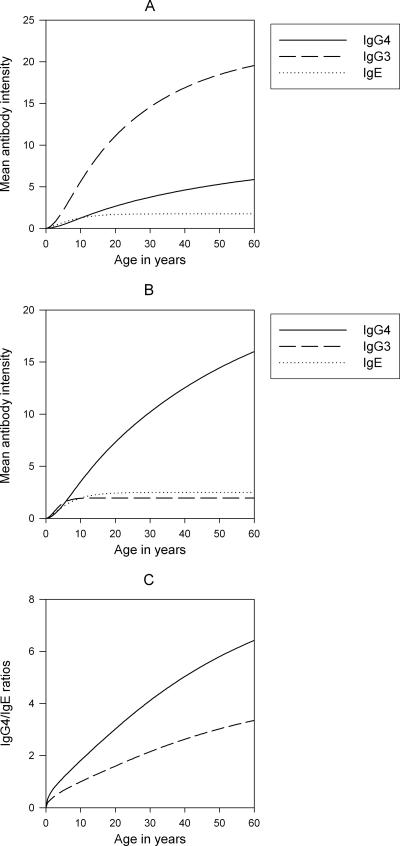
The results from simulations of the constructed mathematical model (see the Appendix) describing the generation of the three basic antibody response types outlined above as a function of both transmission and adult worm intensities are presented in Fig. 4. The results show how these variables interacting at the individual host level can, through simple feedback mechanisms, qualitatively generate age profiles of IgG4-, IgG3-, and IgE-type responses corresponding to those observed in the two study communities (compare Fig. 2 and 4). Thus, the results show that the IgG4-type age responses may be different in a high-transmission community and a low-transmission community primarily as a function of differences in the dynamics of adult worm intensity, specifically the gain of higher mean worm intensities in the former community, leading to higher age patterns of specific IgG4, compared to the opposite situation for the low-transmission community. By contrast, the model simulations show that the IgG3-type age response may be generated largely as a function of transmission intensity, but with higher transmission intensity strongly suppressing the generation of this type of response (Fig. 4). The simulation results for the IgE-type response, on the other hand, show how negative feedback with adult worm intensity may primarily govern the differences in the observed age patterns of this antibody response between communities with different transmission levels (Fig. 2 and 4). The age patterns of the mean IgG4/IgE ratios predicted by the model for a high-transmission community and for a low-transmission community are also shown in Fig. 4C. Qualitatively, these predictions correspond to the observed age patterns for the ratios of these specific antibody levels in Masaika and Kingwede (Fig. 2F) and clearly indicate that the observed increase in the IgG4/IgE ratio with host age in filariasis is a direct function of the contrasting worm intensity-associated positive (IgG4) and -negative (IgE) feedback mechanisms regulating the generation of these specific antibodies in communities where the disease is endemic.
FIG. 4.
Age-specific IgG4, IgG3, and IgE responses in (A) a low-transmission community and (B) a high-transmission community as a function of transmission intensity and worm burden, as simulated by the model described in the appendix incorporating generative mechanisms suggested by the empirical analyses of the data carried out in this study (see text). Panel C shows the predictions of the model for the expected age-specific IgG4/IgE ratios in each of the communities (solid line, high-transmission community; dashed line, low-transmission community) arising from the proposed mechanisms of generation of these antibody types. In contrast to previous observations, the simulated profiles are in accord with the findings of changes in this ratio in the two study populations.
DISCUSSION
Our investigation of the role of specific antibody responses in regulating filarial infection was premised upon finding differences in the levels and age patterns of infection intensity between the study communities. It appeared that there were such differences. The infection level, regardless of whether it was measured by mf or CFA analysis, was found to be markedly higher and to increase with age in the younger hosts and to drop sharply in the oldest host age classes in the high-transmission intensity community (Masaika), in contrast to the situation in the low-transmission intensity community (Kingwede), where the infection intensity was both lower overall and increased with age without reaching a plateau or decreasing among the older hosts. This epidemiological pattern of a significantly higher but peaking infection intensity profile in the high-transmission community compared to a low rising age intensity profile in the low-transmission community has been shown by mathematical models, with a similar age exposure pattern in different communities, to be a function of the occurrence of protective immunity (particularly against larval establishment) in higher-transmission areas (24, 51). By comparison, not only did the subjects in the low-transmission locality harbor less infection due to a lower exposure rate, but the observed occurrence of a linearly increasing age-infection intensity curve was also most likely reflective of the absence of any significant age-dependent host regulation of infection (24, 25, 27).
However, it is apparent that this differential infection pattern was only weakly observed in this study, supporting our previous conclusion that the transmission intensities observed in the current study communities may be below the threshold required to induce strong population-level protective immune responses (25). Age exposure differences also cannot be totally excluded in confounding the present observations (6, 24, 27, 51), although it is plain that such vector biting patterns would have to be considerably different in the two study communities in order to significantly influence the differences observed in the corresponding age profiles of infection.
The immunoepidemiological rationale for comparing age intensities of infection with corresponding age profiles of specific antibody responses in communities with different transmission intensities is that any intercommunity discordance between such profiles, especially in older subjects, is thought to provide not only a more direct sign of the occurrence of acquired immunity (3, 10, 27, 28, 46, 48) but also identification of which of the specific immune components examined may potentially be a marker of such immunity. More specifically, it is hypothesized that the correlation between immune responses and infection intensity changes with both transmission intensity and host age; the association is expected to be positive in young children because both infection and immune responses reflect exposure, but as the hosts age, the association becomes negative as the immune response gained via cumulative exposure starts to regulate worm intensities (3, 28, 51). Implicit in this hypothesis is that this epidemiological age effect of acquired protective immunity should be manifest or should be greater in communities with higher transmission intensities (1, 48).
Age certainly had a significant effect on the amount of all the specific antibodies examined in Masaika, but it appeared to have a differential effect on antibody production in the low-transmission community, Kingwede. Thus, while in both communities host age affected the specific IgG1, IgG3, and IgG4 responses, it did not influence the generation of specific IgG2 and IgE by hosts in Kingwede. Together with the finding that the mean levels of specific IgG1, IgG2, IgG4, and IgE were also significantly lower in Kingwede, this lack of a host age effect on the generation of IgG2 and IgE in this community may reflect the requirement for a higher cumulative transmission or infection intensity threshold in the age generation of these antibodies compared to the other antibody isotypes (Fig. 2).
Despite this difference in the production of antibodies, a notable finding was that when the age patterns of the specific antibody responses examined were assessed singly, none of them appeared to strongly support expectations for a clear role in fostering acquired immunity to filariasis infection in either study community. Thus, in Masaika, the mean levels of specific IgG1, IgG2, and IgE were higher in the youngest age groups but then declined gradually with age. The mean levels of IgG3 were also generally low and changed only slightly with increasing age. By contrast, the mean levels of specific IgG4 increased with age and reached a plateau in the older age groups. Similarly, in Kingwede, while the mean intensities of specific IgG1 and IgG4 appeared to follow a pattern similar to that seen in Masaika, the age-specific mean levels of IgG2, IgG3, and to a lesser extent IgE either increased linearly with age (IgG3) or increased linearly and reached a plateau in older hosts (IgG2 and IgE). Thus, based on simple assessments of age patterns, either the antibody responses examined in this study appeared to mirror the age profile of infection, as in the case of specific IgG4 or IgG2, IgG3, and IgE responses in Kingwede, or the dominant pattern consisted of a higher response in younger age groups when the mean infection levels were low, which appeared to decline with age even as the infection intensities rose in those age classes (as shown by the IgG1, IgG2, and possibly IgE responses in Masaika). Most previous studies have also generally shown that the specific antifilaria antibody levels are higher in younger age groups than in older age groups, although the differences have rarely been significant (4, 11, 12, 42, 47).
A similar counterintuitive finding regarding the levels and age patterns of the specific antibody response to infection also pertains to the patterns observed for the specific IgG3 response in the present study communities. Thus, while in the high-transmission community the levels of IgG3 appeared to change slightly with age, in the low-transmission community the levels were not only remarkably higher but also increased linearly with age. Furthermore, we also found evidence that despite the lower response in the high-transmission village, these antibodies appeared to have an effect on worm intensity that was more negative than the effect when their levels were much higher under low-transmission conditions (Tables 3, 4, and 6; Fig. 4). The reasons for this striking inverse relationship with exposure intensity and the observed between-community impact of this isotype is not clear, but the data suggest either that the effects of this antibody are manifest only at lower levels coupled with a complex down-regulatory role for exposure intensity under high-transmission conditions or that this finding is an artifact of closely linked changes in some other effective component of the immune response. Further studies, perhaps specifically focusing on specific IgG3 responses in parallel with cellular components known to be associated with the IgG3 response (7) in communities with very different transmission intensities, are warranted if we are to successfully dissect the role of IgG3 in regulating filariasis infection (13).
In contrast to the specific IgG1, IgG2, and IgE responses, the mean levels of IgG4 in both communities increased with age, and the rate of increase was higher in the high-transmission community. This finding clearly mirrors previous observations (12, 20) and is perhaps not surprising since IgG4 levels are generally thought to be positively associated with the presence and intensity of infection (4, 13, 19, 29, 42). The converse age response patterns for specific IgG4 and IgE appear to also underlie the finding in this study that the mean IgG4/IgE intensity ratio varied positively with both transmission intensity and increasing host age, an observation contrary to previous suggestions that in lymphatic filariasis this ratio may be a measure of the strength of immunological resistance to infection and hence expected to be low in the presence of resistance and vice versa (18). If the previous suggestions are correct and the IgG4/IgE ratio is indeed an indicator of resistance, then our findings imply that resistance to infection in bancroftian filariasis is not affected by age. Alternatively, as we have suggested previously, this finding may suggest that the IgG4/IgE ratio may not be a good indicator of resistance to filarial infection (13).
The major finding of interest emerging from carrying out the partial correlation analysis of the effects of the examined filaria-specific antibody responses on parasite intensity is that apart from the overwhelmingly positive specific IgG4 response with infection intensity, the responses of all the other isotypes assessed in both communities were generally found to be negatively associated with host infection intensity irrespective of host age (Tables 3 and 4). This pattern persisted regardless of which infection indicator, mf or CFA intensity, was examined and thus appears to be contrary to theoretical expectations regarding the involvement of antibodies in acquired immunity to filariasis, viz., that antibodies should become more negatively associated with infection intensity as hosts age. Closer inspection of the data also revealed a pattern in which the negative associations with infection observed for each of these isotypes increased from the younger age classes to peak among individuals belonging to the fourth or fifth age decades before declining in the oldest age groups. This nonlinearity appears to support theoretical predictions that interpreting immunity-helminth infection correlations is complicated by specific aspects of the immune response to parasitic infection (50) or that immunity to filariasis infection may be more complex than immunity to other helminths (16, 21, 31, 37, 38, 40). For example, one explanation for the nonlinear negative association of antibody response with infection intensity by host age observed in this study could be that immunity fostered by the specific IgG1, IgG2, IgG3, and IgE response to filariasis may be highest when parasite intensities are either low or moderate during early infection but that as parasite intensities increase with host age, these responses (IgG1, IgG2, and IgE [Fig. 2]) or their impact (IgG3) may be actively suppressed, as has been observed for intestinal nematodes (34). Indeed, such immunological down-regulation with increasing duration of exposure to the parasite (30, 32) has been suggested to be important in filariasis in protecting the host from the more pathological outcomes of infection (16, 17). Interestingly, transmission intensity did not appear to overly influence the individual effects of filaria-specific antibodies, although whether the effects differ in areas with transmission intensities higher than that observed in Masaika remains to be investigated (25).
The above findings give the impression that none of the antibodies studied here are particularly protective or are makers of protective immunity in older individuals. A key finding of this study, however, is that this conclusion, arising from considering only the independent effects of antibody responses, may be premature as it manifestly ignores the influence of interactions and joint effects of the different isotypes that may collectively affect infection intensity (2, 26, 40, 46). Such joint or networked effects among immune components are increasingly thought to be a major factor in determining the outcomes of parasitic infections (15), suggesting that accounting for multivariate immunological effects may be essential for any reliable evaluation of the role of specific antibodies in engendering resistance to filarial infection.
Indeed, our attempt to address this problem by using principal component analysis (5, 46) produced two significant outcomes. First, the results indicated that the specific antibody response to CFA infection (and hence presumably to adult worms) may be dominated by three major response types, including (i) a response reflecting a IgG4 type of reactivity, (ii) a response representing a IgG3 type of reactivity, and (iii) a host phenotype in which higher specific production of IgG1, IgG2, and IgE is countered by lower IgG4-IgG3 generation (Table 5). Second, regression analysis of the relationship between host CFA intensity and the scores obtained for each host for each of these three composite responses confirmed findings from a univariate correlation analysis for the occurrence of a positive association of filarial infection with the IgG4 response and a negative association in individuals exhibiting a heightened specific IgG1, IgG2, and IgE response but a lower specific IgG4 and IgG3 response. The analysis, however, also uncovered two further outcomes that were not revealed by simple correlation analysis. First, the results showed that the specific IgG3 response had a contrasting effect on host CFA levels in the two study communities. Thus, despite the markedly higher levels of specific IgG3 produced by individuals from the low-transmission community (Kingwede), the association of this antibody type with CFA appeared to be positive irrespective of the age class. By contrast, the opposite pattern, a much lower but negative response (which became significant in older individuals), was observed for individuals from Masaika. A second important finding revealed by this analysis, in direct contrast to the results obtained from the correlation analysis, was that the combined IgG1, IgG2, and IgE response was also found to be more negatively associated with worm intensity or CFA levels in older hosts. This and the fact that the magnitude of this association was also higher in the high-transmission community thus indicate that these responses have a role in fostering acquired immunity to filarial (adult) parasites. This is despite the fact that their absolute production declined in older hosts (Fig. 2). These findings provide a key new insight regarding the role of humoral immunity in filariasis, viz., that acquired immunity can be generated through these antibodies but that it perhaps is due more to their composite effects than to separate effects.
The objective of using simple mathematical models in this study to describe the population dynamics of antifilaria antibody responses was not to test or validate the developed model but rather to use it to qualitatively evaluate the mechanistic explanations derived from empirical data analyses regarding the roles of exposure and worm intensity in the generation of these antibodies. This work was predicated primarily on the observation that often mechanistic explanations derived from a purely empirical or statistical analysis of data may not generate observed community patterns owing to problems with scale and the effects of nonlinear interactions between suggested variables that could lead to counterintuitive results at the population level (8). The predictions of our model, which incorporated suggested mechanisms for the production of an IgG4-type response, an IgG3-type response, and an IgE-type response, however, show that in the present case the proposed mechanisms can indeed qualitatively explain the differences in the patterns of these antibody responses observed between our low- and high-transmission communities. In particular, given reasonable parameter values (see Table A1 in the Appendix), the results demonstrate that differences in the linearly increasing age profiles of IgG4 between our communities may arise simply as a direct function of differences in adult worm dynamics, whereas in the case of IgG3 the high linearly increasing age pattern in the low-transmission community compared to the much lower response that varied slightly with age in the high-transmission community was largely due to the effects of negative feedback on antibody production by transmission intensity. In the case of the specific IgE-type response, the simulations suggest that the differences observed between the two study communities, particularly the finding that these antibody types were lower in the low-transmission community and only slightly higher in the high-transmission community with a plateau-type relationship with host age (Fig. 2E and 4A and B), were a function of strong down-regulation of antibody production by high adult worm numbers. We were also able to show that the mechanisms underlying the specific IgG4 and IgE responses can explain the contrary finding of a positively increasing pattern of IgG4/IgE ratios observed in our study communities.
TABLE A1.
Parameter definitions and values used in the simulations shown in Fig. 4
| Parameter | Definition | Value (yr−1) |
|---|---|---|
| λ | Larval immigration rate | 5 and 25 for low- and high-transmission communities, respectively |
| μ1 | Mortality rate of adult worms | 0.2 |
| β1 | Rate of reduction of adult worms due to effects of the IgG3 response | 0.0005 |
| β2 | Rate of reduction of adult worms due to effects of the IgE response | 0.005 |
| φ1 | Rate of production of IgG4 | 0.01 |
| μ2 | Decay rate of IgG4 | 0.02 |
| φ2 | Rate of production of IgG3 | 0.01 |
| μ3 | Decay rate of IgG3 | 0.02 |
| β3 | Rate of reduction of IgG3 due to interactions with the adult worm and its products | 0.001 |
| γ | Rate at which IgE antibodies are down-regulated by worm intensity | 0.0075 |
| μ4 | Decay rate of IgE | 0.125 |
| β4 | Rate of reduction of IgE due to interactions with the adult worm and its products | 0.001 |
To conclude, this study has shown that parasite-specific antibody responses can play a role in regulating human filarial infection, perhaps via a form which is dependent on worm transmission intensity and the occurrence of joint effects due to interactions between individual antibody isotypes and possibly between antibodies and other immune components. It also highlights the value of combining novel study designs, statistical analysis methods, and mathematical modeling frameworks if we are to obtain new insights into the complex immunology of host-filarial worm interactions. We suggest that this will be especially critical when future studies are based on an even wider range of immune components (e.g., more refined antigens, principal cytokines, and other cellular components) than those employed in this study. Given the expanding global program to eliminate lymphatic filariasis by reducing parasite transmission, the likely occurrence of complex interactions between exposure intensity, worm burden, and acquired immunity means that carrying out such multicomponent immunoepidemiological studies is also vital to ultimately eliminating W. bancrofti from communities in which it is endemic.
Acknowledgments
We are grateful to the villagers and village helpers in Masaika (Tanzania) and Kingwede (Kenya) for their cooperation in this study. We are also grateful for the dedicated and skilled assistance provided by staff of the Bombo Field Station (Tanzania) and Msambweni Field Station (Kenya), as well as for the technical assistance provided in the laboratory at the DBL-Institute for Health Research and Development (Denmark).
This work received financial support from the International Cooperation with Developing Countries Program of the European Communities (contract ERBIC18CT970257 held jointly by P.E.S., E.M., M.N.M., and B.B.A.E.) and the DBL-Institute for Health Research and Development (Denmark). E.M. was supported by a Medical Research Council fellowship (United Kingdom), and W.G.J. was supported by a fellowship from the Danish Agency for Development Assistance (Danida).
APPENDIX
Mathematical modeling of age patterns of filaria-specific antibody responses.
We modeled the population dynamics of the specific IgG4 (antibody response type 1), IgG3 (type 2), and IgE (type 3) responses as a function of community transmission and host adult worm intensity. Briefly, the filarial adult worm intensity per host (W) in this model increases with age by a constant larval immigration rate (λ) and decreases both at a natural per capita rate (μ1) and at rates (β1 and β2) reflecting the detrimental effects of antibody response types 2 and 3 on worm survival acting proportional to worm intensity in each case. Antibody response type 1 (IgG4) is modeled simply to increase at a rate (φ1) proportional to W and to decrease at a constant natural decay rate (μ2). By contrast, antibody response type 2 (IgG3) is modeled to increase at a rate (φ2) proportional to both λ and W, but the increase is down-regulated in a nonlinear exponential manner as the transmission rate increases. This antibody response type decreases at a rate (μ3) due to natural decay and as a result of (negative) interaction with W given by the term β3WG3. Similarly, antibody response type 3 (IgE) increases with age as a linear function of W, and the increase is then down-regulated in an exponential fashion at higher worm loads. The antibody is removed at a natural decay rate (μ3) and through interaction with W according to the term β4WE. Hence, the full model is:
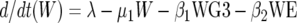 |
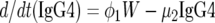 |
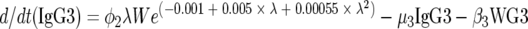 |
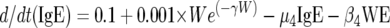 |
Parameter values used in the simulations shown in Fig. 4 are listed in Table A1. All simulations were run by assuming an initial value for W of 1. Note that the parameter values chosen for these simulations were based on an attempt to qualitatively capture the age profiles observed in the two study communities.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Anderson, R. M., and R. M. May. 1985. Herd immunity to helminth infection and implications for parasite control. Nature 315:493-496. [DOI] [PubMed] [Google Scholar]
- 2.Booth, M., J. K. Mwatha, S. Joseph, F. M. Jones, H. Kadzo, E. Ireri, F. Kazibwe, J. Kemijumbi, C. Kariuki, G. Kimani, J. H. Ouma, N. B. Kabatereine, B. J. Vennervald, and D. W. Dunne. 2004. Periportal fibrosis in human Schistosoma mansoni infection is associated with low IL-10, low IFN-gamma, high TNF-alpha, or low RANTES, depending on age and gender. J. Immunol. 172:1295-1303. [DOI] [PubMed] [Google Scholar]
- 3.Bundy, D. A. P., J. E. Lillywhite, J. M. Diddier, I. Simmons, and A. E. Bianco. 1991. Age-dependency of infection status and serum antibody levels in human whipworm (Trichuris trichiura) infection. Parasite Immunol. 13:629-638. [DOI] [PubMed] [Google Scholar]
- 4.Estambale, B. B., P. E. Simonsen, B. J. Vennervald, R. Knight, and J. J. Bwayo. 1994. Bancroftian filariasis in Kwale District of Kenya. II. Humoral immune responses to filarial antigens in selected individuals from an endemic community. Ann. Trop. Med. Parasitol. 88:153-161. [DOI] [PubMed] [Google Scholar]
- 5.Everitt, B. 2005. An R and S-PLUS companion to multivariate analysis. Springer-Verlag, London, United Kingdom.
- 6.Fulford, A. J. C., A. E. Butterworth, R. F. Sturrock, and J. H. Ouma. 1992. On the use of age-intensity data to detect immunity to parasitic infections, with special reference to Schistosoma mansoni in Kenya. Parasitology 105:219-227. [DOI] [PubMed] [Google Scholar]
- 7.Garraud, O., C. Nkenfou, J. E. Bradley, F. B. Perler, and T. B. Nutman. 1995. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J. Immunol. 155:1316-1325. [PubMed] [Google Scholar]
- 8.Haefner, J. W. 1996. Modeling biological systems. Principles and applications. Chapman and Hall, New York, NY.
- 9.Haswell-Elkins, M., M. W. Kennedy, R. M. Maizels, D. B. Elkins, and R. M. Anderson. 1989. The antibody recognition profile of naturally infected humans against Ascaris lumbricoides larval ES antigen. Parasite Immunol. 11:615-627. [DOI] [PubMed] [Google Scholar]
- 10.Hellriegel, B. 2001. Immunoepidemiology—bridging the gap between immunology and epidemiology. Trends Parasitol. 17:102-106. [DOI] [PubMed] [Google Scholar]
- 11.Hitch, W. L., A. W. Hightower, M. L. Eberhard, and P. J. Lammie. 1991. Analysis of isotype-specific antifilarial antibody levels in a Haitian pediatric population. Am. J. Trop. Med. Hyg. 44:161-167. [DOI] [PubMed] [Google Scholar]
- 12.Hitch, W. L., P. J. Lammie, and M. L. Eberhard. 1989. Heightened anti-filarial immune responsiveness in a Haitian pediatric population. Am. J. Trop. Med. Hyg. 41:657-663. [DOI] [PubMed] [Google Scholar]
- 13.Jaoko, W. G., P. E. Simonsen, D. W. Meyrowitsch, B. B. A. Estambale, M. N. Malecela-Lazaro, and E. Michael. 2006. Filarial-specific antibody response in East African bancroftian filariasis: effects of host infection, clinical disease, and filarial endemicity. Am. J. Trop. Med. Hyg. 75:97-107. [PubMed] [Google Scholar]
- 14.Kazura, J. W., M. Bockarie, N. Alexander, R. Perry, F. Bockarie, H. Dagoro, Z. Dimber, P. Hyun, and M. P. Alpers. 1997. Transmission intensity and its relationship to infection and disease due to Wuchereria bancrofti in Papua New Guinea. J. Infect. Dis. 176:242-246. [DOI] [PubMed] [Google Scholar]
- 15.Keil, D., R. W. Luebke, and S. B. Pruett. 2001. Quantifying the relationship between multiple immunological parameters and host resistance: probing the limits of reductionism. J. Immunol. 167:4543-4552. [DOI] [PubMed] [Google Scholar]
- 16.King, C. L. 2001. Transmission intensity and human immune responses to lymphatic filariasis. Parasite Immunol. 23:363-371. [DOI] [PubMed] [Google Scholar]
- 17.King, C. L., M. Connelly, M. P. Alpers, M. Bockarie, and J. W. Kazura. 2001. Transmission intensity determines lymphocyte responsiveness and cytokine bias in human lymphatic filariasis. J. Immunol. 166:7427-7436. [DOI] [PubMed] [Google Scholar]
- 18.Kurniawan, A., M. Yazdanbakhsh, R. van Ree, R. Aalberse, M. E. Selkirk, F. Partono, and R. M. Maizels. 1993. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J. Immunol. 150:3941-3950. [PubMed] [Google Scholar]
- 19.Kurniawan-Atmadja, A., E. Sartono, F. Partono, M. Yazdanbakhsh, and R. Maizels. 1998. Specificity of predominant IgG4 antibodies to adult and microfilarial stages of Brugia malayi. Parasite Immunol. 20:155-162. [PubMed] [Google Scholar]
- 20.Mahanty, S., K. P. Day, M. P. Alpers, and J. W. Kazura. 1994. Antifilarial IgG4 antibodies in children from filaria-endemic areas correlate with duration of infection and are dissociated from antifilarial IgE antibodies. J. Infect. Dis. 170:1339-1343. [DOI] [PubMed] [Google Scholar]
- 21.Maizels, R. M., E. Sartono, A. Kurniawan, F. Partono, M. E. Selkirk, and M. Yazdanbakhsh. 1995. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol. Today 11:50-56. [DOI] [PubMed] [Google Scholar]
- 22.McCullagh, P., and J. A. Nelder. 1991. Generalized linear models, 2nd ed. Chapman & Hall, London, United Kingdom.
- 23.McGarigal, K., S. Cushman, and S. Stafford. 2000. Multivariate statistics for wildlife and ecology research. Springer-Verlag, New York, NY.
- 24.Michael, E., and D. A. Bundy. 1998. Herd immunity to filarial infection is a function of vector biting rate. Proc. R. Soc. Lond. B 265:855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael, E., P. E. Simonsen, M. Malecela, W. G. Jaoko, E. M. Pedersen, D. Mukoko, R. T. Rwegoshora, and D. W. Meyrowitsch. 2001. Transmission intensity and the immunoepidemiology of bancroftian filariasis in East Africa. Parasite Immunol. 23:373-388. [DOI] [PubMed] [Google Scholar]
- 26.Mutapi, F., T. Mduluza, and A. W. Roddam. 2005. Cluster analysis of schistosome-specific antibody responses partitions the population into distinct epidemiological groups. Immunol. Lett. 96:231-240. [DOI] [PubMed] [Google Scholar]
- 27.Mutapi, F., P. D. Ndhlovu, P. Hagan, and M. E. Woolhouse. 1997. A comparison of humoral responses to Schistosoma haematobium in areas with low and high levels of infection. Parasite Immunol. 19:255-263. [DOI] [PubMed] [Google Scholar]
- 28.Needham, C. S., D. A. P. Bundy, J. E. Lillywhite, J. M. Diddier, I. Simmons, and A. E. Bianco. 1992. The relationship between Trichuris trichiura transmission intensity and the age-profiles of parasite-specific antibody isotypes in two endemic communities. Parasitology 105:273-283. [DOI] [PubMed] [Google Scholar]
- 29.Nicolas, L., S. Langy, C. Plichart, and X. Deparis. 1999. Filarial antibody responses in Wuchereria bancrofti transmission area are related to parasitological but not clinical status. Parasite Immunol. 21:73-80. [DOI] [PubMed] [Google Scholar]
- 30.Ottesen, E. A. 1984. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans. R. Soc. Trop. Med. Hyg. 78(Suppl.):9-18. [DOI] [PubMed] [Google Scholar]
- 31.Ottesen, E. A. 1992. The Wellcome Trust Lecture. Infection and disease in lymphatic filariasis: an immunological perspective. Parasitology 104(Suppl.):S71-S79. [DOI] [PubMed] [Google Scholar]
- 32.Ottesen, E. A., P. F. Weller, M. N. Lunde, and R. Hussain. 1982. Endemic filariasis on a Pacific Island. II. Immunologic aspects: immunoglobulin, complement, and specific antifilarial IgG, IgM, and IgE antibodies. Am. J. Trop. Med. Hyg. 31:953-961. [PubMed] [Google Scholar]
- 33.Poulsen, L. K., and T. B. Sorensen. 1993. Elimination of viral infection from blood samples for allergic testing. Allergy 48:207-208. [DOI] [PubMed] [Google Scholar]
- 34.Pritchard, D. I., C. E. Lawrence, P. Appleby, I. A. Gibb, and K. Glover. 1994. Immunosuppressive proteins secreted by the gastrointestinal nematode parasite Heligmosomoides polygyrus. Int. J. Parasitol. 24:495-500. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard, D. I., R. J. Quinnel, A. F. G. Slater, P. G. McKean, D. D. S. Dale, A. Raiko, and A. E. Keymer. 1990. Epidemiology and immunity of Necator americanus infection in a community in Papua New Guinea: humoral responses to excretory-secretory and cuticular collagen antigens. Parasitology 100:317-326. [DOI] [PubMed] [Google Scholar]
- 36.Quinnell, R. J., M. E. Woolhouse, E. A. Walsh, and D. I. Pritchard. 1995. Immunoepidemiology of human necatoriasis: correlations between antibody responses and parasite burdens. Parasite Immunol. 17:313-318. [DOI] [PubMed] [Google Scholar]
- 37.Rajan, T. V. 2005. Natural course of lymphatic filariasis: insights from epidemiology, experimental human infections, and clinical observations. Am. J. Trop. Med. Hyg. 73:995-998. [PMC free article] [PubMed] [Google Scholar]
- 38.Ravindran, B. 2001. Are inflammation and immunological hyperactivity needed for filarial parasite development? Trends Parasitol. 17:70-73. [DOI] [PubMed] [Google Scholar]
- 39.Rwegoshora, R. T., E. M. Pedersen, D. A. Mukoko, D. W. Meyrowitsch, N. Masese, M. N. Malecela-Lazaro, J. H. Ouma, E. Michael, and P. E. Simonsen. 2005. Bancroftian filariasis: patterns of vector abundance and transmission in two East African communities with different levels of endemicity. Ann. Trop. Med. Parasitol. 99:253-265. [DOI] [PubMed] [Google Scholar]
- 40.Satapathy, A. K., E. Sartono, P. K. Sahoo, M. A. Dentener, E. Michael, M. Yazdanbakhsh, and B. Ravindran. 2006. Human bancroftian filariasis: immunological markers of morbidity and infection. Microbes Infect. 8:2414-2423. [DOI] [PubMed] [Google Scholar]
- 41.Simonsen, P. E., and S. K. Dunyo. 1999. Comparative evaluation of three new tools for diagnosis of bancroftian filariasis based on detection of specific circulating antigens. Trans. R. Soc. Trop. Med. Hyg. 93:278-282. [DOI] [PubMed] [Google Scholar]
- 42.Simonsen, P. E., M. M. Lemnge, H. A. Msangeni, P. H. Jakobsen, and I. C. Bygbjerg. 1996. Bancroftian filariasis: the patterns of filarial-specific immunoglobulin G1 (IgG1), IgG4, and circulating antigens in an endemic community of northeastern Tanzania. Am. J. Trop. Med. Hyg. 55:69-75. [DOI] [PubMed] [Google Scholar]
- 43.Simonsen, P. E., D. W. Meyrowitsch, W. G. Jaoko, M. N. Malecela, D. Mukoko, E. M. Pedersen, J. H. Ouma, R. T. Rwegoshora, N. Masese, P. Magnussen, B. B. Estambale, and E. Michael. 2002. Bancroftian filariasis infection, disease, and specific antibody response patterns in a high and a low endemicity community in East Africa. Am. J. Trop. Med. Hyg. 66:550-559. [DOI] [PubMed] [Google Scholar]
- 44.Sokal, R. R., and F. J. Rohlf. 2000. Biometry, 3rd ed. W.H. Freeman and Company, New York, NY.
- 45.Tabachnick, B. G., and L. S. Fidell. 2001. Using multivariate statistics, 4th ed. Allyn and Bacon, Boston, MA.
- 46.Turner, J. D., H. Faulkner, J. Kamgno, F. Cormont, J. Van Snick, K. J. Else, R. K. Grencis, J. M. Behnke, M. Boussinesq, and J. E. Bradley. 2003. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis. 188:1768-1775. [DOI] [PubMed] [Google Scholar]
- 47.Wamae, C. N., S. M. Gatika, J. M. Roberts, and P. J. Lammie. 1998. Wuchereria bancrofti in Kwale District, coastal Kenya: patterns of focal distribution of infection, clinical manifestations and anti-filarial IgG responsiveness. Parasitology 116:173-182. [DOI] [PubMed] [Google Scholar]
- 48.Woolhouse, M. E. 1994. Immunoepidemiology of human schistosomes: taking the theory into the field. Parasitol. Today 10:196-202. [DOI] [PubMed] [Google Scholar]
- 49.Woolhouse, M. E. 1992. Immunoepidemiology of intestinal helminths: pattern and process. Parasitol. Today 8:111. [DOI] [PubMed] [Google Scholar]
- 50.Woolhouse, M. E. 1992. A theoretical framework for the immunoepidemiology of helminth infection. Parasite Immunol. 14:563-578. [DOI] [PubMed] [Google Scholar]
- 51.Woolhouse, M. E., P. Taylor, D. Matanhire, and S. K. Chandiwana. 1991. Acquired immunity and epidemiology of Schistosoma haematobium. Nature 351:757-759. [DOI] [PubMed] [Google Scholar]