Abstract
Dendritic cells (DC) orchestrate innate and adaptive immune responses to bacteria. How Haemophilus ducreyi, which causes genital ulcers and regional lymphadenitis, interacts with DC is unknown. H. ducreyi evades uptake by polymorphonuclear leukocyte and macrophage-like cell lines by secreting LspA1 and LspA2. Many H. ducreyi strains express cytolethal distending toxin (CDT), and recombinant CDT causes apoptosis of DC in vitro. Here, we examined interactions between DC and H. ducreyi 35000HP, which produces LspA1, LspA2, and CDT. In human volunteers infected with 35000HP, the ratio of myeloid DC to plasmacytoid DC was 2.8:1 in lesions, compared to a ratio of 1:1 in peripheral blood. Using myeloid DC derived from monocytes as surrogates for lesional DC, we found that DC infected with 35000HP remained as viable as uninfected DC for up to 48 h. Gentamicin protection and confocal microscopy assays demonstrated that DC ingested and killed 35000HP, but killing was incomplete at 48 h. The expression of LspA1 and LspA2 did not inhibit the uptake of H. ducreyi, despite inactivating Src kinases. Infection of DC with live 35000HP caused less cell surface marker activation than infection with heat-killed 35000HP and lipopolysaccharide (LPS) and inhibited maturation by LPS. However, infection of DC with live bacteria caused the secretion of significantly higher levels of interleukin-6 and tumor necrosis factor alpha than infection with heat-killed bacteria and LPS. The survival of H. ducreyi in DC may provide a mechanism by which the organism traffics to lymph nodes. Partial activation of DC may abrogate the establishment of a full Th1 response and an environment that promotes phagocytosis.
Haemophilus ducreyi is a gram-negative bacterium that causes chancroid, a sexually transmitted disease. Chancroid is marked by the development of papules that evolve into pustules and painful genital ulcers, inguinal lymphadenopathy, and the formation of buboes (5). Although infrequent in Europe and the United States, chancroid is endemic in many developing countries (37). Due to its short duration of infectiousness, H. ducreyi can be maintained in a population only by infecting persons with multiple sex partners, such as sex workers (5). Where sexually transmitted disease treatment services, syndromic treatment of genital ulcer disease, and condom use have been promoted, the prevalence of chancroid has dramatically decreased (13, 27, 37). However, sex work and the lack of health services remain in many resource-poor countries. In addition, chancroid is a public health problem because it facilitates the acquisition and transmission of human immunodeficiency virus type 1 (5, 26, 37). Thus, understanding host responses to H. ducreyi is an important area of investigation.
Dendritic cells (DC) are the key antigen-presenting cells that lead to successful innate and adaptive immune responses to infection (28, 29, 41). Three different subtypes of human DC have been defined: Langerhans cells, interstitial DC, and plasmacytoid DC (24, 28). Human skin, the site of H. ducreyi infection, usually contains the first two types of DC, which are generally referred to as myeloid DC (24). As such, myeloid DC are those likely to interact with H. ducreyi in lesions.
The ability of DC to process and present antigens is dependent on their maturation status. Immature DC are highly phagocytic with weak antigen-presenting cell function, and phagocytosis of particulate antigens or pathogens usually induces DC maturation. Mature DC are potent antigen-presenting cells that express high levels of the surface markers HLA-DR, CD40, CD80, CD83, and CD86 and prime naïve T cells. Microbial pathogens employ multiple strategies to subvert DC function, such as the impairment of DC maturation, cytokine secretion, or migration and the induction of DC apoptosis or necrosis (1, 4, 12, 21, 34, 45). In human volunteers who are experimentally infected with H. ducreyi, CD83-positive and DC lysosome-associated membrane protein (DC-LAMP)-positive cells are found in the epidermis or dermis of pustules (20). However, immature CD1a-positive cells are more abundant in experimental lesions than are cells that express CD83 and DC-LAMP (20), suggesting that H. ducreyi may not fully promote DC maturation.
In experimental pustules and natural ulcers, H. ducreyi is surrounded by polymorphonuclear neutrophils (PMN) and macrophages but is not ingested (2, 3). H. ducreyi secretes two antiphagocytic proteins, LspA1 and LspA2, which prevent the uptake of the organism by PMN-like and macrophage-like cell lines in vitro (42). The expression of LspA1 and LspA2 is required for pustule formation in human volunteers (19). Whether the expression of LspA1 and LspA2 would prevent the uptake of H. ducreyi by DC is unknown.
H. ducreyi also expresses a heat-labile cytolethal distending toxin (CDT) that causes lymphocyte, fibroblast, and epithelial cell death in vitro (6, 35, 40) but is not required for pustule formation in human inoculation experiments (48). Purified recombinant CDT also causes apoptosis of DC in vitro (23, 47). To avoid potential CDT toxicity on DC, Xu and colleagues studied interactions between myeloid DC and gentamicin-killed H. ducreyi bacteria (47). However, interactions between DC and dead bacteria do not always reflect those found between DC and live bacteria (7, 10, 29, 49). To our knowledge, no studies have examined how myeloid DC interact with live H. ducreyi.
In this study, we examined whether myeloid DC are enriched relative to plasmacytoid DC in skin lesions of healthy volunteers infected with H. ducreyi at the pustular stage of disease. We examined the effect of live H. ducreyi on monocyte-derived myeloid DC viability and the ability of myeloid DC to ingest and kill live H. ducreyi. We also explored the impact of infection with H. ducreyi on DC maturation and the production of cytokines.
MATERIALS AND METHODS
Characterization of DC from biopsy samples of H. ducreyi-infected volunteers.
Three adult female volunteers (volunteer numbers 272, 273, and 281), who were infected with H. ducreyi for 6 to 9 days in human inoculation experiments described previously (17, 18), contributed biopsy samples of pustules for flow cytometry. A blood sample was obtained from each subject on the day of biopsy, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque gradient centrifugation. Biopsy samples were minced and processed exactly as described previously (15), except that single cells were collected using a 70-μm sieve. Biopsy cells and PBMC were incubated with fluorescein isothiocyanate (FITC)-conjugated antibodies to lineage cocktail 1 (CD3 clone SK7, CD14 clone MφP9, CD16 clone 3G8, CD19 clone SJ25C1, CD20 clone L27, and CD56 clone NCAM16.2), peridinin chlorophyll protein-conjugated antibodies to HLA-DR (clone G46-6), allophycocyanin (APC)-conjugated antibodies to CD11c (clone S-HCL-3), and phycoerythrin (PE)-conjugated antibodies to CD123 (clone 9F5) (all obtained from BD Biosciences). Cells were washed, suspended in phosphate-buffered saline (PBS) plus 2% paraformaldehyde, and analyzed using a FACSCalibur flow cytometer and BD CellQuest Pro version 4.0.1 software (BD Biosciences).
Generation of myeloid DC from peripheral blood.
Ten healthy adult volunteers (six women and four men) over 18 years of age, who had never been infected with H. ducreyi, contributed blood samples for the isolation of PBMC. Informed consent was obtained from volunteers in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University Indianapolis. PBMC were also isolated from leukopacks obtained from seven anonymous donors from the Indiana Blood Center.
CD14+ cells were isolated from PBMC by positive selection using magnetic CD14 microbeads (Milenyi Biotech) according to the manufacturer's instructions. We routinely achieved 95 to 97% purity, as verified by flow cytometric analysis. Approximately 0.5 × 106/ml to 1 × 106/ml of CD14+ cells were cultured in wells with RPMI 1640 and 5% heat-inactivated (56°C for 30 min) human AB serum or 10% heat-inactivated fetal bovine serum (FBS) for 1 week at 37°C in 5% CO2. Recombinant human interleukin-4 (rhIL-4) and rh granulocyte-macrophage colony-stimulating factor (R&D Systems) were added to the culture on days 0, 2, 4, and 6 (12). After 7 days of culture, the cells were HLA-DR+, CD86+ CD40+, CD3−, and CD14− by flow cytometry.
Bacterial strains and culture conditions.
H. ducreyi 35000HP is a human-passaged variant of strain 35000 and has been reported previously (35). Strain 35000HP/pRB157K expresses green fluorescent protein (GFP) and has been described previously (36). The lspA1 lspA2 double mutant 35000HPΩ12 (19) was transformed with pRB157K and designated 35000HPΩ12/pRB157K. Strain 35000.303 is a cdtC mutant that lacks CDT activity, as described previously (48). 35000HPΩ12, 35000.303, and pRB157K were gifts from Eric Hansen (University of Texas, Southwestern). H. ducreyi was cultivated on chocolate agar plates, in brain heart infusion broth supplemented with hemin, 1% IsoVitaleX, and soluble starch, or in GC (gonococcal) medium supplemented with hemin, 1% IsoVitaleX, and 5% heat-inactivated FBS as described previously (9).
LPS and heat-killed bacteria.
Lipopolysaccharide (LPS) (E. coli O26:B6) was obtained from Sigma-Aldrich. H. ducreyi whole cells were heat killed by incubation at 100°C for 10 min.
Apoptosis, phagocytosis, and gentamicin protection assays.
H. ducreyi was grown to mid-log phase in brain heart infusion broth and washed three times with Hanks balanced salt solution. Live bacteria were centrifuged onto wells containing approximately 105 DC at a multiplicity of infection (MOI) of approximately 20:1 and incubated for 90 min at 35°C in 5% CO2. DC were collected by centrifugation, and nonadherent bacteria were removed by washing with RPMI 1640. Infected DC were incubated in RPMI 1640 containing rhIL-4 and rh granulocyte-macrophage colony-stimulating factor for up to 48 h at 35°C.
To determine whether DC were killed by H. ducreyi, infected and uninfected DC were stained with trypan blue. Infected and uninfected DC were also stained with annexin V-PE apoptosis detection kit I (BD Biosciences) per the manufacturer's instructions and analyzed by flow cytometry as described previously (8). In some experiments, the abilities of 35000HP and 35000.303 to induce apoptosis were compared.
To examine whether H. ducreyi bacteria were ingested and killed by DC, we performed gentamicin protection experiments. The total number of bacteria that were associated with DC after 90 min of incubation was determined by quantitative culture. To kill the extracellular bacteria, infected DC were incubated in RPMI 1640 with gentamicin (50 μg/ml) for 30 min, washed in RPMI 1640, and placed in wells with fresh medium without antibiotics. DC were collected immediately after being washed and, 24 and 48 h later, were lysed with saponin and quantitatively cultured.
To determine the percentage of DC that ingested H. ducreyi, we performed confocal microscopy assays. GFP-expressing 35000HP cells that were either opsonized in 100% autologous serum for 20 min at room temperature or not opsonized were cultured with DC at an MOI of approximately 10:1 for 90 min, 4 h, and 24 h. Infected DC were washed, collected, and centrifuged onto glass slides with a Shandon Cytospin 3 centrifuge (Thermo Electron Corp.) and fixed with 4% paraformaldehyde overnight at 4°C. The slides were blocked with 5% normal goat serum (Sigma) in PBS for 30 min and stained with antibodies to CD45 (clone HI30; BD Biosciences), followed by an indodicarbocyanine (Cy5)-conjugated secondary antibody (Jackson ImmunoResearch). Fifty to 100 DC were optically sectioned by confocal microscopy, and the percentage of DC with associated and internalized bacteria was determined as described previously (36).
To investigate whether the expression of LspA1 and LspA2 prevented phagocytosis of H. ducreyi by DC, 35000HP/pRB157K and 35000HPΩ12/pRB157K were grown in GC broth to mid-log or stationary phase, washed, centrifuged onto wells containing DC at MOIs ranging from 10:1 to 100:1, incubated for 90 min at 35°C, and treated with gentamicin as described above. After fixation with 2% paraformaldehyde for 20 min at room temperature, DC were washed and analyzed for engulfment of GFP-expressing H. ducreyi by flow cytometry.
Western blot analyses.
Strains 35000HP and 35000HPΩ12 were grown to mid-log and stationary phases in GC broth and cocultured with DC at MOIs ranging from 10:1 to 100:1 for 4 h at 35°C. Cell lysates were electrophoresed in 10% acrylamide gels and transferred to nitrocellulose membranes. To detect the active forms of the Src family of protein tyrosine kinases, the membranes were probed with rabbit polyclonal antibodies to the phosphorylated tyrosine 418 of Src (anti-pY418 [α-pY418]; BioSource International Inc.) as described previously (25). To ensure that equal amounts of cellular proteins were analyzed, the mouse monoclonal antibody C4 (ICN Biomedicals Inc.) was used to detect actin.
DC activation and cytokine production.
Myeloid DC were generated as described above except that 10% heat-inactivated FBS was substituted for human AB serum. The immature DC were incubated with medium alone, live H. ducreyi, heat-killed H. ducreyi at an MOI of approximately 10:1, E. coli LPS (200 ng/ml), or a maturation cocktail of tumor necrosis factor alpha (TNF-α) (10 ng/ml), IL-1β (10 ng/ml), and prostaglandin E2 (1 μg/ml) (12). In some wells, DC were incubated simultaneously with live or heat-killed H. ducreyi and LPS. After centrifugation, the culture plates were kept for 90 min at 35°C. To optimize DC activation, the cultures were subsequently incubated at 37°C for an additional 46.5 h.
To measure activation, DC were pelleted, suspended in fluorescence-activated cell sorter buffer (PBS plus 10% fetal calf serum plus 2 mM EDTA), and incubated with conjugated antibodies to the following surface markers: HLA-DR (FITC; clone G46-6), CD40 (APC; clone 5C3), CD80 (PE; clone L307.4), CD83 (PE; clone HB15e), and CD86 (APC; clone 2331) (all obtained from BD Biosciences). Cells were analyzed by flow cytometry. Culture supernatants were collected after 48 h of incubation and assayed for IL-6, IL-10, IL-12p70, and TNF-α levels by enzyme-linked immunosorbent assay (BD Biosciences) according to the manufacturer's instructions.
Statistical analyses of the geometric means of DC surface marker expression and cytokine levels used mixed models of analysis of variance with a random subject effect to account for the correlation among observations of the same individual. Follow-up pairwise tests were adjusted for multiple comparisons with the Tukey-Kramer procedure. P values of <0.05 were considered to be significant.
RESULTS
Myeloid DC are enriched in pustules of H. ducreyi-infected volunteers.
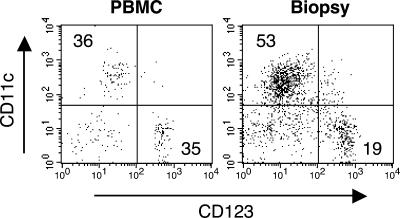
We collected cells from the pustules and peripheral blood of three volunteers experimentally infected with H. ducreyi. Lineage-negative (CD3, CD14, CD16, CD19, CD20, and CD56) HLA-DR+ cells were stained for CD11c and CD123. Representative results for one subject are shown in Fig. 1. A mean of 3,800 DC was isolated from each pustule. The ratio of monocytoid (CD11c+) to plasmacytoid (CD123+) DC in the peripheral blood was 1:1, while that ratio in the pustules was 2.8:1. Since myeloid DC were predominant in lesions, we used monocyte-derived myeloid DC from noninfected volunteers as surrogates to study interactions between H. ducreyi and DC.
FIG. 1.
Fluorescence-activated cell sorter analysis of lineage-negative, HLA-DR+ cells from H. ducreyi-infected skin (right) and peripheral blood (left) obtained at the time of biopsy from one subject. The numbers in the dot plots represent the percentages of cells in the quadrants.
Live H. ducreyi bacteria do not kill myeloid DC.
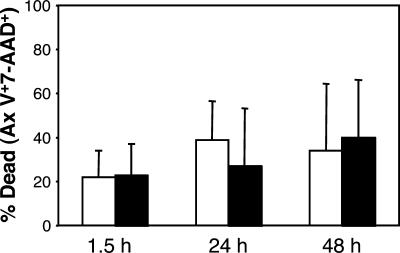
Recombinant H. ducreyi CDT causes apoptosis of DC in vitro (23, 47). Therefore, we examined whether live 35000HP, which produces CDT (48), caused DC death. By trypan blue exclusion, DC infected with live H. ducreyi remained as viable as uninfected DC for up to 48 h (data not shown). To confirm these findings, uninfected DC and infected DC were stained with annexin V-PE and 7-amino-actinomycin D. After 90 min, 24 h, and 48 h of incubation, there was no difference between the number of DC that were apoptotic or necrotic when infected with H. ducreyi and the number of uninfected controls (Fig. 2). Similarly, we found no differences in the abilities of 35000HP and 35000.303, which do not produce active CDT, to cause apoptosis of DC (data not shown). Thus, a CDT-expressing strain of H. ducreyi did not kill DC in this assay over the time course studied.
FIG. 2.
Percentage of DC death after exposure of DC to H. ducreyi (white bars) compared to that for uninfected DC (solid bars) at various time points. Data are expressed as the percentages of annexin V (Ax V)- and/or 7-amino-actinomycin D (7-AAD)-stained cells in the total cell population and represent the means ± SDs for assays performed with DC from four donors.
DC ingest and kill H. ducreyi, but killing is incomplete.
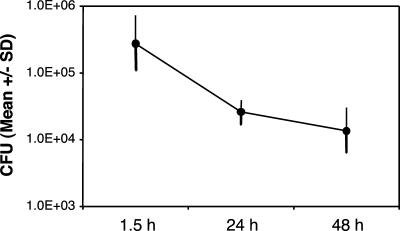
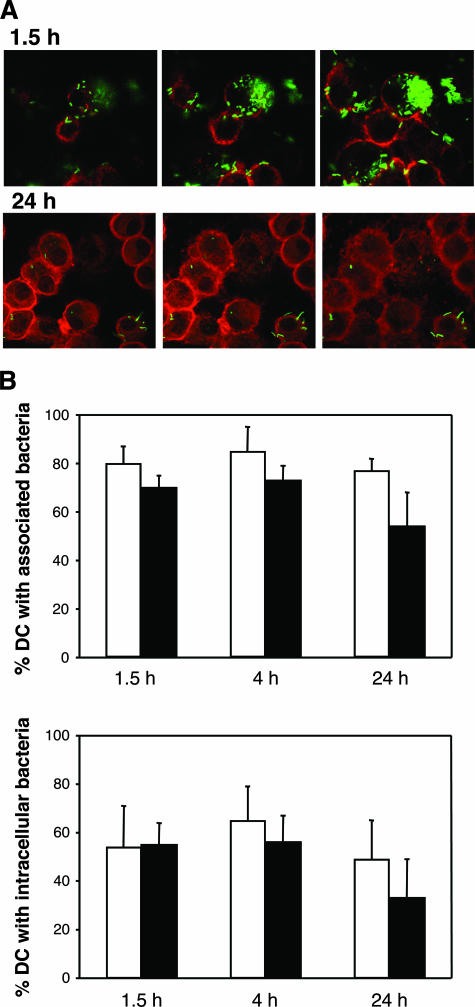
We examined H. ducreyi association with, uptake by, and survival in DC by gentamicin protection and confocal microscopy assays. Of the associated bacteria, 16% ± 20% (mean ± standard deviation [SD]) were internalized (gentamicin protected) after 90 min (Fig. 3). DC killed the internalized bacteria, but 5% of those initially internalized were viable at 48 h (Fig. 3). In the confocal microscopy assay, within 90 min, GFP-expressing 35000HP/pRB157K cells were associated with approximately 70% to 80% of DC and were ingested by more than 50% of DC (Fig. 4A and B). Many DC contained large clumps of bacteria (Fig. 4A). By 24 h, although many DC still contained intracellular bacteria, the bacteria were faint and fewer in number, consistent with bacterial killing (Fig. 4A). Opsonization with autologous serum had little effect on association or uptake (Fig. 4B). Thus, DC ingested and killed H. ducreyi, although killing was incomplete over the time course studied.
FIG. 3.
Numbers of H. ducreyi CFU that were internalized and viable in 104 to 105 DC after 90 min of culture, gentamicin treatment, and subsequent culture in medium without antibiotics. The values represent the means ± SDs for assays performed with DC from six donors.
FIG. 4.
Confocal microscopy of DC infected with GFP-expressing H. ducreyi. (A) Optical sectioning of DC that had been incubated with H. ducreyi for 90 min and 24 h. (B) Percentages of DC that had associated (top) or internalized (bottom) bacteria under opsonized (white bars) and nonopsonized (black bars) conditions. The data represent the means ± SDs for assays performed with DC from three donors.
LspA1 and LspA2 do not prevent uptake of H. ducreyi by DC despite inhibiting Src kinase activation.
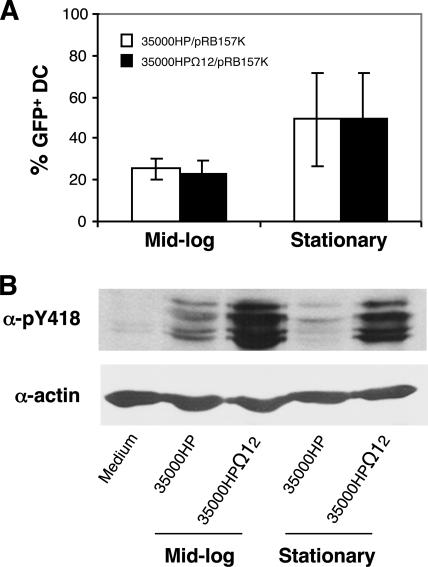
LspA1 and LspA2 inhibit phagocytosis of H. ducreyi by PMN-like and macrophage-like cell lines in vitro by decreasing the activation of the Src family of protein tyrosine kinases and blocking Fcγ receptor-mediated phagocytic signaling (25). To address whether these proteins have any effect on the ability of DC to ingest H. ducreyi, we compared the uptake of 35000HP/pRB157K with that of 35000HPΩ12/pRB157K by flow cytometry. We tested both mid-log- and stationary-phase bacteria for uptake, because the expression of LspA1 and LspA2 is maximized under the latter condition (42). In this assay, approximately 25% of DC infected with mid-log-phase 35000HP/pRB157K or 35000HPΩ12/pRB157K at an MOI of approximately 10:1 were GFP positive after 90 min of coculture (Fig. 5A). Almost 50% of DC incubated with stationary-phase H. ducreyi at an MOI of 50:1 to 100:1 were GFP positive (Fig. 5A). Under all conditions tested, there were no differences between the uptake of 35000HP/pRB157K and that of 35000HPΩ12/pRB157K by DC. Thus, the expression of LspA1 and LspA2 had no effect on the uptake of H. ducreyi by DC (Fig. 5A).
FIG. 5.
Effect of growth phase and expression of LspA1 and LspA2 on H. ducreyi uptake by and Src kinase activation in DC. (A) Percentages of DC containing GFP-expressing bacteria by flow cytometry after incubation with mid-log- and stationary-phase 35000HP/pRB157K or 35000HPΩ12/pRB157K for 90 min. Values are the means ± SDs of the percentages of GFP-positive DC obtained from seven or six donors and cultured with mid-log-phase (MOI of 10) or stationary-phase (MOI of 50 to 100) bacteria, respectively. (B) Western blot of DC incubated with mid-log- or stationary-phase 35000HP or 35000HPΩ12 for 4 h under the conditions described for panel A. Active forms of Src kinases were determined by the α-pY418 antibody. Actin levels were measured as loading controls. Similar results were observed for DC obtained from five donors.
We next examined whether the lack of an effect of LspA1 and LspA2 on the ability of DC to ingest H. ducreyi was due to their inability to inactivate Src kinases in DC. DC were cocultured with 35000HP and 35000HPΩ12 for 4 h, and cell lysates were probed with antibodies (α-pY418) specific to the active form of Src kinases as described previously (25). DC incubated with 35000HP had higher levels of active Src kinases than uninfected DC but considerably lower levels of active Src kinases than DC infected with 35000HPΩ12 (Fig. 5B). These data suggest that LspA1 and LspA2 block the Fcγ receptor-mediated phagocytic pathway in DC and that DC use other mechanisms to take up H. ducreyi.
Effect of H. ducreyi infection on DC activation and cytokine production.
To evaluate the effect of H. ducreyi on DC activation, we measured the geometric mean fluorescence intensities of HLA-DR, CD40, CD80, CD83, and CD86 surface marker expression levels. Preliminary experiments over a 48-h time course indicated that the greatest change in surface marker expression in response to live bacteria occurred by 48 h (data not shown). All subsequent experiments were done after 48 h of culture.
DC were cultured with medium alone, live bacteria, heat-killed bacteria, and/or medium containing E. coli LPS or the proinflammatory maturation cocktail. After 48 h of culture, the expression levels of all markers were significantly higher for DC incubated with heat-killed bacteria, E. coli LPS, and the maturation cocktail than for DC incubated in medium alone (all with P values of <0.05) (Table 1). Although incubation with live bacteria increased DC surface marker expression, the expression level was not significantly different from that of DC cultured in medium alone (Table 1).
TABLE 1.
Effect of H. ducreyi infection on activation of human monocyte-derived DC
| Treatmenta | Mean geometric MFI (SD) of surface marker expressionb
|
||||
|---|---|---|---|---|---|
| HLA-DR | CD40 | CD80 | CD83 | CD86 | |
| Medium alone | 134 (45) | 29 (3) | 17 (6) | 4 (1) | 37 (8) |
| Live H. ducreyi | 202 (32) | 83 (18) | 102 (43) | 15 (4) | 168 (61) |
| HK H. ducreyi | 374 (77)* | 160 (41)* | 257 (71)* | 38 (15)* | 454 (121)* |
| MC | 350 (104)* | 112 (33)* | 135 (43)* | 36 (18)* | 448 (148)* |
| E. coli LPS | 335 (80)* | 117 (37)* | 178 (54)* | 36 (16)* | 351 (97)* |
| Live H. ducreyi + LPS | 199 (37)** | 79 (18) | 93 (30) | 14 (3) | 154 (46)** |
| HK H. ducreyi + LPS | 389 (77)* | 154 (55)* | 271 (88)* | 42 (17)* | 504 (153)* |
DC were cultured for 48 h with medium alone, live H. ducreyi, heat-killed (HK) H. ducreyi, the maturation cocktail (MC), E. coli LPS, or a combination of live or heat-killed bacteria plus LPS.
MFI, mean fluorescence intensity. Values represent data for six donors. *, significant difference compared to medium alone (P < 0.05); **, significant difference compared to E. coli LPS alone (P < 0.05).
The lack of significant induction of surface markers on DC by live H. ducreyi raised the question of whether the bacteria could inhibit activation by DC agonists. After 48 h, DC treated with live bacteria in the presence of LPS had significantly lower levels of HLA-DR and CD86 than DC cultured with LPS (P = 0.04 and P = 0.03, respectively) (Table 1). In contrast, the surface marker expression of DC incubated with heat-killed bacteria and LPS did not differ from that observed with DC incubated with LPS alone (all with P values of >0.05) (Table 1). Therefore, live H. ducreyi partially inhibited DC activation induced by LPS.
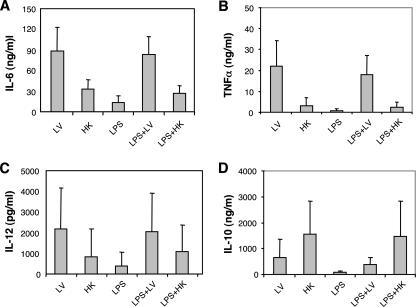
We assayed the secretion of IL-6, IL-10, IL-12p70, and TNF-α in the culture supernatants of DC that were stimulated under conditions corresponding to those described above except for the maturation cocktail. For uninfected DC, the levels of all cytokines tested were below the limits of detection of the assays. Higher levels of IL-6 and TNF-α were secreted by DC cultured with live H. ducreyi than by DC incubated with heat-killed bacteria or LPS (all with P values of <0.001) (Fig. 6A and B). Similarly, DC cultured with live H. ducreyi and LPS secreted higher levels of IL-6 and TNF-α than DC incubated with LPS, LPS and heat-killed H. ducreyi, or heat-killed H. ducreyi (all with P values of <0.009) (Fig. 6A and B). DC infected with live bacteria secreted IL-10 and IL-12, but these levels were not significantly different from those under any of the other conditions tested (Fig. 6C and D). Marked donor-to-donor variation was noted for all cytokine responses measured.
FIG. 6.
Cytokine production by DC exposed to live (LV) or heat-killed (HK) H. ducreyi and LPS singly or in combination after 48 h. Culture supernatants were obtained from DC whose activation levels are reported in Table 1. The levels of IL-6 (A), TNF-α (B), IL-12p70 (C), and IL-10 (D) were assessed by enzyme-linked immunosorbent assay. Data are the means ± SDs of results obtained from six volunteers.
DISCUSSION
In this study, we showed that myeloid DC were enriched relative to plasmacytoid DC in pustules of H. ducreyi-infected volunteers compared to myeloid DC in peripheral blood. Thus, monocyte-derived myeloid DC are reasonable surrogates for the study of interactions between H. ducreyi and DC. In contrast, humans infected by Borrelia burgdorferi or Treponema pallidum have similar proportions of myeloid and plasmacytoid DC in skin lesions and peripheral blood (30, 31). The basis for these findings is unclear but could reflect basic differences among these bacteria, such as the lack of LPS in spirochetes, the relative chronicity of each infection, and the inability of H. ducreyi to disseminate systemically.
Clinical isolates of H. ducreyi form a homogeneous DNA hybridization group and express many of the same surface antigens, suggesting limited diversity within the species (35). However, there are at least two circulating classes of H. ducreyi, which express different proteomes and immunotypes or variants of several outer membrane proteins (33, 46). The oligosaccharide component of the lipooligosaccharide (LOS) of class II strains is truncated compared to that of class I strains (46), but whether their lipid A structures differ has not been determined. To study interactions between DC and H. ducreyi, we used 35000HP, an extensively characterized prototype class I strain that is highly infectious in humans in that the inoculation of as few as 1 CFU causes clinical disease (35). Whether our findings apply to class II H. ducreyi strains is unknown.
Live 35000HP, which expresses CDT, did not cause DC cell death compared to uninfected controls over a 48-h time course. We found no difference in the abilities of 35000HP and 35000.303, which do not express CDT, to cause apoptosis. At concentrations of 0.5 μg/ml to 2 μg/ml, recombinant H. ducreyi CDT causes apoptosis of DC (23, 47). How much CDT is secreted by live 35000HP is unknown. CDT activity is present in cell-free culture supernatants prepared from stationary-phase cultures of 35000HP, which contain at least 108 CFU/ml of bacteria (22, 38). However, 40-fold concentrations of these cytotoxic culture supernatants do not allow the detection of native CDT proteins by CDT-specific monoclonal antibodies in Western blot analysis (22). In our assays, we incubated 106 H. ducreyi bacteria with 105 DC, and the DC killed 95% of the bacteria over the 48-h time course. Thus, the amount of CDT produced by H. ducreyi in our assays was likely several orders of magnitude below the concentrations of recombinant CDT reported to cause apoptosis of DC.
Approximately 50% of the DC ingested GFP-labeled H. ducreyi after 90 min of culture at an MOI of 10:1 in the confocal microscopy assay. However, only 25 to 30% of DC were GFP positive after 90 min of culture with H. ducreyi at a similar MOI in the flow cytometry assay. Confocal microscopy detects the ingestion of as few as one bacterium. We do not know how many GFP-expressing H. ducreyi bacteria need to be ingested by DC to provide a positive signal in the flow cytometry assay, but GFP-positive DC infected with labeled Mycobacterium tuberculosis contain four viable organisms/cell (12). The apparent discrepancy between the confocal microscopy and flow cytometry assays is likely due to the relative sensitivities of the assays.
35000HP expresses the antiphagocytic proteins LspA1 and LspA2, which prevent Fcγ receptor-mediated phagocytosis by PMN-like and macrophage-like cell lines (25). In our assays, both opsonized and nonopsonized 35000HP cells were ingested by DC within 90 min of coculture. The expression of LspA1 and LspA2, even under conditions that maximize their expression, had no effect on the ingestion of H. ducreyi by DC. In contrast, the preincubation of J774A.1 macrophages with live 35000HP for 1 h prevents phagocytic cup development and the uptake of opsonized erythrocytes (25). The incubation of PMN-like and macrophage-like cell lines with live 35000HP reduces the levels of active Src kinases that are involved in Fcγ receptor-mediated phagocytosis, while the incubation of those cell lines with an lspA1 lspA2 double mutant does not (25). Similar results were obtained previously with a DC cell line (25) and with monocyte-derived DC in our assays. Our data suggest that DC use pathways other than Fcγ receptor-mediated phagocytosis to ingest H. ducreyi.
Another receptor by which bacteria enter DC is the DC-specific intercellular adhesion molecule-grabbing nonintegrin (DC-SIGN) (43). DC-SIGN is a C-type lectin that binds carbohydrates that contain mannose or a terminal N-acetylglucosamine (50). The LOS of H. ducreyi strain A77 terminates in N-acetylglucosamine because A77 contains a point mutation in the galactosyltransferase gene, lgtB (39). In contrast, the LOS of 35000HP terminates in N-acetylactosamine, which may be sialylated. A77 adheres to and is phagocytosed by HeLa cells transfected with DC-SIGN (50). However, A77 complemented with 35000HP lgtB expresses LOS similar to that expressed by 35000HP and does not bind to DC-SIGN (50). H. ducreyi does not express mannose-containing carbohydrates. Given its LOS structure, 35000HP is unlikely to be taken up via DC-SIGN. Indeed, multiple attempts to inhibit the ingestion of H. ducreyi by DC with an anti-DC-SIGN antibody were unsuccessful (data not shown). How H. ducreyi is taken up by DC is under investigation in our laboratory.
DC ingested and killed H. ducreyi, but killing was incomplete as 5% of the internalized bacteria were recovered from DC for up to 48 h. In pustules induced by experimental inoculation, some DC express the maturation markers CD83 and DC-LAMP, but the majority of DC are immature CD1a-positive cells (20). H. ducreyi-specific T-cell lines are recovered from pustules of volunteers who are infected for 7 to 14 days (9). These data suggest that some DC mature in lesions, migrate to lymph nodes, and sensitize naïve T cells during experimental infection. In a murine model, DC that acquired FITC-labeled antigens after application to the skin were detected in lymph nodes 24 h later (16). We speculate that in natural chancroid, DC harboring live organisms may migrate to regional lymph nodes and cause the formation of infected buboes.
In vitro infection of myeloid DC with live H. ducreyi resulted in partial activation of DC. Similar results have been shown with Trypanosoma cruzi, Mycobacterium leprae, and Mycobacterium tuberculosis (12, 14, 44). When DC were infected with M. tuberculosis labeled with GFP, M. tuberculosis replicated and GFP-positive DC contained four organisms/cell 2 days after infection (12). After 48 h of M. tuberculosis infection, GFP-negative DC displayed slightly higher but not significantly different levels of activation than GFP-positive DC, likely due to bystander effects (12). In our assays, approximately 50% of DC ingested GFP-labeled H. ducreyi by 90 min. However, the number of GFP-positive DC and the intensity of their staining declined by 24 h because DC killed most of the internalized H. ducreyi. Thus, we cannot determine whether there is a difference in activation between DC that had initially ingested H. ducreyi and those that had not.
Live H. ducreyi also inhibited DC maturation by a potent agonist (LPS); a similar finding was shown for M. tuberculosis (12). Although live H. ducreyi only partially activated DC relative to heat-killed bacteria and LPS, DC incubated with live bacteria secreted significantly higher levels of TNF-α and IL-6 than DC incubated with LPS and heat-killed bacteria. The basis for the dissociation between surface activation and cytokine production by live H. ducreyi is unknown. Immature or partially activated DC may not effectively activate T cells in vivo. The secretion of high levels of IL-6 in the face of partial activation of surface markers may be a mechanism whereby H. ducreyi simultaneously promotes “pathological inflammation” (32) and avoids the establishment of a full Th1 response that promotes phagocytosis of the organism.
The levels of cytokines secreted by DC infected with live bacteria showed marked donor-to-donor variation. In persons who form pustules and in natural ulcers, H. ducreyi is surrounded by phagocytes and is not ingested (3, 35). In reinfection experiments, some volunteers repeatedly resolved all inoculated sites while others repeatedly formed pustules, suggesting that some hosts are able to overcome the antiphagocytic properties of the organism (36). Spontaneous resolution of disease has also been reported in natural infection (11). We have postulated that H. ducreyi causes a proinflammatory response that promotes phagocytosis in resolvers and a tolerizing response that promotes phagocytic failure in pustule formers (36). Our donors were normal, healthy adult volunteers who had not been challenged with H. ducreyi. Whether person-to-person variation in cytokine responses to H. ducreyi by DC is associated with differential host susceptibilities to disease progression is under investigation in our laboratory (15a).
Acknowledgments
This work was supported by grants AI31494, AI27863, and AI059384 (to S.M.S.) from the National Institute of Allergy and Infectious Diseases (NIAID). K.E.B. was supported by NIH grant T32 AI007637 from the NIAID. The human challenge trials were supported by grant MO1RR00750 to the GCRC at Indiana University.
We thank Byron Batteiger and Diane Janowicz for their thoughtful criticism of the manuscript and the volunteers who participated in these studies.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 8 October 2007.
REFERENCES
- 1.Alileche, A., E. R. Serfass, S. M. Muehlbauer, S. A. Porcelli, and J. Brojatsch. 2005. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS Pathogens 1:150-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, M. E., C. A. Townsend, A. R. Ronald, and S. M. Spinola. 2006. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbes Infect. 8:2465-2468. [DOI] [PubMed] [Google Scholar]
- 4.Ben Nasr, A., J. Haithcoat, J. E. Masterson, J. S. Gunn, T. Eaves-Pyles, and G. R. Klimpel. 2006. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J. Leukoc. Biol. 80:774-786. [DOI] [PubMed] [Google Scholar]
- 5.Bong, C. T. H., M. E. Bauer, and S. M. Spinola. 2002. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect. 4:1141-1148. [DOI] [PubMed] [Google Scholar]
- 6.Cope, L. D., S. Lumbley, J. L. Latimer, J. Klesney-Tait, M. K. Stevens, L. S. Johnson, M. Purven, R. S. Munson, Jr., T. Lagergard, J. D. Radolf, and E. J. Hansen. 1997. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 94:4056-4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng, H., D. Zhang, D. Palliser, P. Zhu, S. Cai, A. Schlesinger, L. Maliszewski, and J. Lieberman. 2005. Listeria-infected myeloid dendritic cells produce IFN-beta, priming T cell activation. J. Immunol. 175:421-432. [DOI] [PubMed] [Google Scholar]
- 8.Gelfanova, V., E. J. Hansen, and S. M. Spinola. 1999. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect. Immun. 67:6394-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gelfanova, V., T. L. Humphreys, and S. M. Spinola. 2001. Characterization of Haemophilus ducreyi-specific T-cell lines from lesions of experimentally infected human subjects. Infect. Immun. 69:4224-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gervassi, A., M. R. Alderson, R. Suchland, J. F. Maisonneuve, K. H. Grabstein, and P. Probst. 2004. Differential regulation of inflammatory cytokine secretion by human dendritic cells upon Chlamydia trachomatis infection. Infect. Immun. 72:7231-7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond, G. W., M. Slutchuk, J. Scatliff, E. Sherman, J. C. Wilt, and A. R. Ronald. 1980. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev. Infect. Dis. 2:867-879. [DOI] [PubMed] [Google Scholar]
- 12.Hanekom, W. A., M. Mendillo, C. Manca, P. A. J. Haslett, M. R. Siddiqui, B. Clifton III, and G. Kaplan. 2003. Mycobacterium tuberculosis inhibits maturation of human monocyte-derived dendritic cells in vitro. J. Infect. Dis. 188:257-266. [DOI] [PubMed] [Google Scholar]
- 13.Hanenberg, R. S., W. Rojanapithayakorn, P. Kunasol, and D. C. Sokal. 1994. Impact of Thailand's HIV control programme as indicated by the decline of sexually transmitted diseases. Lancet 344:243-245. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto, K., Y. Maeda, H. Kimura, K. Suzuki, A. Masuda, M. Matsuoka, and M. Makino. 2002. Mycobacterium leprae infection in monocyte-derived dendritic cells and its influence on antigen-presenting function. Infect. Immun. 70:5167-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humphreys, T. L., L. A. Baldridge, S. D. Billings, J. J. Campbell, and S. M. Spinola. 2005. Trafficking pathways and characterization of CD4 and CD8 cells recruited to the skin of humans experimentally infected with Haemophilus ducreyi. Infect. Immun. 73:3896-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Humphreys, T. L., L. Li, X. Li, D. M. Janowicz, K. R. Fortney, Q. Zhao, W. Li, J. McClintick, B. P. Katz, D. S. Wilkes, H. J. Edenberg, and S. M. Spinola. 2007. Dysregulated immune profiles for skin and dendritic cells are associated with increased host susceptibility to Haemophilus ducreyi infection in human volunteers. Infect. Immun. 75:5686-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itano, A. A., and M. K. Jenkins. 2003. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 4:733-739. [DOI] [PubMed] [Google Scholar]
- 17.Janowicz, D., I. Leduc, K. R. Fortney, B. P. Katz, C. Elkins, and S. M. Spinola. 2006. A DltA mutant of Haemophilus ducreyi is partially attenuated in its ability to cause pustules in human volunteers. Infect. Immun. 74:1394-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janowicz, D., N. R. Luke, K. R. Fortney, B. P. Katz, A. A. Campagnari, and S. M. Spinola. 2006. Expression of OmpP2A and OmpP2B is not required for pustule formation by Haemophilus ducreyi in human volunteers. Microb. Pathog. 40:110-115. [DOI] [PubMed] [Google Scholar]
- 19.Janowicz, D. M., K. R. Fortney, B. P. Katz, J. L. Latimer, K. Deng, E. J. Hansen, and S. M. Spinola. 2004. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect. Immun. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janowicz, D. M., K. Tenner-Racz, P. Racz, T. L. Humphreys, C. Schnizlein-Bick, K. R. Fortney, B. Zwickl, B. P. Katz, J. J. Campbell, D. D. Ho, and S. M. Spinola. 2007. Experimental infection with Haemophilus ducreyi in persons who are infected with HIV does not cause local or augment systemic viral replication. J. Infect. Dis. 195:1443-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kikuchi, T., K. Kobayashi, K. Gomi, T. Suzuki, Y. Tokue, A. Watanabe, and T. Nukwira. 2004. Dendritic cells pulsed with live and dead Legionella pneumophila elicit distinct immune responses. J. Immunol. 172:1727-1734. [DOI] [PubMed] [Google Scholar]
- 22.Lewis, D. A., M. K. Stevens, J. L. Latimer, C. K. Ward, K. Deng, R. Blick, S. R. Lumbley, C. A. Ison, and E. J. Hansen. 2001. Characterization of Haemophilus ducreyi cdtA, cdtB, and cdtC mutants in in vitro and in vivo systems. Infect. Immun. 69:5626-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, L., A. Sharipo, E. Chaves-Olarte, M. G. Masucci, V. Levitsky, M. Thelestam, and T. Frisan. 2002. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cell. Microbiol. 4:87-99. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y.-J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 25.Mock, J. R., M. Vakevainen, K. Deng, J. L. Latimer, J. A. Young, N. S. van Oers, S. Greenberg, and E. J. Hansen. 2005. Haemophilus ducreyi targets Src family protein tyrosine kinases to inhibit phagocytic signaling. Infect. Immun. 73:7808-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orroth, K. K., R. G. White, E. L. Korenromp, R. Bakker, J. Changalucha, J. D. F. Habbema, and R. J. Hayes. 2006. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: simulation results. Sex. Transm. Dis. 33:536-544. [DOI] [PubMed] [Google Scholar]
- 27.Paz-Bailey, G., M. Rahman, C. Chen, R. Ballard, H. J. Moffat, T. Kenyon, P. H. Kilmarx, P. A. Totten, S. Astete, M. C. Boily, and C. Ryan. 2005. Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin. Infect. Dis. 41:1304-1312. [DOI] [PubMed] [Google Scholar]
- 28.Rescigno, M. 2002. Dendritic cells and the complexity of microbial infection. Trends Microbiol. 10:425-431. [DOI] [PubMed] [Google Scholar]
- 29.Rescigno, M., F. Granucci, and P. Ricciardi-Castagnoli. 2000. Molecular events of bacterial-induced maturation of dendritic cells. J. Clin. Immunol. 20:161-166. [DOI] [PubMed] [Google Scholar]
- 30.Salazar, J. C., A. R. Cruz, C. D. Pope, L. Valderrama, R. Trujillo, N. G. Saravia, and J. D. Radolf. 2007. Treponema pallidum elicits innate and adaptive cellular immune responses in skin and blood during secondary syphilis: a flow-cytometric analysis. J. Infect. Dis. 195:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salazar, J. C., C. D. Pope, T. J. Sellati, H. M. Feder, Jr., T. G. Kiely, K. R. Dardick, R. L. Buckman, M. W. Moore, M. J. Caimano, J. G. Pope, P. J. Krause, and J. D. Radolf. 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171:2660-2670. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti, P. J., and J. P. Di Santo. 2007. Debugging how bacteria manipulate the immune response. Immunity 26:149-161. [DOI] [PubMed] [Google Scholar]
- 33.Scheffler, N. K., A. M. Falick, S. C. Hall, W. C. Ray, D. M. Post, R. S. Munson, Jr., and B. W. Gibson. 2003. Proteome of Haemophilus ducreyi by 2-D SDS-page and mass spectrometry: strain variation, virulence, and carbohydrate expression. J. Proteome Res. 2:523-533. [DOI] [PubMed] [Google Scholar]
- 34.Semnani, R. T., A. Y. Liu, H. Sabzevari, J. Kubofcik, J. Zhou, J. K. Gilden, and T. B. Nutman. 2003. Brugia malayi microfilariae induce cell death in human dendritic cells, inhibit their ability to make IL-12 and IL-10, and reduce their capacity to activate CD4+ T cells. J. Immunol. 171:1950-1960. [DOI] [PubMed] [Google Scholar]
- 35.Spinola, S. M., M. E. Bauer, and R. S. Munson, Jr. 2002. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 70:1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spinola, S. M., C. T. H. Bong, A. L. Faber, K. R. Fortney, S. L. Bennett, C. A. Townsend, B. E. Zwickl, S. D. Billings, T. L. Humphreys, M. E. Bauer, and B. P. Katz. 2003. Differences in host susceptibility to disease progression in the human challenge model of Haemophilus ducreyi infection. Infect. Immun. 71:6658-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steen, R. 2001. Eradicating chancroid. Bull. W. H. O. 79:818-826. [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens, M. K., J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen. 1999. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect. Immun. 67:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun, S., B. Schilling, L. Tarantino, M. V. Tullius, B. W. Gibson, and R. S. Munson. 2000. Cloning and characterization of the lipooligosaccharide galactosyltransferase II gene of Haemophilus ducreyi. J. Bacteriol. 182:2292-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svensson, L. A., A. Tarkowski, M. Thelestam, and T. Lagergard. 2001. The impact of Haemophilus ducreyi cytolethal distending toxin on cells involved in immune response. Microb. Pathog. 30:157-166. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 133:133-146. [DOI] [PubMed] [Google Scholar]
- 42.Vakevainen, M., S. Greenberg, and E. J. Hansen. 2003. Inhibition of phagocytosis by Haemophilus ducreyi requires expression of the LspA1 and LspA2 proteins. Infect. Immun. 71:5994-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Kooyk, Y., and T. B. H. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 44.Van Overtvelt, L., N. Vanderheyde, V. Verhasselt, J. Ismaili, L. De Vos, M. Goldman, F. Willems, and B. Vray. 1999. Trypanosoma cruzi infects human dendritic cells and prevents their maturation: inhibition of cytokines, HLA-DR, and costimulatory molecules. Infect. Immun. 67:4033-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velan, B., E. Bar-Haim, A. Zauberman, E. Mamroud, A. Shafferman, and S. Cohen. 2006. Discordance in the effects of Yersinia pestis on the dendritic cell functions manifested by induction of maturation and paralysis of migration. Infect. Immun. 74:6365-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White, C. D., I. Leduc, C. Jeter, C. Harris, and C. Elkins. 2005. Haemophilus ducreyi outer membrane determinants, including DsrA, define two clonal populations. Infect. Immun. 73:2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu, T., A. Lundqvist, H. J. Ahmed, K. Eriksson, Y. Yang, and T. Lagergard. 2004. Interactions of Haemophilus ducreyi and purified cytolethal distending toxin with human monocyte-derived dendritic cells, macrophages and CD4+ T cells. Microbes Infect. 6:1171-1181. [DOI] [PubMed] [Google Scholar]
- 48.Young, R. S., K. R. Fortney, V. Gelfanova, C. L. Phillips, B. P. Katz, A. F. Hood, J. L. Latimer, R. S. Munson, Jr., E. J. Hansen, and S. M. Spinola. 2001. Expression of cytolethal distending toxin and hemolysin is not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 69:1938-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaharik, M. L., T. Nayar, R. White, M. Caixia, B. A. Vallance, N. Straka, X. Jiang, J. Rey-Ladino, C. Shen, and R. C. Brunham. 2007. Genetic profiling of dendritic cells exposed to live- or ultraviolet-irradiated Chlamydia muridarum reveals marked differences in CXC chemokine profiles. Immunology 120:160-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, P., S. Snyder, P. Feng, P. Azadi, S. Zhang, S. Bulgheresi, K. E. Sanderson, J. He, J. Klena, and T. Chen. 2006. Role of N-acetylglucosamine within core lipopolysaccharide of several species of gram-negative bacteria in targeting the DC-SIGN (CD209). J. Immunol. 177:4002-4011. [DOI] [PubMed] [Google Scholar]