Abstract
Diseases associated with Chlamydia infection, such as pelvic inflammatory disease and ectopic pregnancy, are due to inflammation-mediated tissue damage and scarring that occur after chronic or repeated infections. The inflammatory chemokine interleukin-8 (IL-8) is produced by Chlamydia-infected cells through an endogenous mechanism of activation, independent of soluble factors in the supernatant. The host signaling pathways necessary for this response are not understood, but the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) has been shown to be activated at similar times as IL-8 mRNA up-regulation. The purpose of this study was to elucidate the MAPK pathways necessary to induce the endogenous IL-8 response to Chlamydia trachomatis infection of epithelial cells. IL-8 induced by infection with C. trachomatis L2 was shown to be dependent on ERK and independent of p38 and Jun N-terminal MAPK by use of chemical inhibitors of the signaling pathways. Persistent ERK activation during IL-8 mRNA production at 24 h postinfection was necessary to maintain the response. C. trachomatis serovar D also induced IL-8 in an ERK-dependent manner. We concluded that IL-8 induced during infection of epithelial cells is dependent on continual activation of ERK by C. trachomatis.
Interleukin-8 (IL-8) is an important inflammatory chemokine associated with immune response-mediated tissue damage (21). Recruitment and activation of neutrophils by IL-8 can cause damage to surrounding tissues (18), yet IL-8 is also an essential part of the initial innate immune response (17). IL-8 induction is transcriptionally controlled through multiple promoter elements (9). NF-κB is primarily associated with inflammatory stimuli; however, mitogen-activated protein kinase (MAPK) signaling pathways are also involved in these processes (4). Pathogen modulation of MAPK pathways changes host cell responses through activation or repression of the signaling pathway, which can ultimately affect immune responses such as IL-8 production (6, 19).
Cytokines produced locally by Chlamydia-infected epithelial cells are postulated to be essential for the progression of the inflammatory response that leads to pathology and disease (22). Inflammatory cytokines are induced by Chlamydia trachomatis infection in vitro and in vivo (3, 16, 20) and include IL-8, a chemotactic attractant and activator of neutrophils that is associated with inflammation-mediated tissue damage (18). The markedly late induction of IL-8 (at 15 h postinfection [hpi]) and its dependence on bacterial growth and protein synthesis implicate direct intracellular interactions of bacteria and the host cell (1). Consistent with this hypothesis, IL-8 induction occurs only within inclusion-containing cells and is independent of exogenous factors in the supernatant before 48 hpi, signifying that chlamydial products within the host cell lead to this inflammatory response (1). This is termed the endogenous IL-8 response and precedes the very late amplification of IL-8 expression by IL-1α following cell lysis at 72 hpi (1, 20).
While inflammatory cytokines are essential components of the host innate immune response to chlamydial infection, how they are induced and maintained is not known. Characterization of the IL-8 promoter revealed that the AP-1 and C/EBP (NFIL6) elements are necessary for the full transcriptional response of IL-8 after C. trachomatis infection (1). The transcription factors that bind to these sites are regulated by the host extracellular signal-regulated kinase (ERK), p38, and Jun N-terminal kinase (JNK) MAPK signaling pathways (10, 13). Consequently, the IL-8 response by the host cell to C. trachomatis infection may be due to bacterium-host interactions causing up-regulation of one or more of these cellular signaling pathways. ERK phosphorylation and activation occur later during C. trachomatis infection and are necessary for host lipid uptake by the bacterium (23). ERK pathway activation is maintained throughout the course of infection during the time that the endogenous IL-8 response occurs in C. trachomatis-infected cells. Thus, ERK is a likely candidate as a cellular signaling pathway through which IL-8 is induced after C. trachomatis infection.
The purpose of this research was to identify the host signaling pathways necessary for and leading to induction of the endogenous IL-8 response. Not only is IL-8 an essential inflammatory factor, but elucidation of the mechanism of its up-regulation will define how Chlamydia growth and bacterial products modulate the host cell response. In this study, we show that IL-8 mRNA induced by C. trachomatis is dependent on the ERK signaling pathway and that ERK activation occurs concurrently with IL-8 mRNA production, suggesting that a late and constant bacterial signal induces this response.
MATERIALS AND METHODS
Cell culture.
HeLa 229 cells, L929 murine fibroblast cells, and Hep2 cells were grown in RPMI 1640 plus l-glutamine (Invitrogen, Carlsbad, CA) with 5% heat-inactivated fetal bovine serum (HyClone, Logan, UT) and 10 μM HEPES (Invitrogen) at 37°C in 5% CO2. Cell lines and Chlamydia stocks were tested for Mycoplasma contamination by PCR (VenorGeM Mycoplasma detection system; Sigma) throughout use and were found to be negative.
Growth of Chlamydia.
C. trachomatis L2/434/Bu was cultured in L929 murine fibroblast cells and isolated as previously described (1). C. trachomatis D/UW-3/Cx was cultured in HeLa 229 cells for 48 h and harvested as described previously (1); however, it was not passed over 30% RenoCal-76 but was suspended directly in sucrose-phosphate-glutamic acid and frozen at −80°C. Chlamydia infections of HeLa and Hep2 cells were performed as described previously (1), using 90% confluent cell monolayers with an approximate multiplicity of infection of 2.
QPCR.
Quantitative PCR (QPCR) analysis was performed according to the method of Buchholz and Stephens (1) and normalized as previously described (15). QPCR to quantify chlamydial genomes was performed according to a previously described method (11), with modification. Briefly, 26-μl reaction mixtures contained 200 nM of each primer, a 400 μM concentration of each deoxynucleoside triphosphate, 150 nM 6-carboxyfluorescein probe, 300 nM Cy5 probe, 5 mM MgCl2, and 2.5 units Platinum Taq (Invitrogen) and were run at 95°C for 10 min, followed by 35 cycles at 95°C (15 s), 55°C (5 s), and 72°C (10 s) on a Smartcycler machine (Cepheid, Sunnyvale, CA). The following primers were used: for 16S RNA, 5′-CGCCTGAGGAGTACACTCGC-3′ and 5′-CCAACACCTCACGGCACGAG-3′; and for beta-globin, 5′-TACCCTTGGACCCAGAGGTTCTTTGA-3′ and 5′-TCAGGATCCACGTGCAGCTTGTCA-3′. The following probes were used: a 16S probe with a 5′ Cy5 fluorophore and a 3′ BHQ2 quencher (5′-CACAAGCAGTGGAGCATGTGGTTTAA-3′) (IDT) and a beta-globin probe with a 5′ 6-carboxyfluorescein fluorophore and a 3′ IBFQ quencher (5′-ATGGCAAGAAAGTGCTCGGTGCCTTT-3′) (IDT). The number of Chlamydia genomes per well was quantified by comparison to a standard curve of genomic L2 DNA, using cycle threshold values.
Immunoblotting.
Cell lysate was harvested with sodium dodecyl sulfate-polyacrylamide gel electrophoresis buffer, boiled, run in 10% sodium dodecyl sulfate-polyacrylamide gels, and transferred to nitrocellulose for probing with antibodies specific to phosphorylated or total protein. Phospho-p44/42 (ERK) MAPK monoclonal antibody, p44/42 MAPK antibody, phospho-MAPKAPK-2 antibody, p38 antibody, SAPK/JNK antibody, and phospho-SAPK/JNK antibody were all obtained from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated goat anti-mouse and horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (heavy plus light chains) secondary antibodies were purchased from Zymed (South San Francisco, CA).
Chemical inhibitors of MAPK pathways.
Inhibitors of the ERK, JNK, and p38 pathways (Calbiochem, San Diego, CA) were diluted in dimethyl sulfoxide and added directly to culture supernatants after infection. The MEK1/2 inhibitor SL327 was used at a final concentration of 1 μM, the JNK II inhibitor SP600125 was used at a final concentration of 1 μM, and the p38 MAPK III inhibitor ML3403 was used at a final concentration of 2 μM, unless otherwise noted.
Statistical methods.
The statistical significance of data was determined using two-tailed Student's t test.
RESULTS
The ERK MAPK signaling pathway is essential for IL-8 up-regulation in response to infection.
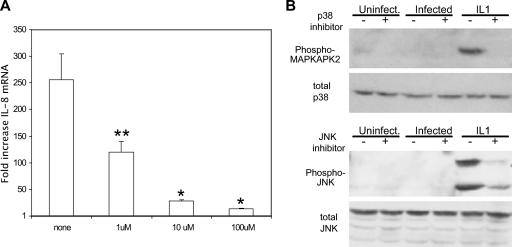
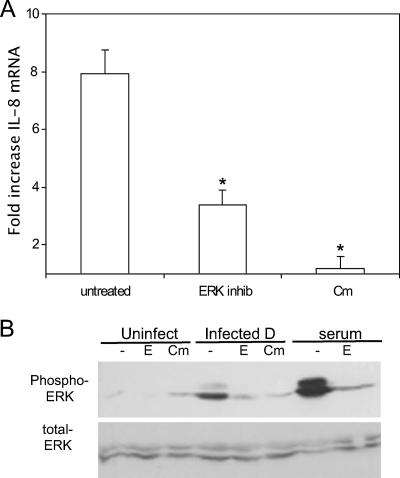
To determine the role of the MAPK signaling pathways in IL-8 up-regulation, a chemical inhibitor specific to each pathway was added to C. trachomatis-infected HeLa cells. It was found that the inhibitor of MEK1/2, the MAPK kinases directly upstream of ERK, reduced the level of IL-8 mRNA, while the inhibitors of the p38 and JNK pathways did not (Fig. 1A). A similar profile was seen in an analysis of IL-8 protein secretion (data not shown). Immunoblotting with antibodies specific to phosphorylated ERK was used to identify ERK activation after C. trachomatis infection and to assess the functionality of the inhibitor. Activation of ERK occurred at 30 hpi, and this phosphorylation was reduced by treatment with the MEK1/2 inhibitor of the ERK pathway (Fig. 1B).
FIG. 1.
Identification of MAPK pathway involvement in IL-8 up-regulation in response to C. trachomatis infection. (A) HeLa cells were infected or incubated in medium alone for 2 h. Fresh medium containing the appropriate MAPK inhibitor (MEK1/2 inhibitor, 1 μM; JNK inhibitor, 1 μM; p38 inhibitor, 2 μM) was then added, and total RNA was isolated at 30 hpi. The relative change in IL-8 mRNA compared to that in uninfected cells with similar inhibitor treatment was determined by QPCR as described in Materials and Methods. Data shown are averages ± standard deviations (SD) for three wells. Data are from one of three experiments. *, P < 0.03 compared to infected cells without inhibitor treatment. (B) HeLa cells were infected with C. trachomatis L2 for 30 h, with or without the MEK1/2 inhibitor of the ERK pathway. Data are from one of three experiments.
In order to show the specificity of the ERK pathway inhibitor in reducing the IL-8 response to infection, increasing concentrations of the chemical inhibitor were used to test for dose-dependent effects. Inhibition of the C. trachomatis-induced IL-8 mRNA response by the MEK1/2 inhibitor was dose dependent (Fig. 2A), while IL-1-induced IL-8 was not inhibited (data not shown). This demonstrated that the inhibitor of the ERK MAPK pathway blocks IL-8 induced by C. trachomatis infection. The JNK and p38 pathways were not detectably active after Chlamydia infection (Fig. 2B) (7), but these pathways could be activated strongly by stimulation of cells with IL-1α (Fig. 2B). Phosphorylation of MAPKAPK2, a substrate of p38 induced by IL-1α, was abolished by the specific chemical inhibitor of the p38 pathway (Fig. 2B). Phosphorylation of JNK induced by IL-1α was similarly reduced by the specific chemical, showing that the dose used was sufficient to functionally inhibit these pathways. The use of increasing doses of JNK and p38 MAPK inhibitors, of 1 and 10 μM and 2 and 20 μM, respectively, did not inhibit IL-8 mRNA induced by C. trachomatis infection (data not shown). Thus, we conclude that the ERK pathway is necessary for the up-regulation of IL-8 mRNA in response to C. trachomatis infection, while the JNK and p38 pathways are not activated or involved in the IL-8 response at this time.
FIG. 2.
Dose-dependent reduction in C. trachomatis-induced IL-8 by an inhibitor of the ERK pathway. (A) Increasing concentrations of the MEK1/2 inhibitor of the ERK MAPK pathway were added 90 min prior to total RNA harvest at 24 hpi. The relative change in IL-8 mRNA was determined as described in the legend to Fig. 1. Data shown are averages ± SD. Data are from one of two experiments. **, P = 0.012; *, P < 0.01 (compared to infected cells without inhibitor treatment). (B) C. trachomatis-infected cells were treated with 2 μM p38 inhibitor or 1 μM JNK inhibitor at 24 hpi. One nanogram (p38) or 4 ng (JNK) IL-1α was added for 20 min prior to harvest at 25.5 hpi. Data are from one of two experiments.
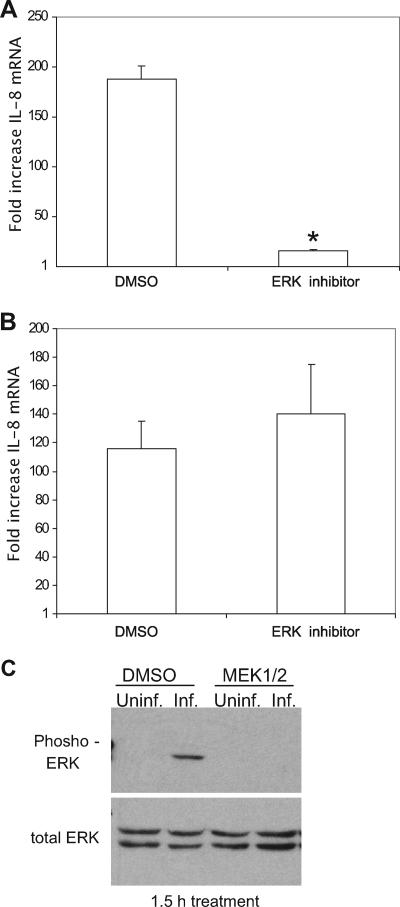
Late ERK MAPK activation is necessary for IL-8 response.
The ERK MAPK pathway is induced by C. trachomatis infection of HeLa cells from 12 hpi and continues throughout the course of infection; however, there is also transient activation of the pathway 10 min after infection (23). To identify when ERK pathway activation is needed to induce the IL-8 response, the MEK1/2 inhibitor of ERK pathway activation was added for 90 min before total RNA was collected at 24 hpi. Inhibition of the ERK pathway for 90 min prior to collecting RNA at 24 hpi reduced IL-8 mRNA levels (Fig. 3A), indicating that signaling through the pathway at this time was essential for the response to C. trachomatis. Treatment for 3 h prior to collection of total RNA at 24 hpi showed similar reductions in IL-8 mRNA levels (data not shown). Inhibiting ERK pathway activity early, i.e., 1 h prior to infection through 2 hpi, and then removing the inhibitor did not significantly affect IL-8 levels induced by infection measured at 24 hpi (Fig. 3B). This demonstrated that there was no role for ERK pathway activation during that time in the IL-8 response produced at 24 hpi. Immunoblots for phosphorylated ERK confirmed that a short, 90-min incubation with the MEK1/2 inhibitor effectively blocked the pathway (Fig. 3C). Together, these data show that ERK pathway activation induced at 10 hpi by C. trachomatis L2 infection (23) is not involved in the IL-8 response quantified at 24 hpi. However, activation of ERK immediately prior to total RNA isolation at 24 hpi was essential. This indicates that continual activation of ERK MAPK is necessary to maintain the IL-8 response.
FIG. 3.
Late ERK pathway activation induces IL-8 response. C. trachomatis-infected HeLa cells were treated with 1 μM MEK1/2 inhibitor for 90 min prior to RNA isolation at 24 hpi (A) or from 1 h prior to 2 hpi, after which the inhibitor was removed, with RNA isolated at 24 hpi (B). The relative change in IL-8 mRNA compared to that in similarly treated uninfected cells was determined as described in the legend to Fig. 1. Data shown are averages ± SD. Data are from one of three experiments. *, P < 0.01. (C) C. trachomatis-infected cells were treated with 1 μM MEK1/2 inhibitor for 90 min prior to collection of lysate at 24 hpi. Data are from one of two experiments. DMSO, dimethyl sulfoxide.
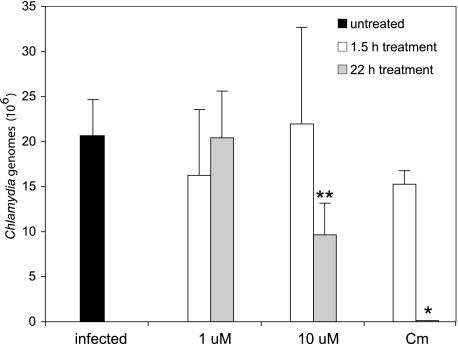
ERK MAPK pathway inhibition does not affect growth of C. trachomatis.
The ERK pathway is necessary for chlamydial uptake of host glycerophospholipids, and blocking activation of the pathway reduces the number of infectious Chlamydia cells (23). IL-8 induced by C. trachomatis infection depends on chlamydial protein synthesis and growth, and thus any effects these inhibitors have that could inhibit growth could be expected to result in a reduced IL-8 response. The reduced level of IL-8 mRNA caused by a short, 90-min incubation with the MEK1/2 inhibitor implies a direct role for ERK in IL-8 induction. Nevertheless, to test whether the ERK pathway inhibitor affected chlamydial growth and thus, indirectly, the level of IL-8, we quantified C. trachomatis 24 h after infection, with or without MEK1/2 inhibitor treatment. While the addition of chloramphenicol, an inhibitor of bacterial protein synthesis, for 22 h blocked C. trachomatis growth, treatment for 90 min did not significantly reduce the number of bacterial genomes (Fig. 4). Treatment with 1 μM MEK1/2 inhibitor and 90 min of treatment with 10 μM MEK1/2 inhibitor did not inhibit the growth of the bacteria (Fig. 4). Twenty-two hours of treatment with 10 μM MEK1/2 inhibitor did cause a small but significant reduction in bacterial numbers (Fig. 4). Thus, the IL-8 response to C. trachomatis infection is inhibited by a concentration of MEK1/2 inhibitor (1 μM) which does not reduce bacterial growth, and thus, we concluded that the ERK pathway directly induces IL-8 production.
FIG. 4.
Measurement of Chlamydia infection by QPCR. HeLa cells were infected with C. trachomatis L2 and treated with 1 μM or 10 μM MEK1/2 inhibitor or 100 μg/ml chloramphenicol immediately after removal of infection (gray bars), or treatment was added 90 min prior to DNA isolation at 24 hpi (white bars). Data shown are averages ± SD for three separate wells. Data are from one of two experiments. **, P < 0.05; *, P < 0.01 (compared to infected cells without inhibitor treatment).
The ERK MAPK pathway is a common mechanism by which C. trachomatis induces IL-8.
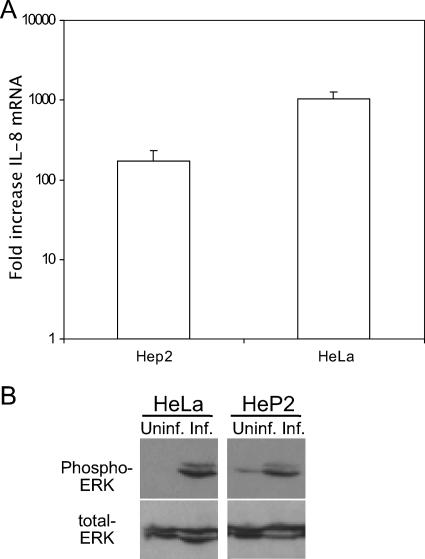
The investigation of IL-8 induction thus far has been with HeLa 229 epithelial cells. However, IL-8 is induced in other cell lines, including primary endocervical cells (20). To show IL-8 production in an additional cell type, Hep2 cells were infected with C. trachomatis L2. IL-8 was up-regulated in Hep2 cells in response to infection (Fig. 5A). Similar to the case for infection of HeLa cells, the ERK MAPK pathway was also activated in Hep2 cells (Fig. 5B). Thus, IL-8 induction and ERK pathway activation by C. trachomatis occur in cell lines other than HeLa epithelial cells.
FIG. 5.
ERK activation in HeP2 cells during C. trachomatis infection. (A) HeLa or HeP2 cells were concomitantly infected with C. trachomatis L2, and total RNA was harvested at 48 hpi. The relative change in IL-8 mRNA was determined as described in the legend to Fig. 1. Data shown are averages ± SD. Data are from one of two experiments. (B) Phosphorylation of ERK protein was determine by immunoblotting of C. trachomatis L2-infected HeLa or HeP2 cells. Data are from one of two experiments.
Investigations of inflammatory cytokine induction by Chlamydia have shown that C. trachomatis L2, D, E, and I induce IL-8 (2, 20). To determine if the mechanisms of IL-8 induction by Chlamydia are similar between C. trachomatis LGV and a non-LGV urogenital biovar, HeLa cells were infected with C. trachomatis serovar D and IL-8 mRNA induction was quantified. Infection with C. trachomatis serovar D was indeed able to stimulate IL-8 production (Fig. 6A). The IL-8 response was significantly reduced by treatment with an inhibitor of the ERK MAPK pathway and with chloramphenicol, which blocks bacterial protein synthesis. Immunoblots specific for the phosphorylated form of ERK demonstrated that infection with serovar D induced activation of the ERK MAPK pathway (Fig. 6B). ERK phosphorylation was inhibited by the chemical inhibitor of the ERK pathway as well as by chloramphenicol treatment (Fig. 6B). We concluded from these data that C. trachomatis serovar D induces IL-8 in a manner dependent on bacterial protein synthesis and the ERK signaling pathway analogous to the mechanism of IL-8 induction by C. trachomatis L2.
FIG. 6.
ERK activation by C. trachomatis serovar D infection. (A) HeLa cells were infected with C. trachomatis serovar D, and total RNA was isolated at 30 hpi. The relative change in IL-8 mRNA compared to that in similarly treated uninfected cells was determined as described in the legend to Fig. 1. Data shown are averages ± SD. *, P < 0.01 compared to infected cells without inhibitor treatment. Data are from one of two experiments. (B) C. trachomatis serovar D-infected HeLa cells were evaluated for ERK phosphorylation at 48 hpi. E, MEK1/2 inhibitor of ERK pathway; Cm, chloramphenicol. Data are from one of three experiments.
DISCUSSION
Disease and pathology from Chlamydia infection are caused by inflammation-associated tissue damage. Chronic production of cytokines by infected epithelial cells may be responsible for the persistent inflammation at the site of infection (22). IL-8 is an attractant and activator of neutrophils associated with early host responses to pathogens (5, 12) that is produced chronically by C. trachomatis-infected cells. The induction of IL-8 by Chlamydia is not specific to HeLa cells but is a broad response to infection. Rasmussen et al. (20) found that IL-8 was stimulated by C. trachomatis L2 in various cell types, including primary endocervical cells (20). Various species and serovars of Chlamydia have been found to induce IL-8, including C. trachomatis serovars D, E, and I and C. psittaci (3, 20). Similar to the response in HeLa cells, activation of the ERK signaling pathway occurs in HeP2 cells (this study) and primary endometrial cells (23). The use of serovar D in this study suggests that the mechanism of IL-8 induction through ERK is consistent among these genital C. trachomatis strains. However, serovar D did not induce equivalent levels of IL-8 to those induced by C. trachomatis serovar L2. This is in part due to lower static infection levels of cell monolayers by serovar D than those obtained using serovar L2 with similar inocula. Even so, this does not rule out inherent differences between the serovars in inducing this host response.
The MAPK (p38, JNK, and ERK) signaling pathways can induce IL-8 transcription, although the contribution of each varies depending on cell type or stimulus (9, 14). The ERK pathway is activated in C. trachomatis-infected epithelial cells at 12 hpi in a manner that can be inhibited by the addition of chloramphenicol (23; data not shown), while the JNK and p38 MAPK pathways are not activated in response to C. trachomatis infection (7). Analogous to the localization of IL-8 only within infected cells (1), it has been shown that phosphorylated ERK protein, which is necessary for lipid acquisition, is found only in infected cells, not in neighboring uninfected cells (23). Activation of the ERK pathway occurs at similar times as IL-8 up-regulation and thus was a likely candidate to be involved in the IL-8 response (1). A short incubation time of only 90 min with the ERK pathway inhibitor was sufficient to block IL-8 production induced by infection. Continuous activation of this pathway is necessary to maintain IL-8 production, similar to the need for constant bacterial protein synthesis in sustaining the response (1). Together, these data suggest that a bacterial product produced during chlamydial growth is responsible for inducing and maintaining the ERK pathway activation that leads to inflammatory IL-8 production.
ERK activation during C. trachomatis infection is necessary for host lipid uptake by bacteria (7, 23). Inhibiting the ERK MAPK pathway reduced the number of inclusion-forming units recovered from C. trachomatis infection (23). Since inhibiting the ERK pathway could reduce growth and because the IL-8 response to Chlamydia infection is dependent on growth, the effect on IL-8 mRNA levels could have been indirect. Although we employed concentrations of the inhibitor that did not cause reductions in the number of inclusion-forming units, as previously shown (23), we nevertheless controlled for an unexpected effect on growth by using two experimental strategies, First, we added the ERK pathway inhibitor for only 90 min prior to collecting RNA to limit the time that it could interfere with chlamydial growth and development. Second, through quantification of Chlamydia genomes, we could compare treated and untreated infections for reduced numbers of Chlamydia bacteria. It was found that a 1 μM concentration of the inhibitor did not affect C. trachomatis growth, as assessed by the number of genomes, at 24 hpi, while 22 h of incubation with 10 μM ERK pathway inhibitor caused a slight reduction in the number of C. trachomatis genomes per host cell compared to an untreated infection. These results are similar to those reported by Su et al. (23). Thus, the reduction in IL-8 mRNA seen using 1 μM ERK pathway inhibitor is due to direct involvement of the signaling pathway in mRNA induction, not to indirect effects of the inhibitor on chlamydial growth.
Pathogenic bacteria can stimulate host signaling pathways by interacting with pathway members or molecules upstream of the signaling pathway (6, 19). During C. trachomatis infection, Ras and Raf induce ERK activation (23). We also found that Raf1 is necessary for phosphorylation of ERK after infection by treating cells with a Raf1 inhibitor (data not shown). The exact bacterium-host interaction upstream of and leading to ERK MAPK pathway activation has not been discerned. As such, it is not known whether the IL-8 response serves the bacteria or is a by-product of ERK involvement in chlamydial acquisition of glycerophospholipids. Nevertheless, ERK involvement in the induction of inflammation is essential for pathogenesis and disease.
Intracellular pathogens must have close associations with their host cells in order to survive and propagate. These host-pathogen interactions are especially vital for the obligate intracellular pathogen Chlamydia, as interactions with the cell occur throughout the developmental cycle and are necessary for viable infectious progeny (8). Interactions with the cell may also lead to host recognition, response to infection, and induction of the innate immune response. Here we have described the host ERK signaling pathway that is stimulated during productive C. trachomatis infection and induces the inflammatory mediator IL-8. Characterizing how C. trachomatis infection alters the host cell response is important for understanding the mechanism by which the bacterium facilitates its intracellular development and the host cell responds to infection.
Acknowledgments
This work was supported by National Institutes of Health grants HL071730, AI042156, and AI032943 and by NIH training grant T32-AI007620.
We thank Carolyn G. Conant for developing the QPCR assay to quantify chlamydial genomes.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Buchholz, K. R., and R. S. Stephens. 2006. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell. Microbiol. 8:1768-1779. [DOI] [PubMed] [Google Scholar]
- 2.Dessus-Babus, S., T. L. Darville, F. P. Cuozzo, K. Ferguson, and P. B. Wyrick. 2002. Differences in innate immune responses (in vitro) to HeLa cells infected with nondisseminating serovar E and disseminating serovar L2 of Chlamydia trachomatis. Infect. Immun. 70:3234-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessus-Babus, S., S. T. Knight, and P. B. Wyrick. 2000. Chlamydial infection of polarized HeLa cells induces PMN chemotaxis but the cytokine profile varies between disseminating and non-disseminating strains. Cell. Microbiol. 2:317-327. [DOI] [PubMed] [Google Scholar]
- 4.Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. [DOI] [PubMed] [Google Scholar]
- 5.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 61:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friebel, A., H. Ilchmann, M. Aepfelbacher, K. Ehrbar, W. Machleidt, and W. D. Hardt. 2001. SopE and SopE2 from Salmonella typhimurium activate different sets of RhoGTPases of the host cell. J. Biol. Chem. 276:34035-34040. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda, E. Y., S. P. Lad, D. P. Mikolon, M. Iacobelli-Martinez, and E. Li. 2005. Activation of lipid metabolism contributes to interleukin-8 production during Chlamydia trachomatis infection of cervical epithelial cells. Infect. Immun. 73:4017-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackstadt, T. 1999. Cell biology, p. 101-138. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, DC.
- 9.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847-855. [PubMed] [Google Scholar]
- 10.Hu, J., S. K. Roy, P. S. Shapiro, S. R. Rodig, S. P. Reddy, L. C. Platanias, R. D. Schreiber, and D. V. Kalvakolanu. 2001. ERK1 and ERK2 activate CCAAAT/enhancer-binding protein-beta-dependent gene transcription in response to interferon-gamma. J. Biol. Chem. 276:287-297. [DOI] [PubMed] [Google Scholar]
- 11.Hybiske, K., and R. S. Stephens. 2007. Mechanisms of Chlamydia trachomatis entry into nonphagocytic cells. Infect. Immun. 75:3925-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 95:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karin, M., Z. Liu, and E. Zandi. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240-246. [DOI] [PubMed] [Google Scholar]
- 14.Li, J., S. Kartha, S. Iasvovskaia, A. Tan, R. K. Bhat, J. M. Manaligod, K. Page, A. R. Brasier, and M. B. Hershenson. 2002. Regulation of human airway epithelial cell IL-8 expression by MAP kinases. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L690-L699. [DOI] [PubMed] [Google Scholar]
- 15.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 16.Magee, D. M., J. G. Smith, C. A. Bleicker, C. J. Carter, L. F. Bonewald, J. Schachter, and D. M. Williams. 1992. Chlamydia trachomatis pneumonia induces in vivo production of interleukin-1 and -6. Infect. Immun. 60:1217-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukaida, N., A. Harada, and K. Matsushima. 1998. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 9:9-23. [DOI] [PubMed] [Google Scholar]
- 18.Mukaida, N., T. Matsumoto, K. Yokoi, A. Harada, and K. Matsushima. 1998. Inhibition of neutrophil-mediated acute inflammatory injury by an antibody against interleukin-8 (IL-8). Inflamm. Res. 47:S151-S157. [DOI] [PubMed] [Google Scholar]
- 19.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen, S. J., L. Eckmann, A. J. Quayle, L. Shen, Y. X. Zhang, D. J. Anderson, J. Fierer, R. S. Stephens, and M. F. Kagnoff. 1997. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J. Clin. Investig. 99:77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sansonetti, P. J., J. Arondel, M. Huerre, A. Harada, and K. Matsushima. 1999. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens, R. S. 2003. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 11:44-51. [DOI] [PubMed] [Google Scholar]
- 23.Su, H., G. McClarty, F. Dong, G. M. Hatch, Z. K. Pan, and G. Zhong. 2004. Activation of Raf/MEK/ERK/cPLA2 signaling pathway is essential for chlamydial acquisition of host glycerophospholipids. J. Biol. Chem. 279:9409-9416. [DOI] [PubMed] [Google Scholar]