Abstract
In this study, we show that stimulation of human airway epithelial cells (HAECs) by Pseudomonas aeruginosa strain PAO1 induces time- and dose-dependent activation of p38 mitogen-activated protein kinase (MAPK). Activated p38 MAPK stayed in the cytoplasm instead of translocating to the nucleus, as shown by cellular fractionation. p38 MAPK was activated when HAECs were incubated with P. aeruginosa strain PAK and Burkholderia cepacia, while little activation was observed with the isogenic flagellin-free strains PAK/fliC and B. cepacia BC/fliC. The presence of Toll-like receptor 5 (TLR5) in 293 cells mediated PAO1-dependent activation of p38 MAPK, and in HAECs p38 MAPK activation was blocked by the overexpression of a dominant negative TLR5. Two inhibitors of p38 MAPK, SB202190 and SB203580, significantly attenuated PAO1-dependent expression of an NF-κB-dependent luciferase reporter gene, suggesting that p38 MAPK activation is required for full activation of NF-κB-dependent signaling. Microarray analysis of NF-κB target genes revealed up-regulation of multiple genes by PAO1 in HAECs. Reverse transcription-PCR and protein expression analysis were used to show that up-regulation of NF-κB-dependent genes induced by PAO1, such as the genes encoding Cox-2 and interleukin-8, was attenuated by SB203580. These results demonstrate a role for p38 MAPK signaling in gene regulation in response to P. aeruginosa via TLR5.
Pseudomonas aeruginosa is an opportunistic pathogen that often infects the lungs of cystic fibrosis (CF) patients (5). Airway epithelial cells are a major site of host defense in the lung and the major sentinel cells against pathogens (12). Host responses are triggered by Toll-like receptors (TLRs), an evolutionarily conserved family of receptors that initiate host responses via recognition of pathogen-associated molecular patterns, such as the bacterial cell components lipopolysaccharide (LPS), pili, and flagellin (10, 35). TLR activation initiates signaling cascades that converge at the NF-κB signaling pathway, which activates the synthesis of proteins such as inflammatory cytokines and β-defensins to elicit host innate immune responses (23).
Recent studies with Caenorhabditis elegans and Arabidopsis thaliana have demonstrated a role for p38 mitogen-activated protein kinase (MAPK) in innate defense against bacterial and fungal pathogens (1, 2, 17, 22). p38 MAPK is a Pro-directed Ser/Thr kinase that is critical in inflammation and the host response to stress signals. Members of the MAPK family have been shown to be activated by P. aeruginosa in several epithelial cell line systems (26, 34). Studies have suggested that inhibition of p38 MAPK might be of clinical use for the control of inflammatory responses in CF patients (20, 24).
In this study, we demonstrated that in primary human airway epithelial cells (HAECs) P. aeruginosa challenge activates p38 MAPK. In addition, we demonstrated that p38 MAPK activation is dependent upon activation of TLR5 by flagellin and that inhibition of p38 MAPK activity diminishes the synthesis of proteins that are involved in the different aspects of epithelial cell functions, including those that are critical in the induction of the innate immune response in the airway.
MATERIALS AND METHODS
Reagents and plasmids.
SB202190, SB203580, SB202474, and PD98059 were purchased from Calbiochem (La Jolla, CA). Lipoteichoic acid (LTA) was purchased from Sigma (St. Louis, MO). Phospho-specific antibodies against Thr180 and Tyr182 dual-phosphorylated p38 MAPK, antibodies against total p38 MAPK (phosphorylation state independent), phospho-specific extracellular signal-regulated kinase (ERK) antibody, histone H3 and cyclooxygenase 2 (Cox-2), and horseradish peroxidase (HRP)-linked anti-rabbit immunoglobulin G antibody were obtained from Cell Signaling Technology (Danvers, MA). Murine TLR5 was cloned from a mouse lung cDNA library (Clontech, Palo Alto, CA) using primers 5′ATACGGATATCATGGCATGTCAACTTGACTTGCTCATAG and 3′AAGGAAAAAAGCGGCCGCCTAGGAAATGGTTGCTATGGTTCGCAACTG. A dominant negative mutant of human TLR5 (DNhTLR5) encoding amino acids 1 to 664 was constructed in pIRESpuro vector (catalog no. 6031-1; Clontech, Palo Alto, CA) by PCR amplification as previously described (35). Construction of DNhTLR2 has been described previously (29). The NF-κB promoter-driven luciferase reporter was generated by insertion of a 1.0-kb promoter region of the human beta-defensin 2 (hBD2) gene into pGL3 vector (Promega, Madison, WI) as described previously (29).
Bacterial strains.
Strains PAO1 and PAK are wild-type, nonmucoid, piliated, motile P. aeruginosa strains. Strain PAK/fliC is a nonmotile derivative of PAK in which the fliC gene encoding flagellin was replaced by homologous recombination with a mutant gene interrupted by a gentamicin resistance cassette (35). Burkholderia cepacia has flagella, while the fliC gene encoding flagellin in B. cepacia BC/fliC is disrupted by a gentamicin resistance cassette (35). All strains were grown in tryptic soy broth (Difco Laboratories, Detroit, MI) supplemented with 10 μg/ml kanamycin until the mid-log phase was reached. The bacterial concentration was about 1 × 108 CFU/ml (optical density at 600 nm of about 0.5), which was verified for each experiment by serial plating. Bacteria were washed, resuspended in phosphate-buffered saline (PBS) (Invitrogen, Carlsbad, CA), and immediately killed by incubation in a 60°C water bath for 30 min. Samples of the preparation were cultured on tryptic soy broth plates to ensure that the killing was complete.
Purification of flagellin.
Purified flagellin protein from PAK was prepared as previously described (35). A PAK culture was collected and sonicated using a Misonix Sonicator 3000 homogenizer (Fisher Scientific, Pittsburgh, PA) briefly to obtain flagellum release with minimal protein or LPS contamination. Bacteria were removed by centrifugation at 16,000 × g for 15 min at 4°C, and the resulting supernatant was centrifuged in an ultracentrifuge with a 42.1 rotor (model L8-70; Beckman, Duarte, CA) at 100,000 × g for 3 h at 4°C to collect the flagella. Concentrations of flagellin protein were determined by the Bradford method with bovine serum albumin as the standard. A mock flagellin preparation was prepared in an identical manner using PAK/fliC.
Cell culture.
Human embryonic kidney 293 cells were cultured in Dulbecco's modified Eagle's medium (Mediatech Cellgro, Virginia) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 IU/ml penicillin, and 100 μg/ml streptomycin. HAECs were prepared by protease digestion as previously described (18). Trachea and central bronchi freshly isolated from human lung that could not be utilized for transplantation were washed three times in 250 ml minimal essential medium supplemented with 0.5 mg/ml dithiothreitol, 10 μg/ml DNase I, 50 μg/ml ceftazidime, 2.5 μg/ml amphotericin B, and 40 μg/ml tobramycin and were digested in 250 ml of 0.1% protease-supplemented minimal essential medium (including DNase and antibiotics but not dithiothreitol). The separated cells in the medium were harvested and grown in bronchial epithelial cell growth medium (Clonetics BioWhittaker, Walkersville, MD).
Transfection.
Transfections with 293 cells or HAECs were performed as previously described (35). Briefly, a LipofectAMINE2000 reagent (Invitrogen, Carlsbad, CA) was used for 293 cells. A total of 0.5 × 106 293 cells were seeded in 24-well plates in 250 μl of Dulbecco's modified Eagle's medium per well. Then 0.5 μg of murine TLR5 plasmid was transfected to each well 24 h before bacterial challenge. Effectene reagents (Qiagen Inc., Valencia, CA) were used for transfection of HAECs. Plasmids including 0.3 μg of hBD2 promoter-driven firefly luciferase and 10 ng of the Renilla luciferase gene were used. One microgram of a DNhTLR5 plasmid was used in some experiments where indicated. Empty pIRES vectors were used to normalize the total DNA content.
Immunocytochemistry.
HAECs grown on glass coverslips were fixed with 4% paraformaldehyde, stained with rabbit phospho-p38 MAPK antibody, and subjected to ABC detection using a Vectastain ABC kit for rabbit immunoglobulin G (Vector Laboratories, Burlingame, CA).
Western blot analysis.
Cells were lysed in protein loading buffer (62.5 mM Tris-HCl [pH 6.8], 2% [wt/vol] sodium dodecyl sulfate, 10% glycerol, 50 mM dithiothreitol, 0.1% [wt/vol] bromophenol blue) and analyzed on a 10% sodium dodecyl sulfate-polyacrylamide gel. The proteins were transferred onto a nitrocellulose membrane and incubated with primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibody. Blots were developed using the enhanced chemiluminescence system (Amersham Pharmacia, Piscataway, NJ) and were exposed to X-ray film. Western blot results were quantified by densitometric analysis using GeneTools (Syngene, Frederick, MD).
Nuclear extraction.
Cellular fractionation was performed as described by Ito et al. (14). In brief, cells were lysed in ice-cold lysis buffer (10 mM Tris-HCl, 50 mM sodium bisulfite, 1% Triton X-100, 10 mM MgCl2, 8.6% sucrose, Complete protease inhibitor mixture [Roche, Germany]) for 20 min at 4°C. The supernatant cytoplasmic fraction was collected after centrifugation at 12,500 rpm with a desktop centrifuge for 10 min. The pellet was repeatedly washed in buffer until the supernatant was clear.
Luciferase assay.
A dual-luciferase assay was performed according to the manufacturer's instructions (Promega, Madison, WI) using a Wallac 1420 multilabel counter (Perkin-Elmer, Wellesley, MA). Data were expressed as the relative luciferase activity, which was determined using the firefly luciferase activity normalized by the Renilla luciferase activity.
Microarray.
A microarray was carried out using an NF-κB target gene array kit (Panomics, Inc., Redwood City, CA). A long sense strand oligonucleotide for each of the 111 human genes that have previously been shown to be regulated by the NF-κB signaling pathway was spotted in duplicate on a nitrocellulose membrane. Biotinylated DNA was spotted along the right and bottom sides of the array membrane as a control. The array experiments were performed according to the manufacturer's instructions. In brief, HAECs were stimulated for 18 h with heat-killed PAO1 bacteria (multiplicity of infection [MOI], 100). In the control experiment PBS was used instead of bacteria. mRNA of HAECs was isolated using biotin-labeled oligo(dT)20 and streptavidin-conjugated magnetic particles (Roche, Germany). Five hundred nanograms of mRNA was used to prepare the biotin-labeled cDNA probe by incorporation of biotin-dUTP into cDNA via reverse transcription (RT) reactions. Each biotin-labeled cDNA probe was hybridized to an array membrane at 42°C overnight in a hybridization incubator (Fisher Scientific, Pittsburgh, PA). The membrane was washed and subjected to further incubation with blocking buffer and subsequently with streptavidin-conjugated HRP. The membrane was developed using chemiluminescence reactions and immediately exposed to Hyperfilm ECL X-ray film (Amersham, Piscataway, NJ) for 1 min. The dots on the array were quantified by densitometric analysis using GeneTools (Syngene, Frederick, MD), and the value for each dot (D) was determined as follows: D = (signal value − background value)/mean value of biotinylated DNA dots. The ratio of the D value for the bacteria to the D value for the control was used to determine the effect of bacterial stimulation on gene regulation. A ratio of >1 indicated up-regulation of a gene.
RT-PCR.
mRNA of HAECs was isolated using biotin-labeled oligo(dT)20 and streptavidin-conjugated magnetic particles (Roche, Germany). A Titan one-tube RT-PCR kit (Roche, Germany) was used to detect target molecules in HAEC mRNA samples. Forty nanograms of mRNA of each sample was added to a reaction mixture. The following primers were used: for Cox-2, 5′TGAGCATCTACGGTTTGCTG and 5′TGCTTGTCTGGAACAACTGC; for dihydrodiol dehydrogenase (DDH1), 5′GAAGTGATCCCAAAAGATGA and 5′CTTTTGACTTGCAGAAATCC; for interleukin-8 (IL-8), 5′GGAAGAAACCACCGGAAGGA and 5′AGAGAGCCACGGCCAGCTT; for IL-1alpha, 5′CAAGTTGTGCTTATCCCATAG and 5′ATAACAGTGGTCTCATGGTTG; for interferon regulatory factor 1 (IRF-1), 5′AGGAAGGGAAATTACCTGAG and 5′TCTGTGAAGACACGCTGTAG; for mitotic arrest-deficient protein (MAD3), 5′GCTGATGTCAACAGAGTTACC and 5′AAACACACAGTCATCATAGGG; for Mn superoxide dismutase (Mn-SOD), 5′GCCTACGTGAACAACCTG and 5′GTCACGTTTGATGGCTTC; for transporter antigen peptide 1 (TAP1), 5′GTGACGGGATCTATAACAACA and 5′CACCAGGTACCACAGAAATAA; for thymic stromal lymphopoietin (TSLP), 5′TGCTACTCAGGCAATGAAG and 5′TTTGGGCTGTGAAATATGA; and for vascular endothelial growth factor C (VEGFC), 5′CTAAATCCTGGAAAATGTGC and 5′TTTCCAATATGAAGGGACAC. The specificity of the primers was tested by performing a BLAST analysis against the gene bank sequences (http://www.ncbi.nlm.nih.gov/BLAST/). A T3 thermocycler PCR system (Biometra Whatman, Germany) was used to initiate the 30-min RT reaction at 50°C, followed by a 20-cycle PCR. PCR products were separated on a 1% agarose gel containing ethidium bromide.
Cytokines.
HAEC culture supernatants were stored at −70°C for analysis of IL-8 using a human IL-8 enzyme-linked immunosorbent assay kit (BD Biosciences, Cockeysville, MD).
Statistical analyses.
Differences were analyzed using one-way analysis of variance and were considered significant when the P value was <0.05. Data were expressed as means ± standard deviations.
Microarray data accession number.
Data from the microarray experiments in this study (PAO1 versus PBS) have been deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE8986.
RESULTS
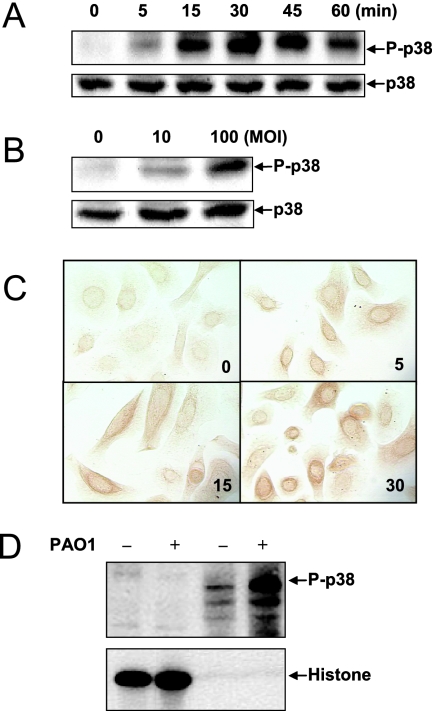
Phosphorylation of the threonine and tyrosine residues in the TGY sequence (residues 180 to 182) of p38 MAPK (P-p38 MAPK) has been shown to be necessary and sufficient for p38 MAPK activation (21). PAO1-dependent activation of p38 MAPK in HAECs was demonstrated after stimulation with heat-killed PAO1, as increased phosphorylation at T180 and Y182 was detected with P-p38 MAPK antibody (Fig. 1). The activation was time dependent, with maximal phosphorylation obtained in 30 min (Fig. 1A). At 30 min, activation of p38 MAPK was dose dependent (Fig. 1B). Immunocytochemistry revealed a time-dependent increase in cytoplasmic P-p38 MAPK which was similar to that shown in Fig. 1A (Fig. 1C). Surprisingly, no significant increase in PAO1-activated p38 MAPK was observed in the nucleus, suggesting that PAO1-activated p38 MAPK did not translocate to the nucleus (Fig. 1C). p38 MAPK translocation after PAO1 challenge was further examined by fractionation of PAO1-stimulated HAECs. Consistent with the immunocytochemistry data, induction of P-p38 MAPK was observed only in the cytoplasmic fraction of HAECs, while no detectable P-p38 MAPK was found in the nuclear extract, as identified by nuclear protein histone H3 (Fig. 1D). The data demonstrate that PAO1 stimulated p38 MAPK signaling and that instead of translocating to the nucleus, P-p38 MAPK stayed in the cytoplasm.
FIG. 1.
p38 MAPK is activated by P. aeruginosa and remains in the cytoplasm of HAECs. (A) Time-response curve for p38 MAPK phosphorylation stimulated by PAO1. HAECs were seeded in 24-well plates and stimulated with medium or heat-killed PAO1 at an MOI of 100 at 37°C. The cell lysates were analyzed by Western blotting using phosphorylated p38 (P-p38) MAPK antibody (upper panel). Variations in sample loading were examined by detection of total p38 MAPK (lower panel). (B) Dose-response curve for p38 MAPK with PAO1 stimulation. HAECs were cultured in 24-well plates and were incubated with heat-killed PAO1 at the MOI indicated for 30 min. Phosphorylated and total p38 MAPK were analyzed by Western blotting. (C) Intracellular localization of P-p38 MAPK in HAECs. HAECs grown on glass coverslips placed in 24-well plates were challenged with heat-killed PAO1 (MOI of 100). Immunocytochemical analysis using P-p38 MAPK antibody was carried out as described in Materials and Methods. The numbers indicate time (in minutes). (D) Subcellular fractionation of P-p38 MAPK in HAECs. HAECs were grown on 10-cm culture plates and incubated with PAO1 (MOI of 100) alone or with medium for 30 min. Cytoplasmic and nuclear extracts were prepared as described in Materials and Methods and subjected to blotting with antibodies against either P-p38 MAPK (upper panel) or histone H3 subunit (lower panel). All images are representative of at least two independent experiments.
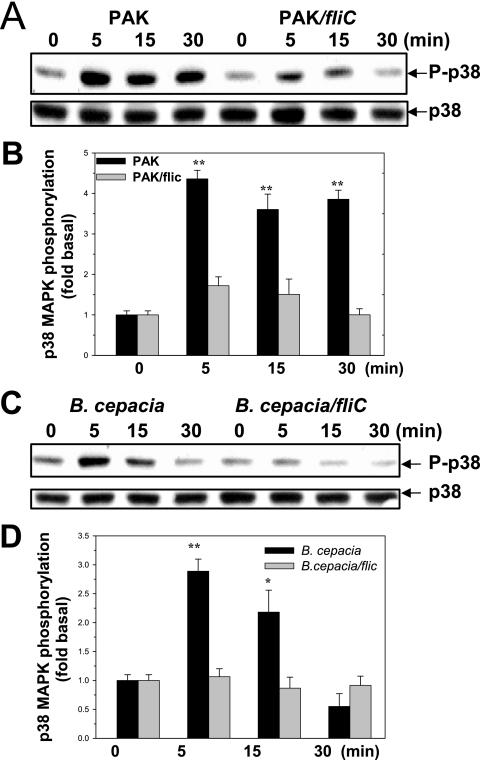
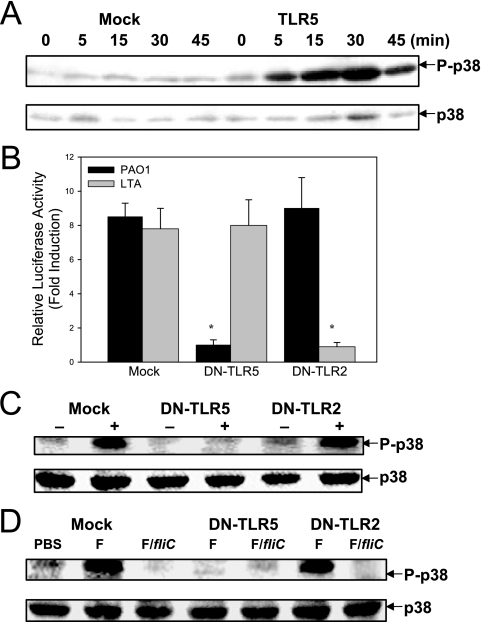
Incubation of HAECs with PAK, but not incubation with the isogenic strain PAK/fliC, a flagellin-free strain, led to significant p38 MAPK activation (Fig. 2A). A similar result was obtained with B. cepacia, where incubation of HAECs with B. cepacia, but not incubation with an isogenic flagellin-free strain, B. cepacia BC/fliC, led to p38 MAPK activation (Fig. 2C). This observation was confirmed by densitometric quantification, which indicated that activation by flagellin-deficient mutants was not statistically significant (Fig. 2B and D). When 293 cells were exposed to PAO1, activation of p38 MAPK was observed in cells that had been transfected with an expression vector for TLR5 but not in mock-transfected cells (Fig. 3A). PAO1-dependent p38 MAPK activation in TLR4-transfected 293 cells was not detected (data not shown). Overexpression of DNhTLRs, which lack the intracellular signaling TIR domain, is used to block the receptor-mediated downstream signaling. The degree of knockdown of TLR5 signaling achieved by overexpression of DNhTLR5 was examined using the NF-κB luciferase assay. As shown in Fig. 3B, overexpression of DNhTLR5 was able to induce complete and selective inhibition of NF-κB activation by TLR5 but not by TLR2 in HAECs. DNhTLR5, but not DNhTLR2, inhibited PAO1-induced p38 MAPK activation, suggesting that TLR5 mediated PAO1-induced p38 MAPK signaling (Fig. 3C). Similar inhibition of purified flagellin protein-induced p38 MAPK was exerted by DNhTLR5 but not by DNhTLR2 (Fig. 3D). These data demonstrate that flagellin, via an interaction with TLR5, is the major mechanism for p38 MAPK activation in HAECs.
FIG. 2.
P. aeruginosa activates p38 MAPK via flagellin protein in HAECs. (A) Effect of PAK and PAK/fliC on the activation of p38 MAPK. HAECs cultured in 24-well plates were incubated with heat-killed PAK or PAK/fliC at an MOI of 100 for the time intervals indicated. Phosphorylated and total p38 MAPK were analyzed by Western blotting. (B) Densitometric quantification of the results shown in panel A. Densitometry of the phosphorylated p38 MAPK was normalized by using the total loading control. The data show the fold increases for a comparison with the PBS control using normalized densitometry. (C) Effect of B. cepacia and B. cepacia BC/fliC on p38 MAPK activation. HAECs cultured in 24-well plates were incubated with heat-killed B. cepacia or B. cepacia BC/fliC (MOI of 100). Phosphorylated and total p38 MAPK were analyzed by Western blotting. (D) Densitometric quantification of the results shown in panel C. The data show the fold increases for a comparison with the PBS control using normalized densitometry. All images are representative, and the values are means and standard deviations from three independent experiments. Two asterisks, P < 0.01 for a comparison with the control; one asterisk, P < 0.05 for a comparison with the control.
FIG. 3.
P. aeruginosa activates p38 MAPK through TLR5 but not through TLR2. (A) TLR5-dependent activation of p38 MAPK by PAO1 in 293 cells. 293 cells were seeded in 24-well plates and transfected with 0.5 μg DNA of an empty vector (Mock) or murine TLR5. Twenty-four hours later, cells were incubated with heat-killed PAO1 at an MOI of 100. Phosphorylated and total p38 MAPK were analyzed by Western blotting. (B) Effect of DNhTLR5 and DNhTLR2 on PAO1- and LTA-stimulated NF-κB activation. HAECs were seeded in 24-well plates and transfected with empty vector (1 μg), DNhTLR5 (1 μg), or DNhTLR2 (1 μg) in combination with plasmids containing the hBD2 promoter-driven luciferase gene (25 ng) and the Renilla luciferase gene (1 ng). Twenty-four hours later, cells were stimulated with medium, heat-killed PAO1 (MOI of 100), or LTA (10 μg/ml) for 18 h. Induction of luciferase activity was measured with the dual-luciferase assay. (C) Effect of DNhTLR5 and DNhTLR2 on PAO1-stimulated p38 MAPK activation in HAECs. HAECs were seeded in 24-well plates and transfected with empty vector (1 μg), DNhTLR5 (1 μg), or DNhTLR2 (1 μg). Twenty-four hours after transfection, cells were incubated with heat-killed PAO1 at an MOI of 100 for 30 min. Phosphorylated and total p38 MAPK were analyzed by Western blotting. (D) Effect of DNhTLR5 and DNhTLR2 on the activation of p38 MAPK by P. aeruginosa flagellin protein. HAECs were seeded in 24-well plates and transfected with empty vector (1 μg), DNhTLR5 (1 μg), or DNhTLR2 (1 μg). Twenty-four hours after transfection, cells were incubated with 1 μg/ml flagellin protein purified from PAK (lanes F) or mock flagellin protein prepared from PAK/fliC (lanes F/fliC) for 30 min. Phosphorylated and total p38 MAPK were analyzed by Western blotting. All images are representative of at least two independent experiments. One asterisk, P < 0.01 for a comparison with the control.
c-Jun N-terminal kinase (JNK) and ERK are two close relatives of p38 MAPK in the MAPK family. Interestingly, we found that ERK was not activated by PAO1 in HAECs, while JNK was activated by heat-killed PAO1 in TLR5-transfected 293 cells but not in mock-transfected 293 cells, and this activation was flagellin dependent in HAECs since unlike PAK, the flagellin-deficient isogenic strain PAK/fliC failed to activate JNK (date not shown). We next focused on the p38 MAPK signaling pathway for a more extensive investigation of its role in gene regulation of the cellular response to P. aeruginosa.
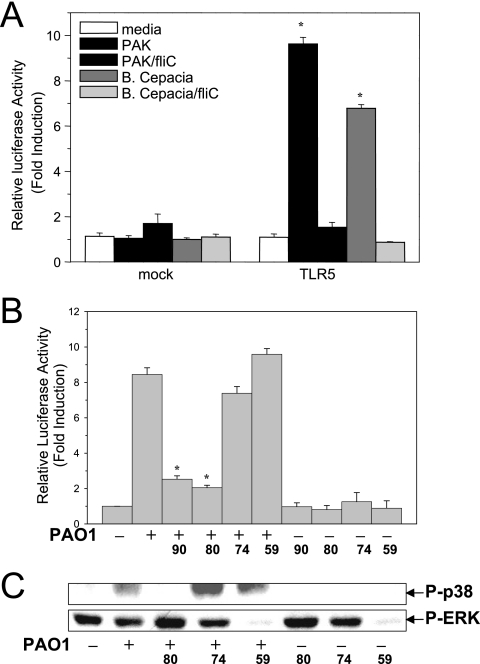
The NF-κB signaling pathway is activated by P. aeruginosa in a manner similar to the manner determined for p38 MAPK. 293 cells were transfected with a luciferase reporter gene controlled by the promoter region of the hBD2 gene that contains three NF-κB binding sites with or without TLR5 (29). As shown in Fig. 4A, P. aeruginosa strain PAK, but not the isogenic flagellin-free strain PAK/fliC, activated NF-κB signaling in the presence of TLR5. Similar results were obtained with the isogenic pair B. cepacia and flagellin-free strain BC/fliC (Fig. 4A).
FIG. 4.
p38 MAPK is required for activation of TLR5-dependent NF-κB signaling. (A) Flagellin activates NF-κB signaling via TLR5. 293 cells grown in 96-well plates were transfected with empty vector (25 ng) (mock) or murine TLR5 DNA (25 ng) in combination with the hBD2 promoter-driven firefly luciferase reporter vector (25 ng) and the Renilla luciferase vector (1 ng). Twenty-four hours later, cells were stimulated with medium, heat-killed PAK, PAK/fliC, B. cepacia, or B. cepacia BC/fliC for 18 h. Induction of luciferase activity in the cells was measured by dual-luciferase assay. (B) p38 MAPK is involved in P. aeruginosa-induced activation of NF-κB signaling in HAECs. HAECs were seeded in 24-well plates and transfected with a DNA mixture containing 0.3 μg of the hBD2 promoter-driven firefly luciferase gene and 10 ng of the Renilla luciferase gene. Twenty-four hours later, cells were incubated with medium, SB202190 (90) (10 μM), SB203580 (80) (10 μM), SB202474 (74) (10 μM), or PD98059 (59) (20 μM) for 30 min. After washing with PBS, the cells were incubated with medium or heat-killed PAO1 (MOI of 100). Eighteen hours later, the dual-luciferase assay was performed to measure the induction of luciferase activity in the cells. (C) PAO1 induces autophosphorylation of p38 MAPK in HAECs. HAECs cultured in 24-well plates were pretreated with medium, SB203580 (10 μM), SB202474 (10 μM), or PD98059 (20 μM) for 30 min. After washing with PBS, the cells were incubated with heat-killed PAO1 (MOI of 100) for 30 min. The cell lysates were analyzed by blotting using phosphorylated p38 (P-p38) MAPK or P-ERK antibodies. Results were obtained from at least three different experiments. One asterisk, P < 0.01 for a comparison with the control.
The inhibitors SB202190 and SB203580 bind specifically to the p38 MAPK ATP binding site to block p38 MAPK activity (20). The concentrations of the inhibitors were selected based on their 50% inhibitory concentrations, and these inhibitors were shown to cause specific inhibition in earlier studies (36). As determined in this study, inhibition of p38 MAPK activity with SB203580 led to significantly attenuated autophosphorylation of p38 MAPK (Fig. 4C). The inhibitors at the selected concentrations had no effect on PAO1-induced JNK activation in HAECs, and no signs of toxicity were observed after treatment with the inhibitors (data not shown). The inhibitors used in our studies had no effect on the basal level of activity of NF-κB or p38 MAPK (Fig. 4B and C). Interestingly, pretreatment of HAECs transfected with the NF-κB-driven luciferase reporter with p38 MAPK inhibitor SB202190 or SB203580 significantly attenuated PAO1-stimulated luciferase activity, while the inactive analogue SB202474 or ERK inhibitor PD98059 had no such effect (Fig. 4B). This observation suggests that p38 MAPK is upstream of NF-κB signaling and is involved in its regulation.
An NF-κB target gene array analysis (Panomics, Inc., Redwood City, CA) was performed to profile the PAO1-dependent expression pattern of 111 NF-kB-regulated genes in HAECs as described in Materials and Methods. Thirty-six genes were found to be up-regulated in response to PAO1 stimulation compared to a PBS control array (ratio, >1), and 15 of these genes showed a >1-fold increase in mRNA level (ratio, >2). Our preliminary gene array data for PAK and PAK/fliC suggested that PAK could induce a set of genes that is similar to the set of PAO1 genes. Interestingly, there appeared to be a lack of signals overall (e.g., IL-8) in response to PAK/fliC, suggesting that flagellin plays a critical role in the induction of the cellular responses in HAECs to P. aeruginosa (data not shown).
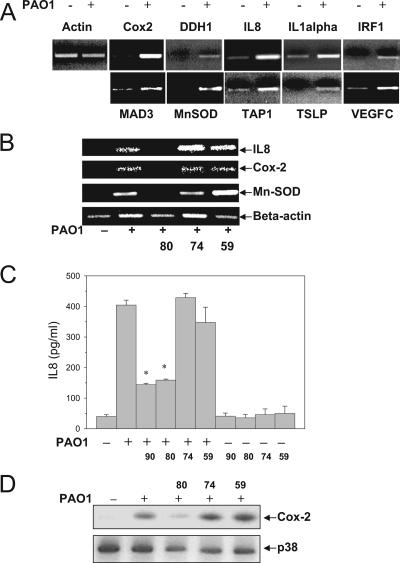
Nine genes were selected as representatives of the up-regulated genes identified in the microarray experiment for further confirmation by RT-PCR. We also included one gene (the gene encoding TSLP) that was not available for the microarray in the RT-PCR experiment. These genes were all confirmed by RT-PCR, and the nine genes identified by the microarray experiment were the genes encoding Cox-2, DDH1, IL-8, IL-1alpha, IRF-1, MAD3, Mn-SOD, TAP1, and VEGFC (Fig. 5A).
FIG. 5.
p38 MAPK mediates gene regulation in HAECs in response to P. aeruginosa stimulation. (A) Gene up-regulation in HAECs after PAO1 stimulation. HAECs were cultured in 10-cm plates until complete confluence and were stimulated with medium or heat-killed PAO1 (MOI of 100) for 18 h. Following a 30-min RT reaction at 50°C, 20 cycles of PCR were performed. Forty nanograms of mRNA was used in each reaction mixture. PCR products were separated on a 1% agarose gel containing ethidium bromide. (B) Inhibition of p38 MAPK activity inhibited PAO1-stimulated gene up-regulation. HAECs were cultured in 10-cm plates until complete confluence. The cells were incubated with medium or SB203580 (10 μM) and then incubated with heat-killed PAO1 (MOI of 100) for 18 h. mRNA was isolated, and RT-PCR was performed to determine the mRNA level of target molecules. (C) Inhibition of p38 MAPK activity significantly attenuated PAO1-stimulated IL-8 protein secretion in HAECs. HAECs were cultured in 24-well plates, incubated with medium, SB202190 (90) (10 μM), SB203580 (80) (10 μM), SB202474 (74) (10 μM), or PD98059 (59) (20 μM) for 30 min, and then incubated with medium or heat-killed PAO1 (MOI of 100) for 18 h. IL-8 in the supernatant (500 μl/well of a 24-well plate) was analyzed using a human IL-8 enzyme-linked immunosorbent assay kit. (D) Inhibition of p38 MAPK blocked PAO1-stimulated Cox-2 protein production in HAECs. HAECs cultured in 24-well plates were incubated with medium, SB203580 (10 μM), SB202474 (10 μM), or PD98059 (20 μM) for 30 min, and then incubated with medium or heat-killed PAO1 (MOI of 100) for 18 h. Cells were lysed directly in protein loading buffer and subjected to blot analysis of the Cox-2 protein expression level using Cox-2 antibodies. All results were confirmed in at least two different experiments. One asterisk, P < 0.01 for a comparison with the control.
As shown in Fig. 5B, pretreatment of HAECs with SB203580 blocked or attenuated the PAO1-dependent increase in mRNA for IL-8, Cox-2, and Mn-SOD. Other than the IL-8, Cox-2, and Mn-SOD genes, we found it difficult to draw firm conclusions concerning the rest of the genes. This was largely due to the technical obstacles created by the overall low expression or high baseline expression of the genes. The effects of PAO1 and p38 MAPK inhibitors on IL-8 mRNA were confirmed by measuring IL-8 secretion. The level of secreted IL-8 was increased 10-fold by treatment with heat-killed PAO1. Pretreatment with SB202190 or SB203580 attenuated IL-8 secretion, while the control compound SB202474 and PD98059 showed no effect (Fig. 5C). None of the inhibitors had an effect on the basal level of IL-8 secretion. We further examined the effects of the inhibitors on PAO1-induced protein expression of the Cox-2 gene, a gene that is similarly regulated, as shown in Fig. 5B. PAO1 induced a substantial level of Cox-2 protein expression in HAECs (Fig. 5D). Similarly, the Cox-2 up-regulation was significantly inhibited by p38 MAPK inhibitor SB203580 (Fig. 5D), but it was not affected by the control chemical SB202474 or the ERK inhibitor PD98059.
DISCUSSION
The evidence described in this paper is the first evidence showing that in primary HAECs flagellin protein is the dominant component in the activation of p38 MAPK signaling upon P. aeruginosa infection; the activation is induced through the interaction of flagellin with TLR5, which subsequently induces multiple genes critically involved in different aspects of the cellular responses of airway epithelial cells.
P. aeruginosa contains multiple cell surface factors, including flagellin, pili, and LPS, which have been found to interact with distinct epithelial membrane proteins, such as asialylated glycolipid receptors, TLRs, or combinations of these proteins (26, 27). Raia et al. showed that LPS, a component derived from P. aeruginosa, activates p38 MAPK in CF nasal epithelial cells (24). Upon stimulation with flagellin-negative strains, there was observable but not statistically significant activation of p38 MAPK, suggesting that a component(s) other than LPS may be more potent in stimulating p38 MAPK activation and thus that it is necessary to use whole P. aeruginosa cells to evaluate the relative significance of cell surface components of P. aeruginosa in p38 MAPK activation (Fig. 2). Our data show that when the flagellin protein is absent, as observed with PAK/fliC and B. cepacia BC/fliC, activation of p38 MAPK is nearly abolished (Fig. 2). These results suggest that instead of LPS, flagellin is the major component that stimulates the cellular response of HAECs. Consistent with the finding of other workers that purified flagellin protein induces strong activation of p38 MAPK signaling, we found that blocking TLR5, but not blocking another receptor, such as TLR2, in HAECs led to significant attenuation of PAO1-induced p38 MAPK activation (28, 35) (Fig. 3B). Delgado et al. recently showed that DNA released from P. aeruginosa can activate p38 MAPK through a non-TLR9-dependent pathway in CF airway epithelial cell lines, which leads to IL-8 secretion (6). Although whether the same pathway also exists in HAECs remains to be further tested, it is possible that the low level of p38 MAPK activation observed in our experiments was caused by contamination of leaked DNA in heat-killed P. aeruginosa preparations, as such a pathway exists in these cells.
Under in vivo conditions, the minor and major p38 MAPK stimulants may work in a coherent way. Although earlier studies suggested that airway epithelial cells lack responsiveness to LPS due to the absence of sufficient coreceptors, such as MD2 and CD14 for TLR4 signaling (3, 13), previous studies by other workers and us have shown that LPS purified from some bacteria can stimulate a minor response in HAECs (7, 35). Studies have suggested that in TLR2 or TLR4 knockout mice, early pulmonary clearance of P. aeruginosa after infection with a moderate dose is not significantly affected (25; unpublished data). Infection with a high dose of PAO1 led to increased mortality and morbidity in TLR4-deficient mice (unpublished data). In addition, using knockout animal models, Feuillet et al. recently demonstrated that the mortality due to P. aeruginosa pneumonia was increased in the absence of both TLR4 and TLR5 (8).
It is not clear what intermediate steps are required for TLR5-medated p38 MAPK signaling. Ge et al. have shown that p38 MAPK can be activated via autophosphorylation of p38 MAPK, which is mediated by transforming growth factor β-activated protein kinase 1-binding protein 1 (TAB1), and TAB1 signaling seemed to be immediately activated by the MyD88-interleukin receptor-associated kinase (IRAK)-tumor necrosis factor receptor-associated factor 6 (TRAF6) signaling cascade (9). Indeed, our previous study using dominant negative molecules demonstrated that MyD88, IRAK, and TRAF6 are indispensable for TLR5-mediated NF-κB activation in HAECs (35). In this study, our data further showed that PAO1 induced autophosphorylation of p38 MAPK (Fig. 4C). These findings suggest that P. aeruginosa is sensed by TLR5 and may subsequently evoke the MyD88-IRAK-TRAF6-TAB1 signaling cascade to induce the p38 MAPK autophosphorylation which activates p38 MAPK. Wu et al. have recently shown that stimulation of polarized HAECs with P. aeruginosa leads to activation of IRAK1 and p38 MAPK, which subsequently results in IL-8 secretion (31).
Studies have shown that P. aeruginosa activates NF-κB and MAPK signaling pathways, which in turn leads to the production of inflammatory cytokines, such as IL-6, IL-8, and tumor necrosis factor alpha (26, 34). However, whether MAPK and NF-κB signaling pathways are independent mechanisms underlying gene regulation remains controversial. Tallant et al. have shown that in intestinal epithelial cells flagellin-induced activation of p38 MAPK, but not NF-κB, is required for maximal IL-8 transcription and mRNA production (28). Delgado et al. showed that in CF airway epithelial cells IL-8 secretion was not due to an increase in NF-κB- or activator protein 1-dependent IL-8 promoter transcription but instead depended on p38 MAPK and ERK (6), while other workers have suggested that in corneal epithelial cells NF-κB is the master regulator of cytokines, including IL-8, and p38 MAPK is partially involved (34). The difference in these observations concerning the signaling pathway used to respond to P. aeruginosa may have reflected the difference in the cell types selected for the studies. Our study, using primary HAECs, showed that inhibition of p38 MAPK activity significantly attenuated NF-κB signaling and the up-regulation of multiple genes regulated by NF-κB (Fig. 4B and Fig. 5), suggesting that p38 MAPK is essential for the complete function of NF-κB signaling in the epithelial cellular response to PAO1. After activation by PAO1, p38 MAPK did not translocate into the nucleus, suggesting that p38 MAPK is unlikely to be involved in the direct phosphorylation of nucleus-located transcription factors, such as activating transcription factor-2 (Fig. 1C and D). So far, little is known about how NF-κB activity is regulated by p38 MAPK. There is still no evidence showing that p38 MAPK interacts directly with NF-κB components. A recent study suggested that p38 MAPK may activate mitogen- and stress-activated protein kinase 1, which in turn can phosphorylate p65 at Ser-276, leading to activation of the NF-κB signaling pathway (16). p38 MAPK phosphorylates many kinases in the cytoplasm, including MAPK-interacting kinase 1/2, MAPK-activated protein kinase 2, mitogen- and stress-activated protein kinase 1, and p38-regulated and -activated kinase, which regulate gene expression by phosphorylating transcription factors such as cyclic AMP-responsive element-binding protein and activating transcription factor-1 (11).
Well-differentiated air-liquid interface HAEC cultures have been extensively used as an epithelium xenograft model for gene transfer research; however, little is known about the intracellular signaling events in this model. Wu et al. showed that p38 MAPK signaling is activated upon challenge of P. aeruginosa using well-differentiated primary human tracheobronchial epithelial cell cultures, suggesting that what we observed in the submerged HEACs provides information for differentiated HAECs (31). Recently, Feuillet et al. (8) showed that epithelium-derived TLR singling pathways, but not bone marrow-derived TLR singling pathways, are involved in the recognition of P. aeruginosa, indicating that the epithelia have a critical role in the host defense against bacterial infection. Thus, we believe that an important future direction of our research is to validate what we have learned from submerged HAECs in well-differentiated air-liquid interface cultures.
Inhibitors of p38 MAPK are under investigation in preclinical and clinical settings for treating a variety of inflammatory diseases (20). In addition to inflammatory responses, our data suggest that a vast array of genes with differential biological functions are up-regulated upon P. aeruginosa infection in HAECs (Fig. 5). Besides inflammatory genes, including the genes encoding IL-8, TSLP, IL-1alpha, and the transcription factor IRF-1 (15, 26, 37), a set of genes associated with tumorigenesis, such as the genes encoding DDH1, TAP1, Mn-SOD, and VEGFC, are also up-regulated (4, 19, 32, 33). Cox-2 has been linked to production of prostaglandin E2, which induces mucus secretion and promotes vasodilation and edema (15, 21, 30). Our findings suggest that the p38 MAPK signaling pathway may serve as a critical therapeutic target and that cell type-specific modulation, especially modulation that can target airway epithelial cells, is desired for managing different manifestations of infectious diseases.
Acknowledgments
We thank Jeffrey N. Weiser for careful reading of the manuscript. We thank Deirdre McMenamin (Animal Model Group) and Peter Bell and Di Wu (Cell Morphology Core) from the Gene Therapy Program for technical assistance.
This work was supported by NIH grant 5R01HL049040-13 and by the Cystic Fibrosis Foundation.
Editor: F. C. Fang
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Alegado, R. A., M. C. Campbell, W. C. Chen, S. S. Slutz, and M. W. Tan. 2003. Characterization of mediators of microbial virulence and innate immunity using the Caenorhabditis elegans host-pathogen model. Cell. Microbiol. 5:435-444. [DOI] [PubMed] [Google Scholar]
- 2.Asai, T., G. Tena, J. Plotnikova, M. R. Willmann, W. L. Chiu, L. Gomez-Gomez, T. Boller, F. M. Ausubel, and J. Sheen. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977-983. [DOI] [PubMed] [Google Scholar]
- 3.Becker, M. N., G. Diamond, M. W. Verghese, and S. H. Randell. 2000. CD14-dependent lipopolysaccharide-induced beta-defensin-2 expression in human tracheobronchial epithelium. J. Biol. Chem. 275:29731-29736. [DOI] [PubMed] [Google Scholar]
- 4.Benlloch, M., S. Mena, P. Ferrer, E. Obrador, M. Asensi, J. A. Pellicer, J. Carretero, A. Ortega, and J. M. Estrela. 2006. Bcl-2 and Mn-SOD antisense oligodeoxynucleotides and a glutamine-enriched diet facilitate elimination of highly resistant B16 melanoma cells by tumor necrosis factor-α and chemotherapy. J. Biol. Chem. 281:69-79. [DOI] [PubMed] [Google Scholar]
- 5.Davies, J. C. 2002. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr, Respir. Rev. 3:128-134. [DOI] [PubMed] [Google Scholar]
- 6.Delgado, M. A., J. F. Poschet, and V. Deretic. 2006. Nonclassical pathway of Pseudomonas aeruginosa DNA-induced interleukin-8 secretion in cystic fibrosis airway epithelial cells. Infect. Immun. 74:2975-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 286:1561-1565. [DOI] [PubMed] [Google Scholar]
- 8.Feuillet, V., S. Medjane, I. Mondor, O. Demaria, P. P. Pagni, J. E. Galan, R. A. Flavell, and L. Alexopoulou. 2006. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. USA 103:12487-12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge, B., H. Gram, F. Di Padova, B. Huang, L. New, R. J. Ulevitch, Y. Luo, and J. Han. 2002. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 295:1291-1294. [DOI] [PubMed] [Google Scholar]
- 10.Greene, C. M., T. P. Carroll, S. G. Smith, C. C. Taggart, J. Devaney, S. Griffin, J. S. O'Neill, and N. G. McElvaney. 2005. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J. Immunol. 174:1638-1646. [DOI] [PubMed] [Google Scholar]
- 11.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar, A. M., H. Harowicz, H. D. Liggitt, P. J. Fink, C. B. Wilson, and S. J. Skerrett. 2005. An essential role for non-bone marrow-derived cells in control of Pseudomonas aeruginosa pneumonia. Am. J. Respir. Cell Mol. Biol. 33:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hybiske, K., J. K. Ichikawa, V. Huang, S. J. Lory, and T. E. Machen. 2004. Cystic fibrosis airway epithelial cell polarity and bacterial flagellin determine host response to Pseudomonas aeruginosa. Cell. Microbiol. 6:49-63. [DOI] [PubMed] [Google Scholar]
- 14.Ito, K., E. Jazrawi, B. Cosio, P. J. Barnes, and I. M. Adcock. 2001. p65-activated histone acetyltransferase activity is repressed by glucocorticoids: mifepristone fails to recruit HDAC2 to the p65-HAT complex. J. Biol. Chem. 276:30208-30215. [DOI] [PubMed] [Google Scholar]
- 15.Jindal, S. K., and R. Agarwal. 2005. Autoimmunity and interstitial lung disease. Curr. Opin. Pulm. Med. 11:438-446. [DOI] [PubMed] [Google Scholar]
- 16.Kefaloyianni, E., C. Gaitanaki, and I. Beis. 2006. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell. Signal. 18:2238-2251. [DOI] [PubMed] [Google Scholar]
- 17.Kim, D. H., R. Feinbaum, G. Alloing, F. E. Emerson, D. A. Garsin, H. Inoue, M. Tanaka-Hino, N. Hisamoto, K. Matsumoto, M. W. Tan, and F. M. Ausubel. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623-626. [DOI] [PubMed] [Google Scholar]
- 18.Klockmann, M. T., H. U. Jahn, S. Hippenstiel, H. J. Kramer, and N. Suttorp. 1998. Interaction of human neutrophils with airway epithelial cells: reduction of leukotriene B4 generation by epithelial cell derived prostaglandin E2. J. Cell. Physiol. 175:268-275. [DOI] [PubMed] [Google Scholar]
- 19.Kloor, M., C. Becker, A. Benner, S. M. Woerner, J. Gebert, S. Ferrone, and M. von Knebel Doeberitz. 2005. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 65:6418-6424. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., J. Boehm, and J. C. Lee. 2003. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2:717-726. [DOI] [PubMed] [Google Scholar]
- 21.Mann, J. R., M. G. Backlund, and R. N. DuBois. 2005. Mechanisms of disease: inflammatory mediators and cancer prevention. Nat. Clin. Pract. Oncol. 2:202-210. [DOI] [PubMed] [Google Scholar]
- 22.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell. Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 23.Philpott, D. J., S. E. Girardin, and P. J. Sansonetti. 2001. Innate immune responses of epithelial cells following infection with bacterial pathogens. Curr. Opin. Immunol. 13:410-416. [DOI] [PubMed] [Google Scholar]
- 24.Raia, V., L. Maiuri, C. Ciacci, I. Ricciardelli, L. Vacca, S. Auricchio, M. Cimmino, M. Cavaliere, M. Nardone, A. Cesaro, J. Malcolm, S. Quaratino, and M. Londei. 2005. Inhibition of p38 mitogen activated protein kinase controls airway inflammation in cystic fibrosis. Thorax 60:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramphal, R., V. Balloy, M. Huerre, M. Si-Tahar, and M. Chignard. 2005. TLRs 2 and 4 are not involved in hypersusceptibility to acute Pseudomonas aeruginosa lung infections. J. Immunol. 175:3927-3934. [DOI] [PubMed] [Google Scholar]
- 26.Ratner, A. J., R. Bryan, A. Weber, S. Nguyen, D. Barnes, A. Pitt, S. Gelber, A. Cheung, and A. Prince. 2001. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J. Biol. Chem. 276:19267-19275. [DOI] [PubMed] [Google Scholar]
- 27.Sadikot, R. T., T. S. Blackwell, J. W. Christman, and A. S. Prince. 2005. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am. J. Respir. Crit. Care Med. 171:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tallant, T., A. Deb, N. Kar, J. Lupica, M. J. de Veer, and J. A. DiDonato. 2004. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, X., Z. Zhang, J. P. Louboutin, C. Moser, D. J. Weiner, and J. M. Wilson. 2003. Airway epithelia regulate expression of human beta-defensin 2 through Toll-like receptor 2. FASEB J. 17:1727-1729. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg, J. B. 2000. Nitric oxide synthase 2 and cyclooxygenase 2 interactions in inflammation. Immunol. Res. 22:319-341. [DOI] [PubMed] [Google Scholar]
- 31.Wu, Q., Z. Lu, M. W. Verghese, and S. H. Randell. 2005. Airway epithelial cell tolerance to Pseudomonas aeruginosa. Respir. Res. 6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, M. D., C. C. Wu, S. H. Chiou, C. F. Chiu, T. Y. Lin, I. P. Chiang, and K. C. Chow. 2003. Reduction of dihydrodiol dehydrogenase expression in resected hepatocellular carcinoma. Oncol. Rep. 10:271-276. [PubMed] [Google Scholar]
- 33.Yu, X. M., C. Y. Lo, W. F. Chan, K. Y. Lam, P. Leung, and J. M. Luk. 2005. Increased expression of vascular endothelial growth factor C in papillary thyroid carcinoma correlates with cervical lymph node metastases. Clin. Cancer Res. 11:8063-8069. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, J., X. Y. Wu, and F. S. Yu. 2005. Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr. Eye Res. 30:527-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, Z., J. P. Louboutin, D. J. Weiner, J. B. Goldberg, and J. M. Wilson. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 73:7151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Z., S. M. Xin, G. X. Wu, W. B. Zhang, L. Ma, and G. Pei. 1999. Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108-15 cells and this is regulated by protein kinase A and protein kinase C. J. Neurochem. 73:1502-1509. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, B., M. R. Comeau, T. De Smedt, H. D. Liggitt, M. E. Dahl, D. B. Lewis, D. Gyarmati, T. Aye, D. J. Campbell, and S. F. Ziegler. 2005. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6:1047-1053. [DOI] [PubMed] [Google Scholar]