Abstract
Vascular endothelium is an exposed target in systemic endovascular Staphylococcus aureus infections. We reported earlier that the proinflammatory and procoagulant activities of primary human umbilical vein endothelial cells (ECs) after binding and ingestion of S. aureus organisms provide the cells effective means for leukocyte-mediated bacterial elimination. Expanding on this, we now show that these ECs exhibit a modest intrinsic capacity for eliminating intracellular S. aureus that was influenced by cytokines relevant to S. aureus infections. Using various EC infection assays, we showed that gamma interferon (IFN-γ), applied to cultures of ECs prior to or after infection with S. aureus, markedly reduced the level of infection, illustrated by lower percentages of S. aureus-infected ECs and less intracellular bacteria per infected cell. IFN-γ-activated ECs had unaltered abilities to bind S. aureus and processed ingested bacteria by a seemingly conventional phagocytic pathway. IFN-γ treatment rescued EC monolayers from severe injury by virulent clinical S. aureus strains or excessive bacterial numbers. Mechanistically, IFN-γ controls S. aureus infection via IFN-γ receptor, most likely through stimulation of intrinsic endothelial antibacterial mechanisms but independent of processes that deprive bacteria of intracellular l-tryptophan or iron. The antibacterial activity of IFN-γ-stimulated ECs coincided with sustained or slightly elevated endothelial proinflammatory responses that supported monocyte recruitment. In conclusion, we identify IFN-γ as a potent regulatory Th1 cytokine possessing exclusive abilities to augment intrinsic antistaphylocccal effector mechanisms in human ECs without ablating the S. aureus-induced proinflammatory EC responses and, as such, coordinating a protective efficacy of ECs against blood-borne S. aureus infection.
Staphylococcus aureus is a predominantly extracellularly growing, highly virulent gram-positive bacterium and the most common cause of community- and hospital-acquired infections. The clinical manifestations range from superficial skin infections to potentially fatal hematogenous infections, such as sepsis, vasculitis, and endocarditis (1, 22, 26). Such infections depend principally on the ability of the bacterium to spread via the bloodstream and to colonize the endothelial cells (ECs) that line the vascular system (1, 21, 22, 26) and, by preference, ECs that line the postcapillary venules (21). Accordingly, a repertoire of secreted staphylococcal adhesins and cell wall-anchored staphylococcal adhesive proteins with extracellular matrix binding properties, such as clumping factor A and fibronectin binding proteins FnBPA and FnBPB, has been described to mediate interactions between S. aureus and ECs (8, 17, 20, 31, 35, 43, 44) or to control bacterial motility on the surface of macrophages and ECs and to modulate the invasion kinetics (30, 39, 42).
The subsequent role ECs play in the control of a hematogenously spread S. aureus infection after the initial bacterial adherence is elusive. Human venous ECs as well as heart valve ECs actively internalize S. aureus organisms (5, 23, 28, 57) by mechanisms that resemble the classic “zipper” model of phagocytosis by professional phagocytes (15). Inside ECs, S. aureus organisms are observed within phagolysosome-like organelles (5, 23, 28) with bactericidal activities (40). Occasionally, bacteria translocate to the cytosol and acquire an indolent “small-colony-variant” phenotype that allows intraendothelial persistence (23, 25, 33, 39, 56). Although the frequency and significance of intraendothelial S. aureus are difficult to determine, the theory resulting from these findings was that ECs contribute to the persistent and recurrent nature of S. aureus infections rather than adding significantly to S. aureus clearance.
In the past decade, however, data pointing to a more influential contribution of ECs to host defense and S. aureus elimination have accumulated. Predisposed by the magnitude of bacterial challenge and specific bacterial characteristics (e.g., type, strain, and virulence), S. aureus-infected human ECs initiate and coordinate production of a variety of proinflammatory mediators, e.g., interleukin-1 (IL-1), IL-6, CXCL8 (IL-8), CCL2 (monocyte chemoattractant protein 1), and CCL5 (RANTES) (24, 45, 47, 59, 60) and orderly express significant levels of the adhesion molecules CD54 (ICAM-1) and CD106 (VCAM-1), tissue factor procoagulant molecule (CD142), and major histocompatibility complex class I (MHC-I) molecules on their cell membrane (5, 14, 24, 46, 53). Concomitantly, S. aureus-infected ECs avidly bind circulating monocytes and granulocytes (5, 54) that, by their adhesion, show enhanced phagocytic killing (29) and tissue factor procoagulant molecule-dependent fibrin deposition (52-54). From these data, it is apparent that S. aureus-infected endothelium, through initiation of coagulation, inflammation, and concordant recruitment of professional phagocytes, has developed a mostly indirect yet very effective mechanism for elimination of cell-associated bacteria. The present study continues work on this subject and aims to uncover more direct mechanisms in human vascular endothelium that limit survival of associated S. aureus organisms and subsequent staphylococcal infections.
The early phase of disseminated infection with S. aureus is characterized by the endogenous production of inflammatory cytokines, such as IL-1, IL-6, IL-18, tumor necrosis factor alpha (TNF-α), and gamma interferon (IFN-γ), which then cooperatively coordinate host resistance (10, 22, 27, 36, 62). Of these, the pleiotropic 17-kDa polypeptide IFN-γ is regarded as a key regulatory cytokine, produced mainly by activated T lymphocytes, and is capable of orchestrating both innate and acquired host resistances against staphylococcal infections (10, 36, 37). In patients with infective endocarditis, a potent modulatory contribution of this cytokine is suggested, as high numbers of IFN-γ-secreting CD8+ T lymphocytes are found underneath the valvular endothelium (58). IFN-γ induces classically activated macrophages and neutrophils, defined by their enhanced phagocytic and microbicidal effector functions and efficient recruitment of effector T lymphocytes (41). However, also in studies with nonprofessional phagocytes, including human ECs, IFN-γ treatment (often in concert with TNF-α) efficiently restricts intracellular growth of obligatory intracellular pathogens such as Pseudomonas aeroginosa (12), Toxoplasma gondii (11), and Candida albicans (19), most likely by mechanisms involving production of reactive oxygen radicals or tryptophan depletion (11, 12, 41).
In an effort to better understand the often recurrent nature and pathogenesis of endovascular S. aureus infections, the aforementioned studies and postulated similarities of ECs with professional phagocytes, with regard to S. aureus phagocytosis, led us to design the present study. In particular, we focused on the question of whether human ECs can be considered potential effector cells in the (local) immune response against blood-borne S. aureus and, as such, display properties that provide defense against the lethal effects of these pathogens. Given the relevance of IFN-γ, a comprehensive investigation to outline the ability of this cytokine to confer resistance to infection with S. aureus in ECs and/or to modulate the inflammatory phenotype of S. aureus-infected EC was set up.
MATERIALS AND METHODS
Cell culture media and chemicals.
Medium 199 (M199) and fetal calf serum were purchased from GIBCO Laboratories (Grand Island, NY), penicillin from Brocades Pharma B.V. (Leiderdorp, The Netherlands), streptomycin from Gist-Brocades N.V. (Delft, The Netherlands), amphotericin B from Squibb B.V. (Rijswijk, The Netherlands), l-glutamin from Flow Laboratories (Irvine, United Kingdom), collagenase type 1A, lysostaphin, cytochalasin E, and l-tryptophan from Sigma-Aldrich Co. (St. Louis, MO), gelatin and trypsin from Difco Laboratories (Detroit, MI), and EDTA from Boehringer Mannheim (Mannheim, Germany). Human serum was isolated from healthy donors and used after inactivation at 56°C for 30 min (HuSi). Iron salt ferric citrate came from BDH Chemicals Ltd. (Poole, United Kingdom) and was used after adjustment to pH 7.4 with 2 M NaOH.
Cytokines and MAbs.
Recombinant human IFN-γ and IL-1α were purchased from Hoechst Marion Roussel S. A. (Romanville Cédex, France) and Hoffmann-La Roche (Nutley, NJ), respectively. The following monoclonal antibodies (MAbs) against human antigens were used: anti-CD11b MAb 2LPM19c from DakoCytomation B. V. (Heverlee, Belgium); anti-CD49d MAb 15A8 from Sanquin-CLB reagents (Amsterdam, The Netherlands); anti-CD29 MAb K20 from Immunotech S. A. (Marseille, France); anti-CD54 MAb MEM112 from Sanbio B. V. (Uden, The Netherlands); anti-CD106 MAb 1G11B1 from Biosource International (Camarillo, CA); and anti-HLA class I MAb W6/32 from F. Koning (Leiden University Medical Center, Leiden, The Netherlands). Anti-CD18-specific hybridomas IB4 were obtained from the American Type Culture Collection (Manassas, VA). For confocal microscopy mouse anti-human early endosomal antigen-1 (EEA-1) MAb 14 from BD Biosciences (Palo Alto, CA) and mouse anti-human CD107A (LAMP-1) MAb H4A3 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), were used. A neutralizing anti-CD119 MAb against IFNGR1, the α subunit of human IFN-γ receptor (IFN-γR), was purchased from Genzyme (Cambridge, MA).
Cell isolation and culture.
Human ECs were isolated from umbilical cord veins by collagenase digestion and cultured on 0.75% (wt/vol) gelatin-coated tissue culture dishes (Falcon; BD Biosciences, Alphen a/d Rijn, The Netherlands) in culture medium, i.e., M199 supplemented with 1,000 U/ml penicillin G, 0.1 mg/ml streptomycin, 100 U/ml amphotericin B, 0.1 mg/ml crude EC growth factor from bovine hypothalamus, 5 U/ml heparin, 1 mM l-glutamin and 5% (vol/vol) heat-inactivated fetal calf serum (FCSi), and 5% (vol/vol) HuSi in a 5% CO2 incubator at 37°C as described previously (4). After 4 to 6 days, these primary cultures were harvested with 0.05% (wt/vol) trypsin and 0.01% (wt/vol) EDTA and subcultured in gelatin-coated tissue culture dishes or onto gelatin-coated 12-mm-diameter glass coverslips (2) in 24-well tissue culture plates (Corning B.V. Life Sciences, Schiphol-Rijk, The Netherlands). After reaching confluence, these secondary cell cultures were used in the experiments and had an average cell density of 800 to 1,000 ECs per mm2 of surface area. In some experiments, these cultures were incubated for different periods of time at 37°C with culture medium supplemented with human recombinant IFN-γ or recombinant IL-1α. These cells are referred to as IFN-γ- or IL-1-activated ECs, respectively.
Human monocytes were isolated from buffy coats of heparinized peripheral blood of healthy donors (Sanquin Blood Bank, Leiden, The Netherlands) by density gradient centrifugation using Ficoll-amidotrizoate (5). The mononuclear cell fraction was washed three times with phosphate-buffered saline (PBS) containing 0.5 U/ml heparin and 2% autologous plasma. Enrichment for monocytes was performed by centrifugal elutriation by using a Beckman J2-21 M/E centrifuge equipped with a JE-6 rotor and a standard separation chamber (Beckman Instruments Inc., Palo Alto, CA) according to the supplier's manual, with minor adjustments. Purity of monocytes was more than 90%. Cell viability was above 97%, as judged by trypan blue exclusion. Monocytes were resuspended in ice-cold M199 with 10% HuSi at the desired cell density and used immediately.
Bacteria.
S. aureus CAPD is a virulent strain isolated from spent dialysis fluid of a continuous ambulatory peritoneal dialysis (hence, CAPD) patient suffering from peritonitis (47). S. aureus 42D is a relatively low-virulence original clinical isolate kept in the laboratory for several years. Both strains have been extensively characterized for their potential to infect and activate human ECs (3, 5, 29, 47, 53, 54). The bacteria were stored at −70°C and routinely grown overnight at 37°C in nutrient broth no. 2, washed in PBS, opsonized with human serum for 30 min in M199 containing 0.1% gelatin to avoid aggregation, and suspended at the desired concentration in M199 plus 10% HuSi, as described previously (5). At the end of each infection experiment, the exact number of bacteria used to infect ECs was determined afterwards by colony counts after plating serial dilutions in PBS on diagnostic sensitivity test agar plates and overnight incubation at 37°C.
To visualize bacteria inside ECs, we used the plasmid pSE1gfp+ (a gift from W. Schneider, Protein Design Labs, Inc., Fremont, CA) to construct green fluorescent protein (GFP)-expressing S. aureus 42D bacteria, according a previously described protocol (38).
Infection of ECs.
Infection of ECs with S. aureus was performed essentially as described elsewhere (5). In brief, confluent monolayers of about 1.8 × 105 to 2.0 × 105 ECs grown on gelatin-coated glass coverslips in 24-well tissue culture plates were washed with culture medium without antibiotics and incubated under various experimental conditions with bacteria in M199 plus 10% HuSi at 37°C in a 5% CO2 incubator. Thereafter, the infected monolayers were washed two times with warm PBS to remove extracellular bacteria and incubated with 10 U/ml lysostaphin for 5 min at room temperature to lyse the remaining cell-bound extracellular S. aureus organisms. Monolayers of ECs with intracellular bacteria were additionally cultured in culture medium in the absence of antibiotics or lysostaphin for set periods of time at 37°C in a 5% CO2 incubator. Culture supernatants were collected, filtered through a 0.2-μm-pore filter to remove cell debris and bacteria, and frozen at −70°C until they were assayed for IL-8 content. Alternatively, the coverslips with the infected cells were rinsed with warm PBS and the cells were fixed in methanol for 15 min and stained with Giemsa stain. Determination of the percentage of infected ECs, i.e., cells with at least one intracellular bacterium, and assessment of the number of intracellular bacteria were done by light microscopy using a standardized counting procedure as described in detail previously (57).
In most experiments, IL-1- or IFN-γ-activated ECs were used. Depending on the specific question, these cytokines were added to the cells at various time points prior to or immediately after infection and lysostaphin treatment.
When the experiments were performed with 35-mm culture dishes, the same infection protocol was used; the bacterial inoculum and concentrations of lysostaphin and cytokines were adjusted proportionally to the number of EC in the culture dish.
Confocal laser scanning microscopy.
Confocal microscopy was conducted to visualize the intracellular localization of S. aureus in ECs. EC monolayers were cultured for 72 h with 500 U/ml IFN-γ and infected with GFP-expressing S. aureus bacteria for 2 h to allow formation and maturation of the phagocytic compartments, washed, and treated with lysostaphin. Infected ECs were then washed with PBS and fixed for 10 min with 4% (wt/vol) paraformaldehyde in PBS with 1.0 mM CaCl2 and 0.5 mM MgCl2 (PBS+) and permeabilized for 15 min in permeabilization medium, i.e., PBS+ containing 0.02% (vol/vol) saponin and 0.5% (wt/vol) bovine serum albumin. Thereafter, cells were consecutively incubated for 60 min, with washes with permeabilization medium between incubations, with 2 μg/ml of primary MAb against EEA-1 or CD107A or a control mouse immunoglobulin G1 (IgG1) MAb, 2 μg/ml rhodamine Red-X-conjugated secondary goat anti-mouse MAb (Jackson Immuno-Research Laboratories, Inc., West Grove, PA). The slides were washed and mounted with Vectashield mounting medium and scanned by using an LSM-510 confocal laser scanning microscope (Zeiss, Jena, Germany) in a multitrack setting. Image analysis was performed using LSM Image Examiner software (Zeiss).
Flow cytometric analysis of EC surface molecules.
Cytokine-stimulated and/or S. aureus-infected ECs were harvested by mild trypsinization, collected in cold PBS plus 1% FCSi (wash buffer), and prepared for fluorescence-activated cell sorter analysis as described previously (5). Briefly, single-cell suspensions were taken under three incubation steps of 30 min on ice: use of wash buffer containing 1% goat serum, use of 1 μg/ml of the appropriate primary MAb, and use of 1 μg/ml of phycoerythrin-conjugated goat anti-mouse Ig (Southern Biotechnology Associates Inc., Birmingham, AL). In between each step, cells were washed with cold wash buffer. At least 5,000 cells were analyzed by flow cytometry on a FACSCaliber flow cytometer (BD Biosciences) using CellQuest software. ECs treated with conjugated MAb alone served as the control to set background fluorescence.
Analysis of chemokine production.
Supernatants of EC cultures, harvested after various treatments, were assayed for production of the chemokines CXCL8 and CCL2 by using the human CXCL8 PeliKine Compact enzyme-linked immunosorbent assay kit (Sanquin, Amsterdam, The Netherlands) and the human monocyte chemoattractant protein 1 Cytoset immunoassay kit (Biosource International). The assays were performed according to the supplier's instructions. Limits for detection of CXCL8 and CCL2 were 8.0 pg/ml and 15.0 pg/ml, respectively.
Monocyte-EC adhesion assay.
Monolayers of (cytokine-activated) ECs grown on gelatin-coated glass coverslips in a 24-well plate were incubated with S. aureus for 1 h and subsequently treated with lysostaphin and washed as described above. The cells were cultured for an additional 23 h at 37°C in culture medium without antibiotics, washed, and then incubated with 2 × 105 to 3 × 105 monocytes per well (i.e., 1 to 2 monocytes per single EC) for 30 min at 37°C in a CO2 incubator. The 1-h and 30-min time points used in this assay for infection and monocyte adhesion, respectively, were selected on the basis of our published studies on the adhesion kinetics (2). Then, nonadherent monocytes were removed by washing the coverslips five times with prewarmed PBS. The cells were fixed with methanol for 15 min and stained with Giemsa stain. The number of EC-adherent monocytes was determined by counting under a light microscope exactly as described previously (2).
In some experiments, the role of CD54- and CD106-mediated adhesion in monocyte-EC interaction was studied. In these experiments, the adhesion assay was as described above but with monocytes that, prior to their addition to the ECs, were incubated for 30 min with ice-cold antibiotic-free culture medium with a panel of MAbs directed against their β1- and β2-integrin molecules, i.e., the receptors for endothelial CD106 and CD54, respectively. A combination of 20 μg/ml of each of the following MAbs was used: anti-CD11b, anti-CD18, anti-CD49d, and anti-CD29. The MAbs remained present during the adhesion assay.
Statistical analysis.
Statistical analyses were conducted by using the paired Student t test (two-tailed) or the Wilcoxon matched-pairs signed-rank test and the SPSS program, version 11, for Windows (GmbH software, Muenchen, Germany). The tests for paired data were used to control for variation in measurements due to the fact that for each experiment, ECs, and in some experiments also monocytes, isolated from different donors were used. The level of significance was set at a P value of <0.05.
RESULTS
EC infection with S. aureus is reduced by IFN-γ in a time- and dose-dependent manner.
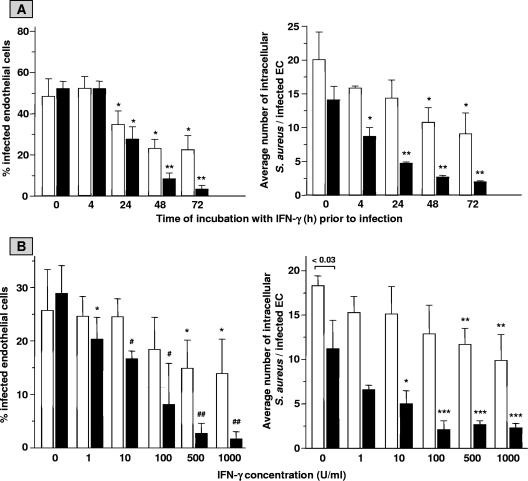
The aptitude of IFN-γ to modulate EC infection with S. aureus was assessed in series of different experiments. First, monolayers of 2 × 105 ECs were incubated for up to 72 h with 500 U/ml IFN-γ, a concentration routinely used by us to activate a variety of EC functions (3), washed, exposed to live S. aureus 42D bacteria for 1 h, and treated with lysostaphin. The degree of infection was analyzed immediately thereafter and after 23 h of subsequent culture of the infected cells. Analysis immediately after bacterial exposure revealed the following. Incubation of control unactivated EC cultures with 4 × 107 S. aureus resulted in 48.3% ± 8.5% (n = 10) of the ECs infected, with 20.1 ± 4.1 (n = 10) intracellular bacteria per infected cell (Fig. 1A). These values confirmed our previous findings (5, 47, 53). A time-dependent reduction of these values was observed for ECs with prior IFN-γ treatment. Already after 24 h of IFN-γ treatment, a significant 39% reduction (P < 0.05 in comparison to the value for control EC cultures [n = 6]) of the percentage of infected ECs was observed (Fig. 1A). A maximal 55% reduction of both the percentage of infected ECs and the average number of intracellular bacteria per infected cell was calculated from cultures treated with IFN-γ for 72 h prior to infection.
FIG. 1.
Time course (A) and dose dependency (B) of the effect of IFN-γ on EC infection with S. aureus. Monolayers of ∼2 × 105 ECs per well were cultured for the indicated periods of time in culture medium with 500 U/ml IFN-γ (A) or cultured for 72 h in culture medium with the indicated concentrations of IFN-γ (B). Thereafter, cell cultures were washed and exposed for 1 h to 4 × 107 (A) or 2 × 107 (B) S. aureus organisms, washed, and treated with lysostaphin. The percentages of infected ECs, i.e., cells with at least one intracellular bacterium, and the average numbers of intracellular bacteria per infected cell were determined immediately (open bars) and after an additional 23 h of culture of the infected monolayers (closed bars). (A) Values represent means ± standard deviations (SD) for 6 to 10 experiments performed on different days and with ECs from three to five different donors. A paired t test was used to evaluate the data. * indicates a P value of <0.05 and ** indicates a P value of <0.001 relative to ECs cultured in medium alone (bars at 0 h). (B) Values represent means ± SD for six experiments with ECs from three different donors. * indicates a P value of <0.04, ** indicates a P value of <0.02, *** indicates a P value of <0.009, # indicates a P value of 0.0005, and ## indicates a P value of <0.0001 in comparison with the respective cultures incubated with medium alone (bars at 0 U/ml).
To establish a possible temporal contribution of IFN-γ to the reduced infection, EC monolayers were exposed to a pulse of IFN-γ for only the first 24 or 48 h of the 72-h culture period before S. aureus exposure. This resulted in less-effective inhibition of EC infection (data not shown), indicating the necessity of the continuous presence of this cytokine in the culture medium in the period prior to infection.
Additional dose-response experiments, in which EC cultures were incubated with increasing concentrations of IFN-γ for 72 h prior to S. aureus infection, revealed that a minimal dose of 500 U/ml is required to significantly reduce EC infection (Fig. 1B).
The data given above are from measurements performed immediately after 1 h of infection. The IFN-γ-induced reduction in infectivity, however, revealed to be much more evident when the infection was evaluated in S. aureus-infected EC cultures that after lysostaphin treatment had been cultured for 23 h (Fig. 1). For example, the left panel of Fig. 1A shows that when IFN-γ was added to EC cultures 72 h before bacterial exposure, the percentage of infected ECs analyzed at 23 h after lysis of extracellular bacteria dropped dramatically from 52.1 ± 3.4 (control EC cultures at 0 h [n = 8]) to 3.2 ± 1.9 (at 72 h [n = 5]), i.e., a 94% reduction. Concomitantly, the average number of intracellular bacteria per infected EC gradually decreased from 14.1 ± 2.0 in control cultures to 2.0 ± 0.2 (i.e., 86% reduction; n = 5) in 72-h IFN-γ-pretreated cultures (Fig. 1A, right panel). Similar findings are presented in Fig. 1B for different concentrations of IFN-γ. When infection was evaluated after 23 h of culture of the infected EC monolayers, a slight 29% reduction (P = 0.038 in comparison to control cultures [n = 6]) of infected ECs with 1 U/ml IFN-γ was detected already (Fig. 1B), and a maximal 91% reduction (P value of <0.0001 in comparison to control cultures [n = 6]) of the percentage of infected cells and a 75% reduction (P value of 0.009 in comparison to control cultures [n = 6]) of the average number of intracellular bacteria per infected cell were achieved following incubation with at least 500 U/ml IFN-γ.
Taken together, these results indicate that ECs following incubation with IFN-γ (e.g., for 24 to 72 h) have increased abilities to limit the amount of intracellular bacteria during S. aureus infection but in particular over time during the postinfection culture period.
Effects of IFN-γ on bacterial binding and internalization.
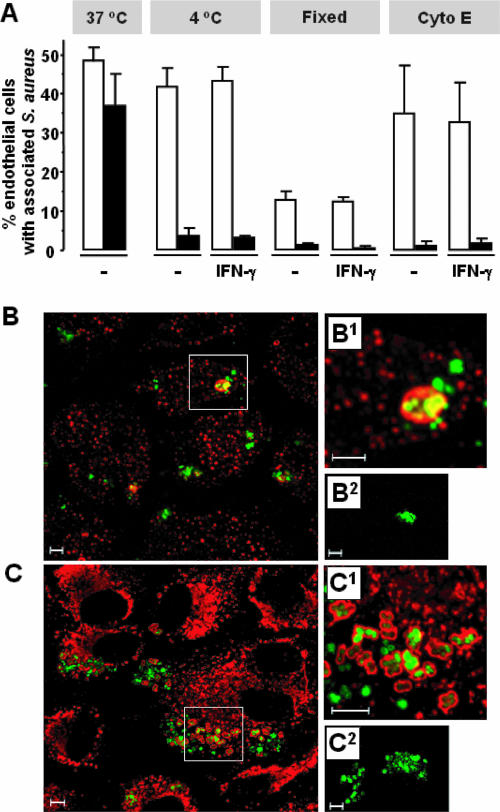
To delineate whether IFN-γ exerts its infection-limiting ability in part by interfering in the binding of S. aureus organisms to the EC surface membrane and their consequent uptake, infection experiments were performed at 4°C in the presence of cytochalasin E to block microfilament-mediated endocytosis or with paraformaldehyde-fixed ECs. These conditions prevent internalization of surface-bound S. aureus and facilitate a precise quantification of the number of extracellular cell surface-associated bacteria. Additional treatment with lysostaphin was applied to ascertain the extracellular localization of the EC-bound bacteria. Despite the finding that paraformaldehyde-fixed ECs have an impaired capacity to bind S. aureus, the results and those obtained at 4°C or with cytochalasin E-treated ECs reveal no differences in the percentages of cells with extracellular bound bacteria between IFN-γ-pretreated ECs and ECs that were not pretreated (Fig. 2A). It was concluded that IFN-γ-activated ECs have normal capacities for binding S. aureus organisms to their cell surface. Additional confocal microscopy revealed the localization of S. aureus in IFN-γ-pretreated ECs inside EEA-1-positive early endosomes (Fig. 2B) as well as CD107A-positive phagolysosomes (Fig. 2C) (55), indicating that bacteria are ingested by a common phagocytic pathway involving phagosome fusion with early endosomes and lysosomes.
FIG. 2.
IFN-γ-activated ECs have unchanged abilities to bind S. aureus and do internalize bacteria. (A) Monolayers of 2 × 105 ECs, cultured for 72 h in culture medium alone (−) or with 500 U/ml IFN-γ (IFN-γ), were exposed to 4 × 107 S. aureus for 30 min at 37°C or 4°C. The infection period was 30 min in order to avoid detachment of EC during culture at 4°C. Alternatively, EC cultures were fixed with 1% (wt/vol) paraformaldehyde in PBS or treated with 2 μg/ml cytochalasin E (Cyto E) for 10 min at 37°C and washed before bacteria were added for 30 min at 37°C. These different infection protocols were followed by a wash in PBS to remove free extracellular bacteria (open bars) or treatment with lysostaphin to lyse also all cell-bound extracellular bacteria (closed bars). Values represent the means ± standard deviations of three experiments with cells from two different donors. (B and C) Representative confocal microscopic images of localization of GFP-expressing S. aureus within early endosomes (EEA-1 positive) or late endosomes/lysosomes (CD107A positive) in ECs. ECs were cultured for 72 h with 500 U/ml IFN-γ and infected with GFP-expressing S. aureus for 2 h and prepared for confocal microscopy analysis. Graphs represent infected ECs labeled with anti-EEA-1 MAb (B1), anti-CD107A MAb (C1), or control mouse IgG1 MAb (B2 and C2) followed by labeling with a rhodamine Red-X-conjugated secondary antibody. The overlay of two channels is shown. Intracellular bacteria appear green; EEA-1- or CD107A-stained vesicles appear red. Bacteria that are in close association with the vesicle membrane appear yellow. Bar = 5 μm.
Further analysis of the kinetics of the IFN-γ-induced limitation of EC infection with S. aureus.
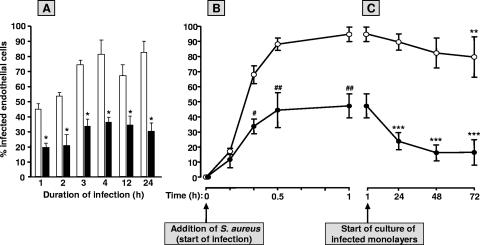
Hitherto, ECs were infected with S. aureus for 1 h and examined immediately thereafter or after a 23-h period of culture of the infected EC monolayers. These time points were chosen from our previous infection experiments (e.g., reference 5). To ascertain the optimal time point for assessment of the effect of IFN-γ on endothelial infection, additional kinetic studies were performed. First, monolayers of 2 × 105 ECs were incubated for 72 h with 500 U/ml IFN-γ, i.e., conditions that gave the maximal effect as concluded from the results shown in Fig. 1, and then exposed to S. aureus for intervals ranging from 1 h to 24 h (Fig. 3A). These experiments were performed with relatively small amounts of bacteria to avoid EC monolayer destruction resulting from the prolonged infection period, as reported by us elsewhere (52, 53). For each of the infection intervals up to 24 h, the data reveal a maximum reduction of about 50 to 60% in the number of infected ECs in IFN-γ-treated EC cultures in comparison to the time-matched control cultures (Fig. 3A). Next, the time course of infection was followed during the 1-h infection period. Again, about 50 to 60% lower percentages of infected ECs were observed after prestimulation with IFN-γ. This reduction was obvious already at 20 min after the start of infection (Fig. 3B). Finally, IFN-γ-treated and untreated control EC cultures were exposed to S. aureus for 1 h, treated with lysostaphin, and then cultured for an additional variable period of time up to 72 h. Figure 3C shows that the culture of untreated control ECs infected with S. aureus was accompanied by a modest gradual reduction of about 16% in the percentage of infected ECs, i.e., the percentage of infected cells declined from 94.7% ± 4.8% (n = 4) at the start of culture (immediately after lysostaphin treatment) to 79.7% ± 13.4% (n = 3) at 72 h of culture. However, for EC cultures treated with IFN-γ prior to infection, this reduction was much more pronounced and followed a different time course (Fig. 3C). In the first 24 h of culture, the percentage of S. aureus-infected cells declined almost 50% from 47.3% ± 7.9% (n = 4) to 23.9% ± 5.6% (n = 3); thereafter, this percentage dropped more slowly to 16.5% ± 8.5% (n = 3) at 72 h of culture. The decrease in the average number of intracellular bacteria per single infected EC following IFN-γ incubation followed a somewhat similar course and declined from 20.6 bacteria per cell immediately after bacterial lysis to 7.0 at 24 h and 4.4 at 72 h of culture of the infected cell cultures (data not shown).
FIG. 3.
Effect of IFN-γ on the course of EC infection with S. aureus. (A) Monolayers of 2 × 105 ECs per well were cultured for 72 h in culture medium alone (open bars) or supplemented with 500 U/ml IFN-γ (closed bars), washed, exposed to about 3 × 107 S. aureus organisms for the indicated time intervals, washed, and treated with lysostaphin followed by immediate determination of the percentage of infected cells. (B) EC monolayers were also cultured for 72 h in culture medium alone (open circles) or with 500 U/ml IFN-γ (closed circles), washed, infected for 1 h with approximately 108 S. aureus organisms, washed, and treated with lysostaphin. The percentage of infected cells was determined at the indicated time points during the 1-h infection period. (C) Some of the infected EC cultures described for panel B were additionally cultured for up to 72 h in plain culture medium (i.e., in the absence of IFN-γ). The percentages of infected cells were determined at the indicated time points during this postinfection culture period. All values represent means ± standard deviations for three (B and C) or four (A) experiments with ECs from different donors. A paired t test was used to evaluate the data. * indicates a P value of <0.04, # indicates a P value of 0.01, and ## indicates a P value of <0.001 relative to ECs cultured in medium alone. ** indicates a P value of <0.05 and *** indicates a P value of <0.001 compared to the respective culture at the start of culture of the infected monolayer.
Together, these experiments indicate that the limiting effect of IFN-γ pretreatment on EC infection is eminent at all time points during as well as after endothelial infection. Furthermore the data suggest that IFN-γ induces or alleviates an antibacterial endothelial response.
IFN-γ reduces infection also when applied to ECs after bacterial exposure.
The above findings with ECs treated with IFN-γ prior to staphylococcal infection prompted us to examine whether IFN-γ is capable also of inducing such response when applied to ECs after S. aureus infection. EC cultures were incubated for 1 h to 6 × 107 S. aureus bacteria to result in about 93.7% infected cells harboring an average of 24.3 intracellular bacteria per cell (Table 1). Without IFN-γ treatment these values progressively declined to 78.9% infected cells with about 11.5 bacteria per cell during the next 72 h of culture, indicating the intrinsic antibacterial potential as also depicted in Fig. 3C. However when the infected EC monolayers were cultured in the presence of IFN-γ, these rather indolent antibacterial responses were much more effective. Under these conditions the percentage of infected cells declined considerably to 28.3% with about 5.2 intracellular bacteria per EC being a reduction of 64.1% and 54.7%, respectively (Table 1).
TABLE 1.
IFN-γ reduces infection when applied to EC cultures after their exposure to S. aureusa
| Time of incubation (h) | % of S. aureus-infected ECs in mediumb:
|
% Reduction in % of S. aureus-infected ECsc | Avg no. of intracellular S. aureus bacteria/infected EC in mediumb:
|
% Reduction in avg no. of intracellular S. aureus bacteria/infected ECc | ||
|---|---|---|---|---|---|---|
| Without IFN-γ | With IFN-γ | Without IFN-γ | With IFN-γ | |||
| 0 | 93.7 ± 3.7 | 24.3 ± 7.7 | ||||
| 1 | 89.7 ± 2.0 | 91.1 ± 2.3 | −1.5 | 24.2 ± 3.1 | 23.2 ± 7.4 | 4.1 |
| 24 | 86.6 ± 6.4 | 69.8 ± 1.6 | 19.4 | 16.8 ± 4.3 | 9.9 ± 1.5f | 41.1 |
| 48 | 81.2 ± 9.1 | 52.2 ± 5.7d | 35.7 | 13.2 ± 4.6 | 6.4 ± 1.5f | 51.5 |
| 72 | 78.9 ± 11.5 | 28.3 ± 3.9e | 64.1 | 11.5 ± 2.2 | 5.2 ± 0.9d | 54.7 |
EC monolayers (2 × 105 cells/well) were exposed to 6 × 107 S. aureus organisms for 1 h, washed, and treated with lysostaphin. The percentage of infected ECs and the average number of intracellular bacteria per infected EC were determined immediately after lysostaphin treatment (0 h) or at the indicated time points during a subsequent culture of the infected EC monolayers in culture medium alone or with 500 U/ml IFN-γ.
Values represent means ± standard deviations for three to four identical experiments with ECs from three different donors.
Percent reduction in the number of S. aureus-infected ECs or average number of intracellular S. aureus per infected EC of IFN-γ-treated cultures in comparison to the cultures treated with medium alone at the respective time points.
P = 0.02 in comparison to the value for the ECs in medium without IFN-γ (paired t test).
P = 0.008 in comparison to the value for the ECs in medium without IFN-γ (paired t test).
P < 0.04 in comparison to the value for the ECs in medium without IFN-γ (paired t test).
Specificity of the IFN-γ effect on endothelial infection with S. aureus.
ECs respond to numerous cytokines (see, e.g., reference 3). We examined whether IFN-γ shares its ability to reduce S. aureus infectivity in ECs with other prominent proinflammatory cytokines. Preincubation of ECs with an optimal dose of 5 ng/ml IL-1 did not reveal the effect seen with IFN-γ (Table 2). In contrast, prolonged exposure to IL-1 modestly increased the percentage of cells infected with S. aureus, although the average number of intracellular bacteria per infected cell was slightly decreased. Similar results were obtained with 500 ng/ml TNF-α (data not shown).
TABLE 2.
Effect of IL-1 on EC infection with S. aureusa
| Time of incubation with IL-1 prior to infection with S. aureus (h) | % of S. aureus-infected ECsb | Avg no. of intracellular S. aureus bacteria/infected ECb |
|---|---|---|
| 0 (no IL-1) | 46.9 ± 8.4 | 15.6 ± 1.5 |
| 4 | 38.1 ± 7.0 | 13.1 ± 3.7 |
| 24 | 47.4 ± 4.5 | 13.9 ± 6.1 |
| 48 | 57.0 ± 9.1 | 14.6 ± 9.0 |
| 72 | 61.4 ± 4.5c | 10.5 ± 2.2d |
EC monolayers (2 × 105 ECs/well) were incubated for the indicated periods of time in culture medium with or without (0 h) 5 ng/ml IL-1, washed, and then exposed for 1 h to 3 × 107 S. aureus organisms, washed, and treated with lysostaphin. Infected EC monolayers were cultured for 23 h and then the percentage of infected ECs and the average number of intracellular bacteria per infected EC were determined.
Values represent means ± standard deviations for three or four experiments with ECs from different donors.
P = 0.009 in comparison to the percentage of infected ECs at 0 h (two-tailed Student's t test).
P = 0.03 in comparison to average number of bacteria per infected EC at 0 h (two-tailed Student's t test).
Next we assessed the involvement of the EC surface receptor for IFN-γ (CD119). Neutralizing anti-CD119 antibodies applied to S. aureus-infected EC cultures 30 min before and during the 72 h postinfection IFN-γ treatment effectively abrogated the infection-limiting effect of IFN-γ. IFN-γ treatment reduced the percentage of infected cells by 54.4% ± 5.2% (n = 3), whereas the reduction in the presence of anti-CD119 antibodies was only 5.0% ± 8.8% (n = 3). The isotype-matched control anti-human MHC-I MAb W6/32 was without any effect (data not shown).
IFN-γ limits EC infection over a wide range of bacterial concentrations and rescues the cells from lysis by virulent S. aureus strains.
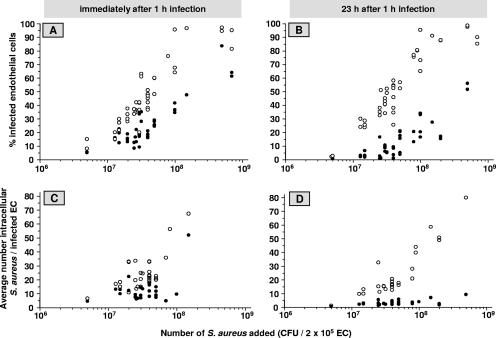
As a result of the studies described above, we conducted all subsequent infection experiments with EC cultures that 72 h prior to the 1 h-bacterial exposure were treated with 500 U/ml IFN-γ. The experiments in which we used different inocula of S. aureus strain 42D to infect the ECs revealed that over a wide range of bacterial concentrations, the percentage of infected cells and the number of intracellular bacteria per infected cell in IFN-γ-pretreated EC cultures were two to five times lower than those in untreated cultures (Fig. 4), the magnitude of reduction being dependent on whether the assessments were performed immediately after the 1-h infection (Fig. 4A and C) or after a subsequent 23-h culture period (Fig. 4B and D).
FIG. 4.
Effect of IFN-γ on EC infection with different inocula of S. aureus. Monolayers (2 × 105 ECs per well) were cultured for 72 h in culture medium alone (open circles) or supplemented with 500 U/ml IFN-γ (closed circles), then exposed to the indicated number of S. aureus organisms for 1 h, washed, and treated with lysostaphin. The percentages of infected ECs (A and B) and the average numbers of intracellular bacteria per infected cell (C and D) were determined immediately (A and C) and after an additional 23 h of culture of the infected monolayers (B and D). Each circle represents a value derived from an individual measurement. In total, values represent data for ECs from 12 to 16 different donors.
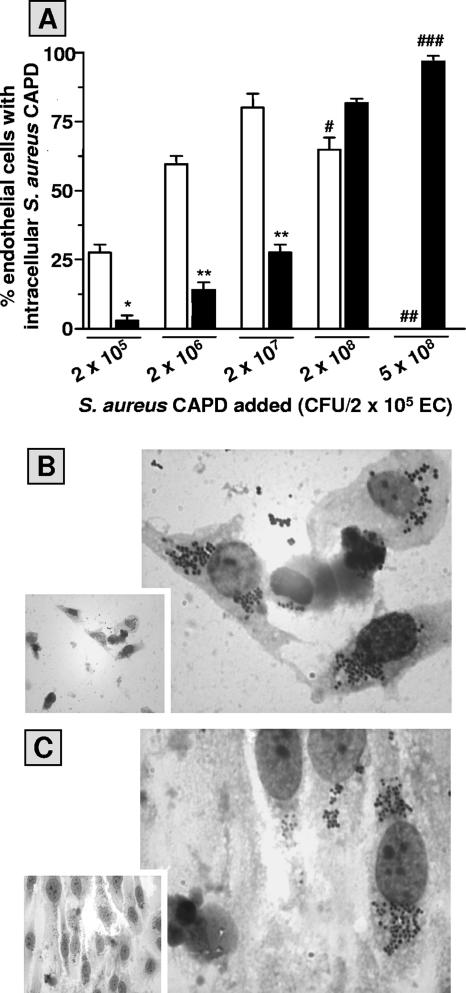
The relatively low-virulence characteristics of S. aureus strain 42D used in this study could explain the successful elimination of these microorganisms by IFN-γ-activated ECs. However, data depicted in Fig. 5A show that pretreating EC monolayers with IFN-γ also markedly reduced the infectivity of the virulent S. aureus strain CAPD. Moreover, IFN-γ treatment prevented lysis and detachment of infected cells and loss of monolayer integrity caused by organisms of this strain (Fig. 5B and C). Similar findings were observed with two virulent clinical S. aureus strains isolated from patients with definite S. aureus infective endocarditis (gifts from V. G. Fowler, Duke University Medical Center, Durham, NC) and the virulent S. aureus strain RN4220, a derivative of S. aureus NCTC8325-4 (57; data not shown).
FIG. 5.
IFN-γ limits EC infection and rescues the cells from injury by virulent S. aureus strain CAPD. (A) Monolayers (2 × 105 ECs per well) were cultured for 72 h in culture medium alone (open bars) or supplemented with 500 U/ml IFN-γ (closed bars), then exposed to the indicated number of S. aureus CAPD for 1 h, washed, and treated with lysostaphin. The percentages of infected ECs were determined after an additional 23 h of culture of the infected monolayers. Values represent means ± standard deviations for three experiments with ECs from different donors. A paired t test was used to evaluate the data. * indicates a P value of 0.0005 and ** indicates a P value of <0.0001 relative to EC cultures in culture medium alone. Microscopic evaluation revealed significant cell damage and apparent loss of monolayer integrity (#), complete loss of cell monolayer (##) (typical micrograph shown in panel B), and no obvious cell damage (###) (typical micrograph shown in panel C).
IFN-γ does not avert the inflammatory response of ECs to infection with S. aureus.
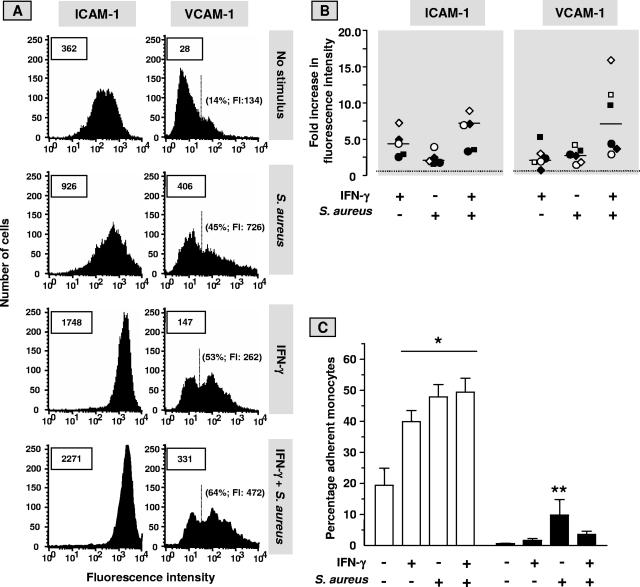
Internalization of S. aureus induces a proinflammatory phenotype in cultured human ECs that, among other features, is accompanied by binding of circulating monocytes that in turn could assist in the control of the bacterial infection, as described previously (5, 54). Based on the results given above, we surmised that the IFN-γ-induced reduction in intracellular bacterial burden would be accompanied by a diminished inflammatory response of EC to infection with S. aureus. In the present study, this was investigated by analysis of EC surface expression of adhesion receptors CD54 and CD106, chemokine production, and monocyte-EC interaction. In line with our previous publications (2, 5), exposure of EC to IFN-γ or S. aureus induced CD106 expression and upregulated the low constitutive expression of CD54 on the cell membrane (Fig. 6A and B). However, contrary to our expectations, the exposure of IFN-γ-pretreated ECs to S. aureus caused an extra, probably superimposed, upregulation of CD54 (P < 0.02, compared to treatment with IFN-γ or S. aureus alone [n = 5]), and CD106 (P < 0.05, compared to treatment with IFN-γ or S. aureus alone [n = 6]) (Fig. 6B). Similar supplementary stimulatory effects were seen with respect to surface expression of MHC-I molecules (data not shown). Concomitantly, the binding of monocytes to EC monolayers, which, by using a combination of neutralizing antibodies against CD18, CD11b, CD29, and CD49d, is almost entirely dependent on molecular interactions between CD54/β2-integrin molecules and CD106/β1-integrin molecules, was the highest when ECs were used after IFN-γ pretreatment and subsequent S. aureus exposure (Fig. 6C). Untreated and IFN-γ-pretreated ECs secreted about similar elevated quantities of CXCL8 or CCL2 in response to exposure to S. aureus. CXCL8 production during 23 h of culture after 1 h of infection with S. aureus amounted to 33.0 ng/ml for untreated ECs and 45.0 ng/ml for IFN-γ-pretreated ECs. CCL2 concentrations in these culture supernatants were 14.9 ng/ml for untreated ECs and 13.3 ng/ml for pretreated ECs. The spontaneous releases of CXCL8 and CCL2 from ECs cultured in the absence of IFN-γ and bacteria were 3.3 ng/ml and 6.3 ng/ml, respectively. Values are means of two independent experiments. Together, these findings indicate that the antibacterial potential of ECs afforded by IFN-γ coincides with a sustained or even elevated capacity for eliciting a proinflammatory response to the presence of intracellular S. aureus that favors monocyte recruitment.
FIG. 6.
IFN-γ does not avert the S. aureus-induced endothelial inflammatory response. Monolayers of ECs, cultured for 72 h in culture medium alone or supplemented with 500 U/ml IFN-γ were exposed to S. aureus (EC to bacteria ratio, 1:100) or incubated with plain medium for 1 h, washed, treated with lysostaphin, and cultured for an additional 23 h in culture medium without antibiotics. Then, the cells were prepared for analysis by fluorescence-activated cell sorter (A and B) or used in the monocyte adhesion experiments (C). (A) A typical analysis by flow cytometry of endothelial CD54 (ICAM-1) and CD106 (VCAM-1) surface expression. Boxed numbers represent the mean fluorescence intensities expressed in arbitrary units of all analyzed ECs. Mean background fluorescence of cells incubated with the phycoerythrin-conjugated MAb ranged only from 15 to 30. The proportions of the cells that expressed VCAM-1 above the background level and their mean fluorescence intensities (FI) are depicted in parentheses. (B) Levels of expression of ICAM-1 and VCAM-1 in five and six separate experiments, respectively, with ECs from different donors were calculated relative to the median level of expression (set at level 1; dotted line) on untreated cells. Identical symbols represent data from the same experiment. Horizontal bars represent the medians of the separate experiments. (C) Monocytes were prepared and preincubated with plain medium (open bars) or with a panel of MAb directed against the β1- and β2-integrin molecules (closed bars) to block their interaction with endothelial VCAM-1 and ICAM-1, respectively, as described in Materials and Methods. Monocytes were allowed to adhere to the prepared EC monolayers for 30 min, and the percentage of EC-bound monocytes was determined thereafter. Data are given as means ± standard deviations for three separate experiments with cells from different donors. A paired t test was used to evaluate the data. *, P < 0.0001 relative to control ECs not treated with IFN-γ, bacteria, and MAb; **, P < 0.05 relative to one of the other EC cultures with MAb present.
Effect of l-tryptophan or iron on the IFN-γ-induced restriction of EC infection with S. aureus.
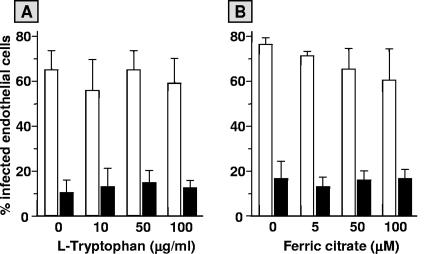
Depletion of the cellular pools of essential growth nutrients like l-tryptophan and iron by IFN-γ in human cells, including ECs, has been reported as an effective antimicrobial mechanism for obligatory intracellular pathogens such as Toxoplasma gondii (11, 13). In an effort to clarify the mechanism underlying the IFN-γ-induced restriction of the infection of ECs with S. aureus in the current study, infection experiments were performed in the presence of excess concentrations of extracellular l-tryptophan or ferric citrate. The addition of these compounds to ECs during pretreatment with IFN-γ and subsequent infection with S. aureus did not abrogate the infection-limiting effect of IFN-γ (Fig. 7).
FIG. 7.
Effect of l-tryptophan or ferric citrate on the IFN-γ induced reduction in S. aureus infection of ECs. Monolayers of 2 × 105 ECs, cultured for 72 h in culture medium alone (open bars) or supplemented with 500 U/ml IFN-γ (closed bars) were exposed to 5 × 107 S. aureus for 1 h, washed, treated with lysostaphin, and cultured for an additional 23 h in culture medium without antibiotics. During cytokine stimulation, infection, and the postinfection culture periods, indicated concentrations of l-tryptophan (A) or ferric citrate (B) were present in the media. Values represent the means ± standard deviations of three (A) or two (B) experiments with ECs from different donors.
DISCUSSION
In disseminated endovascular S. aureus infections, the vascular ECs are known to bind and internalize these pathogens. In this study, we used our in vitro model of endovascular infection to focus on the potential outcomes of such S. aureus-EC interaction in the context of the immune-regulatory Th1 cytokine IFN-γ. We demonstrated that human venous ECs exhibit a modest intrinsic ability to eliminate internalized S. aureus organisms which is considerably enhanced upon stimulation of the cells with IFN-γ. Mechanistically, IFN-γ exerts this infection-limiting ability through the endothelial IFN-γR but independent of concurrent l-tryptophan or iron deprivation. IFN-γ-treated ECs are more resistant to the toxic and damaging effects of clinical S. aureus strains or excessive numbers of less-virulent strains and upon infection display an unrelenting pronounced proinflammatory phenotype enabling binding and recruitment of monocytes. The findings demonstrate the capacity for ECs to eliminate S. aureus and underscore the multifactoral character of the regulatory role of IFN-γ in blood-borne S. aureus infections, in particular when the ECs are the focus of infection.
The competence of ECs to combat internalized S. aureus organisms as well as the fate of intracellular S. aureus are issues that are under debate. Certain S. aureus strains have been reported to rapidly kill ECs upon close contact and consequential expression of the alpha-toxin hemolysin (16, 51). Others report EC survival and persistence of S. aureus within the nutrient-rich cytosol or intracellular lysosomal compartments (25, 56). Our previous studies reveal that certain less-virulent S. aureus strains upon EC interaction and invasion do not severely damage the cells (52, 53) but elicit a procoagulant and proinflammatory EC phenotype that, with the aid of newly recruited professional phagocytes (and activated T lymphocytes), impedes bacterial survival and further spread (5, 18, 47, 54). In the current study, we utilized the same S. aureus strains and showed that human ECs exhibit an intrinsic ability to eliminate internalized S. aureus organisms. In essence, this conclusion is confirmatory to that of a recent study by Schröder and colleagues (40). However, we observed a maximal ∼20% decrease in the percentage of S. aureus-infected ECs after a 72-h-postinfection culture period (Fig. 3C), whereas Schröder et al. reported the elimination of >99% of intracellular S. aureus by ECs within 24 h of culture. This relatively high elimination potency of EC is most likely explained by a concomitant intracellular effect of the bactericidal compounds, gentamicin and lysostaphin, that were added to the culture medium after infection. In studies with human monocytes and granulocytes, these compounds were shown to penetrate the cells at physiologic temperatures and, in sequential synergy with intracellular bactericidal processes, effectively lyse intracellular S. aureus (34, 49, 50). We performed our culture experiments in the absence of antibiotics or lysostaphin to allow unfailing measurements of an intrinsic, albeit modest, endothelial ability to resolve an infection with S. aureus.
These modest abilities to withstand intracellular S. aureus and cell damage caused by virulent S. aureus strains were considerably enhanced upon stimulation of ECs with IFN-γ. The results from the 24- and 48-h pulse experiments reveal the necessity of IFN-γ being present continuously in the EC culture medium prior to S. aureus infection. This could imply that IFN-γ exerts its effect by interference with processes of bacterial adherence or phagocytosis. However, at least three current observations argue against this explanation. First, the experiments performed at low temperature or with cytochalasin E, i.e., two conditions that block bacterial internalization, demonstrated that the number of S. aureus organisms adherent to the EC surface is not altered by prior treatment of the cells with IFN-γ. Second, intracellular S. aureus are found inside ECs after stimulation with IFN-γ in early endosomal EEA-1-positive vacuoles that, similar to those in a recent study with unstimulated ECs (40), underwent maturation and fusion with lysosomes to form CD107A-positive phagolysosomes. Third, IFN-γ efficiently decreases the extent of EC infection also when applied to the cell cultures after their infection with S. aureus. Altogether, we surmise that IFN-γ suppresses endothelial infection levels not by an impediment of bacterial binding to ECs and/or modulation of endothelial phagocytic pathways but most likely by potentiating the antibacterial competence of ECs against intracellular bacteria. It should be noted, however, that a possible additional inhibitory effect of IFN-γ on the actual uptake of membrane-bound S. aureus, on the basis of the current data, cannot be completely ruled out. This awaits further study.
The capacity for reducing the percentage of infected ECs was exclusively found when IFN-γ was used. It required ligation of the IFN-γR, illustrated by the neutralizing effect of anti-IFN-γR MAb, and was not shared with IL-1 and TNF-α, two other potent activators of a proinflammatory EC phenotype (see, e.g., reference 3). Moreover, the data show that IL-1 and TNF-α, in apparent contrast to IFN-γ, increased the susceptibility of ECs to infection with S. aureus, illustrated by the significant increase in the percentage of IL-1- or TNF-stimulated ECs with intracellular S. aureus. These findings are in keeping with and additive to data from previous in vitro and in vivo studies reporting increased contacts between S. aureus and ECs following stimulation with IL-1 or TNF-α (9, 21, 48). The mechanism underlying the effects of these cytokines is currently under investigation.
With regard to the mechanisms by which IFN-γ limits the amount of intracellular S. aureus in ECs, our results exclude a few potential mechanisms postulated by others using different human cell types, including human phagocytes, fibroblasts, retinal epithelial cells, and brain ECs infected with T. gondii, Chlamydia psittaci, or Legionella pneumophila (6, 7, 11, 13, 19, 32). In these studies, IFN-γ inhibits the intracellular replication of these pathogens by limiting the availability of iron and/or induction of IDO (indoleamine 2,3-dioxygenase) that deprives the pathogen from l-tryptophan. We confirmed the induction of IDO mRNA in IFN-γ-activated ECs by using RT-PCR (data not shown), suggesting tryptophan depletion. However, surplus of l-tryptophan or iron in our assays did not reverse the S. aureus infection-limiting effect of IFN-γ. Alternatively, no significant quantities of nitric oxide or hydrogen peroxide could be detected in our ECs upon treatment with IFN-γ (unpublished observations), which is in accord with data obtained by others (61). Although this issue requires further scrutiny, the data so far suggest that IFN-γ controls S. aureus infection in ECs by mechanisms independent of l-tryptophan or iron deprivation and most likely also of released toxic nitrogen or oxygen radicals.
It is well known that, dependent on the magnitude of bacterial challenge, the infection of EC with S. aureus provokes EC activation with aspects of inflammation, coagulation, and enhance interaction with monocytes and neutrophils (5, 53, 54). In this context, we hypothesized that the reduced bacterial presence in IFN-γ-activated ECs, as shown in the current study, might dampen the intensity of EC activation. However, our data indicate that the antibacterial potential of EC afforded by IFN-γ coincides with a sustained or slightly elevated capacity for producing chemokines and cell surface adhesion molecules that support adhesion of monocytes to the EC surface. Apparently, EC activation occurs as a consequence of the interaction of S. aureus with the EC surface, of which the magnitude was not altered by IFN-γ (as discussed above), rather than occurring after internalization of the bacteria by the cells. In essence, this conclusion is in accord with that drawn from our recent study with various recombinant Lactococcus lactis bacteria expressing individual FnBP adhesins from S. aureus showing EC activation relative to the number of bacteria associated with the EC surface membrane (18).
In conclusion, our data show that ECs upon stimulation with the immunoregulatory cytokine IFN-γ acquire different cooperative strategies to aid in a defense against blood-borne S. aureus infections. Through enhanced antibacterial mechanisms, of which the exact nature remains to be further clarified, IFN-γ-activated ECs are more resistant to S. aureus infection. Accordingly, the cells are rescued from S. aureus-induced cell death and support an inflammatory reaction that recruits professional phagocytes to the infected vascular site. As shown by others, these phagocytes may acquire, by their adhesion to the infected ECs, enhanced bacterium-killing properties (29).
Acknowledgments
We thank the coworkers of the Department of Gynaecology at the Leiden University Medical Center (LUMC) for collection of the human umbilical cords and Bep Ravensbergen for assistance in preparation of the EC cultures. We also are grateful to Frans Prins from the Department of Pathology (LUMC) for technical assistance in the confocal microscopy study and Tahar van der Straaten from the Department of Clinical Pharmacy and Toxicology (LUMC) for preparation of GFP-expressing S. aureus.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Alexander, E. H., and M. C. Hudson. 2001. Factors influencing the internalization of Staphylococcus aureus and impacts on the course of infections in humans. Appl. Microbiol. Biotechnol. 56:361-366. [DOI] [PubMed] [Google Scholar]
- 2.Beekhuizen, H., A. J. Corsèl-van Tilburg, and R. van Furth. 1990. Characterization of monocyte adherence to human macrovascular and microvascular endothelial cells. J. Immunol. 145:510-518. [PubMed] [Google Scholar]
- 3.Beekhuizen, H., and J. S. van de Gevel. 1998. Endothelial cell adhesion molecules in inflammation and postischemic reperfusion injury. Transpl. Proc. 30:4251-4256. [DOI] [PubMed] [Google Scholar]
- 4.Beekhuizen, H., and R. van Furth. 1994. Growth characteristics of cultured human macrovascular venous and arterial and microvascular endothelial cells. J. Vasc. Res. 31:230-239. [DOI] [PubMed] [Google Scholar]
- 5.Beekhuizen, H., J. S. van de Gevel, I. J. van Benten, B. Olsson, and R. van Furth. 1997. Infection of human vascular endothelial cells with Staphylococcus aureus induces hyperadhesiveness for human monocytes and granulocytes. J. Immunol. 158:774-782. [PubMed] [Google Scholar]
- 6.Byrd, T. F., and M. A. Horwitz. 1989. Interferon gamma-activated human monocytes downregulate transferring receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J. Clin. Investig. 83:1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlin, J. M., E. C. Borden, and G. I. Byrne. 1989. Interferon-induced indoleamine 2,3 dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J. Interferon Res. 9:329-337. [DOI] [PubMed] [Google Scholar]
- 8.Chavakis, T., K. Wiechmann, K. T. Preissner, and M. Herrmann. 2005. Staphylococcus aureus interactions with endothelium. The role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 94:278-285. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, A. L., J. M. Koomey, S. Lee, E. A. Jaffe, and V. A. Fischetti. 1991. Recombinant human tumor necrosis factor alpha promotes adherence of Staphylococcus aureus to cultured human endothelial cells. Infect. Immun. 59:3827-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culshaw, S., B. P. Leung, J. A. Gracie, C. C. Campbell, D. Thomson, C. Gemmell, F. Y. Liew, and I. B. McInnes. 2005. Prior elevation of IL-18 promotes rapid early IFN-gamma production during staphylococcal infection. Eur. J. Immunol. 35:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Däubener, W., B. Spors, C. Hucke, R. Adam, M. Stins, K. S. Kim, and H. Schroten. 2001. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 69:6527-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Assis, M. C., A. O. Da Costa, T. C. Barja-Fidalgo, and M. C. Plotkowski. 2000. Human endothelial cells are activated by interferon-gamma plus tumour necrosis factor-alpha to kill intracellular Pseudomonas aeruginosa. Immunology 101:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimier, I. H., and D. T. Bout. 1998. Interferon-γ-activated primary enterocytes inhibit Toxoplasma gondii replication: a role for intracellular iron. Immunology 94:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drake, T. A., and M. Pang. 1988. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J. Infect. Dis. 157:749-756. [DOI] [PubMed] [Google Scholar]
- 15.Griffin, F. M., J. A. Griffin, J. E. Leider, and S. C. Silverstein. 1975. Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J. Exp. Med. 142:1263-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslinger-Löffler, B., B. C. Kahl, M. Grundmeier, K. Strangfeld, B. Wagner, U. Fischer, A. L. Cheung, G. Peters, K. Schulze-Osthoff, and B. Sinha. 2005. Multiple virulence factors are required for Staphylococcus aureus-induced apoptosis in endothelial cells. Cell. Microbiol. 7:1087-1097. [DOI] [PubMed] [Google Scholar]
- 17.Hauck, C. R., and K. Ohlsen. 2006. Sticky connections: extracellular matrix protein recognition and integrin-mediated cellular invasion by Staphylococcus aureus. Curr. Opin. Microbiol. 9:5-11. [DOI] [PubMed] [Google Scholar]
- 18.Heying, R., J. S. van de Gevel, Y-A. Que, P. Moreillon, and H. Beekhuizen. 2007. Fibronectin-binding proteins and clumping factor A in Staphylococcus aureus experimental endocarditis: FnBPA is sufficient to activate human endothelial cells. Thromb. Haemost. 97:617-626. [PubMed] [Google Scholar]
- 19.Ibrahim, A. S., S. G. Filler, M. A. Ghannoum, and J. E. Edwards Jr. 1993. Interferon-gamma protects endothelial cells from damage by Candida albicans. J. Infect. Dis. 167:1467-1470. [DOI] [PubMed] [Google Scholar]
- 20.Kerdudou, S., M. W. Laschke, B. Sinha, K. L. Preissner, M. D. Menger, and M. Herrmann. 2006. Fibronectin binding proteins contribute to the adherence of Staphylococcus aureus to intact endothelium in vivo. Thromb. Haemost. 96:183-189. [PubMed] [Google Scholar]
- 21.Laschke, M. W., S. Kerdudou, M. Herrmann, and M. D. Menger. 2005. Intravital fluorescence microscopy: a novel tool for the study of the interaction of Staphylococcus aureus with the microvascular endothelium in vivo. J. Infect. Dis. 19:435-443. [DOI] [PubMed] [Google Scholar]
- 22.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 23.Lowy, F. D., J. Fant, L. L. Higgins, S. K. Ogawa, and V. B. Hatcher. 1988. Staphylococcus aureus—human endothelial cell interactions. J. Ultrastruct. Mol. Struct. Res. 98:137-146. [DOI] [PubMed] [Google Scholar]
- 24.Matussek, A., J. Strindhall, L. Stark, M. Rohde, R. Geffers, J. Buer, E. Kihlström, P.-E. Lindgren, and S. Löfgren. 2005. Infection of human endothelial cells with Staphylococcus aureus induces transcription of genes encoding an innate immunity response. Scand. J. Immunol. 61:536-544. [DOI] [PubMed] [Google Scholar]
- 25.Menzies, B. E., and Y. Kourteva. 1998. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect. Immun. 66:5994-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreillon, P., and Y.-A. Que. 2004. Infective endocarditis. Lancet 363:139-149. [DOI] [PubMed] [Google Scholar]
- 27.Nakane, A., M. Okamoto, M. Asano, M. Kohanawa, and T. Minagawa. 1995. Endogenous gamma interferon, tumor necrosis factor, and interleukin-6 in Staphylococcus aureus infection in mice. Infect. Immun. 63:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa, S. K., E. R. Yurberg, V. B. Hatcher, M. A. Levitt, and F. D. Lowy. 1985. Bacterial adherence to human endothelial cells in vitro. Infect. Immun. 50:218-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owaki, T., A. Meneshian, K. Maemura, S. Takao, D. Wang, K. C. Fuh, G. B. Bulkley, and A. S. Klein. 2000. Endothelial cells potentiate phagocytic killing by macrophages via platelet-activating factor release. Am. J. Physiol. Heart Circ. Physiol. 278:H269-H276. [DOI] [PubMed] [Google Scholar]
- 30.Palmqvist, N., J. M. Patti, A. Tarkowski, and E. Josefsson. 2004. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microb. Infect. 6:188-195. [DOI] [PubMed] [Google Scholar]
- 31.Peacock, S. J., T. J. Foster, B. J. Cameron, and A. R. Berendt. 1999. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology 145:3477-3486. [DOI] [PubMed] [Google Scholar]
- 32.Pfefferkorn, E. R. 1984. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proctor, R. A., J. M. Balwit, and O. Vesga. 1994. Variant subpopulations of Staphylococcus aureus as cause of persistent and recurrent infections. Infect. Agents Dis. 3:302-312. [PubMed] [Google Scholar]
- 34.Pruzanski, W., S. Saito, and D. W. Nitzan. 1983. The influence of lysostaphin on phagocytosis, intracellular bactericidal activity, and chemotaxis of human polymorphonuclear cells. J. Lab. Clin. Med. 102:298-305. [PubMed] [Google Scholar]
- 35.Que, Y. A., J.-A. Haefliger, L. Piroth, P. Francois, E. Widmer, J. M. Entenza, B. Sinha, M. Herrmann, P. Francioli, P. Vandaux, and P. Moreillon. 2005. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J. Exp. Med. 201:1627-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki, S., S. Nishikawa, T. Miura, M. Mizuki, K. Yamada, H. Madarame, Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2000. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect. Immun. 68:2424-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki, S., Y. I. Tagawa, Y. Iwakura, and A. Nakane. 2006. The role of gamma interferon in acquired host resistance against Staphylococcus aureus infection in mice. FEMS Immunol. Med. Microbiol. Immun. 46:367-374. [DOI] [PubMed] [Google Scholar]
- 38.Schneider, W. P., S. K. Ho, J. Christine, M. Yao, A. Marra, and A. E. Hromockyj. 2002. Virulence gene identification by differential fluorescence induction analysis of Staphylococcus aureus gene expression during infection-stimulating culture. Infect. Immun. 70:1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder, A., B. Schröder, B. Roppenser, S. Linder, B. Sinha, R. Fässler, and M. Aepfelbacher. 2006. Staphylococcus aureus fibronectin binding protein-A induces motile attachment sites and complex actin remodeling in living endothelial cells. Mol. Biol. Cell 17:5198-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schröder, A., R. Kland, A. Peschel, C. von Eiff, and M. Aepfelbacher. 2006. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med. Microbiol. Immunol. 195:185-194. [DOI] [PubMed] [Google Scholar]
- 41.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 42.Shinji, H., K. Seki, A. Tajima, A. Uchida, and S. Masuda. 2003. Fibronectin bound to the surface of Staphylococcus aureus induces association of very late antigen 5 and intracellular signaling factors with macrophage cytoskeleton. Infect. Immun. 71:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha, B., and M. Herrmann. 2005. Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb. Haemost. 94:266-277. [DOI] [PubMed] [Google Scholar]
- 44.Sinha, B., P. P. François, O. Nüsse, M. Foti, O. M. Hartford, P. Vaudaux, T. J. Foster, D. P. Lew, M. Herrmann, and K. H. Krause. 1999. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell. Microbiol. 1:101-117. [DOI] [PubMed] [Google Scholar]
- 45.Söderquist, B., J. Källman, H. Holmberg, T. Vikerfors, and E. Kihlström. 1998. Secretion of IL-6, IL-8 and G-CSF by human endothelial cells in vitro in response to Staphylococcus aureus and staphylococcal exotoxins. APMIS 106:1157-1164. [PubMed] [Google Scholar]
- 46.Strindhall, J., P.-E. Lindgren, S. Löfgren, and E. V. Kihlström. 2002. Variations among clinical isolates of Staphylococcus aureus to induce expression of E-selectin and ICAM-1 in human endothelial cells. FEMS Immunol. Med. Microbiol. 18:227-235. [DOI] [PubMed] [Google Scholar]
- 47.Tekstra, J., H. Beekhuizen, J. S. van de Gevel, I. J. van Benten, C. W. Tuk, and R. H. J. Beelen. 1999. Infection of human endothelial cells with Staphylococcus aureus induces the production of monocyte chemotactic protein-1 and monocyte chemotaxis. Clin. Exp. Immunol. 117:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas, P. D., F. W. Hampson, and G. W. Hunninghake. 1988. Bacterial adherence to human endothelial cells. J. Appl. Physiol. 65:1372-1376. [DOI] [PubMed] [Google Scholar]
- 49.van den Broek, P. J., F. A. Dehue, P. C. Leijh, M. T. van den Barselaar, and R. van Furth. 1981. The use of lysostaphin in in vitro assays of phagocyte function: adherence to and penetration into granulocytes. Scand. J. Immunol. 15:467-473. [DOI] [PubMed] [Google Scholar]
- 50.van den Broek, P. J., L. F. Buys, and R. van Furth. 1985. Adherence of lysostaphin to and penetration into human monocytes. Scand. J. Immunol. 21:189-193. [DOI] [PubMed] [Google Scholar]
- 51.Vann, J. M., and R. A. Proctor. 1988. Cytotoxic effects of ingested Staphylococcus aureus on bovine endothelial cells: role of S. aureus alpha-hemolysin. Microb. Pathog. 4:443-453. [DOI] [PubMed] [Google Scholar]
- 52.Veltrop, M. H. A. M., and H. Beekhuizen. 2002. Monocytes maintain tissue factor activity after cytolysis of bacteria-infected endothelial cells in an in vitro model of bacterial endocarditis. J. Infect. Dis. 186:1145-1154. [DOI] [PubMed] [Google Scholar]
- 53.Veltrop, M. H. A. M., H. Beekhuizen, and J. Thompson. 1999. Bacterial species- and strain-dependent induction of tissue factor in human vascular endothelial cells. Infect. Immun. 67:6130-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veltrop, M. H. A. M., J. Thompson, and H. Beekhuizen. 2001. Monocytes augment the bacterial species- and strain-dependent induction of tissue factor activity in bacterium-infected human vascular endothelial cells. Infect. Immun. 69:2797-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieira, O. V., R. J. Botelho, and S. Grinstein. 2002. Phagosome maturation: aging gracefully. Biochem. J. 366:689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.von Eiff, C., G. Peters, and K. Becker. 2006. The small colony variant (SCV) concept—the role of staphylococcal SCVs in persistent infections. Injury 37:S26-S33. [DOI] [PubMed] [Google Scholar]
- 57.Vriesema, A. J. M., H. Beekhuizen, M. Hamdi, A. Soufan, A. Lammers, B. Willekens, O. Bakker, M. H. A. M. Veltrop, J. S. van de Gevel, J. Dankert, and S. A. J. Zaat. 2000. Altered gene expression in Staphylococcus aureus upon interaction with human endothelial cells. Infect. Immun. 68:1765-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamauchi, R., M. Tanaka, N. Kume, M. Minami, T. Kawamoto, K. Togi, T. Shimaoke, S. Takahashi, J. Yamaguchi, T. Nishina, M. Kitaichi, M. Komeda, T. Manabe, S. Yonehara, and T. Kita. 2004. Upregulation of SR-PSOX/CSCL16 and recruitment of CD8+ T cells in cardiac valves during inflammatory valvular heart disease. Artherioscler. Thromb. Vasc. Biol. 24:282-287. [DOI] [PubMed] [Google Scholar]
- 59.Yao, L., F. D. Lowy, and J. W. Berman. 1996. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect. Immun. 64:3400-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao, L., V. Bengualid, F. D. Lowy, J. J. Gibbons, V. B. Hatcher, and J. W. Berman. 1995. Interleukin-8 gene expression in Staphylococcus aureus-infected endothelial cells. Infect. Immun. 63:1835-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, B., M. Centra, G. Liang Cao, R. E. Ratych, J. B. Domachowske, H. L. Maleck, and G. M. Rosen. 1997. Are free radicals responsible for endothelial killing of Staphylococcus aureus? Immunol. Lett. 58:113-120. [DOI] [PubMed] [Google Scholar]
- 62.Zhao, Y.-X., I.-M. Nilsson, and A. Tarkowski. 1998. The dual role of interferon-γ in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology 93:80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]