Abstract
Acute fungal sinusitis (AFS) is a devastating disease of the paranasal sinuses afflicting immunocompromised individuals. Knowledge about this disease is limited to clinical observations because there are no animal models in which to study the pathogenesis of the infection. Our goal was to develop a murine model of AFS and examine the role of neutrophils in host defense within the nasal cavity. Female C57BL/6 mice were depleted of neutrophils using anti-Gr-1 monoclonal antibody from day −1 to day 5 postinfection to initiate a transient neutropenia within the mice. At day 0, Aspergillus fumigatus conidia were administered intranasally. The untreated Aspergillus-exposed group had significant neutrophil recruitment by day 3, but by day 7 the leukocyte numbers had returned to unexposed levels. There was not a significant influx of mononuclear cells at either time point. In contrast, beginning at day 3 postinfection and continuing through day 7, anti-Gr-1-treated mice had increased cellular recruitment consisting of banded neutrophils at day 3 and mature neutrophils at day 7. Hyphal masses developed only in the anti-Gr-1-treated mice (25 to 36%) but only during the period of treatment. When the treatment was discontinued, hyphal masses could no longer be detected in the nasal cavities of these mice. In contrast, cyclophosphamide treatment did not induce neutropenia, and the nasal cavity remained free of hyphal masses. These studies demonstrate the feasibility of using this model to study AFS and implicate neutrophils in protection of the sinuses against acute Aspergillus infection and in clearance of established hyphal masses.
Acute fungal sinusitis (AFS), also known as fulminant sinusitis, is a rapid, invasive disease of the paranasal cavities. It develops primarily in immunocompromised individuals, such as bone marrow transplant and AIDS patients (5, 21). The infection is characterized by rapid hyphal transformation within the paranasal sinus cavities. This is followed by fungal invasion of the surrounding mucosa, musculature, vasculature, and, in severe cases, cranium. Patients who do not receive proper medical attention can suffer from bone and tissue loss and, if left untreated, death.
Recent research about AFS has been limited to clinical observations because there are not currently animal models of this disease. Much of what is understood about the innate response to fungal infections in the nasal cavity is inferred from correlatives drawn from other tissue sites. For example, within the lungs, fungal conidium challenge stimulates leukocyte recruitment into the airspaces. The leukocytes, primarily macrophages and neutrophils, internalize resting or swollen conidia and use mechanisms such as respiratory burst to combat infections (2, 3, 10-12, 22, 24). It is believed that macrophages and neutrophils may have a similar response to fungi within the nasal cavity, although there have been no murine models that demonstrate this (18, 20). Neutrophils help mediate the nasal inflammatory response in rodents coexposed to lipopolysaccharide and ozone, but the role of these cells in an active sinus infection has yet to be evaluated (6, 25).
Prior to these studies, there was no report of an animal model of fungal sinusitis in physically intact animals. Other murine studies focused on the pathology of bacterial sinusitis and used physical alterations of the nasal cavity to cause disease. For example, one model of chronic bacterial rhinosinusitis used a Merocel sponge to cause a blockage within the nasal cavity in order to facilitate an infection (9). Although this study did show bacterial colonization and ensuing inflammation, the infection was not localized to a sinus cavity and aspects of the innate immune response were not addressed. The focus of our studies was to determine whether neutrophils protect the sinus cavity from Aspergillus infection. We did this by developing a novel murine model of AFS. In our studies, the nasal cavities of the mice were not physically altered, but the mice were rendered neutropenic, a common predisposing condition for the development of AFS. The mice were then exposed to Aspergillus fumigatus intranasally to determine if the absence of neutrophils was sufficient for fungal colonization and subsequent development of AFS. The neutropenia was then discontinued to determine whether neutrophils play a role in resolving established hyphal masses.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (Harlan Sprague Dawley, Inc.) were housed under specific-pathogen-free conditions in enclosed filter top cages. Food and sterile water were given ad libitum. The mice were maintained by the Unit for Laboratory Animal Medicine at the University of Michigan (Ann Arbor, MI), and protocols were approved by an animal institutional review board.
Induction of temporary neutropenia within mice.
Temporary neutropenia was established within mice to allow development of an invasive fungal infection. Mice were injected intraperitoneally (i.p.) with 100 μg of anti-Gr-1 antibody (RB6-8C5 ascites; raised at Taconic Biotechnology, Germantown, NY) 1 day prior to intranasal fungal exposure. Three additional i.p. injections were given to the mice at days 1, 3, and 5 postinfection to efficiently deplete the mature neutrophil population in treated mice. Once anti-Gr-1 treatment was discontinued at day 5, mature neutrophils began to return, and by day 7 the numbers corresponded to those in a wild-type, uninfected animal.
Immunosuppression in mice using cyclophosphamide.
Animals were treated with 150 mg/kg of cyclophosphamide (Sigma, St. Louis, MO) via i.p. injection 3 days prior to intranasal exposure. In order to maintain immunosuppression, subsequent injections were then given every 3 days.
Intranasal inoculation of A. fumigatus conidia.
A. fumigatus ATCC 13073 was grown on Sabouraud dextrose agar (Difco) for 14 days. Spores (conidia) were harvested by washing plates with sterile 0.1% Tween 80, followed by filtration of the suspension through two layers of sterile gauze to remove hyphae. For infection, the spores were washed in nonpyrogenic saline (Abbott Laboratories, Chicago, IL), counted with a hemocytometer, and diluted to obtain 108 spores/ml in sterile nonpyrogenic saline in order to prepare the final inoculum, 106 conidia/mouse administered in 5 μl/nostril (total volume, 10 μl/mouse). Prior to intranasal inoculation, mice were anesthetized by i.p. injection of a ketamine-xylazine solution (2.5 mg of ketamine [Fort Dodge Animal Health, Fort Dodge, IA]/mouse plus 0.1 g of xylazine [Lloyd Laboratories, Shenandoah, IA]/mouse).
Nasal leukocyte isolation.
Mice were euthanized with CO2. The heads of the mice were removed, and the sinuses were exposed via a transverse cut along the skull. The samples then were enzymatically digested for 40 min at 37°C with 15 ml of digestion buffer (RPMI 1640, 10% fetal calf serum, antibiotics, 1 mg/ml collagenase [Boehringer Mannheim Biochemicals, Chicago, IL], 30 μg/ml DNase [Sigma Chemical Co., St. Louis, MO]) per sample. Samples were removed from the resulting cell suspensions and dispersed by drawing the cells up and down through the bore of a 10-ml syringe. Each cell suspension was then pelleted, and erythrocytes were lysed by incubation in ice-cold NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, 0.0372% Na2EDTA [pH 7.4]; Sigma). Excess RPMI 1640 was added to make the solution isotonic, and the cells were pelleted and resuspended in complete medium (RPMI 1640, 10% fetal calf serum [Life Technologies], 5 × 10−5 M 2-mercaptoethanol, sodium pyruvate, nonessential amino acids, glutamine, antibiotics [Sigma]). Cell concentrations were determined by counting the cells after trypan blue staining.
Splenocyte isolation.
Spleens were excised, and cells were dispersed with the plunger of a 3-ml syringe. Erythrocytes were lysed using NH4Cl buffer, and cells were resuspended in complete medium (RPMI 1640, 5% fetal calf serum, 2 mmol/liter l-glutamine, 50 μmol/liter 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate).
Histological analysis.
Samples were fixed in 10% neutral buffered formalin and decalcified prior to sectioning (4). Slides were then stained with hematoxylin and eosin for visualization, with Alcian Blue-periodic acid-Schiff stain for visualization of intraepithelial mucosubstances, and with Gomori methenamine silver for visualization of the fungi.
Cytokine and chemokine analysis.
Nasal cavities were excised using microsurgical techniques and kept in RNAlater (Ambion, Austin, TX) at −20°C until RNA isolation was performed. For reverse transcription-PCR, total RNA was isolated from the septa using Trizol reagent (Life Technologies, Gaithersburg, MD) as outlined in the Trizol protocol. After amplification, samples were separated on a 2% agarose gel containing 5 μl/100 ml ethidium bromide (10 mg/ml; Sigma), and bands were visualized and photographed using UV transillumination. The primer sequences used are as follows: for KC, forward primer 5′-TGAGCTGCGCTGTCAGTGCCT-3′ and reverse primer 5′-AGAAGCCAGCGTTCACCAGA-3′; for tumor necrosis factor alpha, forward primer 5′-CCTGTAGCCCACGTCGTAGC-3′ and reverse primer 5′-AGCAATGACTCCAAAGTAGACC-3′; for gamma interferon, forward primer 5′-CTACCTCAGACTCTTTGAAGTCT-3′ and reverse primer 5′-CAGCGACTCCTTTTCCGCTT-3′; for MIP-2, forward primer 5′-GCTGGCCACCAACCACCAGG-3′ and reverse primer 5′-AGCGAGGCACATCAGGTACG-3′; for interleukin-6 (IL-6), forward primer 5′-GACAAAGCCAGAGTCCTTCAGAGAG-3′ and reverse primer 5′-CTAGGTTTGCCGAGTAGATCTC-3′; and for β-actin, forward primer 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ and reverse primer 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′. Kodak Molecular Imaging software was used to quantify the intensity of the bands, and the net intensity was used to for comparisons between groups. Net intensity is the background-subtracted pixel values in the band rectangle.
RESULTS
To determine the effect of neutrophil depletion on the development of AFS, mice were made temporarily neutropenic by administration of anti-Gr-1 at days −1, 1, 3, and 5. Mice were inoculated intranasally with A. fumigatus at day 0 and then analyzed histologically for the presence of hyphal masses (Fig. 1 to 3). The anti-Gr-1 depletion protocol was effective in significantly reducing the amount of neutrophils present in the nasal cavity and spleen following intranasal exposure to A. fumigatus at day 3 postinfection (Fig. 4; see Fig. S1 in the supplemental material).
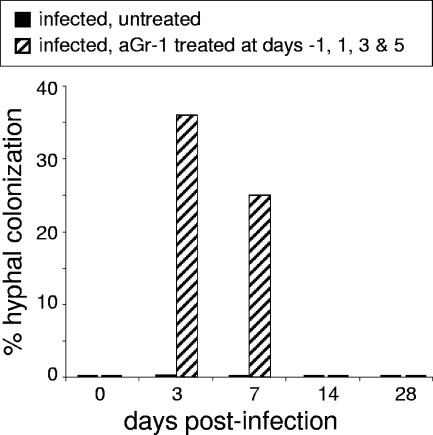
FIG. 1.
Hyphal colonization in untreated and anti-Gr-1 treated mice infected with A. fumigatus. The percent hyphal colonization represents the fraction of animals in which hyphal colonization of the nasal cavity was detected. Histology samples were analyzed for the presence of hyphal masses within the maxillary sinuses and nasal cavity (n ≥ 8/group/time point).
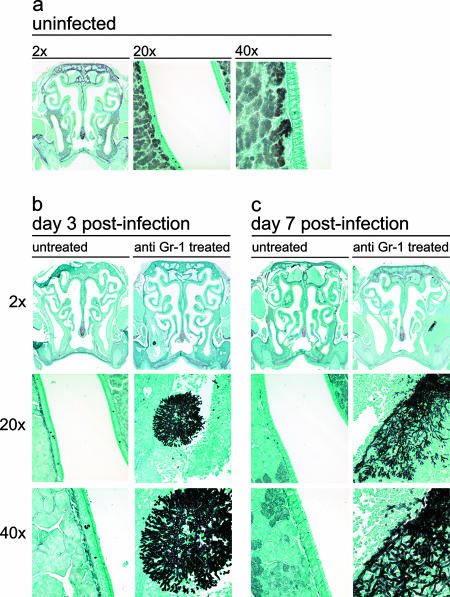
FIG. 3.
Gomori methenamine silver staining of the mouse nasal cavity. Mice were either not treated or treated with anti-Gr-1 and then intranasally infected with A. fumigatus as outlined in the text. At days 3 and 7 postinfection, the sinuses were removed, fixed, sectioned, and stained with Gomori methenamine silver, which stains polysaccharides black, for fungal visualization. (a) Untreated, uninfected control animal. (b) Untreated and anti-Gr-1-treated mice infected with A. fumigatus at day 3 postinfection. (c) Untreated and anti-Gr-1-treated mice infected with A. fumigatus at day 7 postinfection. The 20× and 40× images were derived from the infected maxillary sinus cavities shown in the 2× images of sections.
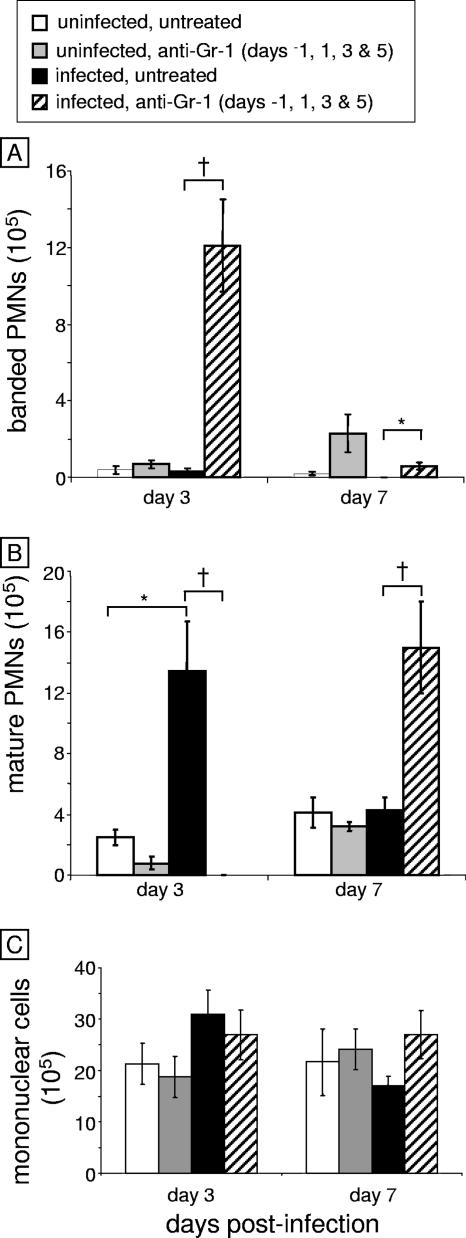
FIG. 4.
Differential analysis of nasal leukocyte recruitment. Following leukocyte isolation from nasal cavity digests, cytospin slides were made and stained with Wright-Giemsa stain to visualize cell populations. (A) Mature neutrophil cell counts. (B) Immature (banded) neutrophil cell counts. (C) Mononuclear cell counts. An asterisk indicates a P value of <0.05 and a dagger indicates a P value of <0.01 as determined by Student's t test (n = 6 mice per group). PMNs, polymorphonuclear leukocytes.
Mice treated with anti-Gr-1 antibody developed invasive hyphal masses that were visible in histology sections. The masses were localized to the maxillary sinus cavities at days 3 and 7. By day 3 postinfection, 36% of treated mice had developed hyphal masses. By day 7, masses were seen in 25% of treated mice (Fig. 1). Conversely, untreated mice infected with A. fumigatus showed no signs of hyphal formation at any time point.
We could not continue to treat mice with anti-Gr-1 antibody past day 7. This is because the mice mounted an immune response (serum sickness) against the anti-Gr-1 monoclonal antibody, which is a rat antibody. We continued to monitor mice after they recovered from the transient neutropenia. At days 14 and 28 postinfection, there were no signs of hyphal colonization in histological sections from either untreated or previously anti-Gr-1-treated mice (data not shown).
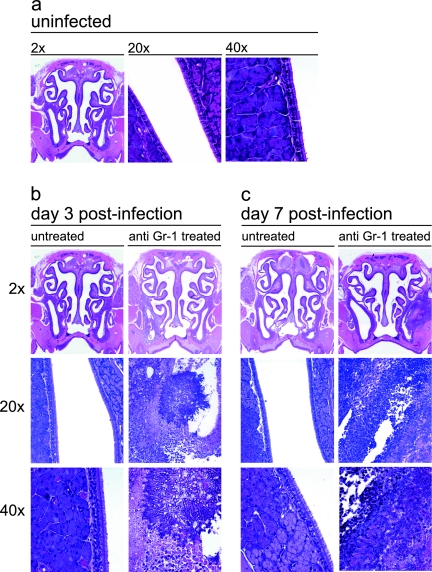
We next examined whether there were changes to the nasal architecture that were caused by hyphal colonization and induction of AFS. All histological analysis was confined to the maxillary sinuses and the areas of the nasal cavity nearest them. Untreated, Aspergillus-exposed mice did not have pathology indicative of AFS (Fig. 2 and 3). All cavities and airways were intact and free of fungal masses, although some minor inflammation was seen in the subepithelial space. In contrast, anti-Gr-1-treated mice had hyphal masses with lesions centered predominantly in the maxillary sinus cavities (Fig. 2 and 3). The lesions were asymmetrical and were found in either the left or right maxillary sinus cavity but never in both cavities. All lesions were characterized by a marked, pyronecrotizing fungal sinusitis with acute rhinitis that varied in severity. At day 3 postinfection, all hyphal masses were localized to the maxillary sinuses and there was some invasion of the underlying tissue, resulting in necrosis and an influx of inflammatory cells (Fig. 2b and 3b).
FIG. 2.
Hematoxylin and eosin staining of the mouse nasal cavity. Mice were either not treated or treated with anti-Gr-1 and then intranasally infected with A. fumigatus as outlined in the text. At days 3 and 7 postinfection, the sinuses were removed, fixed, sectioned, and stained with hematoxylin and eosin to differentially stain leukocytes. (a) Untreated, uninfected control animal. (b) Untreated and anti-Gr-1-treated mice infected with A. fumigatus at day 3 postinfection. (c) Untreated and anti-Gr-1-treated mice infected with A. fumigatus at day 7 postinfection. The 20× and 40× images were derived from the infected maxillary sinus cavities shown in the 2× images of sections.
By day 7, the pathology had worsened, and there was exacerbation of the necrosis and corresponding inflammation. Hyphal invasion into the outer musculature of the cranium was seen, and portions of the initial hyphal mass had become detached. The hyphal fragments then colonized other areas of the nasal cavity, resulting in additional inflammation and drastic architectural changes in both the maxillary sinuses and nasal cavity (Fig. 2c and 3c). At day 7, untreated Aspergillus-exposed mice still had no pathology; all cavities were clear of debris, inflammation, and hyphae.
Mucus production was also disrupted in tissue and mucosa where hyphal masses had become invasive. Using a stain to highlight mucus (Alcian Blue-periodic acid-Schiff stain), there was a significant decrease in mucus production at the sites of hyphal masses in the treated mice at day 3 postinfection. By day 7, there was no mucus production in these sites (see Fig. S2 in the supplemental material).
The next objective was to analyze the kinetics of leukocyte recruitment into the nasal cavity following infection. The nasal cavity was excised and enzymatically digested as outlined in Materials and Methods. The cells in the cellular infiltrates were enumerated and then cytospun onto slides, stained, and analyzed. At day 3 postinfection, there was a significant increase in mature neutrophil recruitment in untreated, infected mice compared to uninfected mice (Fig. 4). In contrast, there were no mature neutrophils at day 3 postinfection in the nasal cavities of anti-Gr-1-treated mice. However, a significant number of banded neutrophils were recruited to the infection within the nasal cavity in anti-Gr-1-treated mice (Fig. 4). By day 7, a small number of banded neutrophils were still present; however, a substantial majority of the infiltrate was now mature neutrophils (the last injection of anti-Gr-1 was on day 5). The number of neutrophils in this group returned to uninfected levels by days 14 and 28 (data not shown). Thus, the kinetics of mature neutrophil influx in both groups correlated with fungal clearance, and if neutrophil influx was prevented by anti-Gr-1 treatment, hyphal masses developed.
Since Gr-1 can also be expressed on a subpopulation of recently recruited monocytes (16), we also analyzed mononuclear cell recruitment in the mice following exposure to Aspergillus. There was no statistically significant change in mononuclear cells in either untreated or anti-Gr-1-treated mice at days 3 and 7 postinfection compared to uninfected mice (Fig. 4). Thus, mononuclear cells are not a major component of the inflammatory infiltrate in the nasal cavity following Aspergillus exposure.
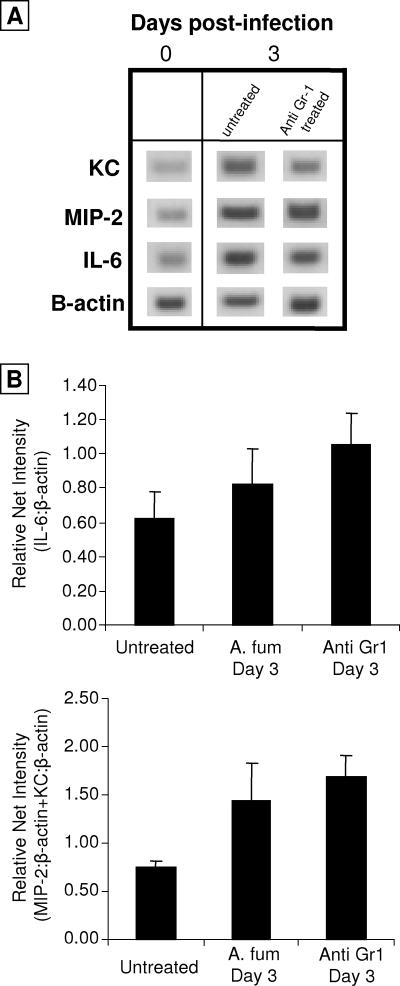
We next examined expression of the CXC chemokines KC (CXCL1) and MIP-2 (CXCL2) and the inflammatory cytokine IL-6 in the nasal cavity at day 3 postinfection (Fig. 5). The levels of expression of KC, MIP-2, and IL-6 were all elevated following Aspergillus infection, and the induction was not affected by treatment with anti-Gr-1. These data demonstrate that mature neutrophils are not required for expression of these cytokines.
FIG. 5.
Nasal cavity chemokine profiles of A. fumigatus-infected mice. Mice were either not treated, infected with A. fumigatus (A. fum), or treated with anti-Gr-1 and infected with A. fumigatus (anti-Gr1). The nasal cavities were excised from mice using microsurgical techniques. RNA was isolated from the samples, and reverse transcriptase PCR was performed in order to analyze localized chemokine expression. (A) After visualization, a representative band was taken from each gel in order to compare groups (n = 4). Kodak Molecular Imaging software was used to quantify the intensity of the bands. (B) Net intensity values expressed as a mean ratio (chemokine/β-actin) for each group (n = 4).
The last objective of these studies was to determine if cyclophosphamide could also induce the development of invasive hyphal masses within the nasal cavities of mice exposed to Aspergillus. Cyclophosphamide depletes circulating T cells, B cells, and mononuclear cells, but it does not interfere with neutrophil development. Mice were treated with 150 mg/kg of cyclophosphamide via i.p. injection 3 days prior to intranasal exposure. Subsequent injections were administered every 3 days in order to maintain immunosuppression. Mice were then analyzed at days 3, 7, 14, and 21 postinfection. Cyclophosphamide did not affect splenic neutrophil numbers at day 3 postinfection, while anti-Gr-1 caused a significant decrease (see Fig. S1 in the supplemental material). At all time points, development of hyphal masses was not seen within the nasal cavities of cyclophosphamide-treated mice (data not shown). In every specimen analyzed, small numbers of leukocytes were seen in the nasal cavities of treated mice; however, hyphal masses were never seen in the sinus or nasal cavities. Thus, protection against AFS depends on a cyclophosphamide-resistant cell population, and since anti-Gr-1 treatment ablated protection, these results suggest that the primary cell involved in host defense in the nasal cavity is the neutrophil.
DISCUSSION
We examined the protective role of neutrophils against A. fumigatus in AFS by developing a novel, reproducible model of the disease. In our studies, mice made temporarily neutropenic were not protected against fungal colonization and subsequent development of AFS. Previous studies of sinusitis pathogenesis have relied on alterations to the nasal architecture or physical impairments to allow disease progression. In the studies described here, the nasal cavity of mice was not altered and the mice were exposed to the fungus through a physiologically relevant route of infection. In both anti-Gr-1-treated and untreated experimental groups, inflammation or fungal colonization was always localized to the nasal cavity and did not disseminate to the lungs (data not shown).
Prior to this investigation, knowledge of the innate immune response against invasive aspergillosis in the nasal cavity was based on clinical observations or extrapolation from murine models of pulmonary disease. Although many correlations can be inferred between AFS and pulmonary invasive aspergillosis, there are significant differences between these diseases. The most noteworthy difference is the formation of the large hyphal masses seen in our model of AFS, which are never seen in pulmonary invasive aspergillosis. Even in neutropenic mice with severe cellular infiltrate, small hyphal fragments are all that is visualized within the lungs of the mice (3, 14). One explanation for this difference is that alveolar macrophages are responsible for ingesting resting conidia, thereby preventing hyphal transformation and germination within the lungs (8, 11, 19, 22, 26). These observations can also account for the inability of cyclophosphamide to induce AFS in our studies. Many murine models of pulmonary invasive aspergillosis use cyclophosphamide in order to immunosuppress the animals and allow initiation of fungal infection. The nasal cavity does not have resident macrophages that reside on the mucosal surfaces within the open air spaces. Without resident airway cells to ingest inhaled resting conidia, hyphal transformation may occur within the nasal cavity. Thus, the nasal cavity must rely on an influx of neutrophils to control hyphal growth.
The results presented here definitively demonstrate that Gr-1+ cells are required for protection of the nasal cavity from Aspergillus infection. In addition to neutrophils, other cells, such as a subpopulation of macrophages and plasmacytoid dendritic cells, also express Gr-1 (16, 17). This study did not exclude the potential positive contribution of these cells to host defense in the upper airways, and we hypothesize that the cells may be extremely important during chronic fungal infection. However, the anti-Gr-1 and cyclophosphamide results clearly demonstrate that Gr-1+ neutrophils are critical for innate host defense in the sinuses during acute fungal exposure.
Expression of the neutrophil chemotactic factors KC and MIP-2 was elevated in the nasal cavity following Aspergillus exposure. Patients with AFS often have elevated levels of the CXC chemokines IL-8, which is a functional homologue of KC, and MIP-2 (23). Increased levels of CXC chemokines following Aspergillus exposure have also been reported in murine models of invasive aspergillosis (3, 12-14). Additionally, transgenic overexpression of KC in the lungs was able to improve the outcome following pulmonary aspergillus infection (15). Thus, it is very likely that CXC chemokines are the major chemotactic signals for neutrophil influx into the nasal cavity following Aspergillus infection.
In conclusion, while much is known about host defense against Aspergillus in the lungs and systemically, relatively little is known about host defense against this mold in the sinuses. Furthermore, little is known about the effect of hyphal colonization on nasal architecture and function. The studies presented here demonstrate the feasibility of this model for studying AFS and implicate neutrophils in protection of the sinuses from Aspergillus infection and in clearance of established hyphal masses. The approach presented here can also be used to study the effect of virus-induced inflammation on the development of AFS. Viral infections can cause inflammation of the ostia, the sinuses, and the surrounding mucosa. Such inflammation has been hypothesized to provide an environment conducive to fungal colonization by disrupting normal regulation of innate immunity and nasal physiology within the paranasal region (1, 7). Thus, using models which do not disrupt the underlying architecture of the nasal cavity holds significant promise for identifying the etiologic factors that lead to fungal sinusitis.
Supplementary Material
Acknowledgments
We thank Galen Toews and Jeffery Terrell for their invaluable scholarly contributions to this study.
This work was supported in part by grants R01AI064479 (to G.B.H.) and T32AI007528 (to T.E.R.) from the National Institute of Allergy and Infectious Diseases.
Editor: A. Casadevall
Footnotes
Published ahead of print on 17 September 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Baraniuk, J. 1994. Physiology of sinusitis, p. 19-39. In H. M. Druce (ed.), Sinusitis: pathophysiology and treatment, vol. 1. Marcel Dekker, Inc., New York, NY. [Google Scholar]
- 2.Braedel, S., M. Radsak, H. Einsele, J. P. Latge, A. Michan, J. Loeffler, Z. Haddad, U. Grigoleit, H. Schild, and H. Hebart. 2004. Aspergillus fumigatus antigens activate innate immune cells via Toll-like receptors 2 and 4. Br. J. Haematol. 125:392-399. [DOI] [PubMed] [Google Scholar]
- 3.Cenci, E., A. Mencacci, C. Fe d'Ostiani, C. Montagnoli, A. Bacci, G. Del Sero, S. Perito, F. Bistoni, and L. Romani. 1998. Cytokine- and T-helper-dependent immunity in murine aspergillosis. Res. Immunol. 149:445-454. (Discussion, 149: 504-505.) [DOI] [PubMed] [Google Scholar]
- 4.Farraj, A. K., J. R. Harkema, and N. E. Kaminski. 2004. Allergic rhinitis induced by intranasal sensitization and challenge with trimellitic anhydride but not with dinitrochlorobenzene or oxazolone in A/J mice. Toxicol. Sci. 79:315-325. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson, B. J. 2000. Definitions of fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 33:227-235. [DOI] [PubMed] [Google Scholar]
- 6.Harkema, J. R., and J. G. Wagner. 2005. Epithelial and inflammatory responses in the airways of laboratory rats coexposed to ozone and biogenic substances: enhancement of toxicant-induced airway injury. Exp. Toxicol. Pathol. 57(Suppl. 1):129-141. [DOI] [PubMed] [Google Scholar]
- 7.Herbert, R. A., and J. R.Leininger. 1999. Nose, larynx and trachea, p. 259-292. In R. Maronpot (ed.), Pathology of the mouse. Cache River Press, Vienna, IL.
- 8.Ibrahim-Granet, O., B. Philippe, H. Boleti, E. Boisvieux-Ulrich, D. Grenet, M. Stern, and J. P. Latge. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71:891-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob, A., B. T. Faddis, and R. A. Chole. 2001. Chronic bacterial rhinosinusitis: description of a mouse model. Arch. Otolaryngol. Head Neck Surg. 127:657-664. [DOI] [PubMed] [Google Scholar]
- 10.Latgé, J. P. 2001. The pathobiology of Aspergillus fumigatus. Trends Microbiol. 9:382-389. [DOI] [PubMed] [Google Scholar]
- 11.Levitz, S. M., M. E. Selsted, T. Ganz, R. I. Lehrer, and R. D. Diamond. 1986. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J. Infect. Dis. 154:483-489. [DOI] [PubMed] [Google Scholar]
- 12.Mehrad, B., T. A. Moore, and T. J. Standiford. 2000. Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts. J. Immunol. 165:962-968. [DOI] [PubMed] [Google Scholar]
- 13.Mehrad, B., R. M. Strieter, T. A. Moore, W. C. Tsai, S. A. Lira, and T. J. Standiford. 1999. CXC chemokine receptor-2 ligands are necessary components of neutrophil-mediated host defense in invasive pulmonary aspergillosis. J. Immunol. 163:6086-6094. [PubMed] [Google Scholar]
- 14.Mehrad, B., R. M. Strieter, and T. J. Standiford. 1999. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 162:1633-1640. [PubMed] [Google Scholar]
- 15.Mehrad, B., M. Wiekowski, B. E. Morrison, S. C. Chen, E. C. Coronel, D. J. Manfra, and S. A. Lira. 2002. Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am. J. Respir. Crit. Care Med. 166:1263-1268. [DOI] [PubMed] [Google Scholar]
- 16.Mordue, D. G., and L. D. Sibley. 2003. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 74:1015-1025. [DOI] [PubMed] [Google Scholar]
- 17.Nakano, H., M. Yanagita, and M. D. Gunn. 2001. CD11c+ B220+ Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeters, D., M. J. Day, and C. Clercx. 2005. An immunohistochemical study of canine nasal aspergillosis. J. Comp. Pathol 132:283-288. [DOI] [PubMed] [Google Scholar]
- 19.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 71:3034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitzurra, L., S. Bellocchio, A. Nocentini, P. Bonifazi, R. Scardazza, L. Gallucci, F. Stracci, C. Simoncelli, F. Bistoni, and L. Romani. 2004. Antifungal immune reactivity in nasal polyposis. Infect. Immun. 72:7275-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pleis, J., and R. Coles. 2003. Summary health statistics for U.S. adults: National Health Interview Survey, 1999. National Center for Health Statistics. Vital Health Stat. 10:1-145. [Google Scholar]
- 22.Roilides, E., H. Katsifa, and T. J. Walsh. 1998. Pulmonary host defences against Aspergillus fumigatus. Res. Immunol. 149:454-465. (Discussion, 149:523-524.) [DOI] [PubMed] [Google Scholar]
- 23.Rudack, C., W. Stoll, and C. Bachert. 1998. Cytokines in nasal polyposis, acute and chronic sinusitis. Am. J. Rhinol. 12:383-388. [DOI] [PubMed] [Google Scholar]
- 24.Schaffner, A., H. Douglas, and A. Braude. 1982. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J. Clin. Investig. 69:617-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner, J. G., J. A. Hotchkiss, and J. R. Harkema. 2001. Effects of ozone and endotoxin coexposure on rat airway epithelium: potentiation of toxicant-induced alterations. Environ. Health Perspect. 109(Suppl. 4):591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldorf, A. R., S. M. Levitz, and R. D. Diamond. 1984. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J. Infect. Dis. 150:752-760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.