Abstract
Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, is an obligate intracellular bacterium that infects neutrophils and neutrophil precursors. Bacterial recognition of P-selectin glycoprotein ligand-1 (PSGL-1) and the α2,3-sialylated- and α1,3-fucosylated-moiety sialyl-Lewis x (sLex), which modifies the PSGL-1 N terminus, is important for adhesion to and invasion of myeloid cells. We have previously demonstrated that A. phagocytophilum organisms of the NCH-1 strain that utilize an sLex-modified PSGL-1-independent means of entry can be enriched for by cultivation in undersialylated HL-60 cells that are unable to construct sLex. Because it was unknown whether other A. phagocytophilum isolates share this ability, we extended our studies to the geographically diverse strains HZ and HGE1. HL-60 A2 is a clonal cell line that is defective for sialylation and α1,3-fucosyltransferase. HL-60 A2 cell surfaces, therefore, not only lack sLex but also are virtually devoid of any other sialic acid- and/or α1,3-fucose-modified glycan. By cultivating HZ and HGE1 in HL-60 A2 cells, we enriched for bacterial subpopulations (termed HZA2 and HGE1A2) that bind and/or infect myeloid cells in the absence of sialic acid and α1,3-fucose and in the presence of antibody that blocks the N terminus of PSGL-1. Thus, multiple A. phagocytophilum isolates share the ability to use sLex-modified PSGL-1-dependent and -independent routes of entry into myeloid cells. HZA2 and HGE1A2 represent enriched bacterial populations that will aid dissection of the complexities of the interactions between A. phagocytophilum and host myeloid cells.
Anaplasma phagocytophilum is the etiologic agent of human granulocytic anaplasmosis, a debilitating and potentially deadly tick-transmitted infection emerging in the United States, Europe, and Asia. The bacterium is also an important veterinary pathogen, causing disease in dogs, sheep, and horses. Clinical symptoms associated with A. phagocytophilum infection include fever, headache, and myalgia. More distinguishing manifestations include leucopenia, thrombocytopenia, and elevated levels of serum hepatic transaminases (3, 6). Within its mammalian host, A. phagocytophilum invades vascular endothelial cells and neutrophils (8), and the invasion of neutrophils is a hallmark of this unusual obligate intracellular pathogen (3, 6, 14). A. phagocytophilum infection of neutrophils and neutrophil precursors is linked to bacterial recognition of P-selectin glycoprotein ligand-1 (PSGL-1), which is expressed at high densities on myeloid cell surfaces, and sialyl-Lewis x (sLex), a tetrasaccharide that modifies the N termini of PSGL-1 and other selectin ligands (7, 9, 11, 13). The known determinants crucial for bacterial binding are α2,3-sialic acid, α1,3-fucose of sLex, and the primary amino acid sequence of the PSGL-1 N terminus (9, 13, 18).
Bacterial adhesion and invasion are complex, often multifactorial processes, and many intracellular pathogens can utilize more than one route of entry. We have recently demonstrated this to be true for the A. phagocytophilum NCH-1 strain (13). Treatments that disrupt A. phagocytophilum interaction with one or more of the known determinants required for adhesion result in various degrees of inhibition but never the abolition of binding or entry (2, 7, 9, 18). We reasoned that A. phagocytophilum likely recognizes at least one additional sLex- and/or PSGL-1-independent receptor. By cultivating NCH-1 in HL-60 sLex(−/low) cells, which are severely undersialylated and consequently cannot construct sLex, we enriched for the subpopulation of NCH-1 organisms (termed NCH-1A) capable of sLex-independent infection. NCH-1A consequently exhibits a lessened dependence on PSGL-1 (13).
NCH-1 was recovered from a patient in Massachusetts (10). It is unknown whether the ability to use one or more sLex-independent receptors is unique to NCH-1 or is shared by other A. phagocytophilum isolates. We, therefore, extended our studies to A. phagocytophilum strains HZ (15) and HGE1 (5), which were isolated from patients in New York and California, respectively. By cultivating HZ and HGE1 in a clonal HL-60 cell line devoid of sialylated and α1,3-fucosylated glycans (HL-60 A2) (7, 13), we successfully enriched for bacteria able to propagate in the absence of these determinants. The selected subpopulations, named HZA2 and HGE1A2, are also less dependent on PSGL-1 for adhesion. This study (i) demonstrates that numerous A. phagocytophilum strains possess the ability to engage multiple receptors for adhesion and entry and (ii) represents an important first step toward identifying the bacterial and host factors involved in the sLex-modified PSGL-1-independent route of entry.
MATERIALS AND METHODS
In vitro cultivation of A. phagocytophilum.
A. phagocytophilum strains HGE1 and HZ were cultured in HL-60 cells (1). HGE1 was a gift from Ulrike Munderloh and Michael Herron of the University of Minnesota (Minneapolis). Ralph Horowitz of New York Medical College (Valhalla) and Yasuko Rikihisa of Ohio State University (Columbus) provided HZ.
Enrichment for HGE1A2 and HZA2 bacteria.
HZ and HGE1 were cultivated in HL-60 sLex(−/low) cells to enrich for bacteria with lessened dependencies on sialic acid (13). To further enrich for sLex-independent bacteria, host cell-free sialic acid-independent organisms recovered from 1.0 × 106 infected HL-60 sLex(−/low) cells were inoculated into 1.0 × 106 sialic acid-deficient and α1,3-fucosyltransferase-defective HL-60 A2 cells (7, 13) (provided by Ulrike Munderloh and Curtis Nelson of the University of Minnesota). By the second passage, the percentages of HZ- or HGE1-infected cells exceeded 90%. The A. phagocytophilum populations able to propagate infection in HL-60 A2 cells were named HZA2 and HGE1A2.
Assessment of A. phagocytophilum binding and infection.
Host cell-free A. phagocytophilum organisms were added to 0.25 × 106 HL-60 or HL-60 A2 cells in Iscove's modified Dulbecco's Eagle medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (IMDM-10). In some experiments, incubations were performed in the presence of 10 μg/ml KPL1 (BD Pharmingen, San Diego, CA) (16), which is a monoclonal antibody (MAb) that blocks A. phagocytophilum's access to the PSGL-1 N terminus (9, 13), or isotype-matched murine immunoglobulin G1 (IgG1). Following incubation for 30 min at 25°C, the cells were washed twice with phosphate-buffered saline and centrifuged at 300 × g for 5 min to remove unbound bacteria. The cells were resuspended in phosphate-buffered saline, transferred to the wells of Teflon-coated slides (Carlson Scientific, Peotone, IL), and examined by immunofluorescence microscopy using rabbit polyclonal anti-A. phagocytophilum antiserum followed by Alexafluor 488-conjugated goat anti-rabbit IgG (Invitrogen) as described in reference 1. To assess infection, the cells were resuspended in 1 ml IMDM-10 following the removal of unbound bacteria and cultivated at 37°C in 5% CO2 for 72 h. At 24-h intervals postinfection, the host cells were examined for the presence of morulae, or intracytoplasmic A. phagocytophilum inclusions, by light microscopic examination of cytofuged samples. A total of 3.5 × 104 A. phagocytophilum-infected host cells in 200 μl IMDM-10 were cytofuged at 70 × g for 2 min using a Cytospin 4 centrifuge (Thermo Electron, Pittsburgh, PA), fixed, and stained using Protocol Hema 3 reagents (ThermoFisher Scientific, Waltham, MA).
Statistical analyses.
Student's t test (unpaired and two-tailed), performed using the Prism 4.0 software package (Graphpad, San Diego, CA), was used to assess statistical significance. Significance was set at a P of <0.05.
RESULTS
A. phagocytophilum HZA2 and HGE1A2 efficiently bind host myeloid cells lacking sialic acid and α1,3-fucose.
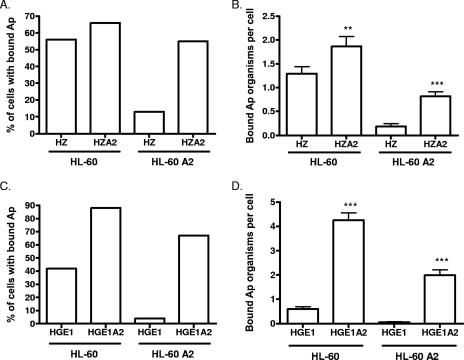
To determine whether the ability to use one or more sLex-independent receptors is unique to NCH-1 (13) or extends to other A. phagocytophilum isolates, we assessed HZ and HGE1. These strains were first cultivated in HL-60 sLex(−/low) cells to enrich for sialic acid-independent organisms (13). To further enrich for sLex-independent bacteria, the derived populations were transferred to the clonal cell line HL-60 A2, which is sialylation defective and fails to transcribe fuc-TVII, the primary α1,3-fucosyltransferase required for sLex construction (7, 13). HL-60 A2 cell surfaces, therefore, not only lack sLex but also are virtually devoid of sialic acid- and/or α1,3-fucose-modified glycans. The selected populations were termed HZA2 and HGE1A2. We compared the efficiencies of HZA2 and HGE1A2 binding to HL-60 A2 cells with those of their HZ and HGE1 parental counterparts. HZ and HZA2 bound to HL-60 cells in comparable percentages, while HZA2 bound to a considerably greater number of HL-60 A2 cells than HZ (Fig. 1A). Significantly greater numbers of HZA2 than HZ organisms adhered to both HL-60 and HL-60 A2 cells (Fig. 1B). Similar results were observed for HGE1 and HGE1A2, though the disparity between the numbers of HGE1 and HGE1A2 organisms bound to HL-60 A2 cells was severalfold greater than the disparity between the numbers of HZ and HZA2 organisms (Fig. 1C and D). HZA2 and HGE1A2 binding to HL-60 A2 cells was less efficient than that to HL-60 cells. Thus, while HZA2 and HGE1A2 bind most effectively to cell surfaces presenting sialic acid and α1,3-fucose, their dependencies on such residues for adherence are strongly diminished relative to those of HZ and HGE1.
FIG. 1.
Binding of A. phagocytophilum (Ap) strains HZ, HZA2, HGE1, and HGE1A2 to HL-60 and HL-60 A2 cells. Host cell-free A. phagocytophilum organisms were incubated with HL-60 or HL-60 A2 cells. After 30 min, unbound bacteria were removed and host cells were examined by immunofluorescence microscopy. (A and C) Percentages of HL-60 and HL-60 A2 cells with bound A. phagocytophilum organisms; (B and D) mean (± standard deviation) numbers of bound A. phagocytophilum organisms per cell. Representative results of one of three experiments are shown. Statistically significant (**, P < 0.01; ***, P < 0.001) values are indicated.
A. phagocytophilum HZA2 and HGE1A2 are less dependent on sialic acid and α1,3-fucose for infection than HZ and HGE1, respectively.
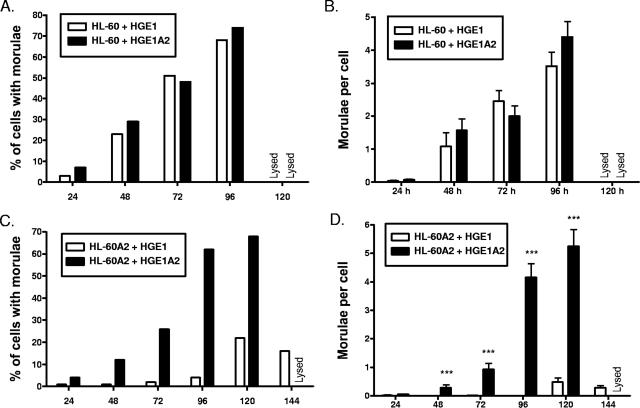
A. phagocytophilum recognition of sLex is an important step that precedes internalization into myeloid cells (7, 18). The propagation of HZA2 and HGE1A2 in HL-60 A2 cells suggests that these bacteria are able to invade by a route that does not depend on α2,3-sialic acid and α1,3-fucose. To directly assess the efficacies of HZA2 and HGE1A2 internalization, host cell-free HZ, HZA2, HGE1, and HGE1A2 organisms were incubated with HL-60 or HL-60 A2 cultures. At 24-h intervals, host cells were monitored for morula development as an indication of bacterial entry and replication. The kinetics of HZA2 infection of HL-60 cells proceeded more rapidly than that of HZ, as higher percentages of cells with HZA2 morulae were observed for all time points (Fig. 2A), significantly more HZA2 morulae per cell were detected at 96 and 120 h (Fig. 2B), and HZA2-infected cultures were lysed by 144 h, whereas HZ-infected cultures were not. The observed disparities between HZ and HZA2 infection were considerably more pronounced for HL-60 A2 cells (Fig. 2C and D). HZ infected a maximum of only 14% of HL-60 A2 cells by 96 h, and then their infection waned, while HZA2 infection reached 65% by 144 h. As much as 36-fold-more bacterial inclusions were detected in HZA2-infected HL-60 A2 cells than in HZ-infected HL-60 A2 cells. HGE1 and HGE1A2 exhibited similar rates of infection in HL-60 cells (Fig. 3A and B), while huge disparities between HGE1- and HGE1A2-infected HL-60 A2 cells were observed for all time points (Fig. 3C and D). HGE1A2 had infected 68.1% of the HL-60 A2 cells, with 5.2 ± 0.6 morulae per HL-60 A2 cell at 120 h, and had lysed host cells by 144 h. In striking contrast, HGE1 reached a maximal infection of only 21.9% of the HL-60 A2 cells, with 0.5 ± 0.1 A. phagocytophilum inclusions per cell. These data demonstrate that HZA2 and HGE1A2 are able to enter and replicate within host myeloid cells via a route that does not involve recognition of sLex or other sialylated and/or α1,3-fucosylated glycans.
FIG. 2.
A. phagocytophilum HZA2 is less dependent on sialic acid and α1,3-fucose for infection than HZ. Host cell-free HZ or HZA2 bacteria were incubated with HL-60 or HL-60 A2 cells for 30 min. Following the removal of unbound organisms, the host cells were cultivated for 144 h, during which time they were examined for the presence of morulae at 24-h intervals as an indication of bacterial internalization and replication. (A) Percentages of HZ- or HZA2-infected HL-60 cells with morulae; (B) mean (± standard deviation) numbers of morulae per HL-60 cell; (C) percentages of HZ- or HZA2-infected HL-60 A2 cells with morulae; (D) mean (± standard deviation) numbers of morulae per HL-60 A2 cell. The percentages and mean numbers of morulae per cell each refer to the total population of cells examined (infected and uninfected). Representative results of one of three experiments are shown. Statistically significant (**, P < 0.01; ***, P < 0.001) values are indicated.
FIG. 3.
A. phagocytophilum HGE1A2 is less dependent on sialic acid and α1,3-fucose for infection than HGE1. Host cell-free HGE1 or HGE1A2 bacteria were incubated with HL-60 or HL-60 A2 cells for 30 min. Following the removal of unbound organisms, the host cells were cultivated for 144 h, during which time they were examined for the presence of morulae at 24-h intervals as an indication of bacterial internalization and replication. (A) Percentages of HGE1- or HGE1A2-infected HL-60 cells with morulae; (B) mean (± standard deviation) numbers of morulae per HL-60 cell; (C) percentages of HGE1- or HGE1A2-infected HL-60 A2 cells with morulae; (D) mean (± standard deviation) numbers of morulae per HL-60 A2 cell. The percentages and mean numbers of morulae per cell refer to the total population of cells examined (infected and uninfected). Representative results of one of three experiments are shown. Statistically significant (***, P < 0.001) values are indicated.
A. phagocytophilum HZA2 and HGE1A2 are less dependent on PSGL-1 for cellular adherence.
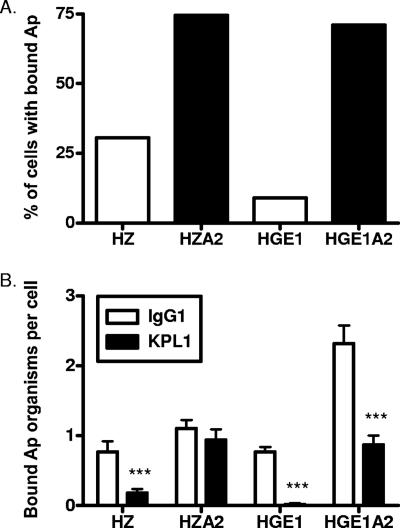
PSGL-1 is an important receptor for A. phagocytophilum adhesion to human myeloid cells. Antibodies targeting the PSGL-1 N terminus can block this event (9, 13). In addition to lowering the dependency of NCH-1A organisms on sLex, cultivation in HL-60 sLex(−/low) cells also enriches for PSGL-1-independent binding (9, 13). To determine whether enrichment of HZA2 and HGE1A2 bacteria in HL-60 A2 cells also selects for PSGL-1-independent binding, these strains, HZ, or HGE1 was incubated with HL-60 cells in the presence of the PSGL-1 N-terminus-targeting MAb KPL1 (16) or an isotype control. KPL1 drastically reduced HZ and HGE1 binding to HL-60 cells, though HGE1 binding was more pronounced (Fig. 4). This is in agreement with our (13) and Herron and colleagues’ (9) previous reports that HGE1 is heavily restricted in its dependence on PSGL-1. HZA2 bound to 2.5-fold-more KPL1-treated cells than HZ, while HGE1A2 bound to 7.7-fold-more KPL1-treated cells than HGE1. These results demonstrate that HZA2 and HGE1A2 bacteria have lessened dependencies on PSGL-1 for adherence but that optimal binding occurs in PSGL-1's presence.
FIG. 4.
A. phagocytophilum HZA2 and HGE1A2 exhibit lessened dependencies on the PSGL-1 N terminus for adhesion. Host cell-free A. phagocytophilum (Ap) organisms were added to HL-60 cells that had been treated with MAb KPL1 or murine IgG1. Incubation was continued in the presence of antibody for 30 min, after which unbound bacteria were removed. (A) Percentages of HL-60 cells with bound A. phagocytophilum organisms as determined by immunofluorescence microscopy. Results are the percentages of isotype-matched, control, MAb-treated cells that were infected. (B) Mean (± standard deviation) numbers of bound A. phagocytophilum organisms per cell. Results correspond to total numbers of bacteria associated with a total of at least 100 cells (infected and uninfected) divided by the cell number. Statistically significant (***, P < 0.001) values are indicated.
DISCUSSION
This study demonstrates that geographically diverse and possibly all A. phagocytophilum strains can infect human myeloid cells using sLex-modified PSGL-1 and/or at least one unknown receptor. The binding of A. phagocytophilum to PSGL-1 initiates a Syk- and ROCK1-dependent signaling cascade that promotes bacterial uptake (17). Whether engagement of the sLex- or PSGL-1-independent receptor initiates this or a separate signaling and entry pathway is unknown. NCH-1A, HZA2, and HGE1A2 are model organisms for investigating the signaling events associated with PSGL-1-independent entry.
Because A. phagocytophilum recognition of human sLex-modified PSGL-1 leads to productive infection (7, 9, 13) and because inhibiting PSGL-1-mediated signaling nearly abolishes the infection of human myeloid cells (17), an important question arises as to the relevance of entry through a different receptor. The answer may lie in the receptors utilized by the bacterium to invade its natural mammalian reservoir, the mouse. The binding of A. phagocytophilum to murine neutrophils occurs optimally through the recognition of sialic acid and absolutely requires α1,3-fucose (2). Remarkably, binding to and infection of murine neutrophils does not require PSGL-1. In fact, A. phagocytophilum cannot even bind to murine PSGL-1 because it lacks a critical peptide sequence found in the human PSGL-1 N terminus (18). This stereospecific difference necessitates that A. phagocytophilum utilize a PSGL-1-independent receptor to invade murine neutrophils. Perhaps NCH-1A, HZA2, and HGE1A2 represent A. phagocytophilum organisms exhibiting upregulated expression of the adhesin required for infecting murine neutrophils. This adhesin would presumably not recognize murine PSGL-1 but would recognize a separate receptor and may function in cooperation with one or more adhesins recognizing sialylated and α1,3-fucosylated glycans (such as sLex) on the surfaces of murine neutrophils. This adhesin might recognize the same or a structurally similar receptor on human myeloid cells, which would account for the lessened dependence of NCH-1A, HZA2, and HGE1A2 on sLex-modified PSGL-1.
A. phagocytophilum infects vascular endothelial cells (8, 12). It is hypothesized that such cells serve as the initial site of infection following tick transmission of A. phagocytophilum and/or serve as a reservoir for the bacterium once its presence in the peripheral bloodstream has waned. Endothelial cell line infection is enhanced following sialidase treatment (8), which indicates that sialylated glycans are not utilized for invasion. Considering these data and those of this study and the fact that the bacterium naturally infects mice and several other mammals (3, 6), it is conceivable that A. phagocytophilum possesses a veritable “Swiss army knife” of sLex-independent adhesins that are either endothelial cell or host myeloid cell specific. These may be used in conjunction with or independently of sLex-specific adhesins to invade myeloid and endothelial cells within A. phagocytophilum's various mammalian hosts.
The HZ genome has been sequenced (4). sLex-modified PSGL-1-independent binding by HZA2 (and HGE1A2 and NCH-1A) may be attributable to upregulated expression of an otherwise underexpressed adhesin. However, the phenotype of these organisms is equally consistent with down-regulation of one or more genes encoding sLex- and/or PSGL-1-targeting adhesins such that sLex- and PSGL-1-independent binding becomes more apparent. Indeed, NCH-1A binding to microspheres coated with a glycosulfopeptide mimicking the sLex-modified PSGL-1 N terminus is reduced relative to that of NCH-1 and HGE1 (13). Nonetheless, HZA2 represents an effective tool that can be used to identify genes that are unique to HZA2 or are differentially expressed between HZ and HZA2. We cannot exclude the possibility that sLex- and PSGL-1-independent binding may be afforded by altered posttranslational modification of a preexisting adhesin. Regardless, HZA2, HGE1A2, and NCH-1A are enriched subpopulations of naturally occurring organisms that will prove useful for dissecting the adhesin repertoire used by A. phagocytophilum to infect myeloid cells.
Acknowledgments
We thank Ulrike Munderloh, Michael Herron, and Curtis Nelson (University of Minnesota) for HGE1 and HL-60 A2 cells and Yasuko Rikihisa (Ohio State University) and Ralph Horowitz (New York Medical College) for HZ.
Our study was supported by NIH grants DK065039 and AI072683 and funding from the National Research Fund for Tick-Borne Diseases.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Carlyon, J. A. 2005. Laboratory maintenance of Anaplasma phagocytophilum, p. 3A.2.1-3A.2.30. In R. Coico, T. F. Kowalik, J. M. Quarles, B. Stevenson, and R. Taylor (ed.), Current protocols in microbiology. J. Wiley and Sons, Hoboken, NJ. [DOI] [PubMed]
- 2.Carlyon, J. A., M. Akkoyunlu, L. Xia, T. Yago, T. Wang, R. D. Cummings, R. P. McEver, and E. Fikrig. 2003. Murine neutrophils require alpha1,3-fucosylation but not PSGL-1 for productive infection with Anaplasma phagocytophilum. Blood 102:3387-3395. [DOI] [PubMed] [Google Scholar]
- 3.Dumler, J. S., K. S. Choi, J. C. Garcia-Garcia, N. S. Barat, D. G. Scorpio, J. W. Garyu, D. J. Grab, and J. S. Bakken. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunning Hotopp, J. C., M. Lin, R. Madupu, J. Crabtree, S. V. Angiuoli, J. Eisen, R. Seshadri, Q. Ren, M. Wu, T. R. Utterback, S. Smith, M. Lewis, H. Khouri, C. Zhang, H. Niu, Q. Lin, N. Ohashi, N. Zhi, W. Nelson, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, J. Sundaram, S. C. Daugherty, T. Davidsen, A. S. Durkin, M. Gwinn, D. H. Haft, J. D. Selengut, S. A. Sullivan, N. Zafar, L. Zhou, F. Benahmed, H. Forberger, R. Halpin, S. Mulligan, J. Robinson, O. White, Y. Rikihisa, and H. Tettelin. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley, J. E., L. Crawford-Miksza, J. S. Dumler, C. Glaser, J. S. Chae, E. Yeh, D. Schnurr, R. Hood, W. Hunter, and J. E. Madigan. 1999. Human granulocytic ehrlichiosis in northern California: two case descriptions with genetic analysis of the Ehrlichiae. Clin. Infect. Dis. 29:388-392. [DOI] [PubMed] [Google Scholar]
- 6.Goodman, J. L. 2005. Human granulocytic anaplasmosis, p. 218-238. In J. L. Goodman, D. T. Dennis, and D. E. Sonenshine (ed.), Tick-borne diseases of humans. ASM Press, Washington, DC.
- 7.Goodman, J. L., C. M. Nelson, M. B. Klein, S. F. Hayes, and B. W. Weston. 1999. Leukocyte infection by the granulocytic ehrlichiosis agent is linked to expression of a selectin ligand. J. Clin. Investig. 103:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herron, M. J., M. E. Ericson, T. J. Kurtti, and U. G. Munderloh. 2005. The interactions of Anaplasma phagocytophilum, endothelial cells, and human neutrophils. Ann. N. Y. Acad. Sci. 1063:374-382. [DOI] [PubMed] [Google Scholar]
- 9.Herron, M. J., C. M. Nelson, J. Larson, K. R. Snapp, G. S. Kansas, and J. L. Goodman. 2000. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science 288:1653-1656. [DOI] [PubMed] [Google Scholar]
- 10.Kolbert, C. P., E. S. Bruinsma, A. S. Abdulkarim, E. K. Hofmeister, R. B. Tompkins, S. R. Telford III, P. D. Mitchell, J. Adams-Stich, and D. H. Persing. 1997. Characterization of an immunoreactive protein from the agent of human granulocytic ehrlichiosis. J. Clin. Microbiol. 35:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEver, R. P. 2004. Interactions of selectins with PSGL-1 and other ligands. Ernst Schering Res. Found. Workshop 44:137-147. [DOI] [PubMed] [Google Scholar]
- 12.Munderloh, U. G., M. J. Lynch, M. J. Herron, A. T. Palmer, T. J. Kurtti, R. D. Nelson, and J. L. Goodman. 2004. Infection of endothelial cells with Anaplasma marginale and A. phagocytophilum. Vet. Microbiol. 101:53-64. [DOI] [PubMed] [Google Scholar]
- 13.Reneer, D. V., S. A. Kearns, T. Yago, J. Sims, R. D. Cummings, R. P. McEver, and J. A. Carlyon. 2006. Characterization of a sialic acid- and P-selectin glycoprotein ligand-1-independent adhesin activity in the granulocytotropic bacterium Anaplasma phagocytophilum. Cell. Microbiol. 8:1972-1984. [DOI] [PubMed] [Google Scholar]
- 14.Rikihisa, Y. 2006. Ehrlichia subversion of host innate responses. Curr. Opin. Microbiol. 9:95-101. [DOI] [PubMed] [Google Scholar]
- 15.Rikihisa, Y., N. Zhi, G. P. Wormser, B. Wen, H. W. Horowitz, and K. E. Hechemy. 1997. Ultrastructural and antigenic characterization of a granulocytic ehrlichiosis agent directly isolated and stably cultivated from a patient in New York state. J. Infect. Dis. 175:210-213. [DOI] [PubMed] [Google Scholar]
- 16.Snapp, K. R., H. Ding, K. Atkins, R. Warnke, F. W. Luscinskas, and G. S. Kansas. 1998. A novel P-selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood 91:154-164. [PubMed] [Google Scholar]
- 17.Thomas, V., and E. Fikrig. 2007. Anaplasma phagocytophilum specifically induces tyrosine phosphorylation of ROCK1 during infection. Cell. Microbiol. 9:1730-1737. [DOI] [PubMed] [Google Scholar]
- 18.Yago, T., A. Leppanen, J. A. Carlyon, M. Akkoyunlu, S. Karmakar, E. Fikrig, R. D. Cummings, and R. P. McEver. 2003. Structurally distinct requirements for binding of P-selectin glycoprotein ligand-1 and sialyl Lewis x to Anaplasma phagocytophilum and P-selectin. J. Biol. Chem. 278:37987-37997. [DOI] [PubMed] [Google Scholar]