Abstract
The search for an efficacious vaccine against malaria is ongoing, and it is now widely believed that to confer protection a vaccine must induce very strong cellular and humoral immunity concurrently. We studied the immune response in mice immunized with the recombinant viral vaccines fowlpox strain FP9 and modified virus Ankara (MVA), a protein vaccine (CV-1866), or a combination of the two; all vaccines express parts of the same preerythrocytic malaria antigen, the Plasmodium berghei circumsporozoite protein (CSP). Mice were then challenged with P. berghei sporozoites to determine the protective efficacies of different vaccine regimens. Two immunizations with the protein vaccine CV-1866, based on the hepatitis B core antigen particle, induced strong humoral immunity to the repeat region of CSP that was weakly protective against sporozoite challenge. Prime-boost with the viral vector vaccines, FP9 followed by MVA, induced strong T-cell immunity to the CD8+ epitope Pb9 and partially protected animals from challenge. Physically mixing CV-1866 with FP9 or MVA and then immunizing with the resultant combinations in a prime-boost regimen induced both cellular and humoral immunity and afforded substantially higher levels of protection (combination, 90%) than either vaccine alone (CV-1866, 12%; FP9/MVA, 37%). For diseases such as malaria in which different potent immune responses are required to protect against different stages, using combinations of partially effective vaccines may offer a more rapid route to achieving deployable levels of efficacy than individual vaccine strategies.
Numerous subunit vaccines have been developed in an effort to create an effective vaccine that will protect against malaria infection. The most advanced and successful of these strategies have focused on the induction of either cellular or humoral immunity to the preerythrocytic stage of Plasmodium falciparum infection. However, only partial protection has thus far been achieved in humans, and this has required subunit vaccines that induce exceptionally strong antibody or T-cell responses.
The circumsporozoite protein (CSP) is a major surface protein of the sporozoite and a target of protective antibodies that can prevent sporozoites from entering hepatocytes (32). Once inside hepatocytes, the same antigen can be targeted by protective T cells that then destroy the infected cell (20). Vaccines designed to induce anti-CSP antibodies (5, 17, 27, 38, 47) or CD8+ T cells (3, 16, 37) have demonstrated some protection against malaria infection in rodent models.
Both of these approaches have been assessed in clinical trials. The leading protein/adjuvant vaccine, RTS,S/AS02 induced very high antibody titers against CSP (42). The efficacy of RTS,S/AS02 has also been evaluated in field studies. In semi-immune adult men in the Gambia, the efficacy during the first 9 weeks of follow-up was estimated to be 71%, but it was 0% over the next 6 weeks (6). A more recent trial in Mozambique has shown that RTS,S conferred partial protection against clinical disease for 18 months with an efficacy of about 30% in one cohort of children studied (2).
A very different approach involves sequential immunization with recombinant viral vectors. The most potent preerythrocytic T-cell-inducing vaccine to date is prime-boost immunization with FP9 and modified virus Ankara (MVA), encoding ME-TRAP, which induces high levels of CD8+ gamma interferon (IFN-γ)-producing T cells and confers some sterile protection (45). Effector and memory populations of IFN-γ T cells induced by this regimen, as measured by ex vivo and cultured enzyme-linked immunospot (ELISPOT) assays, respectively, correlated with protection (22). Recent expert groups have suggested that greater levels of protection than are induced by either of these approaches will be needed for cost-effective vaccine deployment (http://www.malariavaccineroadmap.net). Concurrent induction of high frequencies of T cells and antibodies by vaccines could confer better protection, but evidence is lacking (18). A number of preclinical studies utilizing a prime-boost approach with viral vaccines has demonstrated concurrent induction of T cells and antibodies to CSP (28, 34). However, to achieve maximal levels of protection, three or four immunizations with two different constructs are required, a schedule that would be expensive and difficult to deploy clinically.
By combining two different vaccination strategies, viral vector and protein immunization, in an experimental model and using vaccines that encode part or all of the CSP, we show here concurrent induction of potent T-cell and humoral immunity. More importantly the combination induced long-lasting protection against P. berghei sporozoite challenge at a substantially higher level than the vaccines achieved individually.
We have previously studied various routes and combinations of viral and protein vaccines and found that both route and combination were essential for concurrently inducing potent humoral and cellular responses against a hepatitis B antigen (21). It was determined that the optimal immunization strategy for the malaria study was to combine our standard viral vector prime-boost directly with a subunit protein vaccine. The T-cell-inducing arm of our combination strategy consists of heterologous prime-boost with FP.PbCSP and MVA.PbCSP, respectively (F/M). This regimen induces potent T-cell responses against a CD8+ epitope (Pb9) and some protection from Plasmodium berghei challenge in BALB/c mice (3).
The antibody-inducing component of our strategy involves immunization with the protein CV-1866 (two doses, denoted C/C), which consists of an immunodominant B-cell epitope of P. berghei CSP DPPPNPN (DP4) (14) inserted into a carrier protein, hepatitis B virus core protein. This construct has previously been shown to protect mice from sporozoite challenge by eliciting high-titer antibodies when formulated with various adjuvants (38). Human trials of a similar construct that encodes T- and B-cell epitopes of P. falciparum CSP have demonstrated immunogenicity and safety although no protection following P. falciparum challenge after a single-dose immunization (27, 44).
MATERIALS AND METHODS
Animals and immunizations.
Female BALB/c mice (BMSU, John Radcliffe Hospital, Oxford, United Kingdom) 4 to 6 weeks old were used in all experiments. Prior to immunization, mice were anesthetized intraperitoneally (i.p.) with a solution consisting of 0.19 ml ketamine (Ketaset; Wyeth, United Kingdom), 0.25 ml medetomidine (Domitor; Novartis, Basel, Switzerland), and 2.06 ml water at a rate of 0.3 ml/30 g. To reverse the effects of ketamine-medetomidine, atipamezole (Antisedan; Novartis, Basel, Switzerland) was administered subcutaneously at a rate of 0.15 ml/30 g. Anesthetics and reversal were administered with a U-100 29-gauge needle (BD Consumer Healthcare, Le Pont de Claix. France).
MVA.PbCSP and FP.PbCSP both carry the full sequence of P. berghei CSP (24), including the N-terminal signal sequence and the complete C terminus. The generation of these constructs has been described previously (3, 37). In malaria studies all viruses were administered at 1 × 106 PFU in endotoxin-free phosphate-buffered saline (PBS) or were mixed with 5 μg of CV-1866 and injected bilaterally intradermally (i.d.).
Nonrecombinant viruses MVA and FP only express the lacZ marker gene and are termed Mnr and FPnr, respectively. Their generation has been described previously (25, 37).
CV-1866 was a gift from Apovia Inc. and consists of hepatitis B core (HBc) particles, truncated at position 149 with an introduced C-terminal cysteine, and containing two copies of the P. berghei CS B-cell epitope DP4 inserted in the immunodominant loop of HBc between amino acids D78 and P79. Hybrid particles were expressed and purified as described previously (5). Five or 10 μg of CV-1866 was diluted in endotoxin-free PBS and administered i.d or i.p. with or without FP.PbCSP, MVA.PbCSP, FPnr, or MVnr. In previous studies, we (21) and others (36) have found that inclusion of alum or alhydrogel can have a detrimental effect on immunity induced by hepatitis B virus particles or poxviruses. In preliminary experiments (data not shown), we observed that inclusion of alum with CV-1866 had no effect on immunity, and therefore it was not included in studies presented here.
Peptides.
Peptides used in cellular assays were commercially synthesized by Invitrogen (Paisley, United Kingdom), dissolved in dimethyl sulfoxide at a concentration of 10 or 20 mg/ml, and stored at −20°C. Working stocks were further diluted to 1 mg/ml in PBS and stored at −20°C.
The Kd-restricted immunodominant CD8+ peptide SYIPSAEKI (Pb9) was used to study T-cell immunogenicity. Overlapping peptides of the entire P. berghei CSP were manufactured at crude concentrations. The P. berghei peptides are 15-mers overlapping by 10 amino acids. Each pool contained 11 peptides, except pool 5, where peptide 51 was omitted as this contains the Pb9 epitope.
The peptide used for enzyme-linked immunosorbent assay (ELISA) was a gift from Apovia Inc. The sequence consists of two copies of the P. berghei CS B-cell epitope DP4.
Ex vivo IFN-γ ELISPOT.
Peripheral blood mononuclear cells (PBMC) isolated from venous blood were assayed for IFN-γ production. Briefly, 150 μl of blood from the tail vein was collected into microcentrifuge tubes containing 200 μl of 10 mM EDTA in PBS. Cells were then lysed with 1 ml of red blood cell lysis buffer (Puregene) and centrifuged at 4,000 rpm for 4 min in a benchtop centrifuge. The supernatant was removed, and the cell pellet was resuspended in 0.5 ml red blood cell lysis buffer, incubated for 5 min at room temperature, and centrifuged as before. The supernatant was removed, and the remaining cell pellet was resuspended in complete modified Eagle medium. Cells were plated out, and 5 × 104 naive splenocytes were added to each well to act as presenters for the stimulating antigen. Cells were stimulated for 18 to 20 h with 1 μg/ml Kd-restricted CD8+ peptide SYIPSAEKI (Invitrogen, United Kingdom). Spots were counted using an Autoimmunodiagnostika (AID, Strassberg, Germany) ELISPOT plate reader, and results are presented as spot-forming cells per million cells ± 95% confidence interval.
ELISA.
Serum was collected from tail vein blood samples. Blood was allowed to clot overnight at 4°C and then centrifuged at 12,000 rpm for 3 min in a benchtop centrifuge, and the serum was collected and stored at −20°C. Individual mouse sera were analyzed for anti-DP4 antibodies by an indirect ELISA. Briefly, 96-well Maxisorp plates (Nalge Nunc, Rochester, NY) were coated with 1 μg/ml DP4 and dried overnight at room temperature. Plates were washed with PBS containing 0.05% Tween (PBST) and then blocked with 10% skim milk powder in PBST for 1 h at 37°C. Serum diluted to 1:100 in PBST was added in duplicate wells and serially diluted seven times. Following a 2-h incubation at 37°C, bound antibodies were detected using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Sigma) or biotin-conjugated anti-mouse IgG1 or IgG2a antibody (Pharmingen), followed by incubation with ExtrAvidin (Sigma) for isotype analysis. Plates were developed by adding p-nitrophenyl phosphate substrate (Sigma) dissolved in diethanolamine buffer. Optical densities at 405 nm were measured for each well. End point titers were determined at the x axis intercept of the dilution curve, at twice the absorbance given for naive mouse serum diluted 1:100.
P. berghei challenge.
P. berghei sporozoites (ANKA strain clone 1) were used for all challenges, and their generation has been previously described (16, 37). Briefly, salivary glands were dissected from infected female Anopheles stephensi mosquitoes and homogenized in RPMI 1640 medium (Sigma). Mice were injected intravenously (i.v.) with a stringent challenge of 1,000 live sporozoites (3), unless stated otherwise, and monitored by Giemsa-stained blood films for the presence of malaria parasites from day 5 to 10 postchallenge. Animals were sacrificed on second confirmation of parasitemia.
Antibody avidity.
The procedure for the murine serum ELISA was followed as described above up to the point of sample addition. Test serum was diluted to similar concentrations using PBST and added in duplicate to two columns of the plate, for 16 wells in total. Plates were incubated for 2 h at 37°C and then washed six times with PBST. Sodium thiocyanate (Sigma) ranging in concentration from 3 M to 0.5 M was added to wells in duplicate and PBST to control wells. Plates were incubated for 20 min at room temperature and then washed six times with PBST. Plates were developed and read as for the standard murine serum ELISA. The initial optical density without NaSCN was assumed to represent effective total binding of specific Ig, and subsequent optical densities after treatment with various concentrations of NaSCN were converted to the percentage of the total bound Ig. The avidities of individual sera were determined at the x-axis intercept of the NaSCN dilution curve where 50% of antibody remained bound.
Intracellular cytokine staining.
Splenocytes (1 × 106) in 100 μl of culture medium were incubated in a flat-bottom plate (Nalge Nunc, Rochester, NY) with 0.2 μl of Golgi plug (brefeldin A) (BD, Oxford, United Kingdom) in 50 μl of medium and Pb9 diluted 10-fold from 5 μg/ml to 5 × 10−9 μg/ml for 5 h at 37°C. Plates were then centrifuged at 800 × g for 4 min at 4°C. Supernatant was discarded and cells washed twice with 200 μl PBS. Fc receptors were blocked with 1 μg Fcγ/CD16 (BD, Oxford, United Kingdom) per 100 μl (1 × 106 cells). Cells were incubated for 15 min at 4°C and then washed twice with 160 μl of PBS. Twenty-five microliters of surface antibodies diluted in PBS was added to each well and incubated for 30 min at 4°C. Cells were washed twice with PBS, resuspended in 100 μl of Cytofix/Cytoperm (BD, Oxford, United Kingdom), and incubated for 20 min at 4°C. One hundred microliters of Perm/Wash (BD, Oxford, United Kingdom) was added per well, and then plates were centrifuged at 800 × g for 4 min at 4°C. Cells were washed with 160 μl of Perm/Wash, and then 25 μl of intracellular antibodies diluted in Perm/Wash were added to each well and incubated for 30 min at 4°C. Cells were washed twice with 160 μl of Perm/Wash and resuspended in 1% formaldehyde in PBS. Samples were collected on a FACSCanto (BD, Oxford, United Kingdom) and analyzed with FACS Diva software (BD, Oxford, United Kingdom).
In vivo cytotoxic T-cell assay.
Splenocytes isolated from naive spleens were used as target cells for the in vivo cytotoxicity assay. These cells were resuspended in PBS and split into two populations. One population was pulsed with 5 μg/ml of Pb9 peptide, and both were incubated at 37°C for 30 min. Cells were washed twice in PBS and the Pb9 pulsed population labeled with a high concentration of carboxyfluorescein diacetate N-succinimidyl ester (CFSE) (Sigma) (5 μM) (CFSE-high cells), while the second population was labeled with a low concentration of CFSE (0.5 μM) (CFSE-low cells). Following 30 min of incubation at 37°C, cells were washed twice in PBS and then resuspended in PBS. The two populations were then combined and a total of 20 × 106 cells in 200 μl of PBS injected i.v. into immunized mice. Cells were injected into mice that had previously been immunized with the prime-boost regimen F/M, C/C, or F+C/M+C. Blood was collected at 3, 6, and 24 h and spleens were harvested at 24 h posttransfer. As a negative control, PBMC and splenocytes were also harvested from naive mice that did not receive CFSE-labeled splenocytes. PBMC and splenocytes were analyzed by flow cytometry, and each population was detected by their differential CFSE fluorescence intensities. To calculate specific lysis, the following formulas were used: ratio = (percent CFSE low/percent CFSE high); percent specific lysis = [1 − (ratio unprimed/ratio primed) × 100] (13).
IFA.
The indirect immunofluorescence assay (IFA) was carried out using P. berghei ANKA sporozoites dissected from the salivary glands of infected Anopheles mosquitoes. Sporozoites dissected into PBS were air dried onto multiwell microscope slides (eight wells per slide) and stored at −20°C until use. Test sera (from immunized or naive mice) were diluted twofold from 1:100 to 1:51,200 in PBS and 100 μl of each dilution added in duplicate to microscope wells. Slides were incubated for 2 h at 37°C in a humidified chamber. Slides were dip washed in PBS, and 100 μl of rabbit anti-mouse IgG/fluorescein isothiocyanate conjugate (Sigma) diluted 1:100 in PBS was added to each well. Slides were incubated for 30 min at 37°C in a humidified chamber and then dip washed in PBS. Slides were mounted with aqueous gel mount (Sigma) and a coverslip. Reactivity was observed by fluorescence microscopy. Naive BALB/c serum was used as a negative control. The end point titer was determined to be the last serum dilution at which fluorescent sporozoites were detectable by eye.
Sporozoite neutralization assay.
Sera used in sporozoite neutralization experiments were isolated from immunized mice that were subsequently protected from challenge. This neutralization assay was adapted from an in vitro assay described by Kumar et al. (23). Briefly, sporozoites were dissected from infected female Anopheles stephensi mosquitoes and homogenized in RPMI 1640 medium (Sigma). Sporozoites were then incubated on ice for 20 min with 10 μl of immune or naive serum per 1,000 sporozoites. Sporozoites were then diluted to 250 or 400 sporozoites per 100 μl in RPMI (Sigma) and injected i.v. into female BALB/c mice. Mice were monitored by Giemsa-stained blood films for the presence of malaria parasites from day 5 to 10 postchallenge. Animals were sacrificed on second confirmation of parasitemia.
Depleting antibodies.
All monoclonal antibodies used were rat anti-mouse IgG2b purified from hybridoma supernatants by ammonium sulfate precipitation. The anti-CD4 depleting antibodies used were clones YTA 3.1 and YTS 191.1 (11, 31).
The anti-CD8 depleting antibodies YTS 169.4 and YTS 156 (11) (10) were a gift from Katja Simon (IMM, University of Oxford, United Kingdom). Endotoxin units were <0.5/μg as determined by Limulus assay (Cambrex, Nottingham, United Kingdom). Mice received 100 μg of both antibodies i.p. on days −3 and −1 prior to challenge, which depleted >98% of CD4 and CD8 T cells (data not shown). Depletion was confirmed by fluorescence-activated cell sorting (FACS). Samples were collected on a FACSCanto (BD) and analyzed with FACS Diva software (BD).
Statistical analysis.
When analyzing ELISPOT counts and antibody titers, distributions of these variables were examined graphically to determine whether they were normally distributed. Logarithmic transformation was carried out on antibody titers, since only after this transformation did the data appear to be normally distributed. The observed distributions were compared with the normal distribution using the Kolmogorov-Smirnov test.
When comparing multiple immunization regimens, outcome variables which were approximately normally distributed (log antibody titers) were examined by one-way analysis of variance to determine whether there were differences between the regimens. If this was the case (as indicated by the F statistic, with a P value of <0.05 being considered significant), then multiple t tests were performed comparing regimens, using a Bonferroni correction to compensate for multiple testing.
For data which were not normally distributed, the Kruskal-Wallis test was used to compare regimens. Mann-Whitney tests were used to examine significance of differences between two groups, and the Wilcoxon signed ranks test was used for differences in paired samples.
A P value of <0.05 was considered significant throughout. SPSS for Windows version 12 (SPSS Inc., Chicago, IL) was used for all analyses.
RESULTS
A summary of vaccine regimens and abbreviations used in this paper is presented in Table 1.
TABLE 1.
Immunization regimens
| Immunization | Immunizationa
|
|
|---|---|---|
| Priming (day 0) | Boosting (day 14 or 21) | |
| C/C | CV-1866 i.d. or i.p. | CV-1866 i.d. or i.p. |
| F/M | FP.PbCSP i.d. | MVA.PbCSP i.d |
| F,C/M,C | FP.PbCSP i.d., CV-1866 i.p. | MVA.PbCSP i.d., CV-1866 i.p. |
| F+C/M+C | FP.PbCSP + CV-1866 mixed i.d. | MVA.PbCSP + CV-1866 mixed i.d. |
| Fnr+C/Mnr+C | FP.LacZ + CV-1866 mixed i.d. | MVA.LacZ + CV-1866 mixed i.d. |
Doses and routes for vaccines: 5 μg or 10 μg of CV-1866 i.d. or i.p., respectively; 1 × 106 PFU of recombinant (F and M) and nonrecombinant virus (Fnr and Mnr), both i.d.
CD8+ T-cell responses following vaccination.
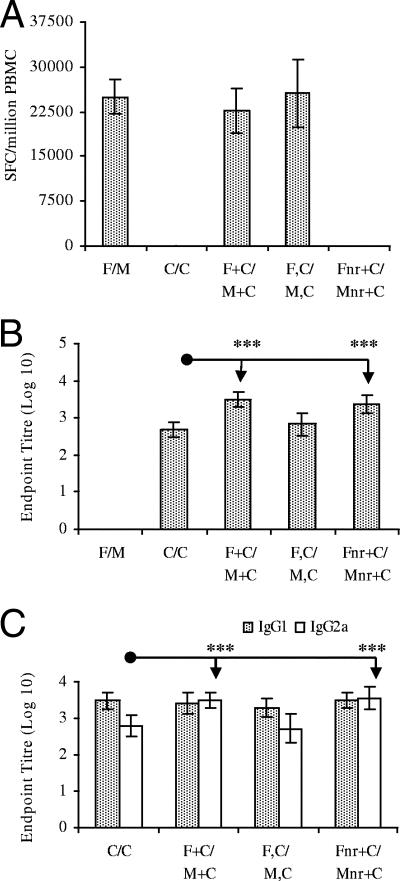
Mice received two vaccinations i.d., unless stated otherwise, 2 weeks apart. Regimens included FP.PbCSP prime and MVA.CSP boost (F/M), CV-1866 twice (C/C), a mixture of FP.PbCSP and CV-1866 at prime and a mixture of MVA.PbCSP and CV-1866 at boost (F+C/M+C), FP.PbCSP i.d. and CV-1866 i.p. at prime and MVA.PbCSP i.d. and CV-1866 i.p. at boost (F,C/M,C), and a mixture of nonrecombinant FP and CV-1866 at prime and a mixture of nonrecombinant MVA and CV-1866 at boost (Fnr+C/Mnr+C). CV-1866 was given at 5 μg i.d. and 10 μg i.p. All viruses were administered at 1 × 106 PFU. Repeat immunization with CV-1866 (C/C) i.d. or i.p (data not shown) and prime-boost with nonrecombinant virus mixed with CV-1866 (Fnr+C/Mnr+C) did not induce any Pb9-specific T cells (Fig. 1A). This was expected, as the only malaria antigen that CV-1866 encodes is the B-cell epitope DP4 from P. berghei. Immunization with F/M, F+C/M+C, and F,C/M,C induced potent levels of CD8+ T cells that did not differ significantly in their magnitude, demonstrating that the combination of the protein vaccine (CV-1866) with FP.PbCSP and MVA.PbCSP at the same (F+C/M+C) or separate (F,C/M,C) sites does not inhibit the induction of malaria-specific T-cell responses (Fig. 1A).
FIG. 1.
Combination of prime-boost and protein immunizations induces potent cell-mediated and humoral immunity. BALB/c mice were immunized with the F/M, C/C, F+C/M+C, F,C/M,C, or Fnr+C/Mnr+C regimen with a 2-week interval between prime and boost. Two weeks after boosting (day 28), blood was collected from the tail vein. PBMC were assayed for CD8+ T-cell responses to Pb9 by ELISPOT assay (A), and sera were assayed for anti-DP4 IgG responses (B) and anti-DP4 isotype profiles (C) by ELISA. Results are from several pooled experiments, except for the F,C/M,C immunized group, where n = 12. ELISPOT assay results are expressed as spot-forming cells (SFC) per million PBMC ± 95% confidence interval. ELISA results are presented as log-transformed end point titers ± 95% confidence interval (n = 12 to 22 per group). ***, P ≤ 0.001.
Antibody responses to the B-cell epitope DP4.
Two immunizations with CV-1866 i.d. (C/C) or concurrent immunization at separate sites with C/C i.p. and F/M i.d (F,C/M,C) induced potent antibody responses to DP4 that did not significantly differ (Fig. 1B). However, mixing and coadministration of recombinant or nonrecombinant virus with CV-1866 (F+C/M+C and Fnr+C/Mnr+C, respectively) induced significantly greater antibody responses to DP4 than repeat i.d. immunization with CV-1866 (C/C) (P < 0.001 for both). The B-cell epitope DP4 encoded in the CV-1866 construct is also present in the recombinant FP and MVA; however, prime-boost immunization with F/M failed to induce detectable levels of anti-DP4 antibodies. Together these results indicate an adjuvant effect of recombinant and nonrecombinant virus on the coadministered protein leading to enhanced antibody responses, an observation we have previously made in a hepatitis B model (21).
The ratio of isotype subclasses IgG1 and IgG2a gives an indication of a Th2 or Th1 bias of humoral responses, respectively (12). Immunization with C/C or coadministration of recombinant virus and protein concurrently but at separate sites (F,C/M,C) induced a Th2-type response (IgG1 bias) (Fig. 1C). Conversely, prime-boost immunization with CV-1866 mixed and immunized with recombinant or nonrecombinant virus (F+C/M+C and Fnr+C/Mnr+C, respectively) significantly increased IgG2a antibodies to DP4 compared to repeat i.d. immunization with CV-1866 (P < 0.001 for both). Mixing and coadministration of virus with protein therefore enhances Th1-type immunity, as demonstrated by an increase in IgG2a.
Protective efficacy of vaccination following malaria challenge.
Coadministration of poxviruses and proteins enhanced cellular and humoral immunity to the encoded malaria antigens. To investigate if this enhanced immunogenicity was efficacious, immunized animals were challenged with P. berghei malaria sporozoites and protection of immunization regimens compared. Protected animals were monitored for parasitemia for up to 14 days postchallenge even though unprotected vaccinees and controls were consistently parasitemic between days 5 and 7 postchallenge. Prime-boost with F/M or repeat immunization with C/C i.d. protected 37% and 12%, respectively, of mice from challenge on day 30 (16 days postboost) (Table 2). However, combining CV-1866 with poxviral vaccines at prime and boost (F+C/M+C) conferred 90% protection from malaria challenge. Immunization with the same vaccines concurrently but at separate sites (F,C/M,C) conferred little protection (16% protected). Combination of the protein vaccine with nonrecombinant vectors (Fnr+C/Mnr+C) also conferred a greater level of protection than protein alone, indicating an adjuvant effect of nonrecombinant virus on the coadministered protein, although protection was maximal if the poxviruses encoded malarial antigens. These and previous results further support the hypothesis that viral vectors adjuvant coadministered protein (21) and that this coformulation works synergistically to confer greater levels of protection than either vaccine regimen alone. The durability of protection following F/M, C/C, and F+C/M+C immunization was also assessed by a long-term challenge schedule. Mice were primed on day 0, boosted on day 21, and challenged on day 86 (Table 2). A second group of 11 F+C/M+C-immunized mice were also challenged on day 190. Overall, F+C/M+C continued to confer 90% protection at days 86 and 190 (65 and 169 days postboost, respectively), demonstrating the durability of combination vaccination.
TABLE 2.
Protection against P. berghei challenge elicited by combination vaccinesa
| Immunization | Day 30 challenge
|
Long term
|
||
|---|---|---|---|---|
| No. protected/no. challenged | Protection (%) | No. protected/no. challenged | Protection (%) | |
| F/M | 6/16 | 37 | 4/18 | 22 |
| C/C | 2/16 | 12 | 8/18 | 44 |
| F+C/M+C | 20/22 | 90 | 25/27 (10/11) | 93 (90) |
| F,C/M,C | 2/12 | 16 | ||
| Fnr+C/Mnr+C | 6/12 | 50 | ||
| Naive | 0/22 | 0 | ||
BALB/c mice were immunized with F/M, C/C, F+C/M+C, F,C/M,C, or Fnr+C/Mnr+C. The interval between prime and boost was 2 or 3 weeks (day 30 and long term schedules, respectively). Immunized and control (naive) animals were challenged intravenously with 1,000 live P. berghei sporozoites on day 30, 86, or 190 and monitored 5 to 14 days postchallenge by Giemsa-stained blood film. Animals were considered protected if no parasites were detected in this time. Results are from several pooled experiments, except for the F,C/M,C-immunized group. Numbers in parentheses are values for day 190.
CD8+ T cells from F/M- or F+C/M+C-immunized mice do not differ in breadth of the response, functional avidity, or in vivo killing.
T cells undergo extensive functional maturation leading to greater responsiveness to antigens, and these high-avidity T cells may be required to protect from disease (15). We measured the avidity maturation of T cells from F/M- and F+C/M+C-vaccinated mice over time following stimulation with various concentrations of the CD8+ peptide Pb9 and overlapping peptides to the whole CSP (40). No specific IFN-γ responses were detected by FACS or ELISPOT assay following stimulation with overlapping peptides to CSP. The functional avidity of splenocytes to the dominant CD8+ epitope Pb9 as determined by ELISPOT assay was not significantly different for splenocytes from mice immunized with F/M or F+C/M+C (6.0 × 104 and 7.2 × 104, respectively [average of three mice per group; P = 0.77]). ELISPOT assay and FACS of peptide-stimulated splenocytes showed similar patterns of functional avidity for Pb9 (data not shown). These results indicated that there was no difference in the avidity of Pb9-specific splenocytes induced by F/M or F+C/M+C immunization.
Experiments to address the in vitro functional avidity of antigen-specific T cells revealed no difference in the Pb9-specific T cells induced by immunization with F/M or F+C/M+C in blood and spleen. However, similar in vitro behavior in response to high concentrations of synthetic peptide epitopes does not guarantee that the T cells would behave similarly in vivo. To address this issue, their capacity to kill Pb9-loaded targets was measured in vivo using an assay described by Coles et al. (13). Naive spleen cells, pulsed with or without Pb9 and labeled with a low or high concentration of CFSE, respectively, were transferred in equal ratios to mice immunized with the three key regimens (F/M, C/C, and F+C/M+C; n = 3 per group) 2 weeks postboost. PBMC and splenocytes were isolated and analyzed for CFSE fluorescence by FACS at various time points after transfer. If the functional avidity of Pb9-specific T cells induced by F/M or F+C/M+C is different, then disparate rates of specific lysis would be induced. No peptide-specific killing was seen in the C/C-immunized mice; the F/M and F+C/M+C groups specifically lysed peptide-loaded targets at similar rates at 6 h, 12 h, and 24 h in the blood (F/M, 53%, 89%, and 100%; F+C/M+C, 57%, 86%, and 100%) and at 24 h in the spleen (100% for both). This result further supports the hypothesis that Pb9-specific T cells are unchanged between F/M and F+C/M+C immunization.
Antibodies induced by C/C and F+C/M+C immunization do not differ significantly in avidity for the B-cell epitope DP4.
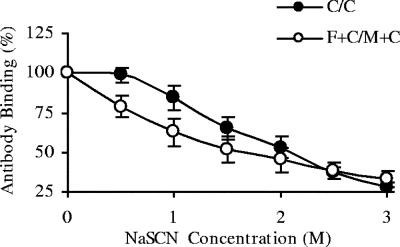
An important parameter for measuring humoral immune responses is antibody affinity. Measuring true antibody affinity is difficult, and therefore measurement of antibody avidity is used as an alternative. Antibody avidity is determined primarily by the rate of dissociation of antibody from its target. Incubation of antibodies bound to their target with a range of sodium thiocyanate concentrations and measuring the concentration at which the antibody-antigen interaction is broken are used as a measure of antibody avidity (29). Therefore, in this experiment, antibodies binding with less avidity to the CSP B-cell epitope DP4 would be disrupted at lower concentrations of NaSCN than for antibodies with greater avidity. The avidity of whole IgG anti-DP4 antibodies induced by C/C or F+C/M+C immunization was assessed. The NaSCN concentrations required to remove 50% of anti-DP4 antibodies were 1.7 M for F+C/M+C and 2.0 M for C/C (Fig. 2). Although there was no significant difference between these (P = 0.15), there was a strong trend for higher avidity in C/C samples, indicating that increased antibody avidity to the peptide was not responsible for the enhanced protection of F+C/M+C.
FIG. 2.
Antibody avidity does not differ significantly between F+C/M+C- and C/C-immunized mice. BALB/c mice were immunized with the C/C or F+C/M+C regimen. The interval between prime and boost was 2 weeks. Two weeks after boosting (day 28), blood was collected from the tail vein, sera were diluted in PBS to similar concentrations, and an avidity ELISA was performed. The rate at which DP4-specific antibodies were dissociated from the DP4 peptide by sodium thiocyanate was used as a measure of antibody avidity. Results are from one experiment and are expressed as the percentages of antibody bound at various NaSCN concentrations. Error bars represent ±95% confidence interval; n = 10.
Antibodies induced by C/C and F+C/M+C immunization bind specifically to P. berghei sporozoites.
All antibody data generated by ELISA in the malaria studies indicate recognition of the linear B-cell epitope DP4. To demonstrate that the antibodies induced by the vaccine regimens C/C and F+C/M+C specifically recognize the parasite they are raised against, an IFA was performed using P. berghei sporozoites as the target. IFA or ELISA using sporozoites as the target is more relevant than peptide ELISA but is not a practical method for assessing numerous serum samples. Samples from one experiment were used to demonstrate that the anti-DP4 antibodies induced by immunization with C/C and F+C/M+C detectable by peptide ELISA would also bind specifically to P. berghei sporozoites (Fig. 3). IFA was more sensitive at detecting antibodies induced by immunization with C/C than ELISA (P = 0.01) but equaled levels detected by ELISA in F+C/M+C sera (Fig. 3). No fluorescence was detected following incubation of F/M or naive sera with sporozoites.
FIG. 3.
Antibodies induced by C/C and F+C/M+C immunization bind specifically to the linear B-cell epitope DP4 and P. berghei sporozoites. BALB/c mice were immunized with the C/C or F+C/M+C regimen. The interval between prime and boost was 2 weeks. Two weeks after boosting (day 28), blood was collected from the tail vein and sera assayed for anti-DP4 specific IgG responses by ELISA and IFA. Results are representative of two experiments and are expressed as log-transformed end point titers ± 95% confidence interval (n = 4). **, P ≤ 0.01.
To further examine the functionality of this binding, P. berghei sporozoites were incubated with sera from mice immunized with F+C/M+C and C/C to see if the vaccine-induced antibodies could neutralize sporozoites and prevent infection in naive mice. Neutralization experiments were based on a method described by Kumar et al. (23). Some protection (50%; n = 6) was observed in mice that received 250 sporozoites incubated with sera from C/C- or F+C/M+C-immunized mice, but infection in control mice was inconsistent (33%; n = 6). Adding anti-CSP antibody to sporozoites cross-links the CSP and causes shedding of the protein, a process termed the CSP precipitation reaction (43). It is possible this phenomenon occurs during the incubation period in vitro, leading to loss of the anti-CS antibodies, without which sporozoites evade clearance through Fc-mediated phagocytosis and complement activation (39). Coinduction of antibodies and T cells is critical for maximum protection from malaria challenge in our experiments. It is possible that antibodies induced by F+C/M+C reduce the sporozoite burden in the liver, allowing vaccine-induced T cells to kill the remaining infected hepatocytes and thereby conferring protection.
CD8+ but not CD4+ T cells are required for maximal protection in F+C/M+C-immunized mice at the time of challenge.
T-cell responses measured prior to challenge represent a dominant CD8+ epitope within the malarial antigen. To determine the roles of CD4+ and CD8+ T cells in the combination vaccine F+C/M+C at the time of challenge, F+C/M+C-immunized mice were depleted of CD4+ or CD8+ T cells prior to challenge and protection rates compared to those for undepleted F+C/M+C-immunized mice (Table 3).
TABLE 3.
P. berghei challenge of vaccinated mice depleted of CD4+ and CD8+ T cellsa
| Immunization | No. protected/no. challenged | Protection (%) |
|---|---|---|
| F+C/M+C | 15/18 | 83 |
| F+C/M+C CD4− | 5/6 | 83 |
| F+C/M+C CD8− | 8/18 | 44 |
| Naive | 0/18 | 0 |
BALB/c mice were immunized with the F+C/M+C regimen with a 2-week interval between prime and boost. Two groups of F+C/M+C-immunized animals were depleted of CD8+ (F+C/M+C CD8−) or CD4+ (F+C/M+C CD4−) T cells on days 10 and 13 postboost. Mice were challenged i.v. with 1,000 live P. berghei sporozoites 14 days after boosting and monitored 5 to 14 days postchallenge by Giemsa-stained blood film. Animals were considered protected if no parasites were detected in this time. Results are from two pooled experiments, except for the CD4-depleted group.
Depletion of CD8+ T cells from F+C/M+C-immunized mice markedly reduced the protective efficacy of the combination vaccine (44%) compared to that for undepleted F+C/M+C-immunized mice (83%). Conversely, depletion of CD4+ T cells from F+C/M+C-immunized mice prior to challenge did not reduce protection compared to that in immunized, undepleted mice. These results demonstrated that CD8+ but not CD4+ T cells play an essential role in protection conferred by the combination vaccine at the time of challenge.
DISCUSSION
Most malaria vaccines to date have focused on the induction of T cells or antibodies as their principal method of conferring immunity. This study demonstrates a method whereby both arms of the immune system are effectively induced against target antigens, leading to enhanced immune responses and protection. The combination of poxviruses and protein, both encoding malarial epitopes, specifically induced strong Th1-type antibody responses to the coadministered protein and potent T-cell immunity for the virally encoded malaria T-cell epitope. Antibody magnitude and isotype to the protein were strongly influenced by mixing and coadministration with poxviruses, both recombinant and nonrecombinant, demonstrating an adjuvant effect of poxviruses on protein.
Poxviruses have been widely used as vaccine vectors because of their ability to target encoded antigens for processing by the major histocompatibility complex class I pathway by directly infecting immune cells, in particular antigen-presenting cells, but also due to their ability to self-adjuvant. Here they not only adjuvant cell responses for the antigen they encode but also appear to adjuvant the coadministered protein, leading to an enhanced/altered antibody response.
MVA and FP induce IFN-γ, which may be responsible for the enhanced Th1-type immunity as demonstrated by increased levels of IgG2a following immunization with the combination vaccine. However, it is unclear if isotype distribution is an important variable in protection from sporozoite challenge. A few studies in the literature that report correlations between protection and immunoglobulin isotype in P. berghei models present contradictory results. Two studies described increased levels of IgG2a in mice following inoculation with either irradiated P. berghei sporozoites or infectious sporozoites which were then cleared of infection by chemotherapy (1, 8). Protection from a subsequent challenge with sporozoites correlated with the increased levels of IgG2a. A separate study correlated sporozoite-specific IgG1 and avidity with protection following immunization with DP4 conjugated with bovine serum albumin (33). The method used in our study to induce DP4-specific antibodies may be more similar to the studies performed elsewhere (1, 8) First, the B-cell epitope is possibly conformationally closer to the native protein on sporozoites than linear peptide, as it is encoded within an immunodominant loop of the carrier protein HBc. Second, helper T-cell epitopes that are induced by sporozoite immunization enhance the antibody responses to CSP (35); although these same epitopes are not present in the construct used in these experiments, the carrier protein HBc has been shown to supply T-cell help to epitopes inserted into the same region of HBc as DP4 (30).
The coinduction of specific cell-mediated and humoral immunity led to increased levels of protection in the malaria challenge model. It is likely that the synergistic levels of protection observed, at least in part, relate to the ability of antibodies to reduce the number of sporozoites entering hepatocytes, thereby enhancing the ability of T cells to clear the liver of parasitized cells. Anti-CSP antibodies may act in several ways to reduce the number of sporozoites that successfully invade the liver. Binding of anti-CSP antibodies inhibits the motility of sporozoites, which is required for hepatocyte invasion (41). These antibodies also inhibit binding of the sporozoite to the hepatocyte plasma membrane in the liver sinusoids (7, 9), thus exposing them to the immune system for longer. CSP plays a role in the invasion of hepatocytes, and therefore anti-CSP antibodies may also physically block invasion of hepatocytes. Unable to invade the liver, sporozoites opsonized by antibodies would be rapidly phagocytosed by macrophages and other polymorphonucleocytes or lysed following complement activation.
Although potent levels of T cells and antibodies are induced by combination vaccination, it is unclear if it is purely the levels of T cells and antibodies that confer protection.
Administration of the two vaccines at the same time but at separate sites (F,C/M,C) induces good T-cell and antibody responses; however, limited protection is conferred. The results with this vaccine regimen support the hypothesis that there is some synergy between the vaccines when they are mixed and administered at the same site (F+C/M+C) as well as concurrent induction of T cells and antibodies leading to the enhanced levels of protection conferred by combination vaccines. Delivery of the two vaccine components into the same site may lead to the same antigen-presenting cells presenting both CD4 and CD8 epitopes, thereby enhancing immunogenicity (4).
Two major differences in antibodies induced by F+C/M+C compared to C/C immunization were revealed, these being significantly higher whole IgG and the subtype IgG2a responses in F+C/M+C-immunized mice. Further analysis of anti-DP4 antibodies induced by C/C or F+C/M+C immunization demonstrated that both regimens induce whole IgG antibodies that specifically bind to P. berghei sporozoites, and avidity to the linear DP4 epitope, as measured by ELISA, was not significantly different between regimens. In these experiments comparison between C/C and F+C/M+C is the critical factor, so peptide ELISA provides acceptable sensitivity. However, a more sensitive measure of induced antibodies may reveal responses that we have been unable to detect preboost. Branched multiple-antigen peptides or linking linear peptides to a carrier molecule can provide a more sensitive detection method than linear peptide ELISA (26).
A recent study by Hidmark et. al (19) demonstrated enhanced antibody responses to protein when coadministered with Semliki Forest virus. Furthermore, these responses were biased towards IgG2a. Analysis of this response showed that viral adjuvanting of protein was independent of Toll-like receptor signaling but completely dependent on IFN-α and -β induced by the virus. In our model, the adjuvant effects of recombinant and nonrecombinant MVA and FP on the coadministered protein CV-1866 may therefore be “simply” due to innate immune responses to the virus (through induction of type I IFNs) rather than the encoded antigens.
The in vivo depletion of CD8+ cells from F+C/M+C- and Fnr+C/Mnr+C-immunized mice together demonstrated the requirement of both T cells and antibodies for maximal protection conferred by F+C/M+C. These two experiments support the hypothesis that anti-DP4 antibodies induced by CV-1866 may lead to destruction of some inoculated sporozoites before they invade the liver, significantly reducing the number of infecting hepatocytes. The substantially reduced number of infected liver cells could then be destroyed by CD8+ T cells induced by the F/M prime-boost part of the vaccination. The use of reverse transcription-PCR to detect differences in the number of sporozoites that manage to infect hepatocytes in animals immunized with the three key regimens could help evaluate this possibility (46).
The enhanced levels of protection conferred by F+C/M+C compared to the level of protection induced by F/M and C/C suggests synergy of the vaccines when combined. Coinduction of T-cell and antibody responses to the encoded malaria antigens confers enhanced protection, and it is possible that the viral adjuvanting of the coadministered protein leading to an increase in IgG and alteration of isotype ratios may be responsible for the synergistic levels of protection induced by F+C/M+C immunization.
We describe here a strategy whereby poxviruses enhance T-cell and antibody responses to a coadministered protein while retaining strong T-cell immunogenicity for the recombinant antigen encoded by the poxvirus. Poxviral adjuvanting of the coadministered protein leads to an enhanced Th1-type antibody response, and along with the coinduction of specific cell-mediated immunity, led to increased levels of durable protection in our malaria challenge model.
Numerous clinical trials have demonstrated the safety and immunogenicity of vaccines designed to induce either cell-mediated or humoral responses against many diseases, including human immunodeficiency virus disease and malaria. By combining existing strategies that have already been shown to be safe and well tolerated in humans, the timeline for development of highly effective vaccines may be much shortened compared to the assessment of novel vectors or adjuvants.
Acknowledgments
This work was supported by the Wellcome Trust. A.V.S.H. is a Wellcome Trust Principal Fellow.
We thank Mark Tunnicliffe for providing P. berghei-infected mosquitoes for challenge studies.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Akanmori, B. D., S. Waki, and M. Suzuki. 1994. Immunoglobulin G2a isotype may have a protective role in Plasmodium berghei NK65 infection in immunised mice. Parasitol. Res. 80:638-641. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, R. J., C. M. Hannan, S. C. Gilbert, S. M. Laidlaw, E. G. Sheu, S. Korten, R. Sinden, G. A. Butcher, M. A. Skinner, and A. V. Hill. 2004. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 172:3094-3100. [DOI] [PubMed] [Google Scholar]
- 4.Bennett, S. R., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 5.Birkett, A., K. Lyons, A. Schmidt, D. Boyd, G. A. Oliveira, A. Siddique, R. Nussenzweig, J. M. Calvo-Calle, and E. Nardin. 2002. A modified hepatitis B virus core particle containing multiple epitopes of the Plasmodium falciparum circumsporozoite protein provides a highly immunogenic malaria vaccine in preclinical analyses in rodent and primate hosts. Infect. Immun. 70:6860-6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 358:1927-1934. [DOI] [PubMed] [Google Scholar]
- 7.Cerami, C., U. Frevert, P. Sinnis, B. Takacs, P. Clavijo, M. J. Santos, and V. Nussenzweig. 1992. The basolateral domain of the hepatocyte plasma membrane bears receptors for the circumsporozoite protein of Plasmodium falciparum sporozoites. Cell 70:1021-1033. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee, S., P. Druilhe, and M. Wery. 1999. Irradiated sporozoites prime mice to produce high antibody titres upon viable Plasmodium berghei sporozoite challenge, which act upon liver-stage development. Parasitology 118:219-225. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee, S., M. Wery, P. Sharma, and V. S. Chauhan. 1995. A conserved peptide sequence of the Plasmodium falciparum circumsporozoite protein and antipeptide antibodies inhibit Plasmodium berghei sporozoite invasion of Hep-G2 cells and protect immunized mice against P. berghei sporozoite challenge. Infect. Immun. 63:4375-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobbold, S., and H. Waldmann. 1986. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation 41:634-639. [DOI] [PubMed] [Google Scholar]
- 11.Cobbold, S. P., A. Jayasuriya, A. Nash, T. D. Prospero, and H. Waldmann. 1984. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature 312:548-551. [DOI] [PubMed] [Google Scholar]
- 12.Coffman, R. L., B. W. Seymour, D. A. Lebman, D. D. Hiraki, J. A. Christiansen, B. Shrader, H. M. Cherwinski, H. F. Savelkoul, F. D. Finkelman, M. W. Bond, et al. 1988. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102:5-28. [DOI] [PubMed] [Google Scholar]
- 13.Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834-838. [DOI] [PubMed] [Google Scholar]
- 14.Eichinger, D. J., D. E. Arnot, J. P. Tam, V. Nussenzweig, and V. Enea. 1986. Circumsporozoite protein of Plasmodium berghei: gene cloning and identification of the immunodominant epitopes. Mol. Cell Biol. 6:3965-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estcourt, M. J., A. J. Ramsay, A. Brooks, S. A. Thomson, C. J. Medveckzy, and I. A. Ramshaw. 2002. Prime-boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int. Immunol. 14:31-37. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, S. C., J. Schneider, C. M. Hannan, J. T. Hu, M. Plebanski, R. Sinden, and A. V. Hill. 2002. Enhanced CD8 T cell immunogenicity and protective efficacy in a mouse malaria model using a recombinant adenoviral vaccine in heterologous prime-boost immunisation regimes. Vaccine 20:1039-1045. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, D. M., T. W. McGovern, U. Krzych, J. C. Cohen, I. Schneider, R. LaChance, D. G. Heppner, G. Yuan, M. Hollingdale, M. Slaoui, et al. 1995. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit vaccine. J. Infect. Dis. 171:1576-1585. [DOI] [PubMed] [Google Scholar]
- 18.Heppner, D. G., R. J. Schwenk, D. Arnot, R. W. Sauerwein, and A. J. Luty. 2007. The dog that did not bark: malaria vaccines without antibodies. Trends Parasitol. 23:293-296. [DOI] [PubMed] [Google Scholar]
- 19.Hidmark, A. S., E. K. Nordstrom, P. Dosenovic, M. N. Forsell, P. Liljestrom, and G. B. Karlsson Hedestam. 2006. Humoral responses against coimmunized protein antigen but not against alphavirus-encoded antigens require alpha/beta interferon signaling. J. Virol. 80:7100-7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman, S. L., D. Isenbarger, G. W. Long, M. Sedegah, A. Szarfman, L. Waters, M. R. Hollingdale, P. H. van der Meide, D. S. Finbloom, and W. R. Ballou. 1989. Sporozoite vaccine induces genetically restricted T cell elimination of malaria from hepatocytes. Science 244:1078-1081. [DOI] [PubMed] [Google Scholar]
- 21.Hutchings, C. L., S. C. Gilbert, A. V. Hill, and A. C. Moore. 2005. Novel protein and poxvirus-based vaccine combinations for simultaneous induction of humoral and cell-mediated immunity. J. Immunol. 175:599-606. [DOI] [PubMed] [Google Scholar]
- 22.Keating, S. M., P. Bejon, T. Berthoud, J. M. Vuola, S. Todryk, D. P. Webster, S. J. Dunachie, V. S. Moorthy, S. J. McConkey, S. C. Gilbert, and A. V. Hill. 2005. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175:5675-5680. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, K. A., G. A. Oliveira, R. Edelman, E. Nardin, and V. Nussenzweig. 2004. Quantitative Plasmodium sporozoite neutralization assay (TSNA). J. Immunol. Methods 292:157-164. [DOI] [PubMed] [Google Scholar]
- 24.Lockyer, M. J., C. S. Davies, A. Suhrbier, and R. E. Sinden. 1990. Nucleotide sequence of the Plasmodium berghei circumsporozoite protein gene from the ANKA clone 2.34L. Nucleic Acids Res. 18:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mockett, B., M. M. Binns, M. E. Boursnell, and M. A. Skinner. 1992. Comparison of the locations of homologous fowlpox and vaccinia virus genes reveals major genome reorganization. J. Gen. Virol. 73:2661-2668. [DOI] [PubMed] [Google Scholar]
- 26.Nardin, E. H., G. A. Oliveira, J. M. Calvo-Calle, and R. S. Nussenzweig. 1995. The use of multiple antigen peptides in the analysis and induction of protective immune responses against infectious diseases. Adv. Immunol. 60:105-149. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira, G. A., K. Wetzel, J. M. Calvo-Calle, R. Nussenzweig, A. Schmidt, A. Birkett, F. Dubovsky, E. Tierney, C. H. Gleiter, G. Boehmer, A. J. Luty, M. Ramharter, G. B. Thornton, P. G. Kremsner, and E. H. Nardin. 2005. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant montanide ISA 720 in a phase I trial. Infect. Immun. 73:3587-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ophorst, O. J., K. Radosevic, M. J. Havenga, M. G. Pau, L. Holterman, B. Berkhout, J. Goudsmit, and M. Tsuji. 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 30.Pumpens, P., and E. Grens. 1999. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 442:1-6. [DOI] [PubMed] [Google Scholar]
- 31.Qin, S., S. Cobbold, H. Tighe, R. Benjamin, and H. Waldmann. 1987. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur. J. Immunol. 17:1159-1165. [DOI] [PubMed] [Google Scholar]
- 32.Rathore, D., R. Nagarkatti, D. Jani, R. Chattopadhyay, P. de la Vega, S. Kumar, and T. F. McCutchan. 2005. An immunologically cryptic epitope of Plasmodium falciparum circumsporozoite protein facilitates liver cell recognition and induces protective antibodies that block liver cell invasion. J. Biol. Chem. 280:20524-20529. [DOI] [PubMed] [Google Scholar]
- 33.Reed, R. C., V. Louis-Wileman, R. L. Wells, A. F. Verheul, R. L. Hunter, and A. A. Lal. 1996. Re-investigation of the circumsporozoite protein-based induction of sterile immunity against Plasmodium berghei infection. Vaccine 14:828-836. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues, M., S. Li, K. Murata, D. Rodriguez, J. R. Rodriguez, I. Bacik, J. R. Bennink, J. W. Yewdell, A. Garcia-Sastre, R. S. Nussenzweig, et al. 1994. Influenza and vaccinia viruses expressing malaria CD8+ T and B cell epitopes. Comparison of their immunogenicity and capacity to induce protective immunity. J. Immunol. 153:4636-4648. [PubMed] [Google Scholar]
- 35.Romero, P. J., J. P. Tam, D. Schlesinger, P. Clavijo, H. Gibson, P. J. Barr, R. S. Nussenzweig, V. Nussenzweig, and F. Zavala. 1988. Multiple T helper cell epitopes of the circumsporozoite protein of Plasmodium berghei. Eur. J. Immunol. 18:1951-1957. [DOI] [PubMed] [Google Scholar]
- 36.Schirmbeck, R., K. Melber, T. Mertens, and J. Reimann. 1994. Antibody and cytotoxic T-cell responses to soluble hepatitis B virus (HBV) S antigen in mice: implication for the pathogenesis of HBV-induced hepatitis. J. Virol. 68:1418-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus. Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 38.Schodel, F., R. Wirtz, D. Peterson, J. Hughes, R. Warren, J. Sadoff, and D. Milich. 1994. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. J. Exp. Med. 180:1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwenk, R., L. V. Asher, I. Chalom, D. Lanar, P. Sun, K. White, D. Keil, K. E. Kester, J. Stoute, D. G. Heppner, and U. Krzych. 2003. Opsonization by antigen-specific antibodies as a mechanism of protective immunity induced by Plasmodium falciparum circumsporozoite protein-based vaccine. Parasite Immunol. 25:17-25. [DOI] [PubMed] [Google Scholar]
- 40.Slifka, M. K., and J. L. Whitton. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2:711-717. [DOI] [PubMed] [Google Scholar]
- 41.Stewart, M. J., R. J. Nawrot, S. Schulman, and J. P. Vanderberg. 1986. Plasmodium berghei sporozoite invasion is blocked in vitro by sporozoite-immobilizing antibodies. Infect. Immun. 51:859-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmons, B. T. Wellde, N. Garcon, U. Krzych, M. Marchand, et al. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 336:86-91. [DOI] [PubMed] [Google Scholar]
- 43.Vanderberg, J., R. Nussenzweig, and H. Most. 1969. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. V. In vitro effects of immune serum on sporozoites. Mil. Med. 134:1183-1190. [PubMed] [Google Scholar]
- 44.Walther, M., S. Dunachie, S. Keating, J. M. Vuola, T. Berthoud, A. Schmidt, C. Maier, L. Andrews, R. F. Andersen, S. Gilbert, I. Poulton, D. Webster, F. Dubovsky, E. Tierney, P. Sarpotdar, S. Correa, A. Huntcooke, G. Butcher, J. Williams, R. E. Sinden, G. B. Thornton, and A. V. Hill. 2005. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine 23:857-864. [DOI] [PubMed] [Google Scholar]
- 45.Webster, D. P., S. Dunachie, J. M. Vuola, T. Berthoud, S. Keating, S. M. Laidlaw, S. J. McConkey, I. Poulton, L. Andrews, R. F. Andersen, P. Bejon, G. Butcher, R. Sinden, M. A. Skinner, S. C. Gilbert, and A. V. Hill. 2005. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus. Ankara. Proc. Natl. Acad. Sci. USA 102:4836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witney, A. A., D. L. Doolan, R. M. Anthony, W. R. Weiss, S. L. Hoffman, and D. J. Carucci. 2001. Determining liver stage parasite burden by real time quantitative PCR as a method for evaluating pre-erythrocytic malaria vaccine efficacy. Mol. Biochem. Parasitol. 118:233-245. [DOI] [PubMed] [Google Scholar]
- 47.Zavala, F., J. P. Tam, P. J. Barr, P. J. Romero, V. Ley, R. S. Nussenzweig, and V. Nussenzweig. 1987. Synthetic peptide vaccine confers protection against murine malaria. J. Exp. Med. 166:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]