Abstract
Acinetobacter baumannii has emerged as a major cause of both community-associated and nosocomial pneumonia, but little is known about the cellular and molecular mechanisms of host defense against respiratory infection with this bacterial pathogen. In this study, we examined the role of neutrophils in host resistance to pulmonary A. baumannii infection in a mouse model of intranasal (i.n.) infection. We found that neutrophils were rapidly recruited to the lungs following i.n. inoculation of the pathogen and declined to baseline level upon clearance of the infection. Depletion of neutrophils using monoclonal antibody RB6-8C5 prior to infection resulted in an acute lethal infection that was associated with enhanced bacterial burdens in the lung (P < 0.05) and extrapulmonary dissemination to the spleen. The increased susceptibility to A. baumannii in neutropenic mice was associated with a delay in the mRNA expression and production of early proinflammatory cytokines such as tumor necrosis factor alpha, interleukin-6, keratinocyte chemoattractant protein, monocyte chemoattractant protein 1, and macrophage inflammatory protein 2 (MIP-2) in the lungs and development of severe bronchopneumonia and lymphoid tissue destruction in the spleen. Moreover, i.n. administration of the neutrophil-inducing chemokine MIP-2 to normal mice induced a pulmonary influx of neutrophils and significantly enhanced the clearance of A. baumannii from the lungs (P < 0.01). These results imply that neutrophils play a critical role in host resistance to respiratory A. baumannii infection.
Acinetobacter baumannii is a ubiquitous gram-negative bacterium that can survive for prolonged periods in the environment. During the last decade, this organism has emerged as a major cause of both community-associated and nosocomial infections worldwide (11, 14). The overall 30-day mortality of Acinetobacter infection can be as high as 49%, with the respiratory tract being implicated as an important portal of entry (18). Moreover, infections with A. baumannii have become increasingly difficult to treat because of its rapid development of resistance to multiple antibiotics (9). Despite its clinical importance, little is known about the cellular and molecular mechanisms of host defense against respiratory A. baumannii infection (9, 17, 26).
Innate immunity plays a crucial role in determining the outcome of respiratory infection with many bacterial pathogens, including A. baumannii (17, 35). In this regard, it has been recently shown that the CD14/Toll-like receptor 4 pathway is important in the control of intranasal (i.n.) A. baumannii infection in mice (17). However, little else is known about the nature of the innate cellular response to A. baumannii infection. Neutrophils play an important role in early control of acute bacterial infections by killing bacterial pathogens through powerful oxidative and nonoxidative mechanisms and through the production of inflammatory and immunoregulatory cytokines and chemokines (24). However, the contribution of neutrophils in host resistance to respiratory A. baumannii infection has not been directly investigated, although some indirect evidence implies that they may play an important role. For instance, clinical studies have shown that A. baumannii is one of the most frequently isolated gram-negative bacteria in neutropenic febrile patients in nosocomial settings (16). Experimental studies have also shown that neutrophils and neutrophil-recruiting chemokines are present at the site of A. baumannii infection (15, 17, 26), and neutrophil granule extract is bactericidal to other species of Acinetobacter (12, 22).
In the current study, as the first step to furthering our understanding of the role of innate immunity against respiratory A. baumannii infection, we determined the importance of neutrophils in the control of i.n. initiated infection with A. baumannii in mice. We found that neutrophils were rapidly recruited to the lungs after infection and that depletion of these cells exacerbated disease, resulting in an acute and lethal outcome. This increased susceptibility was associated with increased bacterial replication and extrapulmonary dissemination of the pathogen and a decrease in the early proinflammatory cytokine responses in the lung.
MATERIALS AND METHODS
Mice.
Eight- to 12-week-old specific-pathogen-free female C57BL/6 and BALB/c mice were purchased from Charles Rivers Laboratories (St. Constant, Quebec). The animals were housed under specific-pathogen-free conditions in a small animal containment level 2 facility and given free access to sterile water and certified mouse chow. The animals were maintained and used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals, and the experimental procedures were approved by the institutional animal care committee.
A. baumannii and i.n. inoculation.
Fresh inocula were prepared for each experiment from a frozen stock of A. baumannii (ATCC 17961; American Type Culture Collection, Manassas, VA). Stock vials of A. baumannii were thawed and regrown in tryptic soy broth medium for 3.5 h at 37°C with rotation (100 rpm), centrifuged at 12,000 × g for 15 min, resuspended in phosphate-buffered saline, and used immediately. Unless otherwise specified, anesthetized mice were inoculated i.n. with approximately 107 A. baumannii organisms in 50 μl saline. Actual inoculum concentrations were determined by plating 10-fold serial dilutions on brain heart infusion agar supplemented with 50 μg/ml streptomycin. The clinical signs of mice were monitored and recorded with following scores: 0, no abnormal clinical sign; −1, ruffled fur but lively; −2, ruffled fur, moving slowly, down, and sick; −3, ruffled fur, squeezed eye, hunched, hardly moving, and very sick; −4, moribund; and −5, dead.
MAb treatment.
For in vivo depletion of neutrophils, mice were treated intraperitoneally (i.p.) either with the rat anti-mouse monoclonal antibody (MAb) RB6-8C5 (RB6) (25 or 250 μg in 200 μl sterile saline), which recognizes the neutrophil surface marker Gr-1, or with an equivalent amount of purified rat immunoglobulin G (rIgG) (Sigma Chemical Co., St. Louis, MO) as described previously (6, 8, 28). Treatments were administered 18 h before and 1 day after initiation of A. baumannii infection. The specificity and efficacy of this MAb have been well established by us and others (3, 7, 8, 10, 23, 25, 30, 31). Treatment with 250 μg of this MAb depleted >95% of circulating neutrophils for at least 2 days as determined by total and differential blood leukocyte counts (7). In other experiments, mice were treated i.p. with hamster anti-mouse MAb 2E2 (200 μg; kindly supplied by the National Cancer Institute, Rockville, MD) (20) or rat anti-mouse MAb R46A2 (500 μg) (4) to neutralize endogenous tumor necrosis factor alpha (TNF-α) or gamma interferon (IFN-γ), respectively, or with MAb TIB210 (4) or 120G8 (29) to deplete CD8+ T cells or plasmacytoid dendritic cells, respectively.
BAL and sample collections.
Groups of five RB6- or rIgG-treated C57BL/6 mice were sacrificed at 0, 4, 24, and 72 h after i.n. inoculation with ∼107 CFU A. baumannii. Blood samples were collected for serum separation. The trachea was exposed through a midline incision and cannulated with a plastic catheter. Lungs were lavaged five times with 1.0 ml phosphate-buffered saline supplemented with 3 mM EDTA as previously described (5). Cytospin slides of 2 × 104 bronchoalveolar lavage (BAL) fluid cells were prepared using a Cytospin 3 centrifuge (Shandon, Pittsburgh, PA) and stained with HemaStat 3 (Fisher, Pittsburgh, PA). Differential cell counts were determined by examining 200 cells, and the total numbers of neutrophils, lymphocytes, and macrophages were calculated. The lavage fluid was centrifuged at 2,450 × g for 7 min, and the supernatant was collected, filter sterilized, and stored at −80°C for cytokine analysis.
Quantitative bacteriology and histopathology.
For bacterial kinetic analysis, lungs and spleen were aseptically removed and homogenized in sterile saline using aerosol-proof homogenizers. Aliquots (100 μl) of 10-fold serial dilutions of the homogenates were cultured, in duplicate, on plates of brain heart infusion agar supplemented with 50 μg/ml streptomycin to quantify the number of viable A. baumannii organisms in the respective organs. For histopathological examination, the lung and spleen were fixed immediately in 10% neutral buffered formalin and processed by standard paraffin embedding methods (Department of Pathology and Laboratory Medicine, University of Ottawa, Ottawa, Ontario). Sections were cut 4 μm thick, stained with hematoxylin-eosin, and examined by light microscopy (19).
Tissue RNA extraction and quantitative reverse transcription-PCR analysis.
For cytokine/chemokine mRNA expression analysis, the lung and spleen were dissected, immersed immediately in RNAlater (QIAGEN, Germantown, MD), and stored at −20°C until extraction.
Relative amounts of cytokine and chemokine mRNAs in the lung and spleen over the course of infection in the two groups of mice were estimated using a real-time PCR-based method essentially as described elsewhere (13, 19). Briefly, total RNA was extracted from tissues, and cDNA was prepared, amplified, and quantified using primers and probes designed with the Primer3 program available at http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi. Mouse housekeeping gene β2 microglobulin mRNA was measured and used to calculate relative expression (2−ΔΔCT). Levels of cytokine and chemokine PCR products were normalized to β2 microglobulin, which was not differentially expressed over the course of infection in RB6- and rIgG-treated mice. Data are presented as the average of relative expression values compared to those in the corresponding tissues of mice killed immediately before infection (i.e., 0 h, uninfected) (19, 21). The differences in ΔCT between RB6- and rIgG-treated mice were compared by the Mann-Whitney U test, based on the method of Yuan et al. (34).
Measurement of serum and BAL fluid cytokines and chemokines.
Serum and BAL fluid levels of cytokines and chemokines were determined using the mouse panel of Fluorokine MAP Multiplex Kits (R & D Systems, Inc. Minneapolis, MN) on a Luminex 100IS system (Luminex, Austin, TX). Undiluted BAL samples and 1:2 diluted serum samples (50 μl) were analyzed as specified by the manufacturer. The analysis was done in duplicate, and the cytokine/chemokine concentrations were calculated against the standards using Beadview software (version 1.03; Upstate) (19).
Statistical analysis.
Data are presented as means ± standard deviations (SD) for each group, unless otherwise specified. Differences in quantitative measurements were assessed by Student's t test or two-way analysis of variance followed by Bonferroni's post hoc multiple-comparison tests when appropriate. The survival rates between groups were analyzed by the log rank test. Differences were considered significant when the P value was <0.05.
RESULTS
Susceptibility of C57BL/6 mice to i.n. A. baumannii inoculation and kinetics of bacterial replication and extrapulmonary dissemination.
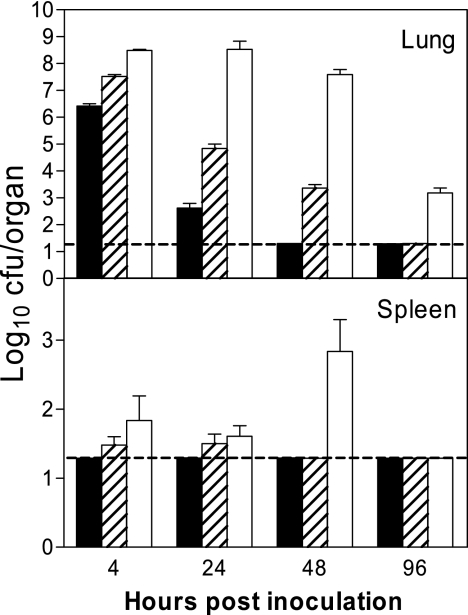
Groups of C57BL/6 mice were inoculated i.n. with 106, 107, or 108 CFU A. baumannii on day 0, and their clinical signs and outcome were monitored. Mice that received 106 CFU A. baumannii showed no clinical signs of infection throughout the experiment, whereas mice inoculated with 107 or 108 CFU A. baumannii developed mild to moderate clinical signs of infections (i.e., ruffled fur, lethargy, and hunched stature) as early as 24 h postinoculation (p.i.), remained symptomatic for the next 48 h, and appeared clinically healthy by 96 h. However, no mouse died of the infection at any challenge dose. Five mice from each group were sacrificed at 0, 4, 24, 48, or 96 h p.i. and used for the determination of bacterial burdens in the lungs and spleens. As can be seen in Fig. 1, the kinetics of the bacterial growth and subsequent clearance of infection in the lungs was related to the dose of initial inoculation. However, regardless of the number of bacteria administered, the number of A. baumannii organisms in the lungs transiently increased at 4 h and then gradually declined over the course of infection, although the rate of decline varied depending on the initial inoculum dosage. Mice that received 106 CFU A. baumannii cleared the infection almost completely by 48 h; the group of mice receiving 108 CFU showed little change in the bacterial burden in the lungs during the first 48 h after inoculation and retained moderate numbers of bacteria by 96 h, whereas the mice that received the lower doses (106 or 107 CFU) showed no cultivable bacteria by this time (Fig. 1). There was no extrapulmonary dissemination detected in the group receiving the lowest dose (106 CFU), but small numbers of viable A. baumannii organisms were cultured from the spleens of the groups receiving the medium (107 CFU) and high (108 CFU) challenge doses for up to 48 h. No viable organisms were cultured from the spleens of any group at 96 h p.i. (Fig. 1). Upon necropsy at 7 days p.i., mice inoculated with the medium and high doses of A. baumannii showed patches or areas of lung consolidation, while the low-dose group showed no abnormal gross or microscopic pulmonary changes by this time (data not shown).
FIG. 1.
Bacterial burdens in the lungs and spleens of C57BL/6 mice inoculated by the i.n. route with 106 (solid bars), 107 (shaded bars) or 108 (open bars) CFU of A. baumannii. The data are presented as mean log10 CFU/organ ± SD (n = 5) and represent one of at least two experiments with similar results. The detection limit (dashed lines) for the bacterial burdens was 1.3 log10 CFU/organ.
Depletion of neutrophils by RB6 MAb treatment exacerbates i.n. A. baumannii infection.
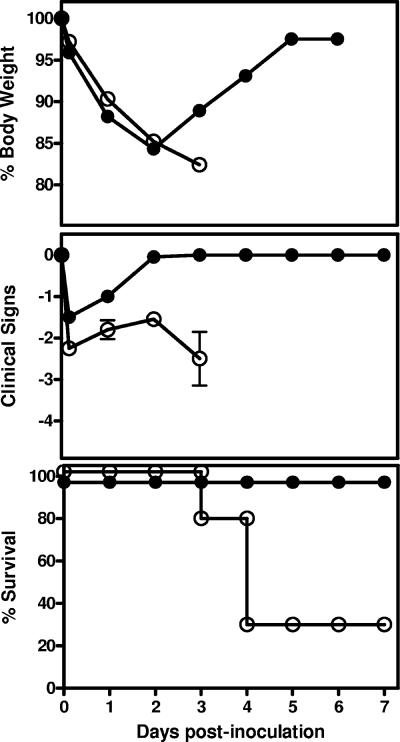
We next determined the potential contribution of neutrophils to host resistance against i.n. A. baumannii infection by depleting these leukocytes in vivo with MAb RB6. Groups of five C57BL/6 mice were treated with 250 μg of RB6 or an equivalent amount of control rIgG 18 h before and 1 day after i.n. inoculation with 107 CFU A. baumannii and their clinical signs monitored. Treatment of uninfected mice with RB6 or rIgG showed no effect on the body weight, and the mice showed no clinical signs of infection throughout the experiment (data not shown). However, RB6-treated challenged mice showed significantly increased susceptibility to infection, with all animals showing more severe clinical signs and substantial loss of body weight, compared with rIgG-treated challenged mice (Fig. 2, top and middle). Moreover, 70% of RB6-treated mice died of infection between 72 and 96 h p.i., while all rIgG-treated mice survived the infection (χ2 = 8.81; P < 0.005) (Fig. 2, bottom). At day 7, the RB6-treated mice that survived the infection remained sick with mild signs of disease (i.e., slightly ruffled fur and moderately reduced activity), whereas all rIgG-treated mice were normal in appearance and activity.
FIG. 2.
Effect of RB6 treatment on the body weight, clinical scores, and survival of mice after i.n. inoculation with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 250 μg of RB6 (open circles) or an equivalent amount of control rIgG (closed circles) at −18 and +24 h after i.n. challenge with 107 CFU of A. baumannii. Body weight, clinical scores, and survival were monitored for 7 days. The data are compiled from two independent experiments with similar results.
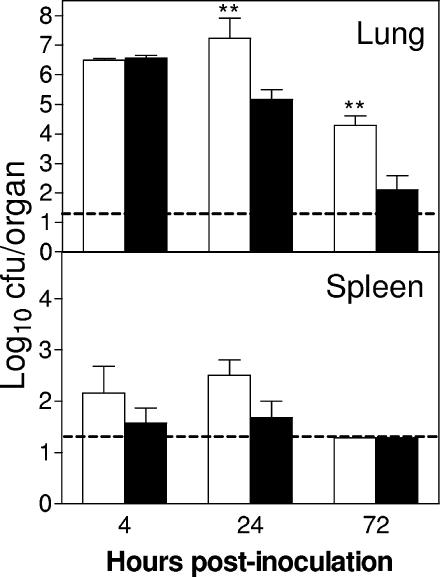
Since neutrophils are phagocytic and potentially bactericidal effector cells, we next determined whether neutrophil depletion increases the bacterial burden in the lung and promotes extrapulmonary dissemination of the pathogen. As shown in Fig. 3, RB6-treated mice showed significantly higher bacterial burdens (P < 0.01) in the lungs than rIgG-treated mice following i.n. inoculation with 107 CFU of A. baumannii, with 100- and 1,000-fold increases at 24 and 72 h p.i., respectively. At day 7, no A. baumannii was isolated from the lungs of rIgG-treated mice, while log10 5.0 CFU of A. baumannii were cultured from the lungs of the remaining RB6-treated mice that survived the infection. Moreover, there were substantially greater numbers of A. baumannii organisms in the spleens of RB6-treated mice than in rIgG-treated mice at 4 and 24 h p.i. (Fig. 3), although the differences did not reach statistical significance. Despite the frequent culture of low numbers of A. baumannii organisms from the spleens of RB6-treated, inoculated mice, only a few organisms were occasionally cultured in blood samples of the same mice (data not shown). The reason for this is not clear, and it could be because (i) the bacteriemia was only very transient, (ii) the level of bacteriemia was too low to be cultured by our method, or (iii) the route of the bacterial spread from lungs to spleens was not hematogenous.
FIG. 3.
Effect of RB6 treatment on bacterial burdens in the lung and spleen following i.n. inoculation with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 250 μg of RB6 (open bars) or an equivalent amount of control rIgG (solid bars) at −18 and +24 h after i.n. challenge with 107 CFU of A. baumannii. Bacterial burdens in the lung and spleen were determined by quantitative bacteriology. The data are presented as mean ± SD (n = 5) and represent one of at least two experiments with similar results. The detection limit (dashed lines) for the bacterial burdens was 1.3 log10 CFU/organ. **, P < 0.01 versus rIgG-treated mice.
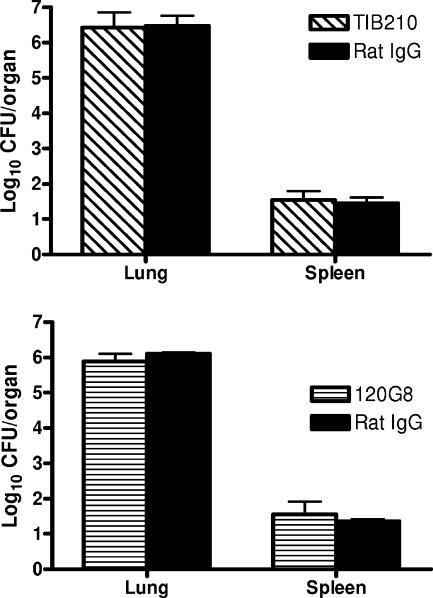
Since it has recently been shown that in addition to neutrophils, other cells (such as a subset of CD8+ T cells and plasmacytoid dendritic cells) also express Gr-1 markers (25, 32, 33), additional experiments were performed to confirm the importance of neutrophils in the observed effect on the enhanced susceptibility in RB6-treated mice. C57BL/6 mice were treated i.p. with low doses (25 μg) of MAb RB6 18 h before i.n. challenge with A. baumannii, since it has previously been shown that this treatment regimen has no adverse effect on other, nonneutrophil Gr-1+ cell populations (25, 33). As can be seen in Fig. 4, the bacterial burdens in the lungs of low-dose-RB6-treated mice were almost identical to those in mice treated with the high dose (250 μg). Moreover, the effect of low and high doses of RB6 on the enhanced susceptibility of mice to i.n. A. baumannii infection was also observed in similarly treated BALB/c mice (Fig. 4). Furthermore, mice depleted of CD8+ T cells or plasmacytoid dendritic cells showed similar susceptibility to i.n. A. baumannii infection as rIgG-treated mice (Fig. 5). These data suggest that depletion of neutrophils with MAb RB6 enhances local growth (in the lung) of A. baumannii with mild systemic dissemination (in the spleen) in mice.
FIG. 4.
Effect of RB6 treatment dosage on bacterial burdens in the lung following i.n. inoculation with A. baumannii. Groups of five C57BL/6 or BALB/c mice were treated by i.p. injection with either 250 μg (open bars) or 25 μg (hatched bars) of RB6 or an equivalent amount of control rIgG (solid bars) at 18 h before i.n. challenge with 2.6 × 107 CFU of A. baumannii. Bacterial burdens in the lung were determined by quantitative bacteriology. The data are presented as mean ± SD (n = 5), and the detection limit for the bacterial burdens was 1.3 log10 CFU/lung. **, P < 0.01 versus rIgG-treated mice.
FIG. 5.
Effect of depletion of CD8+ T cells or plasmacytoid dendritic cells on bacterial burdens in the lung following i.n. inoculation with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 500 μg of MAb TIB210 (top), 150 μg of MAb 120G8 (bottom), or an equivalent amount of control rIgG at 18 h before i.n. challenge with 2.5 × 107 CFU of A. baumannii. Bacterial burdens in the lung were determined by quantitative bacteriology. The data are presented as mean ± SD (n = 5), and the detection limit for the bacterial burdens was 1.3 log10 CFU/lung.
Histopathological observation.
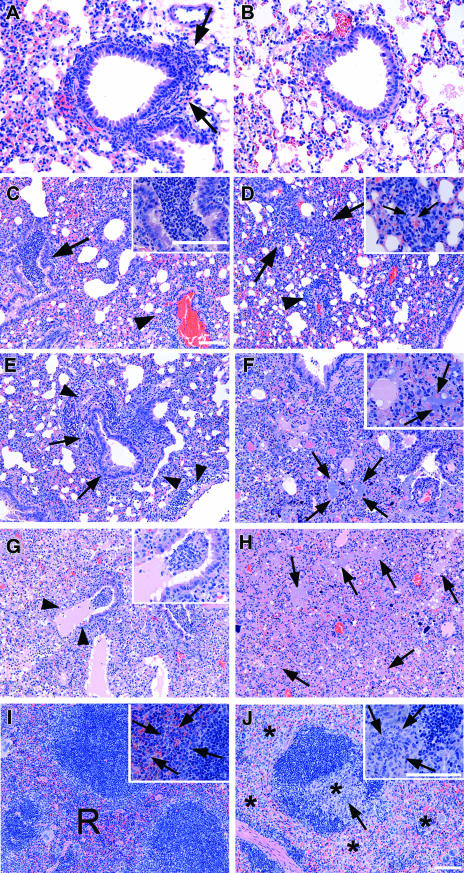
To further elucidate the role of neutrophils in host resistance against i.n. A. baumannii infection, the lungs and spleens were collected from RB6- or rIgG-treated mice at various time points after i.n. challenge with 107 CFU A. baumannii. There was no remarkable change in the lungs or spleens from RB6- or rIgG-treated mice at 0 h. At 4 h, rIgG-treated mice showed neutrophil infiltration in the perivascular and peribronchial areas (Fig. 6A) and cellular exudates in the lumens of large airways. In contrast, RB6-treated mice showed no remarkable change at 4 h despite the presence of nearly 107 bacteria in the lungs (Fig. 6B). At 24 h, rIgG-treated mice showed severe bronchopneumonia consisting of large numbers of neutrophils in the lumens of bronchi and large bronchioles and moderately severe infiltration of mixed neutrophils and mononuclear cells in the perivascular and peribronchial areas (Fig. 6C). The lung lesions in the RB6-treated mice at this time point were mainly interstitial and consisted primarily of perivascular accumulations of mononuclear cells (Fig. 6D). In addition, by 24 h of infection, the RB6-treated mice showed the infiltration of small numbers of neutrophils in the red pulps and interfollicular areas of the spleen. By 72 h, the lesions seen in the lungs from rIgG-treated mice were much more variable than at earlier time points. Overall, the lungs were highly consolidated in many areas. In some areas, there was an extensive perivascular and, to a lesser extent, peribronchial accumulation of lymphoid cells admixed with some neutrophils (Fig. 6E) and small to moderate numbers of neutrophils in the lumens of associated airways. In other areas, the lungs showed complete consolidation associated with the presence of large numbers of neutrophils admixed with many mononuclear cells and large numbers of bacteria (Fig. 6F). The spleens from these mice showed a moderate expansion of the red pulp, due to the increase in the numbers of macrophages and reticuloendothelial cells, and the infiltration of small numbers of neutrophils in the interfollicular areas (Fig. 6I). The lungs from RB6-treated mice killed at 72 h showed extensive bronchopneumonia with the presence of large numbers of neutrophils, fibrinous exudates, and cellular debris in the lumens of many airways (Fig. 6G). Some affected airways showed the erosion of the epithelial mucosa. In the severely affected areas, the lungs were completely consolidated, with the presence of large numbers of bacteria and necrotic cellular debris (Fig. 6H) but with little mononuclear cell response. Many lymphoid follicles in the spleens of these mice showed a moderate degree of lymphocyte depletion and infiltration of neutrophils in both the red pulp and the lymphoid follicles (Fig. 6J).
FIG. 6.
Histopathological findings for the lungs and spleens from control rIgG- and RB6-treated mice killed at 4 (A and B), 24 (C and D), and 72 (E to J) h after i.n. inoculation with 107 CFU A. baumannii. (A and B) The lung from an rIgG-treated mouse killed at 4 h (A) shows early neutrophil infiltration in the peribronchial areas (arrows), whereas the lung from a RB6-treated mouse killed at the same time (B) shows no remarkable change. (C) The lung from an rIgG-treated mouse killed at 24 h shows severe bronchopneumonia with the presence of large numbers of neutrophils in the lumen of bronchi (arrow) and large bronchioles and moderately severe infiltration of mixed neutrophils and mononuclear cells in the perivascular and peribronchial areas (arrowhead). Inset, higher magnification showing the presence of large numbers of neutrophils in the lumen of a bronchus. (D) The lung from a RB6-treated mouse killed at 24 h shows a predominantly interstitial pneumonitis (arrows) and perivascular accumulation of mononuclear cells (arrowhead). Inset, higher magnification showing the accumulation of mononuclear cells around a small blood vessel (arrows). (E and F) The lungs from rIgG-treated mice killed at 72 h show extensive perivascular (arrowheads) and moderate peribronchial (arrows) accumulation of lymphoid cells admixed with some neutrophils (E) and complete consolidation together with the presence of large numbers of neutrophils admixed with many mononuclear cells and large numbers of bacterial colonies (arrows) (F). Inset, higher magnification showing the presence of mixed inflammatory cells and bacterial colonies (arrows). (G and H) The lungs from RB6-treated mice killed at 72 h show severe acute bronchopneumonia with the erosion of the epithelial mucosa (arrowheads and inset) (G) and complete consolidation with the presence of large numbers of bacteria (arrows) and necrotic cellular debris but with little mononuclear cell response (H). (I) The spleen from an rIgG-treated mouse killed at 72 h shows moderate expansion of the red pulp (R) and the infiltration of small numbers of neutrophils in the interfollicular areas (arrows within inset). (J) The spleen from an RB6-treated mouse killed at 72 h shows a moderate degree of lymphocyte depletion (*) and infiltration of neutrophils in both the red pulp and the lymphoid follicles (arrow). Inset, higher magnification showing the infiltration of neutrophils in the lymphoid follicle (arrows) and the depletion of lymphocytes. Hematoxylin and eosin staining was used. Bars, 40 μm.
Effect of depletion of neutrophils on the cellular composition in the lung after i.n. A. baumannii inoculation.
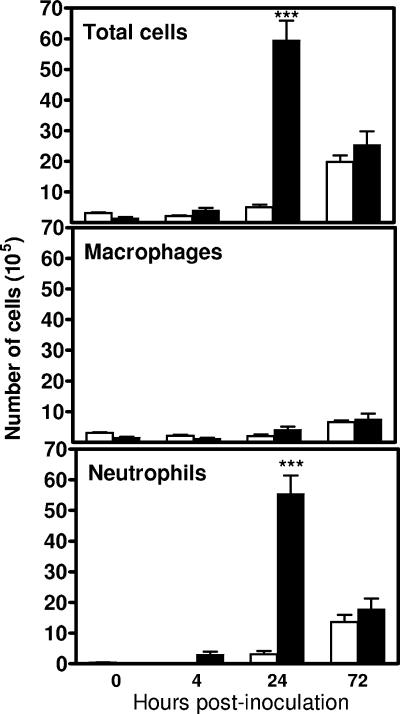
To determine the kinetics of pulmonary neutrophil recruitment, groups of five RB6- and rIgG-treated mice were killed at 0, 4, 24, and 72 h after i.n. inoculation with 107 CFU A. baumannii. The lungs were lavaged and total and differential cell counts in the BAL fluid determined. There was no significant difference in the total BAL cells between RB6- and rIgG-treated mice at 0 h (3.10 × 105 ± 0.49 × 105 cells versus 1.78 × 105 ± 0.59 × 105 cells), with 99% being macrophages. i.n. inoculation of rIgG-treated mice with 107 CFU A. baumannii induced a rapid recruitment of neutrophils into the lung, which was evident as early as 4 h, peaked at 24 h, and remained highly elevated at 72 h, with 67.3, 93.0, and 71.0% of BAL fluid cells, respectively, being neutrophils (Fig. 7). RB6 treatment remarkably reduced both the percentage and the absolute numbers of neutrophils in the bronchoalveolar space at 4 h p.i., although there was no substantial effect on the total number of BAL fluid cells. By 24 h, the RB6-treated mice had significantly (∼10-fold) fewer total BAL fluid cells and neutrophils (P < 0.005), whereas the total number of alveolar macrophages was not significantly affected (Fig. 7). By 72 h, the total and differential BAL fluid cells were comparable for RB6- and rIgG-treated mice. The numbers of lymphocytes or eosinophils in the BAL fluid throughout the course of the experiment in both groups of mice were negligible.
FIG. 7.
Effect of RB6 treatment on the composition of cell populations in the BAL fluid from mice i.n. inoculated with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 250 μg of RB6 (open bars) or an equivalent amount of control rIgG (solid bars) at −18 and +24 h after i.n. challenge with 107 CFU of A. baumannii. At the indicated times, mice were exsanguinated, their lungs were lavaged, and total and differential cell counts were determined. Error bars indicate SD. ***, P < 0.005 versus control rIgG-treated mice.
Effect of depletion of neutrophils on pulmonary and systemic cytokine/chemokine responses to i.n. A. baumannii challenge.
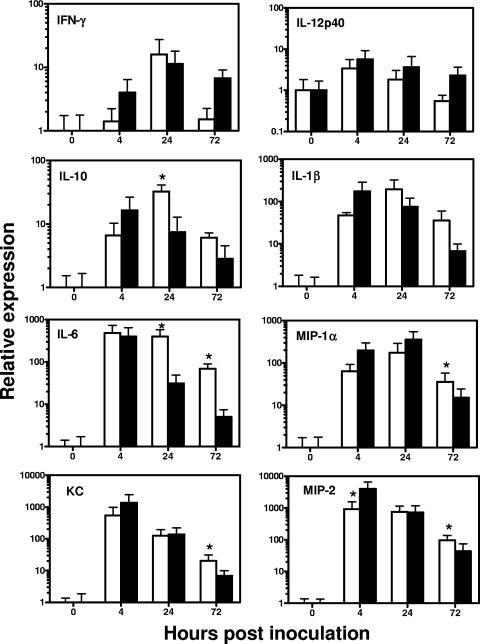
To examine the effect of neutrophil depletion on pulmonary and systemic cytokine/chemokine responses after i.n. A. baumannii infection, total RNA was extracted from lungs and spleens from RB6- or rIgG-treated mice over the course of infection and assessed for mRNA expression of a selected panel of proinflammatory cytokines/chemokines by real-time reverse transcription-PCR (Fig. 8). Among the tested cytokines and chemokines, there were substantial increases in interleukin-1β (IL-1β), IL-6, keratinocyte chemoattractant protein [KC], macrophage inflammatory protein 1 α [MIP-1α], MIP-2, monocyte chemoattractant protein 1 (MCP-1), and TNF-α mRNA expression and a moderate increase in IL-10 mRNA expression in the lungs of both RB6- and rIgG-treated mice over the course of infection, but with different kinetics (Fig. 8). The mRNA expression for most of these cytokines and chemokines peaked at 4 h and subsided substantially or returned to baseline by 72 h in the lungs of rIgG-treated mice. In contrast, there was a delay in the peak mRNA expression of those cytokines and chemokines in the lungs of RB6-treated mice (Fig. 8). On the other hand, there was no change in the mRNA expression of IL-12p35 and p40 over the course of infection, and the IFN-γ mRNA expression was only transiently increased at 24 h by similar magnitudes in both the RB6- and rIgG-treated mice. There was very little change in the mRNA expression levels of the tested cytokines or chemokines in the spleens of RB6- or rIgG-treated mice over the course of infection except that the RB6-treated mice showed transient increases (∼5-fold) in the expression of IL-6, IL-10, KC, MIP-2, and MCP-1 at 24 h only (data not shown).
FIG. 8.
Relative levels of proinflammatory cytokine and chemokine mRNA expression in the lungs of RB6 (open bars)- and control rIgG (solid bars)-treated mice i.n. inoculated with A. baumannii. Groups of C57BL/6 mice (n = 5) were treated by i.p. injection with 250 μg of MAb RB6 or an equivalent amount of control rIgG at −18 and 24 h after i.n. inoculation with 2 × 107 CFU of A. baumannii, and the lungs were collected at 0, 4, 24, and 72 h. Relative levels of cytokine and chemokine mRNA expression were determined by real-time PCR analysis as described in Materials and Methods. Results shown are the average of relative expression values with the 95% confidence interval determined using cDNA from five mice. *, P < 0.05 versus control rIgG-treated mice.
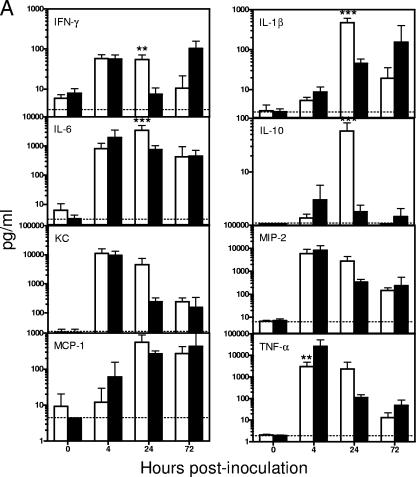
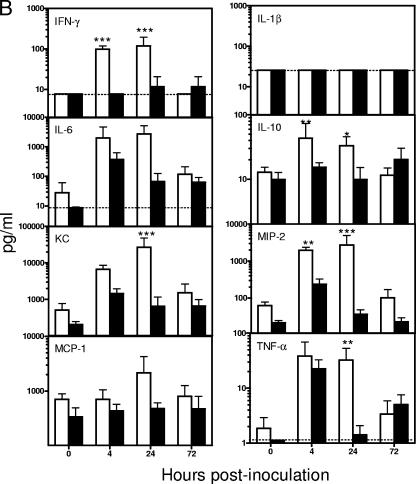
In addition to tissue cytokine/chemokine mRNA levels, the serum and BAL fluid levels of the corresponding cytokine and chemokine proteins were determined over the course of the infection. There was no significant difference in the levels of all cytokine/chemokines examined in the BAL fluid between RB6- and rIgG-treated mice at 0 h. Overall, the BAL fluid cytokine/chemokine levels in both RB6- and rIgG-treated mice increased substantially following the i.n. A. baumannii challenge, with the majority of the assayed cytokine/chemokines reaching their peak levels at 4 or 24 h p.i. (Fig. 9A). In agreement with the observed tissue mRNA expression patterns, the BAL fluid levels of IFN-γ, IL-1β, IL-6, IL-10, KC, MIP-2, MCP-1, and TNF-α in RB6-treated mice were either lower than or comparable to those in rIgG-treated mice at 4 h p.i. (Fig. 9A). By 24 h, most of these cytokines/chemokines were moderately or significantly higher in the BAL fluid of RB6-treated mice than in that of rIgG-treated mice (Fig. 9A). By 72 h p.i., the BAL fluid levels of most cytokines/chemokines of RB6-treated mice were once again lower than or comparable to those in rIgG-treated mice. On the other hand, the serum cytokine/chemokine levels in rIgG-treated mice changed little over the course of infection except that the levels of IL-6 and TNF-α were significantly increased at 4 h p.i. (Fig. 9B). In contrast, most of the cytokines and chemokines tested (IFN-γ, IL-6, IL-10, KC, MIP-2, MCP-1, and TNF-α) showed significantly higher increases at 24 h p.i. in RB6-treated than in rIgG-treated mice (overall, P < 0.05) (Fig. 9), probably reflecting the stimulation of transient extrapulmonary dissemination of this pathogen in RB6-treated mice.
FIG. 9.
Effect of RB6 treatment on cytokine and chemokine levels in sera (A) and BAL fluid (B) from mice i.n. inoculated with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 250 μg of RB6 (open bars) or an equivalent amount of control rIgG (solid bars) at −18 and +24 h after i.n. challenge with 107 CFU of A. baumannii. Blood and BAL samples were collected at 0, 4, 24, and 72 h. Cytokine and chemokine levels in the serum and BAL fluid were determined using the mouse panel of Fluorokine MAP Multiplex Kits (R & D Systems, Inc., Minneapolis, MN) on a Luminex 100 IS instrument. Data are expressed as mean ± SD for five mice at each time point. The detection limits of the assays were 2.5 to 15 pg/ml, as indicated by dotted lines. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (versus control rIgG-treated mice).
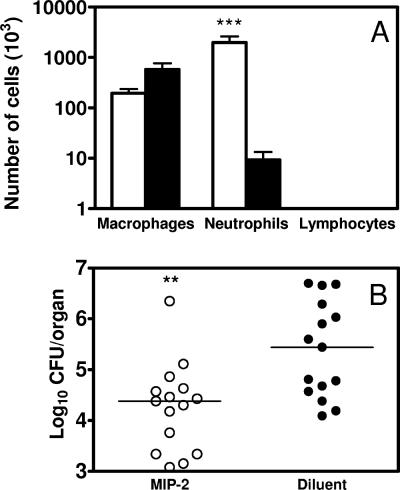
i.n. MIP-2 treatment induces pulmonary neutrophil recruitment and enhances host resistance to i.n. A. baumannii inoculation.
The preceding experiments demonstrated that neutrophil depletion increased A. baumannii burdens in the lungs and delayed local and systemic cytokine/chemokine responses (Fig. 2 to 9). We next examined whether or not enhanced early recruitment of neutrophils by i.n. administration of the neutrophil-inducing chemokine MIP-2 would decrease pulmonary A. baumannii growth in normal mice. As shown previously by others (10), a single i.n. administration to naive mice of 1 μg of recombinant murine MIP-2 induced a significant influx of neutrophils into the lungs at 24 h (P < 0.005) without significantly affecting the total numbers of lymphocytes or macrophages therein (Fig. 10A). To determine the effect of MIP-2 treatment on the pulmonary clearance of A. baumannii, groups of C57BL/6 mice were treated i.n. with either 2.5 μg MIP-2 or 0.1% bovine serum albumin (BSA) diluent at −24 h and −4 h and then were challenged i.n. with 2.4 × 107 CFU of A. baumannii at 0 h. Both MIP-2- and diluent-treated mice were sacrificed at 24 h p.i., and the bacterial burdens in the lung and spleen were determined. Following i.n. challenge with A. baumannii, MIP-2-treated mice showed significantly reduced bacterial burdens in the lungs of the majority of mice at 24 h (P < 0.01) (Fig. 10B). As expected, 0.1% BSA diluent treatment did not alter the cellular composition of the BAL fluid and had no effect on bacterial burdens (Fig. 10).
FIG. 10.
(A) i.n. MIP-2 administration results in recruitment of neutrophils to bronchoalveolar spaces. Groups of C57BL/6 mice were instilled by the i.n. route with either 1.0 μg of recombinant murine MIP-2 (open bars) or 0.1% BSA diluent (solid bars). The mice were killed 24 h later, their lungs were lavaged, and total and differential cell counts were determined. The data are presented as mean ± SD (n = 5). ***, P < 0.005 versus mice treated with diluent. (B) Effect of i.n. MIP-2 administration on A. baumannii burden in the lungs. Groups of C57BL/6 mice were treated i.n. with either 2.5 μg of recombinant murine MIP-2 (open bars) or diluent (solid bars) in 50 μl at 24 and 4 h before i.n. challenge with 2 × 107 CFU A. baumannii. At 24 h, the mice were killed, lung homogenates were prepared from individual mice, and CFU were determined. The data are compiled from three independent experiments with similar results; each individual dot represents the value from a single mouse, and the horizontal line represents the median value for the group (n = 15). **, P < 0.01 versus diluent-treated mice.
DISCUSSION
A. baumannii is an emerging bacterial pathogen, which rapidly develops multiple drug resistance and is responsible for the majority of nosocomial pulmonary infections (9, 11, 14). Interactions between A. baumannii and the host innate immune system likely govern the extent of bacterial proliferation and local host tissue inflammatory response following pulmonary challenge with the pathogen. As demonstrated by others (17, 26), we found that normal C57BL/6 mice were capable of clearing A. baumannii within 72 h after i.n. inoculation with 107 CFU of the pathogen. This suggests that, under normal circumstances, innate immune defenses in the lungs can effectively control A. baumannii infection and prevent the development of severe disease. Moreover, we found that the recruitment of large numbers of neutrophils and the predominantly neutrophilic inflammatory response in the major airways and lung parenchyma of infected mice resembled the suppurative bronchopneumonia previously observed in human patients (1, 2) and correlated well with the control and eradication of A. baumannii growth in the lungs and spleens.
Neutrophils are crucial in host defense against a wide array of respiratory pathogens. However, their contributions are not the same for all infections or even for different routes of infection with the same pathogen (3, 7, 8, 10, 23-25, 30, 31). To our knowledge, there are no published reports on the in vivo contribution of neutrophils to A. baumannii-associated pneumonia. In this study, we used a mouse model of i.n. challenge, which mimics one of the natural routes of A. baumannii exposure in clinical settings (15), to examine the role of neutrophils in host defense against acute respiratory A. baumannii infection. Our results showed for the first time that neutrophils play an important role in host defense against respiratory infection with this pathogen. We found that neutrophils were normally rapidly recruited to the lungs after i.n. A. baumannii challenge. Additionally, depletion of neutrophils prior to challenge converted a self-limiting infection into a rapidly lethal one that was associated with loss of control of bacterial replication at the primary site of implantation and subsequent, although mild, extrapulmonary dissemination of the pathogen to the spleen. Moreover, i.n. administration of the neutrophil-recruiting chemokine MIP-2 to immunocompetent mice enhanced the clearance of A. baumannii from the lungs and prevented its systemic dissemination.
The MAb RB6 used to deplete mice of neutrophils in this study recognizes the Gr-1 marker on cell surface of mature eosinophils and neutrophils and specifically depletes these granulocytes in vivo (32). Additionally, other investigators have reported intermediate expression of Gr-1 on other cells, such as a small population of CD8+ cells and monocytes/dendritic cells (25, 33). However, we believe that the increased susceptibility to i.n. A. baumannii infection in RB6-treated mice in our study was most likely associated with the depletion of neutrophils rather than other Gr-1+ cells because (i) low doses of RB6, which have no effect on nonneutrophil Gr-1+ cells, resulted in a similar increase in the bacterial burden as high-dose treatment (Fig. 4); (ii) depletion of CD8+ cells or plasmacytoid dendritic cells in mice by MAb treatments had no effect on the bacterial burden after i.n. A. baumannii challenge (Fig. 5); and (iii) the recruitment of alveolar macrophages was unaffected by treatment with 250 μg of RB6 in this study (Fig. 7).
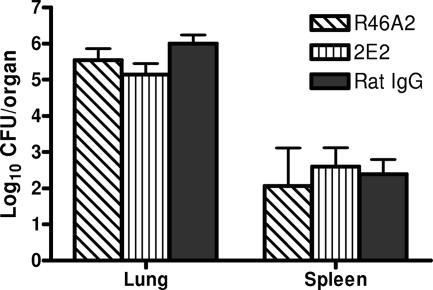
Although our study has shown that neutrophils are important in host defense against respiratory A. baumannii infection, their precise mechanisms of action remain unknown. In this regard, neutrophils are potent effectors of the innate immune response and contribute to protection in other bacterial infections through their direct antimicrobial capacity and the production of cytokines and chemokines that instruct the recruitment and activation of other immune cells (24). In the present study, the differences in the bacterial burdens in control and neutropenic mice at the primary site of infection and in the extrapulmonary tissue were detected as early as 4 h p.i. and peaked at 24 h (Fig. 3). Previous studies have shown that i.n. A. baumannii infection induces local production of moderate amounts of TNF-α, IL-1β, IL-6, MCP-1, MIP-2, and KC at 4 and 24 h p.i. (17, 26). Here we show that, in addition to these cytokines, the mRNA expression and production of IFN-γ and IL-10 were also transiently induced in the lungs at 24 h after i.n. A. baumannii infection. Overall, the magnitude of mRNA expression in the lung and spleen and cytokine levels in the BAL fluid and serum were similar in pattern to the bacterial burdens in the tissue, which peaked at 4 h in rIgG-treated mice and at 24 h in RB6-treated mice (Fig. 3, 8, and 9). The transient changes in cytokine/chemokine mRNA expression and levels in the serum and spleen during respiratory A. baumannii infection reflect the nature of infection, since A. baumannii was only transiently present in the spleens in neutrophil-depleted mice (Fig. 3). However, as with other studies, the cellular sources of these cytokines and chemokines remain to be elucidated. Although NK cells are likely to be the major source of IFN-γ secretion seen in this study, other pulmonary cells, such as alveolar epithelial cells, may also secret this cytokine, as recently reported for Mycobacterium tuberculosis infection (27).
Depletion of neutrophils substantially decreased levels of TNF-α, IL-6, IL-10, and MCP-1 in the lung at 4 h p.i. (Fig. 9), implying that neutrophils play a critical role in the generation of the early proinflammatory cytokine responses in the lungs. In this regard, previous studies have demonstrated that neutrophils can produce many of these cytokines (3), and decreases in TNF-α, IL-6, and other cytokine levels after RB6-induced neutropenia have been reported in other models of infection (3, 23). The differences in cytokine levels in the lungs between RB6- and rIgG-treated mice over the course of the infection show a more complicated picture. At 24 h p.i., RB6-treated mice showed significantly stronger pulmonary cytokine/chemokine responses than rIgG-treated mice, probably reflecting the fact that the depletion of neutrophils at the beginning of infection exacerbated pulmonary bacterial burdens and low levels of extrapulmonary bacterial dissemination in neutropenic mice (Fig. 3), which might stimulate the production of proinflammatory cytokines in infected tissues. By 72 h p.i., most RB6-treated mice were in the terminal stage of the infection and consequently showed substantially lower cytokine/chemokine levels in the BAL fluid than rIgG-treated mice despite the presence of significantly higher numbers of A. baumannii organisms in their lungs. Nevertheless, the delayed or reduced cytokine response seen in RB6-treated mice does not appear to directly contribute to the enhanced susceptibility to i.n. A. baumannii infection, since in vivo neutralization of endogenous TNF-α or IFN-γ in these mice failed to exacerbate the infection (Fig. 11). These data imply that an early direct bactericidal effect by the neutrophils may be primarily responsible for their role in host defense against this pathogen. In this regard, it has been previously shown that granule extracts from human and rat neutrophils and purified human defensin, a potent neutrophil antimicrobial peptide in vitro, are bactericidal to another Acinetobacter species, A. calcoaceticus (12, 22). This hypothesis is further supported by the finding that enhancement of pulmonary neutrophil recruitment with i.n. administration of neutrophil-inducing chemokine MIP-2 resulted in significant reductions in the bacterial burdens in the lung following i.n. A. baumannii inoculation (Fig. 10). Overall, these data demonstrate that neutrophils are an essential component of the protective innate immune response to respiratory A. baumannii infection. The identification of neutrophils as a key element of host defense against this pathogen is likely to have implications for the clinical management of this infection, since Acinetobacter is frequently isolated from neutropenic febrile patients in nosocomial settings (16).
FIG. 11.
Effect of neutralization of endogenous TNF-α or IFN-γ on bacterial burdens in the lung following i.n. inoculation with A. baumannii. Groups of five C57BL/6 mice were treated by i.p. injection with either 500 μg of IFN-γ neutralization MAb R46A2 or TNF-α neutralization MAb 2E2 or an equivalent amount of control rIgG at 18 h before i.n. challenge with 2.0 × 107 CFU of A. baumannii. Bacterial burdens in the lungs and spleens were determined by quantitative bacteriology. The data are presented as mean ± SD (n = 5), and the detection limit for the bacterial burdens was 1.3 log10 CFU/lung.
Acknowledgments
This work was supported by the National Research Council Canada.
We thank Tom Devecseri for assistance in the preparation of photomicrographs and Hongda Shen for technical assistance in part of this study.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Anstey, N. M., B. J. Currie, and K. M. Withnall. 1992. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis. 14:83-91. [DOI] [PubMed] [Google Scholar]
- 2.Bick, J. A., and J. D. Semel. 1993. Fulminant community-acquired Acinetobacter pneumonia in a healthy woman. Clin. Infect. Dis. 17:820-821. [DOI] [PubMed] [Google Scholar]
- 3.Bliss, S. K., L. C. Gavrilescu, A. Alcaraz, and E. Y. Denkers. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W., J. A. Harp, and A. G. Harmsen. 1993. Requirements for CD4+ cells and gamma interferon in resolution of established Cryptosporidium parvum infection in mice. Infect. Immun. 61:3928-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, W., E. A. Havell, L. L. Moldawer, K. W. McIntyre, R. A. Chizzonite, and A. G. Harmsen. 1992. Interleukin 1: an important mediator of host resistance against Pneumocystis carinii. J. Exp. Med. 176:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlan, J. W., R. KuoLee, H. Shen, and A. Webb. 2002. Different host defences are required to protect mice from primary systemic vs pulmonary infection with the facultative intracellular bacterial pathogen, Francisella tularensis LVS. Microb. Pathog. 32:127-134. [DOI] [PubMed] [Google Scholar]
- 8.Conlan, J. W., and R. J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fournier, P. E., and H. Richet. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 42:692-699. [DOI] [PubMed] [Google Scholar]
- 10.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynes, R., and J. R. Edwards. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 41:848-854. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald, G. I., and T. Ganz. 1987. Defensins mediate the microbicidal activity of human neutrophil granule extract against Acinetobacter calcoaceticus. Infect. Immun. 55:1365-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated proteciton against infection-stimulated immunpathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joly-Guillou, M. L. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11:868-873. [DOI] [PubMed] [Google Scholar]
- 15.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim, M., W. Khan, B. Farooqi, and I. Malik. 1991. Bacterial isolates in neutropenic febrile patients. J. Pak. Med. Assoc. 41:35-37. [PubMed] [Google Scholar]
- 17.Knapp, S., C. W. Wieland, S. Florquin, R. Pantophlet, L. Dijkshoorn, N. Tshimbalanga, S. Akira, and T. van der Poll. 2006. Differential roles of CD14 and Toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am. J. Respir. Crit. Care Med. 173:122-129. [DOI] [PubMed] [Google Scholar]
- 18.Kuo, L. C., C. C. Lai, C. H. Liao, C. K. Hsu, Y. L. Chang, C. Y. Chang, and P. R. Hsueh. 2007. Multidrug-resistant Acinetobacter baumannii bacteraemia: clinical features, antimicrobial therapy and outcome. Clin. Microbiol. Infect. 13:196-198. [DOI] [PubMed] [Google Scholar]
- 19.KuoLee, R., G. Harris, J. W. Conlan, and W. Chen. 2007. Oral immunization of mice with the live vaccine strain (LVS) of Francisella tularensis protects mice against respiratory challenge with virulent type A F. tularensis. Vaccine 25:3781-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lean, I. S., S. Lacroix-Lamande, F. Laurent, and V. McDonald. 2006. Role of tumor necrosis factor alpha in development of immunity against Cryptosporidium parvum infection. Infect. Immun. 74:4379-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 22.Loeffelholz, M. J., and M. C. Modrzakowski. 1988. Antimicrobial mechanisms against Acinetobacter calcoaceticus of rat polymorphonuclear leukocyte granule extract. Infect. Immun. 56:552-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks, M., T. Burns, M. Abadi, B. Seyoum, J. Thornton, E. Tuomanen, and L. A. Pirofski. 2007. Influence of neutropenia on the course of serotype 8 pneumococcal pneumonia in mice. Infect. Immun. 75:1586-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer-Scholl, A., P. Averhoff, and A. Zychlinsky. 2004. How do neutrophils and pathogens interact? Curr. Opin. Microbiol. 7:62-66. [DOI] [PubMed] [Google Scholar]
- 25.Mednick, A. J., M. Feldmesser, J. Rivera, and A. Casadevall. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 33:1744-1753. [DOI] [PubMed] [Google Scholar]
- 26.Renckens, R., J. J. Roelofs, S. Knapp, A. F. de Vos, S. Florquin, and T. van der Poll. 2006. The acute-phase response and serum amyloid A inhibit the inflammatory response to Acinetobacter baumannii pneumonia. J. Infect. Dis. 193:187-195. [DOI] [PubMed] [Google Scholar]
- 27.Sharma, M., S. Sharma, S. Roy, S. Varma, and M. Bose. 2007. Pulmonary epithelial cells are a source of interferon-gamma in response to Mycobacterium tuberculosis infection. Immunol. Cell Biol. 85:229-237. [DOI] [PubMed] [Google Scholar]
- 28.Sjostedt, A., J. W. Conlan, and R. J. North. 1994. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 62:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smit, J. J., B. D. Rudd, and N. W. Lukacs. 2006. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 203:1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swain, S. D., T. W. Wright, P. M. Degel, F. Gigliotti, and A. G. Harmsen. 2004. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect. Immun. 72:5722-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 32.Tepper, R. I., R. L. Coffman, and P. Leder. 1992. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science 257:548-551. [DOI] [PubMed] [Google Scholar]
- 33.Tvinnereim, A. R., S. E. Hamilton, and J. T. Harty. 2004. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J. Immunol. 173:1994-2002. [DOI] [PubMed] [Google Scholar]
- 34.Yuan, J. S., A. Reed, F. Chen, and C. N. Stewart, Jr. 2006. Statistical analysis of real-time PCR data. BMC Bioinformatics 7:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]