Abstract
The filarial nematode Onchocerca volvulus is the causative organism of river blindness. Our previous studies demonstrated an essential role for endosymbiotic Wolbachia bacteria in corneal disease, which is characterized by neutrophil infiltration into the corneal stroma and the development of corneal haze. To determine the role of Toll-like receptors (TLRs) in neutrophil recruitment and activation, we injected a soluble extract of O. volvulus containing Wolbachia bacteria into the corneal stromata of C57BL/6, TLR2−/−, TLR4−/−, TLR2/4−/−, and TLR9−/− mice. We found an essential role for TLR2, but not TLR4 or TLR9, in neutrophil recruitment to the cornea and development of corneal haze. Furthermore, chimeric mouse bone marrow studies showed that resident bone marrow-derived cells in the cornea can initiate this response. TLR2 expression was also essential for CXC chemokine production by resident cells in the cornea, including corneal fibroblasts, and for neutrophil activation. Taken together, these findings indicate that Wolbachia activates TLR2 on resident bone marrow-derived cells in the corneal stroma to produce CXC chemokines, leading to neutrophil recruitment to the corneal stroma, and that TLR2 mediates O. volvulus/Wolbachia-induced neutrophil activation and development of corneal haze.
Onchocerca volvulus is endemic in at least 27 sub-Saharan African countries and in Yemen (3). A recent study concluded that the incidence of onchocerciasis has been underreported, and rapid epidemiological mapping indicated that an estimated 37 million individuals are infected with this parasite and another 90 million are at risk (3). Adult males and females are present in collagenous nodules in subcutaneous tissues and release millions of first-stage larvae (microfilariae) into the skin over their 10- to 15-year life span. Microfilariae migrate throughout the skin and can penetrate ocular tissues, including the cornea, resulting in chronic sclerosing keratitis. Studies of posttreatment reactions in infected individuals indicate that most clinical manifestations occur in response to degenerating microfilariae and the release of endosymbiotic Wolbachia bacteria, as indicated by elevated Wolbachia DNA and elevated proinflammatory cytokines in the blood (12, 28, 42). Wolbachia spp. belong to the family Rickettsiaceae and, in addition to infecting insects and other arthropods, are present in the hypodermis of all larval and adult filarial stages and in the uterus of adult female worms (33, 40). Since neutrophils are abundant in untreated nodules containing Wolbachia but not in nodules from antibiotic-treated individuals (7), it is likely that Wolbachia bacteria are important in recruiting neutrophils to the tissues. Neutrophils are also evident around microfilariae in the skin of chronically infected individuals (18, 25).
Wolbachia bacteria appear to be responsible for the early stages of corneal inflammation in a murine model of river blindness, as intrastromal injection of isolated Wolbachia or filarial extracts containing Wolbachia induces neutrophil recruitment to the corneal stroma and the development of corneal haze, whereas filarial extracts injected in the absence of Wolbachia do not cause keratitis (17, 37). Overall, results of studies with animal models and infected individuals are consistent with a sequence of events in which microfilariae in the cornea die and degenerate, releasing Wolbachia bacteria and bacterial products into resident cells in the cornea, including macrophages and dendritic cells. Activation of these cells by Wolbachia results in production of chemotactic cytokines, which mediate neutrophil recruitment from peripheral vessels to the central corneal stroma. Our studies focus on the role of neutrophils in this process, as these cells are the earliest recruited to the corneal stroma and because blockade of adhesion molecules or chemokine receptors inhibits neutrophil recruitment and development of corneal abnormalities (16, 17, 19, 27).
Microbial products activate mammalian cells through a series of pathogen recognition molecules, including Toll-like receptors (TLRs). Receptor binding induces dimerization of the cytoplasmic Toll-interleukin-1 (IL-1) receptor domains of TLR molecules, followed by adaptor molecule recruitment, kinase phosphorylation, NF-κB translocation, and transcription of proinflammatory and chemotactic cytokines. We demonstrated an essential role for the common adaptor molecule myeloid differentiation factor 88 (MyD88) in Onchocerca/Wolbachia-induced keratitis (17), and we recently showed that filarial extracts containing Wolbachia activate peritoneal macrophages through TLR2, TLR6, MyD88, and MyD88 adaptor-like protein (Mal) (23). Given these findings and the possibility that Wolbachia DNA can activate TLR9, the current study utilized TLR2−/−, TLR4−/−, TLR2/4−/−, and TLR9−/− mice to examine the role of resident corneal cells and neutrophils in Onchocerca/Wolbachia-induced keratitis.
MATERIALS AND METHODS
Sources of mice.
TLR2−/−, TLR4−/−, and TLR9−/− mice were provided by S. Akira, Osaka, Japan; TLR2/4−/− mice were generated by crossing single-gene-knockout mice; and all strains were backcrossed to a C57BL/6 background. Control C57BL/6 mice and C57BL/6-TgN (ACTbEGFP; also termed eGFP-C57BL/6) mice were purchased from Jackson Laboratory (Bar Harbor, ME). C57BL/6-TgN mice were crossed to TLR2−/− mice to generate eGFP-TLR2−/− mice.
Preparation of O. volvulus extract and Wolbachia bacteria.
Subcutaneous nodules containing adult O. volvulus worms from infected individuals in the Ivory Coast were processed as described previously (23). Briefly, nodule material was removed by digestion in a collagenase, trypsin, and elastase solution (BioWhittaker, Walkersville, MD), and worms were homogenized with a glass mortar and sonicated for 15 min. Soluble extract was collected after centrifugation for 10 min at 3,000 rpm at 4°C. A Limulus lysate assay (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) was performed, and endotoxin contamination was 0.75 endotoxin unit/ml. The protein concentration of the worm extract was determined by running a bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL) according to the manufacturer's directions and was aliquotted at 1 mg/ml.
Wolbachia bacteria were isolated from filarial nematodes as described previously (24, 33). Briefly, adult male and female Onchocerca ochengi worms were homogenized in 0.001% Nonidet P-40 under sterile conditions and filtered to remove large debris, and nematode nuclei were removed by centrifugation at 350 × g for 25 min. Wolbachia bacteria were recovered after the supernatant was centrifuged at 4,100 × g at 4°C for 25 min and were quantified using PCR to amplify the single-copy Wolbachia surface protein (WSP) gene (33) and by immunofluorescence staining.
Murine model of O. volvulus/Wolbachia-induced keratitis.
The mouse model of keratitis has been described previously (17, 23). Briefly, mice were anesthetized prior to formation of a small incision by use of a 25-gauge needle. Four microliters of Hanks buffered salt solution (HBSS), O. volvulus extracts containing Wolbachia (OvAg; 4 μg), or 10,000 Wolbachia bacteria were injected into the corneal stroma by using a 30-gauge Hamilton syringe. Mice were treated in accordance with the regulations of the Association for Research in Vision and Ophthalmology.
Generation of green fluorescent protein-positive (GFP+) and TLR2 chimeric mice.
Bone marrow cells were recovered by standard methods and suspended at 1 × 106 cells/ml. Recipient mice were irradiated with 1,200 rad of whole-body radiation in two doses, at 3 hours apart, and were injected intravenously with 2 × 105 to 5 × 105 bone marrow cells as described previously (9). Chimeric mice were used after 2 weeks, which allowed time for reconstitution and a response to corneal trauma (9).
Flow cytometric analysis of corneas.
Corneas were excised using a 2-mm trephine, and residual iris material was removed. Corneas were incubated in 100 μl phosphate-buffered saline-20 mM EDTA for 30 min at 37°C and washed in phosphate-buffered saline, and the epithelial layer was removed. The corneal stroma was minced with a scalpel and incubated in 100 μl Blendzyme (Roche) for 1 h at 37°C. Cell suspensions were filtered, washed with 2 ml fluorescence-activated cell sorter (FACS) buffer (HBSS without Mg, 1% fetal bovine serum [FBS], 1 mM EDTA), incubated with 1 μg Fc block for 15 min on ice, washed and stained with the labeled antineutrophil antibody NIMP/R14 (0.5 μg) for 30 min on ice in the dark, and finally washed twice and suspended in 250 μl FACS buffer. Cell profiles were acquired on a BD LSR II flow cytometer immediately after staining. The total volume was acquired for estimation of total cell numbers. Corneal cells were gated on forward versus side scatter, and the total number of neutrophils was calculated using WinList 5.0 (Verity Software House, Inc., Topsham, ME).
Immunohistochemistry.
Eyes were snap frozen in liquid nitrogen, and 5-μm sections were fixed in 4% formaldehyde for 30 min. Sections were washed with 0.05 M Tris-buffered saline (TBS; pH 7.6) and incubated for 2 h with NIMP/R14 (1.91 μg/ml) in 1% fetal calf serum-TBS as described previously (26, 30). Sections were washed as described above and incubated with fluorescein isothiocyanate-conjugated rabbit anti-rat antibody (Vector Laboratories, Burlingame, CA) diluted 1:200 in 1% fetal calf serum-TBS for 45 min. After a wash, the slides were mounted in Vectashield (Vector Laboratories, Burlingame, CA). The number of neutrophils in the corneal stroma per section was counted at a magnification of ×400 by fluorescence microscopy.
In vivo confocal microscopy.
In vivo analysis of corneal stromal haze was evaluated using a ConfoScan3 microscope system (Nidek Technologies, Fremont, CA) as described previously (26). Briefly, anesthetized mice were immobilized on a platform, and images were captured using NAVIS software (Lucent Technologies, Murray Hill, NJ) and stored as a stack. For analysis of corneal haze, the intensity of each image was recorded in Excel and exported to Prism (GraphPad Software, CA), and the area under the curve was calculated and compared with that for naïve, completely transparent corneas.
CXC chemokine production in the cornea.
Corneas were excised using a 2-mm trephine and sonicated for 90 seconds with a 50% duty cycle in a VibraCell sonicator (Sonics and Materials, Danbury, CT), and CXC chemokines were detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's directions (R&D Systems, Minneapolis, MN). Absorption was measured at 450 nm on a VersaMax microplate reader, and concentrations were calculated using SoftMaxPro software 4.7.1 (Molecular Devices).
Corneal fibroblasts.
The MK/T-1 cell line was derived from primary mouse corneal fibroblasts that were transfected with a human telomerase transcriptase (h-TERT) (15, 32). Cells were maintained in low-glucose Dulbecco's modified Eagle's medium containing 10% FBS and 50 μg/ml hygromycin (Invitrogen, Carlsbad, CA) at 37°C with 5% CO2.
Neutrophil isolation.
Neutrophils were isolated from the peritoneal cavity as previously described (16, 32, 39). Briefly, mice were given two intraperitoneal injections of 1% casein. Three hours after the second casein injection, peritoneal cells were collected, washed, and added to a Percoll gradient solution (GE Healthcare Biosciences, Piscataway, NJ). After centrifugation at 31,500 rpm in an ultracentrifuge (Beckman-Coulter, Fullerton, CA) with a 50Ti rotor for 20 min at 4°C, the neutrophil cell layer was examined by Diff-Quik staining (VWR, Bridgeport, NJ). This preparation routinely gave >98% neutrophil purity. Cells (1 × 105 per well) were preincubated in granulocyte-macrophage colony-stimulating factor for 2 h at 37°C and overnight in OvAg (10 μg/ml). Supernatants were collected and assayed for the presence of chemokines by ELISA.
For flow cytometric analysis, isolated neutrophils were stimulated at 1 × 105/200 μl with Wolbachia for 18 h before harvest of the cells. Cells were collected in FACS buffer (HBSS without Ca or Mg, 1% FBS, 1 mM EDTA). After an Fc block for 15 min on ice, cells were stained with 1 μg L-selectin (CD62L, MEL-14, and phycoerythrin [PE]-Cy7; eBioscience) and NIMP/R14 (AF660; conjugated with a Molecular Probes conjugation kit) for 30 min on ice. Cells were washed and resuspended in 500 μl FACS buffer. Data were acquired on a BD LSR II flow cytometer with FACS DIVA as the acquisition software, and analysis was carried out using WinList. Cells were first gated on forward versus side scatter, and then NIMP/R14-positive cells were analyzed for L-selectin expression.
Statistical analysis.
The Student t test was performed for statistical analysis. P values of <0.05 were considered significant.
RESULTS
TLR2, but not TLR4, is essential for O. volvulus-induced neutrophil recruitment to the corneal stroma and development of corneal haze.
Previous studies demonstrated that neutrophils are the predominant cell type infiltrating the corneal stroma after injection of isolated Wolbachia or filarial extracts containing Wolbachia and are associated with keratitis (16, 17, 37). To determine the role of TLR2 and TLR4 in neutrophil recruitment to this site, C57BL/6, TLR2−/−, TLR4−/−, and TLR2/4−/− mice were injected intrastromally with OvAg. After 24 h, corneas were dissected, single-cell suspensions were prepared, and the number of neutrophils in the cornea was determined by flow cytometry. As shown in Fig. 1A, corneas of C57BL/6 and TLR4−/− mice injected with OvAg had a pronounced total neutrophil infiltrate, with a mean of 4,000 NIMP-positive cells, with no significant difference between these mouse strains. In contrast, neutrophil recruitment to the cornea was ablated in TLR2−/− and TLR2/4−/− mice injected with OvAg, and there was no significant difference in numbers of NIMP-positive cells between TLR2−/− and TLR2/4−/− mice. Neutrophil numbers in TLR2−/− and TLR2/4−/− mice were significantly lower (<0.001) than those in C57BL/6 and TLR4−/− mice.
FIG. 1.
O. volvulus-induced keratitis in TLR2−/−, TLR4−/−, and TLR2/4−/− mice. OvAg was injected into the corneal stromata of C57BL/6, TLR2−/−, TLR4−/−, and TLR2/4−/− mice. After 18 h, mice were sacrificed and corneas were examined as described in the text. (A) Single-cell suspensions were prepared from the corneas, and total neutrophils were detected by flow cytometry using the monoclonal antibody NIMP-R14. (B) Five-micrometer-thick corneal sections were immunostained using the same antibody, and cells were counted directly. (C) Corneas were examined by in vivo confocal microscopy. Values were exported to Excel and graphed, and the area under the curve was used to represent corneal haze. Statistically significant differences were seen between OvAg-injected C57BL/6, TLR2−/−, and TLR2/4−/− mice (P < 0.0001). Data points represent individual corneas, and the experiment is representative of four repeat experiments.
To confirm these findings and to determine if neutrophils were present in the central cornea, eyes were processed for immunohistochemistry, and the number of neutrophils per 5-μm section was determined by direct counting as described previously (17). Figure 1B shows that neutrophils in OvAg-injected C57BL/6 and TLR4−/− corneas were elevated compared with those in saline controls, whereas neutrophil numbers in the corneas of OvAg-injected TLR2−/− and TLR2/4−/− mice were significantly lower. Furthermore, those neutrophils present in TLR2−/− and TLR2/4−/− mice were only in the peripheral cornea, whereas neutrophils were distributed throughout the corneal stromata of C57BL/6 and TLR4−/− mice (data not shown).
Because neutrophil infiltration is also associated with the development of corneal haze, as measured by in vivo confocal microscopy (17), we also evaluated corneal haze in these mice. Figure 1C shows that consistent with the neutrophil numbers, C57BL/6 and TLR4−/− mice developed pronounced corneal haze compared with TLR2−/− and TLR2/4−/− mice. Taken together, these data clearly demonstrate that (i) TLR2 expression is essential for neutrophil recruitment to the corneal stroma and development of stromal haze; (ii) TLR4 has no effect on neutrophil recruitment and stromal haze; and (iii) as there were no significant differences between TLR2−/− and TLR2/4−/− mice, TLR4 does not have coreceptor activity with TLR2.
TLR2, but not TLR9, mediates keratitis induced by isolated Wolbachia bacteria.
To determine if TLR2 has a role in keratitis induced by isolated Wolbachia bacteria, corneas of C57BL/6 and TLR2−/− mice were injected with 10,000 Wolbachia bacteria isolated from O. ochengi (17) and examined for neutrophil infiltration and stromal haze after 24 h. As shown in Fig. 2A, neutrophils were recruited to the corneal stromata of C57BL/6 mice after injection of Wolbachia but not HBSS, whereas neutrophil infiltration was ablated in TLR2−/− mice. Corneal haze was also elevated in C57BL/6 mice, but not TLR2−/− mice, injected with Wolbachia. These results demonstrate an essential role for TLR2 in Wolbachia-induced neutrophil recruitment to the corneal stroma and development of corneal haze.
FIG. 2.
Wolbachia-induced keratitis in TLR2−/− and TLR9−/− mice. (A) Corneas of C57BL/6 and TLR2−/− mice were injected with 10,000 Wolbachia bacteria. After 18 h, the numbers of neutrophils in corneal sections were determined by immunohistochemistry, and corneal haze was examined by in vivo confocal microscopy. Data points represent individual corneas, and comparison between C57BL/6 and TLR2−/− mice was statistically significant for neutrophils and corneal haze (P < 0.01). (B) C57BL/6 and TLR9−/− corneas were injected with 4 μl HBSS, 10 μg/ml OvAg, or 10,000 Wolbachia bacteria. After 18 h, corneal haze was measured and the number of neutrophils per 5-μm section was determined by immunohistochemistry. Data points represent individual corneas. No statistically significant differences were noted between C57BL/6 and TLR9−/− mice for any parameter (P > 0.05).
Since TLR9 is activated by bacterial DNA and is functional in the murine cornea (26), we also examined if this receptor has a role in O. volvulus/Wolbachia-induced keratitis. C57BL/6 and TLR9−/− mice were injected intrastromally with OvAg or Wolbachia as described above, and neutrophil infiltration and corneal haze were assessed after 24 h. As shown in Fig. 2B, neutrophil recruitment to the corneal stroma and corneal haze values for TLR9−/− mice were not significantly different from those for C57BL/6 mice, indicating that TLR9 is not essential for development of O. volvulus/Wolbachia-induced keratitis.
TLR2 mediates O. volvulus/Wolbachia-induced CXC chemokine production by resident cells in the cornea.
Because TLR2, rather than TLR4 or TLR9, is essential for O. volvulus/Wolbachia-induced keratitis, we focused on understanding the role of TLR2 in the inflammatory process. To this end, we next examined the role of TLR2 in CXC chemokine production. Our previous studies showed that CXCR2 has an essential role in O. volvulus keratitis and that CXCL1/KC neutralization inhibits neutrophil recruitment to the cornea in a related murine model of keratitis (19, 32).
To determine if OvAg-induced CXC chemokine production in the cornea is regulated by TLR2, C57BL/6 and TLR2−/− mice were injected with O. volvulus or saline. After 6 h, which is the peak time of CXC chemokine production and is prior to neutrophil infiltration (19), corneas were dissected; the corneal stroma was separated from the epithelial layer after a brief incubation in EDTA, after which the stroma was sonicated and centrifuged; and chemokines in the supernatant were measured by ELISA.
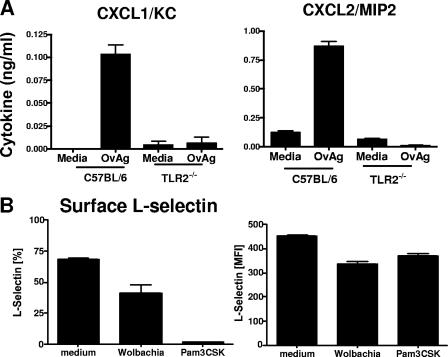
As shown in Fig. 3A and B, KC/CXCL1 and macrophage inflammatory protein 2 (MIP-2)/CXCL2 were elevated in O. volvulus-injected C57BL/6 corneas compared with control, saline-injected corneas. In contrast, KC/CXCL1 and MIP-2/CXCL2 production was ablated in similarly injected TLR2−/− corneas, thereby demonstrating an essential role for TLR2 in CXC chemokine production by resident cells in the corneal stroma.
FIG. 3.
CXC chemokine production by corneal fibroblasts and whole corneas. (A and B) Corneas from C57BL/6 and TLR2−/− mice were injected with 4 μg OvAg or HBSS (4 μl). After 6 h, corneas were dissected and sonicated, and CXCL1/KC and CXCL2/MIP-2 were measured by ELISA. (C) Corneal fibroblasts (MK-T cells) were incubated with 2, 10, or 50 μg/ml OvAg, with Wolbachia bacteria, or with 200 ng/ml Pam3Cys or LPS, and CXCL1/KC in supernatants was measured after 4 h of incubation. Results are shown as means plus standard errors of the means. The experiment was repeated twice with similar results.
The first cells that Wolbachia bacteria are likely to encounter in the corneal stroma are keratocytes, which produce the collagen and proteoglycans that comprise the stromal matrix and are by far the most numerous cell type in the corneal stroma. Under conditions of trauma, inflammation, or wound healing, keratocytes rapidly differentiate into fibroblasts, which can secrete proinflammatory and chemotactic cytokines (13, 31). To examine the role of corneal fibroblasts in CXC chemokine production, we used murine corneal fibroblasts (MK-T cells) that had been immortalized using a telomerase vector and which could produce chemokines in response to lipopolysaccharide (LPS) (15, 32). To determine if fibroblasts produce CXC chemokines after exposure to Wolbachia, MK-T cells were incubated with OvAg containing Wolbachia, with isolated Wolbachia bacteria, or with the TLR2 ligand Pam3CysK or the TLR4 ligand LPS.
As shown in Fig. 3C, corneal fibroblasts produced CXCL1/KC in a dose-dependent manner in response to OvAg and in response to isolated Wolbachia bacteria. Considering this together with the TLR2-dependent response in whole corneas, we concluded that corneal fibroblasts are a major source of CXC chemokines in response to Wolbachia.
TLR2 expression on bone marrow-derived cells mediates neutrophil recruitment.
The predominant cells in the corneal stroma are keratocytes, which produce collagen and proteoglycans that comprise the matrix. However, the corneal stroma also contains resident macrophages, dendritic cells, and other CD34+ cells originating from the bone marrow (8, 20, 38). To evaluate the role of bone marrow-derived cells in TLR2-dependent O. volvulus/Wolbachia-induced keratitis, we generated chimeric mice in which recipient C57BL/6 or TLR2−/− animals were irradiated and reconstituted with bone marrow cells from eGFP-C57BL/6 or eGFP-TLR2−/− mice. O. volvulus extracts containing Wolbachia were injected into the corneal stroma, and after 24 h, infiltration of GFP+ cells into the corneas was examined by fluorescence microscopy and quantified by image analysis, and the number of neutrophils in 5-μm corneal sections was determined as described above. We predicted that if bone marrow-derived cells are essential for initiating neutrophil infiltration, we would detect these cells in corneas of TLR2−/− mice reconstituted with C57BL/6 but not TLR2−/− bone marrow cells. Conversely, we predicted that if resident keratocytes/fibroblasts are essential for neutrophil recruitment, we would detect neutrophils in the corneal stromata of C57BL/6 mice given TLR2−/− donor bone marrow cells after OvAg injection.
Figure 4A shows GFP+ cells in naïve C57BL/6 corneas 2 weeks after reconstitution with bone marrow-derived cells, confirming previous observations that GFP+ bone marrow-derived cells can home to this site (9, 34). No differences in GFP+ cells were detected among naïve recipients of C57BL/6 versus TLR2−/− bone marrow cells (data not shown). Figure 4A (center panel) shows an increased cellular infiltrate in saline-injected corneas (trauma control); however, OvAg-injected corneas had an intense GFP+ corneal infiltrate (lower panel).
FIG. 4.
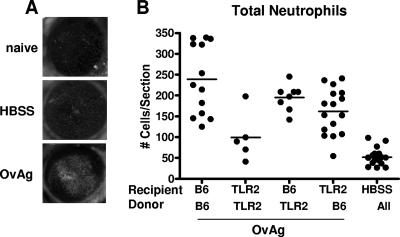
O. volvulus-induced keratitis in TLR2−/− bone marrow chimeric mice. Bone marrow cells from donor C57BL/6 EGFP+ and TLR2−/− mice were used to reconstitute sublethally irradiated recipient C57BL/6 or TLR2−/− mice. After 2 weeks, chimeric mice were injected intrastromally with OvAg or saline (HBSS) and, 18 h later, were examined by fluorescence microscopy. Corneal sections were examined for neutrophils by immunohistochemistry. (A) Representative corneas from irradiated C57BL/6 mice reconstituted with C57BL/6 EGFP+ bone marrow cells. After 2 weeks, mice were either left untreated (naïve) or examined 24 h after injection with either saline (HBSS) or OvAg. (B) Total neutrophils per corneal section. Data points represent individual corneas; these data points were combined from three repeat experiments.
To determine the role of TLR2 on bone marrow-derived cells versus resident cells in neutrophil recruitment, we assessed neutrophil numbers in bone marrow chimeras after O. volvulus injection. As shown in Fig. 4B, C57BL/6-C57BL/6 chimeras and TLR2−/−/TLR2−/− chimeras largely reflected the parent strains, as neutrophil infiltration in C57BL/6-C57BL/6 chimeras was significantly elevated over that in TLR2−/−/TLR2−/− chimeras. In contrast, neutrophil infiltration in C57BL/6-TLR2−/− chimeras and TLR2−/−-C57BL/6 chimeras was elevated compared with that in HBSS (trauma) controls and TLR2−/−/TLR2−/− chimeras; however, recruitment was not significantly different from that in C57BL/6-C57BL/6 chimeras. These findings demonstrate that TLR2 expression on resident bone marrow-derived cells or on resident corneal fibroblasts in the corneal stroma is sufficient to initiate neutrophil infiltration in response to parasite extracts containing Wolbachia.
O. volvulus/Wolbachia-induced neutrophil activation is TLR2 dependent.
Once recruited to the corneal stroma, it is likely that TLR2 expressed on neutrophils also contributes to the inflammatory response by contributing to neutrophil infiltration. Our previous studies showed that neutrophils ingest Wolbachia bacterium cells in the corneal stroma and that neutrophils produce CXCL1 and CXCL2 after stimulation with Wolbachia (16, 17, 35).
To determine if TLR2 expression on neutrophils mediates neutrophil activation and CXC chemokine production in response to O. volvulus or Wolbachia, neutrophils were isolated from the peritoneal cavities of C57BL/6 and TLR2−/− mice and incubated with OvAg, and chemokine production was measured by ELISA (16, 17). L-selectin shedding, which is a marker of TLR-induced neutrophil activation (22, 36), was examined by flow cytometry.
As shown in Fig. 5A, O. volvulus extracts containing Wolbachia stimulated KC/CXCL1 and MIP-2/CXCL2 production by C57BL/6 neutrophils but not by TLR2−/− neutrophils, thereby demonstrating an essential role for TLR2 in O. volvulus/Wolbachia-induced chemokine production by neutrophils. As an additional marker of neutrophil activation, we examined L-selectin shedding, reflected as decreased surface expression detected by flow cytometry. As shown in Fig. 5B, the percentage of neutrophils expressing surface L-selectin was significantly reduced after incubation with Wolbachia or the TLR2 agonist Pam3Cys. Similarly, the mean fluorescence intensity of surface L-selectin was significantly reduced by incubation with Wolbachia or Pam3Cys. Expression of CD11b was not affected under similar conditions (data not shown). Together with TLR2-dependent chemokine production, these findings indicate that neutrophils are activated in response to Wolbachia and that TLR2 is an important mediator of neutrophil activation.
FIG. 5.
CXC chemokine production and L-selectin expression on peritoneal neutrophils. Neutrophils were purified to 98% purity from peritoneal lavage cells and stimulated for 6 h with 10 μg/ml OvAg or culture medium alone. (A) CXCL1/KC and CXCL2/MIP-2 in the culture supernatants were measured by ELISA (P < 0.001 between OvAg-stimulated neutrophils from C57BL/6 versus TLR2−/− mice). (B) Surface L-selectin expression was measured by flow cytometry of gated populations of NIMP-R14+ cells to determine the percentage of positive neutrophils and the mean fluorescence intensity (MFI). Data are means plus standard errors of the means for triplicate wells and are representative of three repeat experiments (P < 0.01 between medium control and Wolbachia- or Pam3Cys-treated cells.).
DISCUSSION
Results from the current study add to our understanding of the pathogenesis of O. volvulus/Wolbachia-induced keratitis by demonstrating that TLR2 is the essential receptor for neutrophil recruitment to the corneal stroma and for development of corneal haze and that TLR2 is effective on at least two levels: (i) the production of CXC chemokines by resident cells in the corneal stroma and (ii) the activation of neutrophils in the corneal stroma. Furthermore, we found that TLR2 expression on resident bone marrow-derived cells and on resident nonmyeloid cells is essential for these events to occur. Since bone marrow-derived cells such as macrophages and dendritic cells populate the normal cornea (8, 21, 34, 38), we concluded that these cells initiate the response to Wolbachia.
TLR2 signaling requires the formation of a heterodimer with either TLR1 or TLR6 (1, 2). Recruitment of adaptor molecules to the Toll-IL-1 receptor region of the heterodimer initiates a series of kinase phosphorylation steps, followed by NF-κB translocation to the nucleus and expression of proinflammatory and chemotactic cytokines (1, 2). Our recent study showed that TLR6 is a coreceptor for Wolbachia responses in macrophages and that cytokine responses to Wolbachia require the adaptor molecules MyD88 and Mal, and we found no role for MyD88-independent adaptor molecules (23). We also demonstrated a requirement for MyD88 in Wolbachia-induced keratitis (17) and showed that TLR2 is important in the development of Th1-associated, but not Th2-associated, responses (11). The role of TLR2 and MyD88 is also consistent with macrophage tolerance in a murine model of lymphatic filariasis (43). These results and our previous studies are consistent with the following sequence of events in O. volvulus/Wolbachia-induced keratitis: (i) TLR2/MyD88-dependent CXC chemokine production by corneal fibroblasts and bone marrow-derived cells in the corneal stroma; (ii) recruitment of neutrophils from peripheral vessels to the corneal stroma and their migration through the corneal stroma; (iii) TLR2/MyD88 activation of neutrophils in the corneal stroma, resulting in further production of CXC chemokines and exacerbated neutrophil infiltration; and (iv) disruption of normal corneal clarity and development of stromal haze.
The results of the current study also demonstrate that TLR4 and TLR9 are not required for O. volvulus/Wolbachia-induced keratitis. Furthermore, there were no differences in neutrophil recruitment or corneal haze between TLR2−/− and TLR2/4−/− mice, indicating that TLR4 does not have a coreceptor role in neutrophil recruitment and development of stromal haze. Although we and others previously implicated TLR4 as an important receptor (6, 29, 37, 41), the current study used TLR4−/− mice on a C57BL/6 background rather than LPS-hyporesponsive C3H/HeJ mice, and we used more stringently prepared filarial extracts. The absence of TLR4 responsiveness is also consistent with the absence of the enzymes associated with LPS biosynthesis in the Brugia malayi/Wolbachia genomes (14, 44).
Given that TLR2-Wolbachia interactions appear to be the stimulus for host cell responses and development of corneal inflammation, it will be interesting to identify Wolbachia ligands. To date, the only reported Wolbachia protein with TLR2 activity is WSP, which is abundantly expressed in insect and filarial Wolbachia bacteria and is conserved among filarial Wolbachia (6, 10). Brattig and colleagues reported that recombinant WSP activates dendritic cells and macrophages through TLR2 (6), and we reported that WSP is readily detected in the cornea after injection of microfilariae (16). Moreover, WSP induces IL-8 production in canine peripheral blood neutrophils (5) and also inhibits apoptosis of human neutrophils (4). Future studies will examine the role of WSP and other putative ligands in O. volvulus/Wolbachia-induced keratitis.
Acknowledgments
This work was supported by NIH grants EY10320 (E.P.), EY11373 (E.P.), AI-07024 (I.G.-F.), and K08 AI054652 (A.G.H.) and by grant DA1024/1-1 from the German Research Foundation (K.D.). These studies were also funded by the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation. M.T. thanks the Wellcome Trust for senior fellowship support and the EC (ICA4-CT2002-10051).
We thank Michael Harling for expert technical assistance. TLR2−/−, TLR4−/−, and TLR9−/− mice were generously provided by S. Akira, Osaka, Japan.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 17 September 2007.
REFERENCES
- 1.Akira, S. 2006. TLR signaling. Curr. Top. Microbiol. Immunol. 311:1-16. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Basanez, M. G., K. Razali, A. Renz, and D. Kelly. 2007. Density-dependent host choice by disease vectors: epidemiological implications of the ideal free distribution. Trans. R. Soc. Trop. Med. Hyg. 101:256-269. [DOI] [PubMed] [Google Scholar]
- 4.Bazzocchi, C., S. Comazzi, R. Santoni, C. Bandi, C. Genchi, and M. Mortarino. 2007. Wolbachia surface protein (WSP) inhibits apoptosis in human neutrophils. Parasite Immunol. 29:73-79. [DOI] [PubMed] [Google Scholar]
- 5.Bazzocchi, C., C. Genchi, S. Paltrinieri, C. Lecchi, M. Mortarino, and C. Bandi. 2003. Immunological role of the endosymbionts of Dirofilaria immitis: the Wolbachia surface protein activates canine neutrophils with production of IL-8. Vet. Parasitol. 117:73-83. [DOI] [PubMed] [Google Scholar]
- 6.Brattig, N. W., C. Bazzocchi, C. J. Kirschning, N. Reiling, D. W. Buttner, F. Ceciliani, F. Geisinger, H. Hochrein, M. Ernst, H. Wagner, C. Bandi, and A. Hoerauf. 2004. The major surface protein of Wolbachia endosymbionts in filarial nematodes elicits immune responses through TLR2 and TLR4. J. Immunol. 173:437-445. [DOI] [PubMed] [Google Scholar]
- 7.Brattig, N. W., D. W. Buttner, and A. Hoerauf. 2001. Neutrophil accumulation around Onchocerca worms and chemotaxis of neutrophils are dependent on Wolbachia endobacteria. Microbes Infect. 3:439-446. [DOI] [PubMed] [Google Scholar]
- 8.Brissette-Storkus, C. S., S. M. Reynolds, A. J. Lepisto, and R. L. Hendricks. 2002. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 43:2264-2271. [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson, E. C., J. Drazba, X. Yang, and V. L. Perez. 2006. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Investig. Ophthalmol. Vis. Sci. 47:241-248. [DOI] [PubMed] [Google Scholar]
- 10.Casiraghi, M., O. Bain, R. Guerrero, C. Martin, V. Pocacqua, S. L. Gardner, A. Franceschi, and C. Bandi. 2004. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: evidence for symbiont loss during evolution. Int. J. Parasitol. 34:191-203. [DOI] [PubMed] [Google Scholar]
- 11.Daehnel, K., I. Gillette-Ferguson, A. G. Hise, E. Diaconu, M. J. Harling, F. P. Heinzel, and E. Pearlman. 2007. Filaria/Wolbachia activation of dendritic cells and development of Th1-associated responses is dependent on Toll-like receptor 2 in a mouse model of ocular onchocerciasis (river blindness). Parasite Immunol. 29:455-465. [DOI] [PubMed] [Google Scholar]
- 12.Debrah, A. Y., S. Mand, S. Specht, Y. Marfo-Debrekyei, L. Batsa, K. Pfarr, J. Larbi, B. Lawson, M. Taylor, O. Adjei, and A. Hoerauf. 2006. Doxycycline reduces plasma VEGF-C/sVEGFR-3 and improves pathology in lymphatic filariasis. PLoS Pathog. 2:e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillmore, R. A., S. E. Nelson, R. N. Lausch, and J. E. Oakes. 2003. Differential regulation of ENA-78 and GCP-2 gene expression in human corneal keratocytes and epithelial cells. Investig. Ophthalmol. Vis. Sci. 44:3432-3437. [DOI] [PubMed] [Google Scholar]
- 14.Foster, J., M. Ganatra, I. Kamal, J. Ware, K. Makarova, N. Ivanova, A. Bhattacharyya, V. Kapatral, S. Kumar, J. Posfai, T. Vincze, J. Ingram, L. Moran, A. Lapidus, M. Omelchenko, N. Kyrpides, E. Ghedin, S. Wang, E. Goltsman, V. Joukov, O. Ostrovskaya, K. Tsukerman, M. Mazur, D. Comb, E. Koonin, and B. Slatko. 2005. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 3:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gendron, R. L., C. Y. Liu, H. Paradis, L. C. Adams, and W. W. Kao. 2001. MK/T-1, an immortalized fibroblast cell line derived using cultures of mouse corneal stroma. Mol. Vis. 7:107-113. [PubMed] [Google Scholar]
- 16.Gillette-Ferguson, I., A. G. Hise, H. F. McGarry, J. Turner, A. Esposito, Y. Sun, E. Diaconu, M. J. Taylor, and E. Pearlman. 2004. Wolbachia-induced neutrophil activation in a mouse model of ocular onchocerciasis (river blindness). Infect. Immun. 72:5687-5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillette-Ferguson, I., A. G. Hise, Y. Sun, E. Diaconu, H. F. McGarry, M. J. Taylor, and E. Pearlman. 2006. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect. Immun. 74:2442-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutierrez-Pena, E. J., J. Knab, and D. W. Buttner. 1996. Neutrophil granule proteins: evidence for the participation in the host reaction to skin microfilariae of Onchocerca volvulus after diethylcarbamazine administration. Parasitology 113:403-414. [DOI] [PubMed] [Google Scholar]
- 19.Hall, L. R., E. Diaconu, R. Patel, and E. Pearlman. 2001. CXC chemokine receptor 2 but not C-C chemokine receptor 1 expression is essential for neutrophil recruitment to the cornea in helminth-mediated keratitis (river blindness). J. Immunol. 166:4035-4041. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah, P., S. O. Huq, Y. Liu, Q. Zhang, and M. R. Dana. 2003. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 74:172-178. [DOI] [PubMed] [Google Scholar]
- 21.Hamrah, P., Y. Liu, Q. Zhang, and M. R. Dana. 2003. The corneal stroma is endowed with a significant number of resident dendritic cells. Investig. Ophthalmol. Vis. Sci. 44:581-589. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi, F., T. K. Means, and A. D. Luster. 2003. Toll-like receptors stimulate human neutrophil function. Blood 102:2660-2669. [DOI] [PubMed] [Google Scholar]
- 23.Hise, A. G., K. Daehnel, I. Gillette-Ferguson, E. Cho, H. F. McGarry, M. J. Taylor, D. T. Golenbock, K. A. Fitzgerald, J. W. Kazura, and E. Pearlman. 2007. Innate immune responses to endosymbiotic Wolbachia bacteria in Brugia malayi and Onchocerca volvulus are dependent on TLR2, TLR6, MyD88, and Mal, but not TLR4, TRIF, or TRAM. J. Immunol. 178:1068-1076. [DOI] [PubMed] [Google Scholar]
- 24.Hise, A. G., I. Gillette-Ferguson, and E. Pearlman. 2004. The role of endosymbiotic Wolbachia bacteria in filarial disease. Cell. Microbiol. 6:97-104. [DOI] [PubMed] [Google Scholar]
- 25.Hoerauf, A., D. W. Buttner, O. Adjei, and E. Pearlman. 2003. Onchocerciasis. BMJ 326:207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, A. C., F. P. Heinzel, E. Diaconu, Y. Sun, A. G. Hise, D. Golenbock, J. H. Lass, and E. Pearlman. 2005. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Investig. Ophthalmol. Vis. Sci. 46:589-595. [DOI] [PubMed] [Google Scholar]
- 27.Kaifi, J. T., E. Diaconu, and E. Pearlman. 2001. Distinct roles for PECAM-1, ICAM-1, and VCAM-1 in recruitment of neutrophils and eosinophils to the cornea in ocular onchocerciasis (river blindness). J. Immunol. 166:6795-6801. [DOI] [PubMed] [Google Scholar]
- 28.Keiser, P. B., S. M. Reynolds, K. Awadzi, E. A. Ottesen, M. J. Taylor, and T. B. Nutman. 2002. Bacterial endosymbionts of Onchocerca volvulus in the pathogenesis of posttreatment reactions. J. Infect. Dis. 185:805-811. [DOI] [PubMed] [Google Scholar]
- 29.Kerepesi, L. A., O. Leon, S. Lustigman, and D. Abraham. 2005. Protective immunity to the larval stages of Onchocerca volvulus is dependent on Toll-like receptor 4. Infect. Immun. 73:8291-8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khatri, S., J. H. Lass, F. P. Heinzel, W. M. Petroll, J. Gomez, E. Diaconu, C. M. Kalsow, and E. Pearlman. 2002. Regulation of endotoxin-induced keratitis by PECAM-1, MIP-2, and Toll-like receptor 4. Investig. Ophthalmol. Vis. Sci. 43:2278-2284. [PubMed] [Google Scholar]
- 31.Kumagai, N., K. Fukuda, Y. Fujitsu, Y. Lu, N. Chikamoto, and T. Nishida. 2005. Lipopolysaccharide-induced expression of intercellular adhesion molecule-1 and chemokines in cultured human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 46:114-120. [DOI] [PubMed] [Google Scholar]
- 32.Lin, M., E. Carlson, E. Diaconu, and E. Pearlman. 2007. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J. Leukoc. Biol. 81:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGarry, H. F., G. L. Egerton, and M. J. Taylor. 2004. Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Mol. Biochem. Parasitol. 135:57-67. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura, T., F. Ishikawa, K. H. Sonoda, T. Hisatomi, H. Qiao, J. Yamada, M. Fukata, T. Ishibashi, M. Harada, and S. Kinoshita. 2005. Characterization and distribution of bone marrow-derived cells in mouse cornea. Investig. Ophthalmol. Vis. Sci. 46:497-503. [DOI] [PubMed] [Google Scholar]
- 35.Pearlman, E., and I. Gillette-Ferguson. 2007. Onchocerca volvulus, Wolbachia and river blindness. Chem. Immunol. Allergy 92:254-265. [DOI] [PubMed] [Google Scholar]
- 36.Sabroe, I., E. C. Jones, L. R. Usher, M. K. Whyte, and S. K. Dower. 2002. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol. 168:4701-4710. [DOI] [PubMed] [Google Scholar]
- 37.Saint Andre, A., N. M. Blackwell, L. R. Hall, A. Hoerauf, N. W. Brattig, L. Volkmann, M. J. Taylor, L. Ford, A. G. Hise, J. H. Lass, E. Diaconu, and E. Pearlman. 2002. The role of endosymbiotic Wolbachia bacteria in the pathogenesis of river blindness. Science 295:1892-1895. [DOI] [PubMed] [Google Scholar]
- 38.Sosnova, M., M. Bradl, and J. V. Forrester. 2005. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells 23:507-515. [DOI] [PubMed] [Google Scholar]
- 39.Sun, Y., A. G. Hise, C. M. Kalsow, and E. Pearlman. 2006. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect. Immun. 74:5325-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor, M. J., C. Bandi, and A. Hoerauf. 2005. Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 60:245-284. [DOI] [PubMed] [Google Scholar]
- 41.Taylor, M. J., H. F. Cross, and K. Bilo. 2000. Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor, M. J., W. H. Makunde, H. F. McGarry, J. D. Turner, S. Mand, and A. Hoerauf. 2005. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: a double-blind, randomised placebo-controlled trial. Lancet 365:2116-2121. [DOI] [PubMed] [Google Scholar]
- 43.Turner, J. D., R. S. Langley, K. L. Johnston, G. Egerton, S. Wanji, and M. J. Taylor. 2006. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 177:1240-1249. [DOI] [PubMed] [Google Scholar]
- 44.Wu, M., L. V. Sun, J. Vamathevan, M. Riegler, R. Deboy, J. C. Brownlie, E. A. McGraw, W. Martin, C. Esser, N. Ahmadinejad, C. Wiegand, R. Madupu, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, J. F. Kolonay, W. C. Nelson, Y. Mohamoud, P. Lee, K. Berry, M. B. Young, T. Utterback, J. Weidman, W. C. Nierman, I. T. Paulsen, K. E. Nelson, H. Tettelin, S. L. O'Neill, and J. A. Eisen. 2004. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2:E69. [DOI] [PMC free article] [PubMed] [Google Scholar]