Abstract
Chemokines play an important role in inflammation and infection due to their ability to recruit cells of innate and adaptive immunity. Here we examined mouse macrophage chemokine responses during intracellular infections with high- and low-virulence Toxoplasma gondii strains. The high-virulence type I strain RH induced a large panel of CC-type chemokines, whereas responses elicited by strains PTG (type II) and M7741 (type III) were much weaker. Strikingly, the T. gondii-induced chemokine response occurred independently of signaling through the Toll-like receptor adaptor MyD88. Instead, production of chemokines during infection was heavily dependent upon phosphoinositide-3-kinase signaling pathways. Because infection with type I strains such as RH results in an uncontrolled proinflammatory cytokine response, we hypothesize that this virulence phenotype is a consequence of early strong induction of chemokines by type I, but not type II or III, Toxoplasma strains.
The intracellular apicomplexan parasite Toxoplasma gondii is a major opportunistic pathogen in immunocompromised populations worldwide and a cause of abortion in humans and animals (16, 24). During acute infection, the parasite invades a wide variety of cell types, including innate immune cells such as macrophages and dendritic cells (10, 33). Entry into host cells involves parasite actin-based motility and regulated discharge of apical organelles (40, 52). Inside the cell, the parasite establishes a nonfusogenic parasitophorous vacuole that remains segregated from host endosomal and lysosomal pathways (8). In most cases, the parasite progresses to establish a persistent, asymptomatic infection associated with formation of quiescent cysts in tissues of the skeletal muscles and central nervous system.
Toxoplasma is known for its ability to trigger vigorous interleukin-12 (IL-12) responses by innate immune cells, including macrophages, dendritic cells, and neutrophils (51). In turn, this results in strong cell-mediated immunity that is associated with gamma interferon (IFN-γ)-producing CD4+ Th1 and CD8+ T-cell effectors (15). In animals lacking IL-12 or IFN-γ, Toxoplasma causes a lethal infection that is marked by uncontrolled parasite replication and widespread dissemination (50, 59). Nevertheless, these cytokines must be tightly regulated through the action of mediators such as IL-10 to avoid inflammatory pathology that can itself be lethal to the host (20, 56). Thus, the ability to establish long-term asymptomatic infection in which both the host and parasite coexist is dependent upon an appropriate balance between cytokines with opposing functions.
In Europe and North America, the population structure of Toxoplasma is dominated by three lineages, designated strain types I, II, and III (12, 22, 53, 54). These strain types appear to have emerged by rapid clonal expansion from a limited number of crosses between ancestral strains occurring approximately 10,000 years ago (7, 55). The present-day strain types possess differing virulence phenotypes. Strains of type I are uniformly lethal in mice prior to establishment of cyst-associated persistence, whereas type II and III strains are less virulent and can establish chronic infections. There is evidence that type I parasites also cause more severe disease in humans (6, 17, 21, 26). Recently, parasite injection of polymorphic rhoptry kinases has been linked to the virulence properties of Toxoplasma (47, 57).
Parasite strain type exerts an important influence on the immune response to T. gondii infection. The prototypic type I strain, RH, causes overproduction of proinflammatory cytokines and widespread apoptosis that is likely to contribute to death during acute infection (18, 39). In contrast, the less virulent type II strain ME49 induces lower levels of cytokines such as IL-12 and IFN-γ in vivo, and infection is associated with progression to a chronic stage. Paradoxically, the ME49 strain induces larger amounts of IL-12 than does RH during in vitro infection of murine bone marrow-derived macrophages (44). At least in part, this appears to be because ME49 engages MyD88 signaling pathways, leading to IL-12 induction during macrophage infection, whereas the RH strain relies on MyD88-independent signaling to elicit low-level macrophage IL-12 production (31).
Because of the importance of chemokines in the initiation of immunity to infection, we compared Toxoplasma strains RH (type I), PTG (type II; derived from ME49), and M7741 (type III) for their relative abilities to trigger expression of this class of molecules and their receptors. Strikingly, RH infection induced a vigorous and long-lasting chemokine response, in contrast to the type II and type III strains. In bone marrow-derived macrophages, parasite triggering of the chemokine response did not depend on the Toll-like receptor (TLR) adaptor molecule MyD88 but, instead, required intact Gi protein and phosphoinositide-3-kinase (PI 3-kinase) signaling pathways. The ability of RH to trigger rapid, strong, and long-lasting proinflammatory chemokine responses may explain the ability of this parasite strain to cause cytokine overproduction that leads to pathology and death in infected animals.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice between 6 and 8 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). MyD88−/− mice on a partially backcrossed 129/Ola × C57BL/6 background were originally constructed by S. Akira (Osaka University, Osaka, Japan) and were provided by E. Pearlman (Case Western Reserve University). The animals were bred as heterozygous crosses, and F1−/− progeny were identified by reverse transcription-PCR. F1+/+ littermates were used as controls in these experiments. Female mice between 5 and 12 weeks of age were used for experiments. All animals were housed under specific-pathogen-free conditions at the Cornell University College of Veterinary Medicine animal facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Care.
Parasites.
Tachyzoites of the virulent T. gondii RH strain (ATCC 50174; American Type Culture Collection, Manassas, VA) served as the representative type I strain. The low-virulence strains PTG (ATCC 50841) and M7741 (ATCC 50859) served as representative type II and III strains, respectively. In addition, type I strains MOR and ENT and type II strains CC and DEG (all from ATCC) were used in some studies. Parasites were maintained by biweekly passage on human foreskin fibroblast medium consisting of Dulbecco's modified Eagle's medium (Mediatech Inc., Herndon, VA), 1% heat-inactivated bovine growth serum (HyClone, Logan, UT), penicillin (100 U/ml; Invitrogen Life Technologies, Grand Island, NY), and streptomycin (0.1 mg/ml; Invitrogen). Parasite cultures were tested every 4 to 6 weeks for Mycoplasma contamination, using a commercial PCR-enzyme-linked immunosorbent assay (ELISA)-based kit (Roche Applied Science, Mannheim, Germany). Tachyzoites were filtered prior to use to remove host cell debris (5-μm pore size; Corning Life Sciences, Acton, MA).
Bone marrow-derived macrophage preparation.
Macrophages were prepared from flushed femur and tibia bone marrow cells and generated as previously described (34). On day 5, nonadherent cells were discarded, and adherent cells were washed and resuspended in medium consisting of Dulbecco's modified Eagle's medium (Mediatech), 10% heat-inactivated bovine growth serum (HyClone), 0.1 mM nonessential amino acids (HyClone), 1 mM sodium pyruvate, 1 mM HEPES, penicillin (100 U/ml), and streptomycin (0.1 mg/ml).
In vivo experimental setup.
Mice were inoculated intraperitoneally (i.p.) with 1.0 × 106 RH, PTG, or M7741 tachyzoites. Peritoneal exudate cells were collected by lavage with ice-cold phosphate-buffered saline (PBS) for total RNA extraction at 6 and 18 h postinfection.
In vitro experimental setup.
Macrophages were allowed to adhere at 3.0 × 106 cells per well in six-well tissue culture plates (Falcon, Franklin Lakes, NJ) for 2 h. Each independent experiment consisted of a medium control and cells infected with RH, PTG, or M7741 tachyzoites (6:1 ratio of parasites to cells) for 6 and 18 h. In some cases, cells were stimulated with ultrapure Salmonella enterica serovar Minnesota lipopolysaccharide (LPS; List Biological Laboratories, Campbell, CA) at 100 ng/ml for 6 h. In some experiments, cells were preincubated for 2 h with 50 ng/ml of pertussis toxin or 50 ng/ml of wortmannin (both purchased from Calbiochem, La Jolla, CA) prior to infection with parasites for 6 h. Plates containing cells and parasites were briefly centrifuged to synchronize tachyzoite and macrophage contact. At the termination of the experiments, cells were collected for total RNA extraction.
Gene array analysis.
Gene array analysis was performed as previously described (34). Briefly, total RNA was isolated using RNeasy mini kits (Qiagen, Valencia, CA). A commercial pathway-focused oligonucleotide array (OMM-022; mouse chemokine and receptor microarray) was obtained from SuperArray Bioscience Corp. (Frederick, MD), and analyses were performed using a chemiluminescence-based detection system following the manufacturer's recommendation. Images were captured on X-ray film (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom), and the data sets were scanned and analyzed using ScanAlyze and Microsoft Excel software, respectively. Background adjustment was performed by subtracting the lowest measured value on the membrane from the values for all genes. Data were normalized against the positive control glyceraldehyde-3-phosphate dehydrogenase housekeeping gene to obtain the processed data sets. Changes were calculated as normalized ratios of the experimental processed data sets to the medium or PBS control processed data sets. Thresholds were set to select genes whose values were upregulated >3 standard deviations from the mean value for all genes.
Immunoblotting.
Immunoblotting was performed as previously described (30). Briefly, macrophages at 2 × 106 cells per well (24-well plate) were lysed with reducing sodium dodecyl sulfate sample buffer and sheared three times through a 27-gauge needle. Samples were boiled (5 min) and briefly centrifuged (18,000 × g) prior to being separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose membranes (Whatman GmbH, Dassel, Germany). The membranes were blocked in 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO) in Tris-buffered saline (TBST; pH 7.6) containing 5% nonfat dry milk (Nestle USA, Solon, OH) at room temperature for 1 h. Membranes were then incubated with an antibody (Ab) specific for phospho-Tyr705 STAT3 (Cell Signaling Technology, Beverly, MA) overnight at 4°C in TBST containing 5% bovine serum albumin. After washing of the membranes, Ab binding was detected with a horseradish peroxidase-conjugated secondary Ab (Cell Signaling Technology) in combination with enhanced chemiluminescence fluorescence (Lumi-GLO; Cell Signaling Technology). A specific horseradish peroxidase-conjugated anti-actin Ab (Santa Cruz Biotechnology, Santa Cruz, CA) was used to confirm an equivalent protein concentration in each sample.
Cytokine ELISA.
Levels of IL-12p40 were measured as described previously (31). A commercially obtained Ab pair was used to measure CCL17 according to the manufacturer's directions (R&D Systems, Minneapolis, MN).
RESULTS
Type I T. gondii strain RH induces high levels of chemokine CCL17/TARC in bone marrow-derived macrophages.
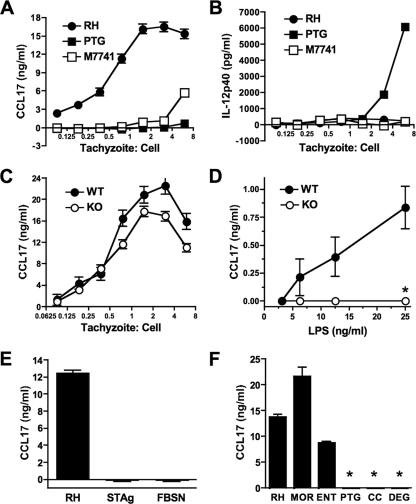
Chemokines and chemokine receptors are key to orchestrating cell recruitment and initiating immunity during in vivo infection. Thymus and activated regulated chemokine (TARC)/CCL17 is involved in recruitment of activated and memory T cells (11, 36). We found a strong parasite strain-specific dependence of induction of this chemokine during Toxoplasma infection of bone marrow-derived macrophages. In particular, RH (type I strain) induced a robust CCL17 response, whereas PTG (type II) and M7741 (type III) elicited little or no production of this chemokine (Fig. 1A). In sharp contrast, infection with PTG induced strong IL-12p40 production, whereas RH and M7741 induced minimal amounts of this cytokine (Fig. 1B). T. gondii strain-specific IL-12 production during macrophage infection has been reported previously (31, 44), but strain-specific CCL17 production is a novel finding. Because of the importance of the TLR-MyD88 signaling pathway in innate immune recognition of microbes, including Toxoplasma (14, 19, 38, 41, 49, 60), we assessed the involvement of the TLR adaptor MyD88 in parasite-induced CCL17 production. As shown in Fig. 1C, the absence of MyD88 had little effect on RH-induced CCL17 production. The slightly lower levels of CCL17 produced by MyD88 null macrophages shown in Fig. 1C were not reproduced in repeat experiments. In contrast, LPS induced a weaker CCL17 response that was dependent upon MyD88 (Fig. 1D). Toxoplasma-induced CCL17 production required intact parasites, since a soluble tachyzoite lysate, as well as filtered supernatants from a lysed culture of infected fibroblasts, elicited no CCL17 (Fig. 1E). Therefore, MyD88-independent induction of CCL17 is likely to require active infection rather than the release of soluble molecules from either tachyzoites themselves or infected host cells. In Fig. 1F, we show that two additional type I strains, MOR and ENT, induced levels of CCL17 similar to those induced by RH. Furthermore, two additional type II strains, CC and DEG, did not induce detectable amounts of this chemokine.
FIG. 1.
Infection with Toxoplasma type I (RH), but not type II (PTG) or III (M7741), tachyzoites induces MyD88-independent CCL17 production. Bone marrow-derived macrophages were incubated for 18 h with RH, PTG, and M7741, and supernatants were collected for analysis of CCL17 (A) and IL-12p40 (B). (C) Wild-type (WT) and MyD88 knockout (KO) mice were infected with strain RH tachyzoites, and CCL17 levels were assessed 18 h later. (D) Wild-type and knockout cells stimulated with LPS. (E) Wild-type macrophages were incubated with live RH strain parasites (3:1 ratio of tachyzoites to cells), soluble tachyzoite lysate (STAg; 40 μg/ml), or cell-free supernatant from a culture of infected fibroblasts containing egressed parasites (FBSN; diluted 1:1). (F) Ability of RH, MOR, and ENT (type I strains) and PTG, CC, and DEG (type II strains) to induce CCL17 production. This experiment employed a 1.5:1 ratio of tachyzoites to cells. *, <60 pg/ml. The experiments were repeated two to four times with similar results.
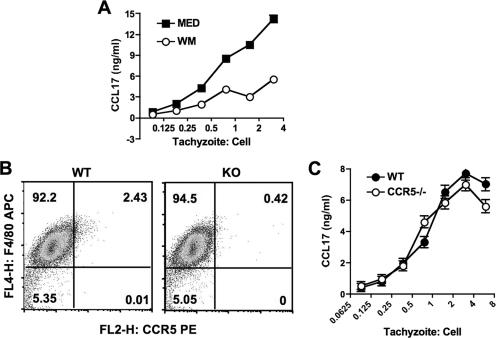
We recently reported that infection with T. gondii induces PI 3-kinase-dependent signaling in infected macrophages (32). As shown in Fig. 2A, we assessed CCL17 production in the presence of wortmannin, a potent and specific inhibitor of PI 3-kinase activity (58). As shown in the figure, inhibition of PI 3-kinase signaling substantially blocked the release of CCL17. Chemokine receptor-mediated signaling involves PI 3-kinase, and CCR5 is implicated in recognition of T. gondii (1). Accordingly, we examined the involvement of this chemokine receptor in CCL17 production. As shown in Fig. 2B, wild-type macrophages expressed low but detectable levels of CCR5 compared to CCR5−/− cells. During infection with RH tachyzoites, wild-type and knockout macrophages produced equivalent amounts of CCL17, arguing against involvement of CCR5 (Fig. 2C).
FIG. 2.
Macrophage CCL17 response during Toxoplasma RH infection depends upon PI 3-kinase signaling. (A) Bone marrow-derived macrophages were preincubated for 60 min with wortmannin (50 ng/ml) and then infected with RH strain parasites. Supernatants were collected for ELISA 18 h later. (B) CCR5 expression in wild-type (WT) and CCR5 knockout (KO) macrophages. The mean fluorescence intensities were 8.1 and 7.3 for wild-type and knockout macrophages, respectively. (C) RH-induced CCL17 production in wild-type and CCR5 knockout macrophages. These experiments were repeated twice with the same results.
Infection of macrophages with type I T. gondii elicits a panel of MyD88-independent CC motif chemokines.
We employed a pathway-specific microarray gene profiling system to assess the expression of 114 genes related to chemokines during infection with the three Toxoplasma strain types. The complete gene listing can be found on the manufacturer's website (www.superarray.com; OMM-022). The CC and CXC motif chemokines and their receptors are included in the array. Other genes important in regulating chemokines as well as their receptors are also contained in this array.
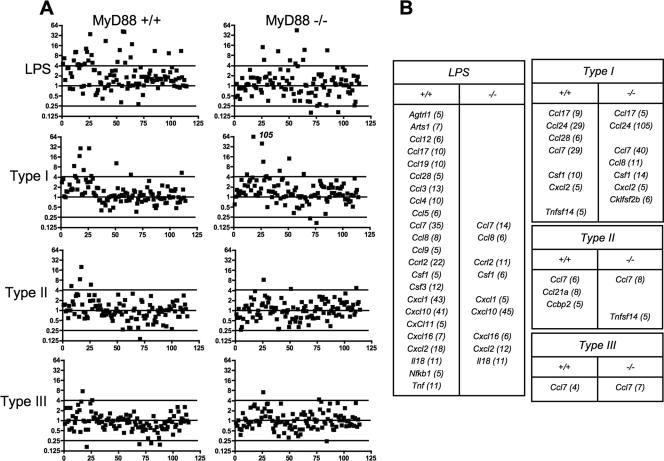
We examined responses in wild-type and MyD88 knockout macrophages, comparing upregulation of chemokines and related genes during infection with the three strain types relative to expression in uninfected cells. In this analysis, the standard deviation for the data set with the least amount of variation was calculated (type III strain infection of MyD88−/− cells). We then considered any value of >3 standard deviations from a value of 1 (no change relative to noninfected cells) as showing significant upregulation. At 6 h postexposure, LPS induced a broad spectrum of chemokines and related genes (Fig. 3 and data not shown). Of 23 genes upregulated by LPS, 9 were upregulated in the absence of MyD88 signaling. This finding is consistent with a recent report that found that an unexpectedly large proportion of LPS-responsive genes were upregulated independently of this TLR adaptor molecule in a genome-wide screen (4).
FIG. 3.
Chemokine response patterns during early T. gondii infection or LPS stimulation of wild-type and MyD88 knockout macrophages. Bone marrow-derived macrophages were stimulated with LPS (100 ng/ml) or infected with type I (RH), type II (PTG), or type III (M7741) tachyzoites, and samples were collected at 6 h postinfection for analysis. A 6:1 ratio of parasites to cells was used, yielding >80% infection. (A) Response patterns of 114 chemokine, chemokine receptor, and related genes. Values are expressed relative to those for nonstimulated cells. The horizontal line defines 3 standard deviations from the mean for all genes for the sample showing the least amount of change (type III infection of MyD88−/− macrophages). (B) Identities of genes upregulated by parasites and LPS. Numbers in parentheses indicate x-fold increases relative to levels in nonstimulated cells.
Type I (RH) infection induced a response at 6 h that consisted of upregulation of Ccl7, Ccl17, Ccl24, and Ccl28 as well as Csf1 and Tnfsf14 (Fig. 3 and data not shown). With the exception of Ccl28 and Tnfsf14, the responses were intact or even greater in the absence of MyD88 signaling. In contrast, infection with type II and III strains induced a weaker response. The divergent chemokine induction during infection with type I versus type II and III strains cannot be explained by differences in intracellular parasite numbers, because the initial infection rates were equivalent among the parasite strain types. Furthermore, strain differences in parasite replication rates do not account for distinct chemokine responses because cells were collected for analysis prior to the first round of parasite division.
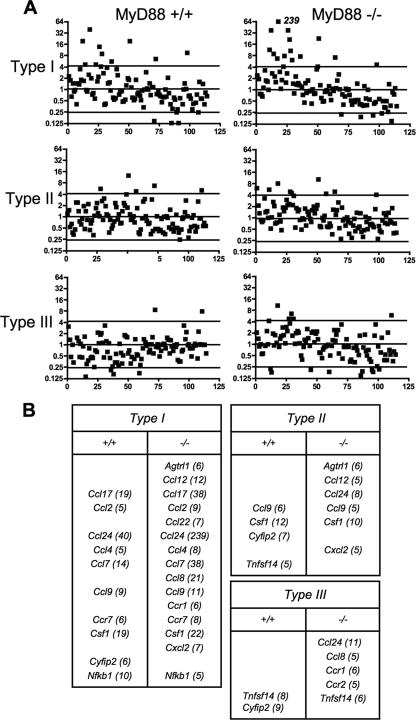
Chemokine responses 18 h after infection with Toxoplasma strain types I, II, and III were also examined (Fig. 4 and data not shown). At this time point, the RH strain induced a larger panel of CC-type chemokines. In common with the 6-h time point, these responses were equivalent or greater in MyD88−/− macrophages. Furthermore, some chemokines (e.g., CCL8) were upregulated during infection of knockout, but not wild-type, macrophages. The 18-h chemokine response during infection with the type II and type III strains remained highly restricted in wild-type macrophages. Nevertheless, MyD88 null cells appeared to be slightly more responsive. For example, Ccl24 was upregulated in MyD88−/− but not MyD88+/+ macrophages during infection with PTG and M7741. Although we detected low-level CCL17 protein production during M7741 infection (Fig. 1), this was not apparent in the chemokine array (Fig. 4). This may be because the low levels of CCL17 protein produced during type III infection may not be detectable in the gene arrays. Alternatively, the samples for the gene arrays were collected at a single time point (18 h), and it is possible that CCL17 gene transcription was switched off at this time. In contrast, the supernatants contained the total amount of chemokine that was produced over 18 h.
FIG. 4.
Chemokine response patterns during late T. gondii infection of wild-type and MyD88−/− macrophages. The experiment was set up as described in the legend to Fig. 1, with samples collected at 18 h postinfection.
In sum, the response to T. gondii is skewed towards CC-type chemokine induction, type I infection induces a stronger and broader response pattern than that for strain types II and III, and the majority of these response are independent of TLR-MyD88 signaling.
Gi-dependent PI 3-kinase signaling controls the CC chemokine response during type I infection.
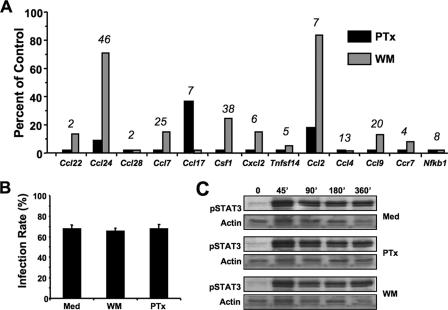
We next assessed the extent to which the RH-induced CC chemokine response was dependent upon PI 3-kinase signaling. Strikingly, preincubation of macrophages with wortmannin potently inhibited the ability of type I parasites to induce CC chemokines (Fig. 5A). The Gi protein-coupled receptor family is involved in the activation of PI 3-kinase signaling pathways. Therefore, we examined the effect of pertussis toxin, a bacterial compound that uncouples Gi protein-coupled receptor signaling, on RH-induced CC chemokine induction. Use of this inhibitor also had dramatic inhibitory effects on CC chemokine induction (Fig. 5A). Nevertheless, it is interesting that two chemokine genes, namely, Ccl24 and Ccl2, were less sensitive to PI 3-kinase inhibition, despite being blocked by pertussis toxin. We confirmed that neither pertussis toxin nor wortmannin blocked the ability of parasites to infect macrophages by assessing the percentage of parasite-positive cells at 6 h postinfection (Fig. 5B). In addition, neither inhibitor affected phosphorylation of STAT3 during tachyzoite infection (Fig. 5C), confirming that pertussis toxin and wortmannin do not cause generalized nonresponsiveness of cells at the concentrations employed in these experiments.
FIG. 5.
Inhibitory effects of blocking Gi protein and PI 3-kinase signaling on type I Toxoplasma infection. (A) Macrophages were preincubated for 2 h with pertussis toxin (PTx) or wortmannin (WM) and then infected with RH strain parasites at a 6:1 ratio of tachyzoites to cells. Samples were collected at 6 h postinfection for analysis. Values are expressed relative to responses in the absence of inhibitors. The x-fold increase of each gene in the absence of inhibitors is shown above the bars for each gene. (B) Infection rates of macrophages in the presence and absence (Med) of inhibitors. Cells were preincubated with 50 ng/ml each of wortmannin and pertussis toxin, and type I infection was carried out at a 6:1 ratio of parasites to cells. (C) Phosphorylation of STAT3 in the presence and absence of pertussis toxin and wortmannin. Cells were pretreated with inhibitors and infected with RH tachyzoites, and samples were collected for Western blot analysis at the indicated time points.
In vivo chemokine response during infection.
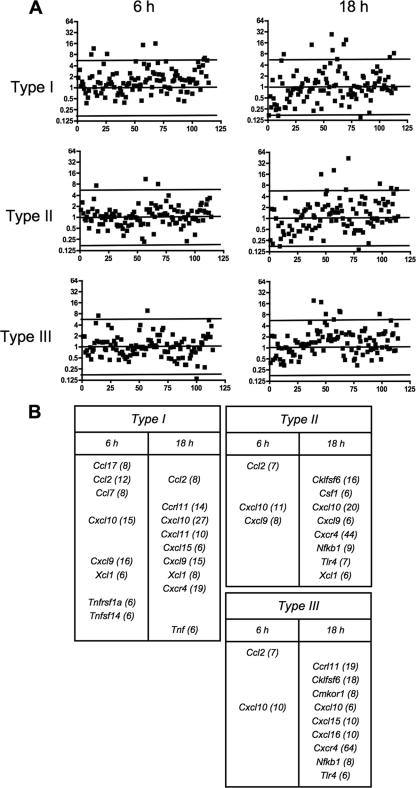
We next examined the chemokine responses to the three parasite strains in an in vivo model of infection consisting of tachyzoite inoculation into the mouse peritoneal cavity. As shown in Fig. 6, type I infection induced a relatively large panel of chemokines and chemokine-related genes at 6 h postinfection, including both CC and CXC types. In contrast, both type II and type III infections induced a highly restricted response at this time point. At 18 h postinoculation, the expression pattern during type II and type III infection diversified (Fig. 6 and data not shown). Several CXC-type chemokines were upregulated, a pattern that was evident for each of the three strain types.
FIG. 6.
Chemokine response patterns during in vivo infections with Toxoplasma strain types I, II, and III. Tachyzoites (106) were inoculated i.p. into mice (three per group), and peritoneal cells were collected at 6 and 18 h postinjection. RNAs were prepared and chemokine gene expression analyzed relative to that in cells from mice injected with PBS alone. (A) Patterns of chemokine, chemokine receptor, and related genes expressed during infection. (B) Identities of genes upregulated during infection. Numbers in parentheses indicated x-fold upregulation relative to the levels in noninfected control animals.
Therefore, although the in vivo response is complicated by the fact that multiple cell types can be expected to enter and exit the peritoneal cavity during infection with the different parasite strains, the responses were similar to the in vitro responses in that type I infection induced more vigorous chemokine production. However, the responses were dissimilar in that T. gondii elicited a CXC-type chemokine response at later time points in infection.
DISCUSSION
Toxoplasma strain type is emerging as an important determinant of the host immune response during infection with this parasitic pathogen (31, 44, 46, 48). In mouse infections, type I parasites rapidly induce death that appears to be the result of a proinflammatory cytokine shock response in combination with an overwhelming parasite burden (18, 39). In contrast, mouse infections with Toxoplasma strain types II and III result under most circumstances in parasite persistence that is associated with the formation of cysts in tissues of the skeletal muscles and central nervous system. During in vitro infection of macrophages, type II T. gondii elicits more robust IL-12 production than the relatively small amounts triggered by type I infection (44). This may be the result of preferential engagement of TLR/MyD88 signaling pathways by type II tachyzoites (31). A recent study also implicated type I ROP16 in strain-specific induction of STAT3/6 as a determinant of IL-12 induction (48). It is not clear why infection with RH results in small amounts of IL-12 during in vitro infection of macrophages but in high levels during in vivo infection. Possibly, during in vivo RH infection, much of the IL-12 derives from noninfected cells as a result of the massive proinflammatory response and accompanying tissue destruction. It is also possible that in other cell types, such as tissue dendritic cells, RH induces larger amounts of IL-12 than do type II strains.
Vigorous IL-12 production by type II T. gondii during early infection may control the parasite, resulting in host survival and the formation of cysts by parasites that escape the host immune response (44). However, the results of this study show that type I tachyzoites, unlike strain types II and III, elicit a vigorous CC chemokine response during early stages of infection both in vivo and in vitro. Therefore, it is possible that chemokine-dependent recruitment of innate immune effector cells during early infection may account for the proinflammatory effects of type I infection.
The chemokine responses we observed were dependent upon Gi protein-coupled receptor signaling during RH infection, inasmuch as they were extremely sensitive to pertussis toxin. We also recently found that protein kinase B activation in macrophages is dependent upon Gi protein signaling (32). Gi protein-dependent signaling is reported to contribute to dendritic cell IL-12 production during stimulation with soluble Toxoplasma lysate preparations, a response mediated through the chemokine receptor CCR5 (1). Nevertheless, at least for Toxoplasma-triggered CCL17, the chemokine receptor CCR5 is not involved. Chemokines are known to induce other chemokines (3). For example, CXCL8 can trigger CCL2. Therefore, it is possible that exposure of macrophages to high-virulence type I parasites triggers chemokine release, which, in turn, induces more chemokines.
The TLR/MyD88 signaling pathway is important in innate responses to infection. For Toxoplasma, TLRs 2, 9, and 11 have been implicated in recognition, although parasite ligands have so far only been identified for TLR11 (14, 19, 38, 41, 49, 60). Here we show that the macrophage chemokine response to type I T. gondii infection overall does not require MyD88 and is therefore likely to be independent of TLR recognition. In fact, several chemokine responses, most notably that of CCL24 during type I infection of macrophages, were greater in the absence of MyD88. The ability of MyD88 signaling to act as a molecular brake on a subset of MyD88-independent genes has been noted previously, although the underlying mechanics are unclear (4).
Regardless, MyD88-independent chemokine induction by Toxoplasma is similar to our previous data showing that low levels of macrophage IL-12 production during RH infection do not require MyD88 (31). However, unlike the chemokine response reported here, macrophage IL-12 production does not proceed through a Gi protein-coupled receptor-PI 3-kinase pathway. Recently, host cell Ca2+ and protein kinase C were shown to be important in the IL-12 response during Toxoplasma infection of macrophages, suggesting an alternate pathway to induction of this cytokine (37).
The chemokine family of proteins is comprised of up to 40 distinct molecules (45). The CXC chemokines generally target neutrophils and T lymphocytes. The CC chemokines, shown here to be induced preferentially during macrophage infection with Toxoplasma, mainly target monocytes, eosinophils, and basophils. A number of chemokines and their receptors have been shown to be involved in resistance to T. gondii. Mice deficient in CCR1, a receptor for CCL3 and several related chemokines, display abnormal neutrophil trafficking and increased susceptibility to infection (28). In mice lacking CCR2, a receptor for CCL2, CCL7, and CCL12, recruitment of a novel population of Gr-1+ monocytes to the site of infection fails to occur. This is associated with an inability to control parasite multiplication at the site of infection and with early host mortality (43). The CCR5 molecule, a receptor for several chemokines, including CCL3, CCL4, and CCL5, has been implicated in recognition of Toxoplasma cyclophilin-18 and contributes to dendritic cell IL-12 production (2). More recently, CCR5 was shown to play an important role in NK cell recruitment and resistance during T. gondii infection (29). The CXCR2 receptor is involved in recognition of CXCL8 and similar molecules and is involved in neutrophil recruitment to sites of infection (35). Mice lacking this receptor also have increased susceptibility to Toxoplasma, and this is associated with decreased Th1 responses and increased parasite numbers during chronic infection (13). The CXCL10 chemokine is required for resistance to T. gondii, and depletion of this molecule prevents T-cell recruitment and effector function at sites of infection (27). Collectively, these studies underscore the importance of chemokines and their receptors in orchestrating inductive and effector phases of immunity during infections with Toxoplasma and other microbial pathogens.
In this study, we found that strain RH triggers high levels of CCL17 independently of MyD88. We also observed a similar parasite strain-specific, MyD88-independent CCL22 response by ELISA (data not shown). These chemokines are the only known ligands for the receptor CCR4 (3). We do not presently know the biological role of CCR4 in the response to Toxoplasma. CCR4 and its chemokine ligands were originally implicated in promoting Th2 responses, such as allergic airway inflammation and granuloma formation (23, 25). Also, Th2 cells have been reported to express high levels of CCR4 (5). However, CCR4−/− mice have diminished proinflammatory responses and are resistant to the effects of LPS challenge (9). Furthermore, macrophages from CCR4−/− mice deviate towards an alternatively activated phenotype (42). Therefore, the role of CCR4 in the immune response is complex, and future work will be required to determine its function in immunity to Toxoplasma.
The findings reported here reveal that Toxoplasma strain type exerts a major influence on chemokine induction. Production of a diverse spectrum of chemokines by type I, but not type II or III, tachyzoites may account for the hyperinflammatory phenotype of T. gondii strains such as RH. More restricted chemokine induction induced by type II and III parasites may result in a weaker, nonpathological response that facilitates the establishment of persistent infection. Defining the molecular basis for these parasite strain-specific effects is an important area for investigation.
Acknowledgments
We thank S. Akira for permission to use MyD88−/− mice.
This work was supported by PHS grant AI50617.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnage, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 2.Aliberti, J., J. G. Valenzuela, V. B. Carruthers, S. Hieny, J. Andersen, H. Charest, C. Reis e Sousa, A. Fairlamb, J. M. Ribeiro, and A. Sher. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat. Immunol. 4:485-490. [DOI] [PubMed] [Google Scholar]
- 3.Allen, S. J., S. E. Crown, and T. M. Handel. 2007. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 25:787-820. [DOI] [PubMed] [Google Scholar]
- 4.Bjorkbacka, H., K. A. Fitzgerald, F. Huet, X. Li, J. A. Gregory, M. A. Lee, C. M. Ordija, N. E. Dowley, D. T. Golenbock, and M. W. Freeman. 2004. The induction of macrophage gene expression by LPS predominantly utilizes MyD88-independent signaling cascades. Physiol. Genomics 19:319-330. [DOI] [PubMed] [Google Scholar]
- 5.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boothroyd, J. C., and M. E. Grigg. 2002. Population biology of Toxoplasma gondii and its relevance to human infection: do different strains cause different disease? Curr. Opin. Microbiol. 5:438-442. [DOI] [PubMed] [Google Scholar]
- 7.Boyle, J. P., B. Rajasekar, J. P. Saeij, J. W. Ajioka, M. Berriman, I. Paulsen, D. S. Roos, L. D. Sibley, M. W. White, and J. C. Boothroyd. 2006. Just one cross appears capable of dramatically altering the population biology of a eukaryotic pathogen like Toxoplasma gondii. Proc. Natl. Acad. Sci. USA 103:10514-10519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carruthers, V., and J. C. Boothroyd. 2007. Pulling together: an integrated model of Toxoplasma cell invasion. Curr. Opin. Microbiol. 10:83-89. [DOI] [PubMed] [Google Scholar]
- 9.Chvatchko, Y., A. J. Hoogewerf, A. Meyer, S. Alouani, P. Juillard, R. Buser, F. Conquet, A. E. Proudfoot, T. N. Wells, and C. A. Power. 2000. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 191:1755-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courret, N., S. Darche, P. Sonigo, G. Milon, D. Buzoni-Gatel, and I. Tardieux. 2006. CD11c and CD11b expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 12.Darde, M. L., B. Bouteille, and M. Pestre-Alexandre. 1992. Isoenzyme analysis of 35 Toxoplasma gondii isolates and the biological and epidemiological implications. J. Parasitol. 78:786-794. [PubMed] [Google Scholar]
- 13.Del Rio, L., S. Bennouna, J. Salinas, and E. Y. Denkers. 2001. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167:6503-6509. [DOI] [PubMed] [Google Scholar]
- 14.Del Rio, L., B. A. Butcher, S. Bennouna, S. Hieny, A. Sher, and E. Y. Denkers. 2004. Toxoplasma gondii triggers MyD88-dependent and CCL2(MCP-1) responses using distinct parasite molecules and host receptors. J. Immunol. 172:6954-6960. [DOI] [PubMed] [Google Scholar]
- 15.Denkers, E. Y., and R. T. Gazzinelli. 1998. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin. Microbiol. Rev. 11:569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayer, R., J. P. Dubey, and D. S. Lindsay. 2004. Zoonotic protozoa: from land to sea. Trends Parasitol. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 17.Fuentes, I., J. M. Rubio, C. Ramirez, and J. Alvar. 2001. Genotypic characterization of Toxoplasma gondii strains associated with human toxoplasmosis in Spain: direct analysis from clinical samples. J. Clin. Microbiol. 39:1566-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavrilescu, L. C., and E. Y. Denkers. 2001. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J. Immunol. 167:902-909. [DOI] [PubMed] [Google Scholar]
- 19.Gazzinelli, R. T., and E. Y. Denkers. 2006. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat. Rev. Immunol. 6:895-906. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-γ, and TNF-α. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 21.Grigg, M. E., J. Ganatra, J. C. Boothroyd, and T. P. Margolis. 2001. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J. Infect. Dis. 184:633-639. [DOI] [PubMed] [Google Scholar]
- 22.Howe, D. K., and L. D. Sibley. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human diseases. J. Infect. Dis. 172:1561-1566. [DOI] [PubMed] [Google Scholar]
- 23.Jakubzick, C., H. Wen, A. Matsukawa, M. Keller, S. L. Kunkel, and C. M. Hogaboam. 2004. Role of CCR4 ligands, CCL17 and CCL22, during Schistosoma mansoni egg-induced pulmonary granuloma formation in mice. Am. J. Pathol. 165:1211-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joynson, D. H., and T. J. Wreghitt. 2001. Toxoplasmosis: a comprehensive clinical guide. Cambridge University Press, Cambridge, United Kingdom.
- 25.Kawasaki, S., H. Takizawa, H. Yoneyama, T. Nakayama, R. Fujisawa, M. Izumizaki, T. Imai, O. Yoshie, I. Homma, K. Yamamoto, and K. Matsushima. 2001. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 166:2055-2062. [DOI] [PubMed] [Google Scholar]
- 26.Khan, A., C. Su, M. German, G. A. Storch, D. B. Clifford, and L. D. Sibley. 2005. Genotyping of Toxoplasma gondii strains from immunocompromised patients reveals high prevalence of type I strains. J. Clin. Microbiol. 43:5881-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, I. A., J. A. MacLean, F. S. Lee, L. Casciotti, E. DeHaan, J. D. Schwartzman, and A. D. Luster. 2000. IP-10 is critical for effector T cell trafficking and host survival in Toxoplasma gondii infection. Immunity 12:483-494. [DOI] [PubMed] [Google Scholar]
- 28.Khan, I. A., P. M. Murphy, L. Casciotti, J. D. Schwartzman, J. Collins, J.-L. Gao, and G. R. Yeaman. 2001. Mice lacking the chemokine receptor CCR1 show increased susceptibility to Toxoplasma gondii infection. J. Immunol. 166:1930-1937. [DOI] [PubMed] [Google Scholar]
- 29.Khan, I. A., S. Y. Thomas, M. M. Moretto, F. S. Lee, S. A. Islam, C. Combe, J. D. Schwartzman, and A. D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, L., B. A. Butcher, and E. Y. Denkers. 2004. Toxoplasma gondii interferes with lipopolysaccharide-induced mitogen-activated protein kinase activation by mechanisms distinct from endotoxin tolerance. J. Immunol. 172:3003-3010. [DOI] [PubMed] [Google Scholar]
- 31.Kim, L., B. A. Butcher, C. W. Lee, S. Uematsu, S. Akira, and E. Y. Denkers. 2006. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J. Immunol. 177:2584-2591. [DOI] [PubMed] [Google Scholar]
- 32.Kim, L., and E. Y. Denkers. 2006. Toxoplasma gondii triggers Gi-dependent phosphatidylinositol 3-kinase signaling required for inhibition of host cell apoptosis. J. Cell Sci. 119:2119-2126. [DOI] [PubMed] [Google Scholar]
- 33.Lambert, H., N. Hitziger, I. Dellacasa, M. Svensson, and A. Barragan. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell. Microbiol. 8:1611-1623. [DOI] [PubMed] [Google Scholar]
- 34.Lee, C. W., S. Bennouna, and E. Y. Denkers. 2006. Screening for Toxoplasma gondii-regulated transcriptional responses in lipopolysaccharide-activated macrophages. Infect. Immun. 74:1916-1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, J., G. Cacalano, T. Camerato, K. Toy, M. W. Moore, and W. I. Wood. 1995. Chemokine binding and activities mediated by the mouse IL-8 receptor. J. Immunol. 155:2158-2164. [PubMed] [Google Scholar]
- 36.Lieberam, I., and I. Forster. 1999. The murine beta-chemokine TARC is expressed by subsets of dendritic cells and attracts primed CD4+ T cells. Eur. J. Immunol. 29:2684-2694. [DOI] [PubMed] [Google Scholar]
- 37.Masek, K. S., J. Fiore, M. Leitges, S. F. Yan, B. D. Freedman, and C. A. Hunter. 2006. Host cell Ca2+ and protein kinase C regulate innate recognition of Toxoplasma gondii. J. Cell Sci. 119:4565-4573. [DOI] [PubMed] [Google Scholar]
- 38.Minns, L. A., L. C. Menard, D. M. Foureau, S. Darche, C. Ronet, D. W. Mielcarz, D. Buzoni-Gatel, and L. H. Kasper. 2006. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J. Immunol. 176:7589-7597. [DOI] [PubMed] [Google Scholar]
- 39.Mordue, D. G., F. Monroy, M. La Regina, C. A. Dinarello, and L. D. Sibley. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J. Immunol. 167:4574-4584. [DOI] [PubMed] [Google Scholar]
- 40.Morisaki, J. H., J. E. Heuser, and L. D. Sibley. 1995. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell Sci. 108:2457-2464. [DOI] [PubMed] [Google Scholar]
- 41.Mun, H.-S., F. Aosai, K. Norose, M. Chen, L.-X. Piao, O. Takeuchi, S. Akira, H. Ishikura, and A. Yano. 2003. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Parasitol. 15:1081-1087. [DOI] [PubMed] [Google Scholar]
- 42.Ness, T. L., J. L. Ewing, C. M. Hogaboam, and S. L. Kunkel. 2006. CCR4 is a key modulator of innate immune responses. J. Immunol. 177:7531-7539. [DOI] [PubMed] [Google Scholar]
- 43.Robben, P. M., M. LaRegina, W. A. Kuziel, and L. D. Sibley. 2005. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. J. Exp. Med. 201:1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robben, P. M., D. G. Mordue, S. M. Truscott, K. Takeda, S. Akira, and L. D. Sibley. 2004. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 172:3686-3694. [DOI] [PubMed] [Google Scholar]
- 45.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18:217-242. [DOI] [PubMed] [Google Scholar]
- 46.Saeij, J. P., J. P. Boyle, and J. C. Boothroyd. 2005. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 21:476-481. [DOI] [PubMed] [Google Scholar]
- 47.Saeij, J. P., J. P. Boyle, S. Coller, S. Taylor, L. D. Sibley, E. T. Brooke-Powell, J. W. Ajioka, and J. C. Boothroyd. 2006. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314:1780-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saeij, J. P., S. Coller, J. P. Boyle, M. E. Jerome, M. W. White, and J. C. Boothroyd. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 50.Scharton-Kersten, T. M., T. A. Wynn, E. Y. Denkers, S. Bala, L. Showe, E. Grunvald, S. Hieny, R. T. Gazzinelli, and A. Sher. 1996. In the absence of endogenous IFN-γ mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J. Immunol. 157:4045-4054. [PubMed] [Google Scholar]
- 51.Sher, A., C. Collazzo, C. Scanga, D. Jankovic, G. Yap, and J. Aliberti. 2003. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunol. Res. 27:521-528. [DOI] [PubMed] [Google Scholar]
- 52.Sibley, L. D. 2003. Perfecting an intracellular life style. Traffic 4:581-586. [DOI] [PubMed] [Google Scholar]
- 53.Sibley, L. D., and J. C. Boothroyd. 1992. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature 359:82-85. [DOI] [PubMed] [Google Scholar]
- 54.Sibley, L. D., and D. K. Howe. 1996. Genetic basis of pathogenicity in toxoplasmosis. Curr. Top. Microbiol. Immunol. 216:4-15. [DOI] [PubMed] [Google Scholar]
- 55.Su, C., D. Evans, R. H. Cole, J. C. Kissinger, J. W. Ajioka, and L. D. Sibley. 2003. Recent expansion of Toxoplasma through enhanced oral transmission. Science 299:414-416. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, Y., A. Sher, G. Yap, D. Park, L. Ellis Neyer, O. Liesenfeld, M. Fort, H. Kang, and E. Gufwoli. 2000. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164:5375-5382. [DOI] [PubMed] [Google Scholar]
- 57.Taylor, S., A. Barragan, C. Su, B. Fux, S. J. Fentress, K. Tang, W. L. Beatty, H. E. Hajj, M. Jerome, M. S. Behnke, M. White, J. C. Wootton, and L. D. Sibley. 2006. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314:1776-1780. [DOI] [PubMed] [Google Scholar]
- 58.Wymann, M. P., G. Bulgarelli-Leva, M. J. Zvelebil, L. Pirola, B. Vanhaesebroack, M. D. Waterfield, and G. Panayotou. 1996. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 16:1722-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yap, G. S., M. H. Shaw, Y. Ling, and A. Sher. 2006. Genetic analysis of host resistance to intracellular pathogens: lessons from studies of Toxoplasma gondii infection. Microbes Infect. 8:1174-1178. [DOI] [PubMed] [Google Scholar]
- 60.Yarovinsky, F., D. Zhang, J. F. Anderson, G. L. Bannenberg, C. N. Serhan, M. S. Hayden, S. Hieny, F. S. Sutterwala, R. A. Flavell, S. Ghosh, and A. Sher. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626-1629. [DOI] [PubMed] [Google Scholar]