Abstract
To investigate the relevance of zinc in host-pathogen interactions, we have constructed Salmonella enterica mutant strains in which the znuA gene, which encodes the periplasmic component of the ZnuABC high-affinity Zn2+ transporter, was deleted. This mutation does not alter the ability of Salmonella to grow in rich media but drastically reduces its ability to multiply in media deprived of zinc. In agreement with this phenotype, ZnuA accumulates only in bacteria cultivated in environments poor in zinc. In spite of the nearly millimolar intracellular concentration of zinc, we have found that znuA is highly expressed in intracellular salmonellae recovered either from cultivated cells or from the spleens of infected mice. We have also observed that znuA mutants are impaired in their ability to grow in Caco-2 epithelial cells and that bacteria starved for zinc display decreased ability to multiply in phagocytes. A dramatic reduction in the pathogenicity of the znuA mutants was observed in Salmonella-susceptible (BALB/c) or Salmonella-resistant (DBA-2) mice infected intraperitoneally or orally. This study shows that the amount of free metals available for bacterial growth within the infected animal is limited, despite the apparent elevated concentration of free metals within cells and in plasma and suggests that Salmonella exploits the ZnuABC zinc transporter to maximize zinc availability in such conditions. These results shed new light on the complex functions of zinc in vertebrate and bacterial physiology and pave the way for a better comprehension of pathogenic mechanisms in Salmonella infections.
The ability of bacteria to colonize specific environments relies on their ability to obtain adequate supplies of the nutrients that are indispensable for their growth. Of particular relevance for human and animal health is to understand how bacterial pathogens face the problem of nutrient limitation in the infected host, an environment where several essential elements are not freely available for infectious microorganisms (44). Well-studied examples are the strategies adopted by pathogens to obtain iron within their host. In fact, iron availability in eukaryotes is strictly controlled by metal-binding proteins (i.e., ferritin, transferrin, and lactoferrin) which prevent its reactivity and limit the uptake ability by invasive microorganisms (40, 42, 43). Moreover, growth of intracellular bacteria is also controlled by specific pumps which remove iron from the bacterium-containing phagosomes (19, 48). As iron plays crucial catalytic roles in a large number of bacterial proteins, an adequate supply of this transition metal is necessary for bacterial survival and multiplication. Therefore, different pathogenic bacteria have evolved sophisticated strategies to acquire and utilize host iron, including the production of molecules (siderophores, hemophores, and membrane-associated pumps) characterized by an extraordinarily elevated iron affinity (40, 42, 43). The outcome of the competition for iron between the host cell and the microorganism is considered one of the most important factors determining the ability of pathogens to multiply and cause disease.
Although iron is traditionally considered the most important trace metal involved in host-pathogen interactions, other transition metals, such as zinc, manganese, and copper, are required by all living organisms. Some recent studies have suggested that the efficient uptake of these divalent metals could also play a critical role during infection and have a major role in virulence (23, 53). In particular, there is growing attention being paid to the relevance of the mechanisms of zinc uptake for the successful outcome of bacterial infections (23). Zinc plays essential catalytic and/or structural roles in enzymes of all six classes as well as in transcription and replication factors (11). The relevance of zinc for living systems is highlighted by a recent bioinformatics analysis which has suggested that about 10% of the human proteome might be constituted by zinc-binding proteins (1) and by its elevated intracellular concentration that, in bacteria, is close to 0.2 mM (37).
Several proteins involved in zinc homeostasis in gram-negative bacteria have been identified in recent years (5, 23). These studies have shown that under conditions of metal starvation, adequate zinc recruitment is ensured by the high-affinity Zn2+ uptake system encoded by the znuABC genes which were initially reported in Escherichia coli (38, 39). This system belongs to the family of ATP-binding cassette (ABC) transporters and is constituted by three proteins: ZnuA, ZnuB, and ZnuC. ZnuB is the membrane permease and ZnuC is the ATPase component of the transporter, whereas ZnuA is a soluble periplasmic metallochaperone which efficiently captures zinc in this cellular compartment (3, 8) and then delivers the metal to the transmembrane component of the transporter. Genetic and biochemical studies have shown that ZnuABC expression is repressed in cells containing adequate concentrations of zinc by the metallated form of Zur, a metalloregulatory DNA-binding protein with femtomolar sensitivity to free intracellular zinc (36, 37, 39). A few investigations carried out with different bacterial species have suggested that the integrity of the znuABC operon is essential to ensure the ability of different bacteria (Haemophilus influenzae, Haemophilus ducreyi, Brucella abortus, Pasteurella multocida, Neisseria gonorrhoeae, Salmonella enterica serovar Typhimurium) to grow in media devoid of zinc or within the infected host (7, 9, 22, 27, 28, 30, 33, 52). These observations suggest that the amount of metals available for bacterial growth within the infected animal is limited, analogous to what is known for iron. This hypothesis, however, is not yet proven and is in apparent contradiction with the elevated zinc concentration present in eukaryotic tissues.
To assess the role of the ZnuABC transporter in bacterial virulence, we have analyzed the expression of ZnuA and its contribution to virulence of Salmonella enterica serovar Typhimurium and serovar Enteritidis. Our results indicate that the ZnuABC transporter plays a very important role in the regulation of host-Salmonella interactions, due to the very poor zinc availability in intracellular environments.
MATERIALS AND METHODS
Media and reagents.
Salmonella enterica strains were cultured in Luria-Bertani (LB) broth (10 g Bacto tryptone per liter, 5 g yeast extract per liter, 10 g NaCl per liter) solidified by the addition of 1.5% (wt/vol) agar when needed. For growth under zinc limiting conditions, Vogel-Bonner minimal medium E (MM) was employed (0.04 g anhydrous MgSO4 per liter, 2 g citric acid per liter, 10 g anhydrous K2HPO4 per liter, 3.5 g NaNH4HPO4 · 4H2O per liter, 2 g glucose per liter). The following antibiotics and concentrations were used: kanamycin monosulfate, 50 mg/liter; sodium ampicillin, 50 mg/liter; and chloramphenicol, 30 mg/liter. Liquid cultures were grown in a Lab-Line Orbit Environ-Shaker, and growth was monitored by measuring the optical density at 600 nm with a Perkin-Elmer Lambda 9 spectrophotometer. N,N,N′,N′-Tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) was purchased from Sigma Aldrich, solubilized in dimethyl sulfoxide as a 5 mM stock solution, and stored at −20°C. 2,2′-Bipyridyl, EDTA, FeCl2, ZnSO4, CuSO4, and MgCl2 were purchased from BDH. Zinquin ethyl ester (Sigma Aldrich) was solubilized in dimethyl sulfoxide as a 5 mM stock solution and stored at −20°C in the dark. Restriction enzymes, DNA polymerase, and T4 DNA ligase were from New England Biolabs.
Bacterial strains, plasmids, and construction of mutants.
The bacterial strains used in this work are listed in Table 1. The Salmonella enterica serovar Typhimurium strain ATCC 14028 (18) and the S. enterica serovar Enteritidis strain LK5 (originally obtained from S. Maloy, San Diego State University, San Diego, CA) were a kind gift of Lionello Bossi (Centre de Génétique Moléculaire, CNRS, 91198 Gif-sur-Yvette, France).
TABLE 1.
Bacterial strains used in this work
| S. enterica strain | Relevant genotype | Source or reference |
|---|---|---|
| Serovar Typhimurium | ||
| ATCC 14028 | Wild type | L. Bossi |
| MA7223 | ilvI3305::Tn10dTac-cat- 43::3xFLAG-kan | 47 |
| MA7225 | ilvI3305::Tn10dTac-cat- 43::3xFLAG-kan sodCI::3xFLAG-kan | 47 |
| SA123 | znuA::kan | This work |
| SA150 | znuA::cam | This work |
| SA176 | znuA (scar) | This work |
| SA140 | ilvI3305::Tn10dTac-cat- 43::3xFLAG-kan znuA::3xFLAG-kan | This work |
| Serovar Enteritidis | ||
| LK5 | Wild type | L. Bossi |
| SA157 | znuA::kan | This work |
Deletion of znuA in S. enterica serovar Typhimurium strain ATCC 14028 was obtained by knocking out the gene using a PCR product by the method of Datsenko and Wanner (15). Briefly, a fragment containing the kanamycin resistance cassette was amplified from plasmid pKD4 using oligonucleotides oli119 and oli120 (the plasmids and oligonucleotides used are listed in Table 2) and electroporated in strain ATCC 14028 carrying pKD46. Recombinants were selected and tested for their inability to grow in a zinc-depleted medium (LB plates containing 1 mM EDTA). Insertion of the kanamycin resistance cassette in znuA was confirmed by PCR using oligonucleotides oli124 and K1. Phage P22 HT 105/1 int-201 was used to transduce the mutation into a clean background as previously described (46). The serovar Typhimurium znuA::kan strain was named SA123. The same procedure was employed to obtain a znuA::cam mutated allele, by using plasmid pKD3 instead of pKD4 for the amplification of the PCR fragment. After electroporation of the fragment into strain ATCC 14028(pKD46), recombinants were selected on LB plates containing chloramphenicol and checked by PCR using oligonucleotides oli124 and K3. The mutated allele was P22 transduced into a clean background, and the resulting strain was named SA150. The kanamycin or chloramphenicol resistance cassette inserted in the znuA gene was subsequently flipped out by introducing the pCP20 plasmid, which expresses the FLP recombinase (15), in strain SA123 or strain SA150.
TABLE 2.
Oligonucleotides and plasmids used in this work
| Oligonucleotide or plasmid | Sequence or description | Reference |
|---|---|---|
| Oligonucleotides | ||
| oli119 | GGAAGCCTTTATGGAGAAGTCGGTCAGGAATATCCCTGATTGTAGGCTGGAGCTGCTTCG | This work |
| oli120 | CGCGCTATCTCTGGGGAGAGCCAAAGATGCATGTTATATTCATATGAATATCCTCCTTAG | This work |
| oli124 | AAACCACGCGTACAAGCGTT | This work |
| K1 | CAGTCATAGCCGAATAGCCT | 47 |
| K3 | AGCTCACCGTCTTTCATTGC | 47 |
| SalznuAfor | ATAGAATTCCGGGGCTCAATTCAAG | This work |
| SalznuArev | TTTAAGCTTAATCTCCTTTCAGGCAGCT | This work |
| Plasmids | ||
| pKD46 | Lambda red functions | 15 |
| pKD4 | Kanamycin cassette template | 15 |
| pCP20 | FLP recombinase function | 15 |
| pSUB11 | 3xFLAG-kanamycin cassette template | 47 |
| pSEZnuAEc | Plasmid constitutively expressing E. coli ZnuA | 3 |
| pEMBL18 | Cloning vector | 16 |
| pPznuA | Plasmid expressing serovar Typhimurium ZnuA from its own promoter | This work |
A P22 lysate growth on strain SA123 was used to transduce the znuA::kan allele into S. enterica serovar Enteritidis strain LK5. Transductants were selected for kanamycin resistance and zinc requirement in growth media. The serovar Enteritidis znuA::kan strain was named SA157.
ZnuA epitope tagging was achieved by adding 3xFLAG sequence to the 3′ terminus of the znuA gene as described previously (46). A fragment containing the 3xFLAG epitope and a kanamycin resistance cassette was amplified using oligonucleotides oli113 and oli114 and plasmid pSUB11 as a template and electroporated in strain ATCC 14028(pKD46); transformants were selected, and the recombination was confirmed by PCR using oligonucleotides oli124 and K1. For an internal standard for Western blot analysis, the 3xFLAG epitope-tagged chloramphenicol acetyltransferase protein, inserted into the ilvIH operon and under the control of the constitutive Tac promoter, was transduced from strain MA7223 (47), obtaining strain SA140 (znuA::3xFLAG ilvI::Tn10dTac-cat::3xFLAG).
Cloning of S. enterica serovar Typhimurium znuA gene.
The znuA sequence was amplified by PCR from S. enterica serovar Typhimurium ATCC 14028 chromosome using oligonucleotides SalznuAfor and SalznuArev. The amplified DNA fragment contains 112 nucleotides upstream of the GTG start codon, thus including the promoter region, and 21 nucleotides downstream of the stop codon. The fragment obtained (about 1,100 bp) was digested with EcoRI and HindIII and cloned into vector pEMBL18, previously restricted with the same enzymes. The ligation mixture was used to transform commercial E. coli DH5α competent cells (Invitrogen), and transformants were selected on LB plates containing ampicillin. The znuA-containing plasmid (pPznuA) was then moved by electroporation into serovar Typhimurium znuA-deleted strains SA123 and SA150.
Cell cultures and in vitro infection studies.
Eukaryotic cell lines were cultured at 37°C in humidified air with 5% CO2. The murine macrophage-like cell lines J774 and TIB-63 were purchased from the American Type Culture Collection. The J774 cell line was maintained in Dulbecco's modified Eagle's medium (Sigma) containing 4.5 g glucose per liter, 2 mM l-glutamine, and 10% fetal calf serum; the TIB-63 cell was grown in RPMI 1640 medium (Sigma) containing 4.5 g glucose per liter, 2 mM l-glutamine, 1.5 g sodium bicarbonate per liter, 10 mM HEPES, 1 mM sodium pyruvate, and 10% fetal calf serum. The human monocyte line THP-1 was maintained in RPMI 1640 medium containing nonessential amino acids (Sigma), 1 mM sodium pyruvate, 2 mM l-glutamine, and 10% fetal calf serum and differentiated for 48 h by adding phorbol myristate acetate (Sigma) (20 ng/ml). The human colon epithelial cell line Caco-2 was grown in Dulbecco's modified Eagle's medium containing 1 g glucose per liter, nonessential amino acids, 4 mM l-glutamine, and 10% fetal calf serum.
In the intracellular survival experiments, approximately 105 cells/ml were infected with S. enterica serovar Typhimurium at a multiplicity of infection of 100:1 for 30 min, washed with phosphate-buffered saline (PBS), and supplemented with fresh medium containing gentamicin (100 μg/ml) in order to kill extracellular bacteria (17). At 1, 3, 24, and 48 h postinfection, cells were washed twice with PBS and lysed in a cold Triton X-100 solution (0.5% in PBS). Serial dilution of the cellular lysates were plated on LB agar to determine the number of viable intracellular bacteria by CFU counts. In each experiment, the intracellular survival assay was carried out three times, and the intracellular viability data reported are the averages of at least three independent experiments. For expression studies of epitope-tagged ZnuA, the cells were lysed 24 h postinfection, and the lysates were harvested and prepared for Western blot analysis.
Measurements of intracellular labile zinc.
Semiconfluent 25-cm2 flasks of Caco-2 (colonic epithelial cells) and differentiated THP-1 (human monocytes) were treated for 2 h at 37°C with 100 μM TPEN in order to deplete intracellular labile zinc or not treated with TPEN (24). The Zn-specific fluorophore Zinquin was added to each flask (25 μM for 30 min). Cells were washed extensively in PBS and lysed with PBS containing 0.5% Triton X-100; cellular debris was pelleted by centrifugation, and supernatants were collected for fluorometric analysis. Emission spectra of Zn(II)-Zinquin complexes were obtained with a Perkin-Elmer LS 50B spectrofluorometer (excitation wavelength of 364 nm). Fluorescence of the different samples was normalized according to the protein concentration determined by the method of Lowry et al. (32), using bovine serum albumin as a standard.
Western blot analysis.
To analyze the accumulation of ZnuA in bacteria cultivated in synthetic media, aliquots of bacterial cultures, corresponding to approximately 5 × 108 cells, were harvested, lysed by resuspending bacteria in sample buffer containing sodium dodecyl sulfate (SDS) and β-mercaptoethanol, and boiled for 5 min at 100°C. ZnuA accumulation in intracellular salmonellae was analyzed as described previously (47). Proteins were run on an SDS-polyacrylamide gel and blotted onto a nitrocellulose membrane (Hybond ECL; Amersham). The epitope-flagged proteins were revealed by incubation of the membrane with a suitable dilution of mouse anti-FLAG immunoglobulin G (Anti-FLAG M2; Sigma) as the primary antibody and anti-mouse horseradish peroxidase-conjugated immunoglobulin G (Bio-Rad) as the secondary antibody, followed by the enhanced chemiluminescence (ECL) reaction (Amersham).
Mouse infection studies.
In the survival studies, groups of at least five BALB/c (itys) or DBA-2 (ityr) mice were utilized. Aliquots of bacterial cultures grown overnight in LB medium were diluted in sterile PBS (for intraperitoneal infections) or 10% sodium bicarbonate (for oral infections) at the desired concentrations. In experiments involving intraperitoneal infection, animals were infected with doses ranging from 10 to 6,250 CFU/mouse, while the doses used in oral infections were between 105 and 109 CFU/mouse. Mouse mortality was monitored daily. Kaplan-Meier analysis, carried out by GraphPad Prism 4, was used to determine the statistical significance of differences in survival of mice. P values of <0.05 were considered significant.
For the analysis of epitope-tagged ZnuA accumulation from bacteria colonizing the spleens of mice, BALB/c mice were infected with 2,000 CFU/mouse and sacrificed when they were terminally ill. The spleens were removed and homogenized. The cellular extracts were prepared for SDS-polyacrylamide gel electrophoresis and Western blot analyses as previously described (47).
RESULTS
znuA is required for Salmonella enterica growth in poor zinc environments.
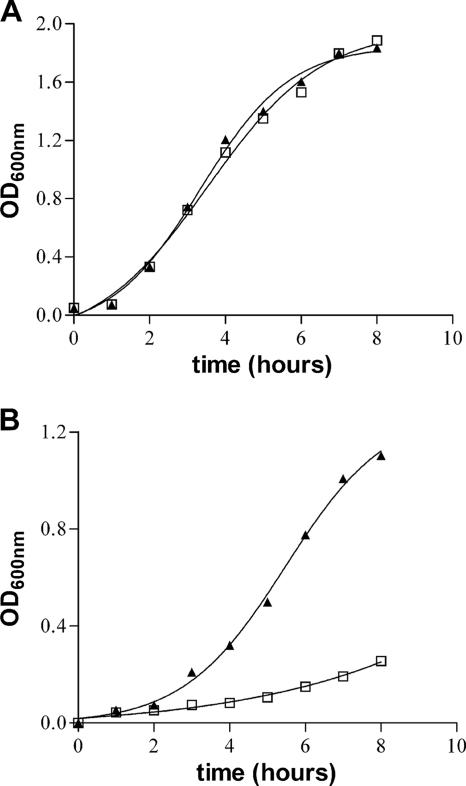
To investigate the role of the ZnuABC transporter in S. enterica, znuA deletion mutants of Salmonella serovar Typhimurium strain ATCC 14028 and serovar Enteritidis strain LK5 were produced as described in Materials and Methods. Figure 1 shows that the absence of the znuA gene does not alter the ability of the bacteria to grow in rich media (LB) but significantly affects serovar Typhimurium growth in a minimal medium (MM) where the zinc concentration is below the micromolar range (37). Similar results were obtained with serovar Enteritidis (data not shown). To analyze the role of ZnuABC for bacterial growth in metal-depleted media, we have also investigated the ability of Salmonella to grow in rich medium (LB) supplemented with the chelating agent EDTA. Table 3 shows that after a 24-h incubation at 37°C, the ability of the wild-type strains to form colonies on LB plates is not affected by the presence of up to 2 mM EDTA. In contrast, growth of znuA mutant strains SA123 and SA157 was heavily affected by the presence of this chelating agent. EDTA is a rather unspecific chelating agent, able to sequester zinc and several other divalent metals. Therefore, similar experiments were carried out in the presence of TPEN, a chelating agent with significant zinc specificity and low affinity for Mg2+ and Ca2+. Also, in the presence of TPEN, the growth of the znuA mutant strain proved to be severely impaired compared to the wild-type strain (Table 3). The significant ability of TPEN to permeate membranes likely explains its higher toxicity with respect to EDTA. The specificity of the observed phenotype is confirmed by the complementation with plasmid pPznuA, which produces serovar Typhimurium ZnuA under control of its own promoter, or with plasmid pSEZnuAEc, which constitutively produces E. coli ZnuA (3). However, it should be noted that, irrespective of the concentration of the chelating agent, growth of the complemented strains on LB plates was slower than that of wild-type Salmonella. As SDS-polyacrylamide gel electrophoresis analysis of Salmonella extracts revealed that ZnuA is the most abundant protein in bacteria containing pPznuA or pSEZnuAEc (data not shown), we believe that the growth disadvantage of the complemented strains should be attributed to toxic effects of protein overproduction. These results were confirmed using the SA150 and SA176 mutants instead of SA123 (data not shown), showing that the growth defect does not depend on the kanamycin cassette insertion into the chromosome or on serovar Enteritidis znuA mutant SA157 (data not shown), thus demonstrating that the observed phenotype is not specific to S. enterica serovar Typhimurium. Furthermore, the critical relevance of the ZnuABC transporter for bacterial growth under zinc limiting conditions was also outlined by an in vitro competition assay. The wild-type strain and znuA mutant strain (SA123) of serovar Typhimurium were grown separately and then mixed at an approximate 1/1 ratio before inoculation in LB medium or MM. After 24 h of growth at 37°C, bacteria were serially diluted and plated onto LB plates. The exact numbers of bacteria of each strain in the inoculum and in the cultures grown for 24 h were calculated by replica plating of cells on LB plates with or without kanamycin. The competitive index (CI) was calculated as follows: (percentage of strain A recovered/percentage of strain B recovered)/(percentage of strain A inoculated/percentage of strain B inoculated). The CI of each set of assays was analyzed statistically by using Student's t test. Three experiments were done for each strain on each medium. The median CI for bacteria grown on LB was 1.22, and the median CI for bacteria grown on MM was 0.031. The P values were 0.547 and 0.005 for bacteria grown on LB and bacteria grown on MM, respectively. The results indicate that there was no selective pressure in LB medium, whereas the wild-type strain outcompeted the znuA::kan mutant when bacteria were grown under zinc-limiting conditions (MM). All together, these results suggests that an adequate zinc supply is crucial for bacterial survival and multiplication in vitro and that a functional ZnuA protein is required for efficient zinc uptake in environments where this metal is scarcely available.
FIG. 1.
Growth curves of S. enterica serovar Typhimurium in synthetic media. Wild-type serovar Typhimurium (triangles) and znuA::kan SA123 mutant (squares) were grown in LB medium (A) and minimal medium (B). Overnight cultures of both strains grown in LB were diluted 1:500 in fresh LB medium or MM, and the optical density at 600 nm (OD600nm) was registered every hour during exponential growth.
TABLE 3.
Salmonella growth on LB plates
| Chelating agent and concn | Growth of serovar Typhimurium ATCC 14028 strainsa
|
||
|---|---|---|---|
| WT | SA123 | SA123(pPznuA) | |
| EDTA | |||
| 0 | + | + | + |
| 0.2 mM | + | +/− | + |
| 0.5 mM | + | − | + |
| 1 mM | + | − | + |
| 2 mM | + | − | + |
| TPEN | |||
| 0 | + | + | + |
| 0.01 mM | + | +/− | + |
| 0.02 mM | + | +/− | + |
| 0.08 mM | + | − | + |
| 0.15 mM | +/− | − | +/− |
Bacteria were grown overnight in LB medium and then streaked on LB plates containing the indicated amounts of EDTA or TPEN. WT, wild type. Symbols: +, growth; −, no growth; +/−, weak growth.
Expression of znuA is up-regulated when zinc is scarcely available.
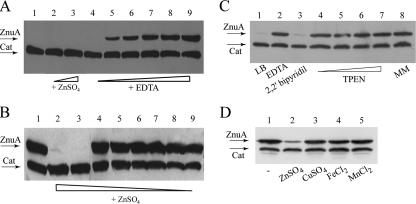
Zinc-dependent accumulation of ZnuA was monitored by introducing a 3xFLAG epitope-encoding tail at the 3′ end of the chromosomal copy of the znuA gene, thus obtaining production of a transporter tagged at its C terminus. The SA140 znuA epitope-tagged strain displayed a growth rate comparable to that of the wild type either in minimal medium or in LB supplemented with EDTA, thus indicating that the ZnuA-flagged protein is fully functional (data not shown). The concomitant presence of the constitutively transcribed epitope-tagged cat allele, moved by general transduction from strain MA7223 (47), allowed us to easily analyze znuA expression by Western blotting. Strain SA140 (ilvI3305::Tn10dTac-cat-43::3xFLAG-kan znuA::3xFLAG-kan) was grown in overnight in LB medium (Fig. 2A) and minimal medium (Fig. 2B) supplemented with increasing amounts of ZnSO4 or the divalent ion chelator EDTA. As shown in Fig. 2, ZnuA accumulation was negligible in the presence of ZnSO4 amounts greater than 0.1 μM, while znuA was induced in media containing a lower zinc concentration, achieved by growing the strain either in minimal medium or in LB medium supplemented with EDTA at concentrations ranging from 0.4 mM to 1.5 mM. These findings corroborate the hypothesis that ZnuA is necessary to optimize zinc uptake under metal shortage.
FIG. 2.
Zinc-dependent ZnuA accumulation in S. enterica serovar Typhimurium (SA140). (A) Bacteria were grown in LB medium (lane 1) supplemented with 5 μM (lane 2) and 10 μM (lane 3) ZnSO4 and in LB supplemented with EDTA at the following concentrations: 0.2 mM (lane 4), 0.4 mM (lane 5), 0.6 mM (lane 6), 0.8 μM (lane 7), 1 mM (lane 8), and 1.5 mM (lane 9). (B) Bacteria were grown in minimal medium (lane 1) or in minimal medium supplemented with various concentrations of ZnSO4. Minimal medium was supplemented with the following concentrations of ZnSO4: 1 μM (lane 2), 0.5 μM (lane 3), 0.1 μM (lane 4), 0.05 μM (lane 5), 0.01 μM (lane 6), 0.005 μM (lane 7), 0.001 μM (lane 8), and 0.0005 μM (lane 9). (C) Bacteria were grown in LB medium (lane 1) supplemented with 1 mM EDTA (lane 2), 0.2 mM 2,2′-bipyridyl (lane 3), and TPEN at the following concentrations: 0.05 mM (lane 4), 0.1 mM (lane 5), 0.15 mM (lane 6), and 0.2 mM (lane 7). The bacteria in lane 8 were grown in minimal medium. (D) Bacteria were grown in minimal medium. When the cultures reached an optical density at 600 nm of 0.5, the medium was supplemented with 3 μM ZnSO4 (lane 2), 3 μM CuSO4 (lane 3), 3 μM FeCl2 (lane 4), and 3 μM MnCl2 (lane 5), and bacteria were grown for 3 hours before harvesting. Lane 1 shows ZnuA accumulation in bacteria grown in standard minimal medium.
To further investigate ZnuA metal specificity, the SA140 strain was grown in the presence of different chelating agents. As shown in Fig. 2C, ZnuA accumulated at high levels in bacteria grown in LB medium containing EDTA or TPEN, but not in the presence of the iron chelator 2,2′-bipyridyl. The concentration of 2,2′-bipyridyl used in this experiment was sufficient to induce high-level accumulation of IroB and SodA, two well-characterized fur-dependent iron-responsive genes (4, 12; data not shown). Finally, the effect of different transition metals on ZnuA accumulation was analyzed in bacteria grown in minimal medium (Fig. 2D). ZnuA accumulation was drastically repressed by the addition of zinc to bacteria, whereas copper, iron, and manganese had no effect, thus demonstrating the high metal selectivity of ZnuA.
ZnuA contributes to Salmonella multiplication within eukaryotic cells and accumulates in intracellular environments.
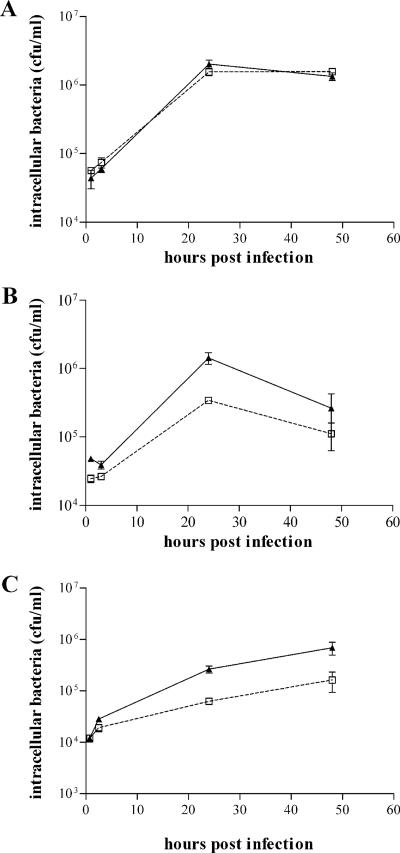
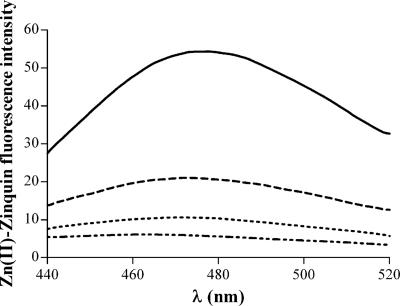
We have compared the invasiveness and intracellular survival of the wild type and znuA mutant of S. enterica serovar Typhimurium in different cell lines, including J774 and TIB-63 macrophages, differentiated THP-1 monocytes, and epithelial Caco-2 cells. Bacterial entry and multiplication in cultured macrophages or monocytes (J774, TIB-63, and THP-1) were only marginally affected by the lack of znuA (Fig. 3A and data not shown), in good agreement with a previous study carried out with a znuC mutant (7). However, the invasive efficiency and bacterial survival in the earliest hours postinfection were somewhat affected by precultivation of the strains in zinc-depleted medium (LB containing 1 mM EDTA), as shown in Fig. 3B. A slightly more consistent decrease in bacterial multiplication was observed in epithelial cells (Fig. 3C), even if both wild-type and znuA strains were grown in rich medium. These experimental results suggest that the intracellular pool of labile zinc could vary within different cell lines. To prove this hypothesis, THP-1 and Caco-2 cells were incubated with the zinc-specific fluorophore Zinquin, whose fluorescent signal greatly increases upon binding of zinc (24, 54). For a control, cells were incubated with TPEN before the addition of Zinquin to remove the labile intracellular concentration of zinc (24). Figure 4 shows that Zinquin fluorescence from either Caco-2 or THP-1 cells pretreated with TPEN was very low. Zinquin fluorescence from Caco-2 cells was modestly affected by preincubation with TPEN, suggesting that most of the intracellular zinc is stably bound by intracellular proteins and is not available for Salmonella growth. In contrast, Zinquin fluorescence from THP-1 cells was significantly higher than that obtained from cells incubated with TPEN, indicating that these cells contain significant amounts of labile zinc.
FIG. 3.
Intracellular growth of Salmonella strains. Intracellular growth of the znuA mutant strain SA123 (squares) compared to wild-type serovar Typhimurium (triangles) in differentiated THP-1 human monocytes (A and B) and Caco-2 colon epithelial cells (C). Prior to infection, bacteria were grown in LB medium (A and C) or in LB supplemented with 1mM EDTA (B). The reported CFU/ml values are the means ± standard deviations (error bars) of at least three independent experiments.
FIG. 4.
Zn(II)-Zinquin-dependent fluorescence of THP-1 and Caco-2 cells. Emission spectra of Zn(II)-Zinquin complexes from cellular lysates of differentiated THP-1 cells (solid black line), THP-1 cells precultivated with 100 μM TPEN (dashed line), Caco-2 cells (dotted line), and Caco-2 cells precultivated with 100 μM TPEN (dotted-dashed line). Zn(II)-Zinquin fluorescence intensity on the y axis is shown in arbitrary florescence units normalized to the protein concentration of the samples.
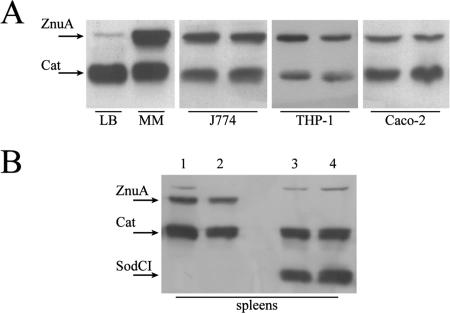
ZnuA accumulation has been analyzed in J774, THP-1, and Caco-2 cells in comparison with its accumulation in LB and minimal media (Fig. 5A). In all cell types, the enzyme is expressed at levels significantly higher than the level that accumulates in bacteria grown in LB medium. znuA expression was also analyzed in bacteria extracted from the spleens of mice infected with S. enterica serovar Typhimurium SA140 strain (Fig. 5B). Also, in this case, the periplasmic zinc transporter, as well as other virulence factors, such as the Cu,Zn superoxide dismutase SodCI, is clearly induced (46). Although the contribution of ZnuA to bacterial entry and multiplication within eukaryotic cells is not always straightforward, its accumulation in intracellular conditions in either in vitro or in vivo infections strongly suggests that intracellular environments have little available zinc.
FIG. 5.
ZnuA accumulation in intracellular salmonellae. (A) ZnuA accumulation in bacteria grown in rich medium (LB) and under zinc-limiting conditions (MM) compared to ZnuA accumulation in bacteria extracted from infected macrophages (J774), differentiated monocytes (THP-1), and colon epithelial cells (Caco-2). Each gel shows the results from two independent experiments. (B) Accumulation of ZnuA in bacteria harvested from spleen homogenates of BALB/c mice infected with strain SA140 (znuA::3xFLAG cat::3xFLAG) (lanes 1 and 2) or strain MA7225 (sodCI::3xFLAG cat::3xFLAG) (lanes 3 and 4). Each lane shows the epitope-flagged proteins recovered from a different mouse.
A functional ZnuABC transporter is essential for Salmonella pathogenicity.
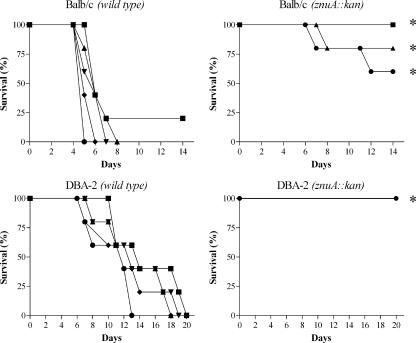
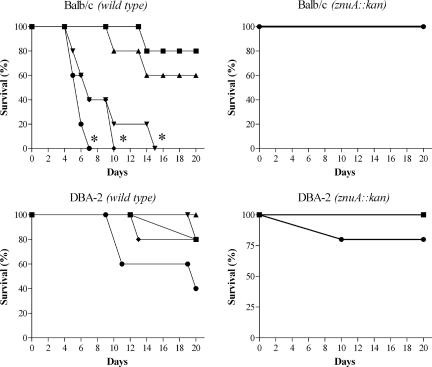
Even if the survival of a znuA mutant was only slightly affected compared to the wild-type strain in a cellular in vitro model, we decided to analyze whether the lack of a functional high-affinity zinc transporter could influence Salmonella pathogenicity in a mouse model. Since activation of different virulence factors, such as those encoded by Salmonella pathogenicity island 1 and 2 (SPI-1 and SPI-2), rely on different environmental conditions (25), oral or parenteral deliveries of wild-type S. enterica serovar Typhimurium and strain SA123 were performed either in susceptible (BALB/c) or resistant (DBA-2) mice. The survival of mice, monitored after infections, is depicted in Fig. 6 and 7. Infection of mice with wild-type serovar Typhimurium induced high mortality rates, irrespective of the delivery route or the genetic background of the recipient mice. In contrast, strain SA123 showed a marked degree of attenuation under all the condition tested. In particular, when SA123 was injected by the intraperitoneal route either in BALB/c or DBA-2 mice, the mortality curves at each dose were significantly different (P ≤ 0.01) from the mortality curves observed in mice injected with different doses of the wild-type strain. When strain SA123 was injected by the oral route in BALB/c mice, the mortality curves at the highest doses were significantly different (P ≤ 0.01) from the mortality curves observed in mice injected with similar doses of the wild-type strain. Finally, when strain SA123 was injected by the oral route in DBA-2 mice, the mortality curves at each dose were not significantly different (P > 0.05) from the mortality curves observed in mice injected with similar doses of the wild-type strain, even if the difference between the mortality rates of mice injected with the highest dose was somewhat significant. Similar results were obtained in experiments carried out with the SA150 or SA176 strain (data not shown), thus confirming that the introduction of a kanamycin resistance cassette within the znuA mutant strain does not contribute to the observed phenotype. Attempts to restore the virulence of the znuA mutant by complementation with multicopy plasmids expressing znuA were unsuccessful due to toxicity of the overproduced protein. In fact, the possibility that the lack of complementation could be due to plasmid loss was excluded by competition experiments carried out in BALB/c mice, as described by Uzzau et al. (46). When mice were intraperitoneally inoculated with a mixture of bacteria containing equal amounts of the SA150 and SA123 strains, no differences in spleen colonization between the two strains was observed in animals sacrificed 4 days after the infection. In contrast, when the experiment was carried out with a mixture of strain SA150 transformed with plasmid pPznuA and strain SA123, only kanamycin-resistant bacteria were identified in the spleens of infected mice (data not shown). We hypothesize that the high-level accumulation of ZnuA in cells containing multiple copies of the znuA gene could be due to titration of the intracellular pool of Zur, the transcriptional repressor of znuABC.
FIG. 6.
Survival of BALB/c and DBA-2 mice infected intraperitoneally with different doses of S. enterica serovar Typhimurium wild-type and SA123 strains. Infection doses were 10 CFU/mouse (▪), 50 CFU/mouse (▴), 250 CFU/mouse (▾), 1,250 CFU/mouse (♦), and 6,250 CFU/mouse (•). Asterisks indicate mortality curves showing significant differences between the wild-type and mutant strains.
FIG. 7.
Survival of BALB/c and DBA-2 mice infected orally with different doses of S. enterica serovar Typhimurium wild-type and SA123 strains. Infection doses were 105 CFU/mouse (▪), 106 CFU/mouse (▴), 107CFU/mouse (▾), 108 CFU/mouse (♦), and 109 CFU/mouse (•). Asterisks indicate mortality curves showing significant differences between the wild-type and mutant strains.
In order to verify whether the transporter contributes to the virulence of other Salmonella serotypes, serovar Enteritidis LK5 SA157 mutant strain was used in similar experiments. The degree of attenuation displayed by this mutant strain was comparable to that obtained with serovar Typhimurium SA123 strain (data not shown).
DISCUSSION
A few investigations carried out in recent years with different bacteria have suggested an important role for the ZnuABC Zn2+ uptake system for growth in environments poor in zinc and for virulence of gram-negative pathogens (7, 9, 22, 27, 28, 30, 33, 52). These studies, however, have not analyzed the molecular bases of the contribution of ZnuABC to bacterial virulence. To clarify the role of this high-affinity metal uptake system in bacterial physiology and of metal availability in host-parasite interactions, we have focused on the intracellular facultative pathogen S. enterica. Our results demonstrate that the growth of S. enterica serovar Typhimurium and serovar Enteritidis strains lacking the periplasmic component of this transporter is drastically impaired in media containing low levels of zinc or chelating agents able to sequester divalent ions. The observed effect of EDTA on the growth of Salmonella znuA mutants is evidently due to a lack of capability to recruit zinc, since introducing a plasmid expressing recombinant ZnuA (Table 3) or supplementing the medium with zinc (data not shown) restores microbial multiplication. Using a strain bearing an epitope-tagged copy of the chromosomal znuA gene, we have been able to monitor expression of the gene in bacteria cultivated in different environments. These experiments have shown that ZnuA is not expressed in a complex media, such as LB broth, where zinc concentration is elevated (37). In contrast, the epitope-tagged form of ZnuA accumulates under all the conditions in which the znuA mutants exhibit a growth defect, i.e., in minimal medium or in LB supplemented with EDTA. The ZnuA-tagged strain has also been used to analyze the intracellular expression of znuA in bacteria growing within cultured cells or within macrophages recovered from the spleens of infected mice. Interestingly, under all the intracellular conditions analyzed, ZnuA accumulated at levels that were comparable to those observed in bacteria cultured in media devoid of zinc. This result is not trivial, as zinc is very abundant within cells and different investigations have established that its intracellular concentration is close to the millimolar range in either bacterial or eukaryotic cells (37). The in vitro titration of ZnuA expression in minimal medium reported here (Fig. 2B) shows that gene transcription is induced only when bacteria are cultivated in the presence of zinc concentrations below 0.5 μM. Therefore, the observation that ZnuA accumulates at significant levels in intracellular salmonellae strongly suggests that within host cells zinc availability for microorganisms is much lower than that expected from the quantization of total zinc and that bacteria are starved for zinc, in spite of the very high total zinc concentration in eukaryotic cells (between 0.1 and 0.5 mM [6, 37]). This apparent contradiction may have different explanations. One possibility is that most of the zinc present inside the eukaryotic cells could be accumulated in vesicular sites (13), which are not accessible to bacteria. Alternatively, it is possible that nearly all the intracellular zinc is tightly bound to proteins and is not easily available to invading bacteria. This last hypothesis is appealing, and it is consistent with previous studies of the E. coli metalloproteins regulating the uptake and efflux of zinc (37). In fact, whereas the intracellular concentration of zinc in E. coli is in the millimolar range, Zur, the protein regulating the transcriptional activity of znuABC, and ZntR, the protein regulating the expression of the ZntA efflux pump, show femtomolar sensitivity to zinc, indicating that there is not a consistent pool of free zinc within the cell (37). Recent studies have also shown that ZntR and Zur are highly sensitive to extracellular zinc variations (51) and that the half-life of ZntR is enhanced when intracellular zinc levels are high (41), thus emphasizing the dynamic nature of intracellular zinc (10).
Interestingly, a well-known feature of the acute-phase response which follows infection by gram-negative bacteria or the administration of lipopolysaccharide is a complex body redistribution of zinc; the plasmatic zinc concentration rapidly decreases, in association with its accumulation in liver and, to a lesser extent, in other tissues (21). This response is mediated by a cytokine cascade involving tumor necrosis factor alpha, interleukin-1, and interleukin-6 and increased synthesis of acute-phase proteins, including metallothionein (13, 31). Different explanations have been suggested for the decreased serum zinc concentrations. Some authors have suggested that this is an adaptive response intended to deprive invading pathogens of zinc (29), but this hypothesis has not been firmly proved. As a matter of fact, the mean concentration of zinc in human plasma is <15 μmol/liter (14), which is about 50-fold lower than that present inside cells, but recent studies have established that about 70% of total plasma zinc is not stably bound to proteins (54). Such a labile pool of zinc could be easily available to infecting microorganisms, and it is likely that the decrease in serum or plasma zinc concentration is due to reduction of this labile pool of metal. Considering the importance of ZnuABC for the growth of Salmonella in zinc-depleted media, we have therefore analyzed the consequence of inactivation of this transporter on Salmonella virulence. We have found that the Salmonella strains lacking ZnuA display a dramatic reduction in pathogenicity in mice, independent of the route of infection (intraperitoneal or oral) and of the genetic background of the mice enrolled in the experiments (BALB/c and DBA-2).
One of the most important characterized differences between resistant and susceptible mice is that predicted protein sequence analysis of natural resistance-associated macrophage protein 1 (NRAMP1) between Ityr (DBA-2) and Itys (BALB/c) strains revealed a single mutation resulting in a glycine-to-aspartic acid substitution at position 169 (34), which results in a complete lack of function of NRAMP1 in susceptible mice (49). Since NRAMP1 is involved in the removal of divalent metal ions from bacterium-containing vacuoles (20, 35), our results suggest that NRAMP1 has a negligible role in removing zinc from such subcellular compartments. These results demonstrate that the ZnuABC transporter is absolutely required for full bacterial virulence, thus signifying that, in spite of the apparently high zinc concentration present in all parts of the body, bacteria rely on this high-affinity zinc uptake system to efficiently obtain zinc in the infected host.
Although S. enterica is able to replicate either outside and inside eukaryotic cells, during infection it mainly resides within the intracellular vacuoles of professional phagocytes. Nonetheless, transient extracytoplasmic phases likely occur during the dissemination and colonization within the infected host. Our results cannot discriminate between the relative importance of low zinc availability in extracellular environments (a consequence of the acute-phase response) and within eukaryotic cells (due to the extraordinary chelating ability of intracellular environments), but we suggest that both these phenomena contribute to the reduction in pathogenicity of znuA mutants. In fact, it is worth noting that inactivation of the ZnuABC transporter not only reduces pathogenicity of bona fide intracellular pathogens, such as S. enterica and B. abortus (7, 27, 28, 52), but also of H. ducreyi (30), which is not able to replicate and survive efficiently within phagocytes (2). Furthermore, in agreement with a previous study (7), we have observed that, despite the fact that ZnuA consistently accumulates in intracellular bacteria, the survival of S. enterica serovar Typhimurium within different phagocytic cell lines is not clearly affected by the absence of the znuA gene. This result is in good agreement with our observation that THP-1 cells contain significant amounts of labile zinc (Fig. 4). In contrast, we have found that the serovar Typhimurium znuA mutant strain is impaired in its ability to replicate in Caco-2 cells, where there is very little labile zinc. These data suggest that there is a good correlation between the ability of the znuA mutant strain to replicate in intracellular environments and the presence of a pool of labile zinc. Moreover, this observation suggests that depending on the activation of different SPI-1 and SPI-2 factors, the routes of cell invasion might be affected differently by zinc shortage. However, it should be noted that B. abortus znuA mutants were reported to be less able than the wild-type strain to grow within macrophages (27, 52). These contradictory results could be explained by differences in the zinc-sequestering ability of various phagocytic cells or by differences in intracellular trafficking between Salmonella and Brucella, which might replicate in vesicular systems characterized by dissimilar availability of labile zinc. However, when phagocytosis experiments were carried out with bacteria precultivated in zinc-depleted media, the znuA mutant strain displayed a significant reduction in intracellular growth compared to the wild-type strain. This observation suggests that bacteria precultivated in zinc-rich media might use a portion of their intracellular pool of zinc to support a short period of growth within eukaryotic cells but that this possibility is lost in bacteria starved for zinc. We hypothesize that the large reduction in pathogenicity observed in infected animals is the consequence of a prolonged zinc starvation within the host, which may be the consequence of zinc shortage in intracellular or extracellular environments. In support of this hypothesis, we have observed that significant numbers of salmonellae (although lower than those found in animals infected with the wild-type strains) can be recovered from the spleens of animals infected with znuA mutant strains but that the ability to multiply within the host sharply declines a few days after the infection (P. Pasquali, S. Ammendola, C. Pistoia, P. Petrucci, M. Tarantino, C. Valente, G. R. Tilio, and A. Battistoni, unpublished data).
Our investigation of the role of the ZnuABC transporter in S. enterica adds some new hints to understand the complex interplay between zinc availability, host immunity, and bacterial pathogenicity. Up to now, there has been some resistance in considering zinc a trace element able to limit bacterial growth within the host (6, 45, 50). This can be largely explained by the common knowledge that the zinc concentration in cellular compartments or in plasma is quite high. Moreover, it is well established that zinc deficiency is associated with depression of the innate immune system and enhanced susceptibility to a wide range of bacterial and viral agents (26, 45, 50), thus emphasizing the importance of zinc in host physiology. Our results, however, show that bacteria are starved for zinc when growing inside eukaryotic cells, despite the apparent abundance of this metal ion, and that bacteria possessing a functional ZnuABC Zn2+ uptake system clearly have an advantage over strains that lack znuA. These findings strongly suggest that there is stringent control of zinc availability in eukaryotic tissues which limits the ability of bacterial pathogens to multiply within the infected host.
In conclusion, our findings clearly indicate that the high-affinity transporter ZnuABC contributes to Salmonella enterica virulence and that this virulence factor exerts its prominent role in environments poor in zinc or in which zinc is not fully available, such as the intracellular compartment which appears to be characterized by a remarkable zinc sequestration ability.
Acknowledgments
This work was partially supported by a Murst-Cofin project to A.B. and by an intramural ISS research project to P. Pasquali (30F7).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 8 October 2007.
REFERENCES
- 1.Andreini, C., L. Banci, I. Bertini, and A. Rosato. 2006. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 5:196-201. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, M. E., M. P. Goheen, C. A. Townsend, and S. M. Spinola. 2001. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 69:2549-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berducci, G., A. P. Mazzetti, G. Rotilio, and A. Battistoni. 2004. Periplasmic competition for zinc uptake between the metallochaperone ZnuA and Cu,Zn superoxide dismutase. FEBS Lett. 569:289-292. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnason, J., C. M. Southward, and M. G. Surette. 2003. Genomic profiling of iron-responsive genes in Salmonella enterica serovar Typhimurium by high-throughput screening of a random promoter library. J. Bacteriol. 185:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blencowe, K., and P. Morby. 2003. Zn(II) metabolism in prokaryotes. FEMS Microbiol. Rev. 27:291-311. [DOI] [PubMed] [Google Scholar]
- 6.Brown, K. H. 1998. Effect of infections on plasma zinc concentration and implications for zinc status assessment in low-income countries. Am. J. Clin. Nutr. 68:425S-429S. [DOI] [PubMed] [Google Scholar]
- 7.Campoy, S., M. Jara, N. Busquets, A. M. Pérez de Rozas, I. Badiola, and J. Barbé. 2002. Role of the high-affinity zinc uptake znuABC system in Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 70:4721-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra, B. R., M. Yogavel, and A. Sharma. 2007. Structural analysis of ABC-family periplasmic zinc binding protein provides new insights into mechanism of ligand uptake and release. J. Mol. Biol. 367:970-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, C.-Y., A. Stephan, and C. Morse. 2001. Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol. Lett. 202:67-71. [DOI] [PubMed] [Google Scholar]
- 10.Chivers, P. T. 2007. A galvanizing story—protein stability and zinc homeostasis. J. Bacteriol. 189:2953-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman, J. E. 1998. Zinc enzymes. Curr. Opin. Chem. Biol. 2:222-234. [DOI] [PubMed] [Google Scholar]
- 12.Compan, I., and D. Touati. 1993. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J. Bacteriol. 175:1687-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousins, R. J., J. P. Liuzzi, and L. A. Lichten. 2006. Mammalian zinc transport, trafficking, and signals. J. Biol Chem. 281:24085-24089. [DOI] [PubMed] [Google Scholar]
- 14.Cousins, R. J. 1989. Systemic transport of zinc, p. 79-93. In C. F. Mills (ed.), Zinc in human biology. Springer-Verlag, London, England.
- 15.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dente, L., G. Cesareni, and R. Cortese. 1983. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 11:1645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 18.Fields, P. I., R. V. Swanson, C. G. Haidaris, and F. Heffron. 1986. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc. Natl. Acad. Sci. USA 83:5189-5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 20.Forbes, J. R., and P. Gros. 2003. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102:1884-1892. [DOI] [PubMed] [Google Scholar]
- 21.Gabay, C., and I. Kushner. 1999. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340:448-454. [DOI] [PubMed] [Google Scholar]
- 22.Garrido, M. E., M. Bosch, R. Medina, M. Llagostera, A. M. Pérez de Rozas, I. Badiola, and J. Barbe. 2003. The high-affinity zinc-uptake system znuABC is under control of the iron-uptake regulator (fur) gene in the animal pathogen Pasteurella multocida. FEMS Microbiol. Lett. 221:31-37. [DOI] [PubMed] [Google Scholar]
- 23.Hantke, K. 2005. Bacterial zinc uptake and regulators. Curr. Opin. Microbiol. 8:196-202. [DOI] [PubMed] [Google Scholar]
- 24.Ho, L. H., R. E. Ruffin, C. Murgia, L. Li, S. A. Krilis, and P. D. Zalewski. 2004. Labile zinc and zinc transporter ZnT4 in mast cell granules: role in regulation of caspase activation and NF-κB translocation. J. Immunol. 172:7750-7760. [DOI] [PubMed] [Google Scholar]
- 25.Hueffer, K., and J. E. Galán. 2004. Salmonella-induced macrophage death: multiple mechanisms, different outcomes. Cell. Microbiol. 6:1019-1025. [DOI] [PubMed] [Google Scholar]
- 26.Ibs, K. H., and L. Rink. 2003. Zinc-altered immune function. J. Nutr. 133:1452S-1456S. [DOI] [PubMed] [Google Scholar]
- 27.Kim, S., K. Watanabe, T. Shirahata, and M. Watarai. 2004. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J. Vet. Med. Sci. 66:1059-1063. [DOI] [PubMed] [Google Scholar]
- 28.Kim, S., M. Watarai, Y. Kondo, J. Erdenebaatar, S. Makino, and T. Shirahata. 2003. Isolation and characterization of mini-Tn5Km2 insertion mutants of Brucella abortus deficient in internalization and intracellular growth in HeLa cells. Infect. Immun. 71:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klasing, K. C. 1984. Effect of inflammatory agents and interleukin 1 on iron and zinc metabolism. Am. J. Physiol. 247:901-904. [DOI] [PubMed] [Google Scholar]
- 30.Lewis, D. A., J. Klesney-Tait, S. R. Lumbley, C. K. Ward, J. L. Latimer, C. A. Ison, and E. J. Hansen. 1999. Identification of the znuA-encoded periplasmic zinc transport protein of Haemophilus ducreyi. Infect. Immun. 67:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuzzi, J. P., L. A. Lichten, S. Rivera, R. K. Blanchard, T. B. Aydemir, M. D. Knutson, T. Ganz, and R. J. Cousins. 2005. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 102:6843-6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry, H. O., N. J. Rosebrough, L. A. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 33.Lu, D., B. Boyd, and C. A. Lingwood. 1997. Identification of the key protein for zinc uptake in Haemophilus influenzae. J. Biol. Chem. 272:29033-29038. [DOI] [PubMed] [Google Scholar]
- 34.Malo, D., K. Vogan, S. Vidal, J. Hu, M. Cellier, E. Schurr, A. Fuks, N. Bumstead, K. Morgan, and P. Gros. 1994. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites Genomics 23:51-61. [DOI] [PubMed] [Google Scholar]
- 35.Nevo, Y., and N. Nelson. 2006. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta 1763:609-620. [DOI] [PubMed] [Google Scholar]
- 36.Outten, C. E., D. A. Tobin, J. E. Penner-Hahn, and T. V. O'Halloran. 2001. Characterization of the metal receptor sites in Escherichia coli Zur, an ultrsensitive zinc(II) metalloregulatory protein. Biochemistry 40:10417-10423. [DOI] [PubMed] [Google Scholar]
- 37.Outten, C. E., and T. V. O'Halloran. 2001. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 292:2488-2492. [DOI] [PubMed] [Google Scholar]
- 38.Patzer, S., and K. Hantke. 1998. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol. Microbiol. 28:1199-1210. [DOI] [PubMed] [Google Scholar]
- 39.Patzer, S., and K. Hantke. 2000. The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J. Biol. Chem. 275:24321-24332. [DOI] [PubMed] [Google Scholar]
- 40.Payne, S. M. 1993. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1:66-69. [DOI] [PubMed] [Google Scholar]
- 41.Pruteanu, M., S. B. Neher, and T. A. Baker. 2007. Ligand-controlled proteolysis of the Escherichia coli transcriptional regulator ZntR. J. Bacteriol. 189:3017-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 43.Schaible, U. E., and S. H. Kaufmann. 2004. Iron and microbial infection. Nat. Rev. Microbiol. 2:946-953. [DOI] [PubMed] [Google Scholar]
- 44.Schaible, U. E., and S. H. Kaufmann. 2005. Nutritive view on the host-pathogen interplay. Trends Microbiol. 13:373-380. [DOI] [PubMed] [Google Scholar]
- 45.Shankar, A. H., and A. S. Prasad. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68:447S-463S. [DOI] [PubMed] [Google Scholar]
- 46.Uzzau, S., L. Bossi, and N. Figueroa-Bossi. 2002. Differential accumulation of Salmonella [Cu, Zn] superoxide dismutases SodCI and SodCII in intracellular bacteria: correlation with their relative contribution to pathogenicity. Mol. Microbiol. 46:147-156. [DOI] [PubMed] [Google Scholar]
- 47.Uzzau, S., N. Figueroa-Bossi, S. Rubino, and L. Bossi. 2001. Epitope tagging of chromosomal genes in Salmonella. Proc. Natl. Acad. Sci. USA 98:15264-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vidal, S., P. Gros, and E. Skamene. 1995. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J. Leukoc. Biol. 58:382-390. [DOI] [PubMed] [Google Scholar]
- 49.Vidal, S. M., E. Pinner, P. Lepage, S. Gauthier, and P. Gros. 1996. Natural resistance to intracellular infections: Nramp1 encodes a membrane phosphoglycoprotein absent in macrophages from susceptible (Nramp1 D169) mouse strains. J. Immunol. 157:3559-3568. [PubMed] [Google Scholar]
- 50.Walker, C. F., and R. E. Black. 2004. Zinc and the risk for infectious disease. Annu. Rev. Nutr. 24:255-275. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto, K., and A. Ishihama. 2005. Transcriptional response of Escherichia coli to external zinc. J. Bacteriol. 187:6333-6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, X., T. Becker, N. Walters, and D. W. Pascual. 2006. Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infect. Immun. 74:3874-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaharik, M. L., and B. B. Finlay. 2004. Mn2+ and bacterial pathogenesis. Front. Biosci. 9:1035-1042. [DOI] [PubMed] [Google Scholar]
- 54.Zalewski, P., A. Truong-Tran, S. Lincoln, D. Ward, A. Shankar, P. Coyle, L. Jayaram, A. Copley, D. Grosser, C. Murgia, C. Lang, and R. Ruffin. 2006. Use of a zinc fluorophore to measure labile pools of zinc in body fluids and cell-conditioned media. BioTechniques 40:509-520. [DOI] [PubMed] [Google Scholar]